Abstract
Twenty-four subjects with suspected ischaemic heart disease underwent a treadmill exercise stress test (TEST). Nine individuals developed ischaemia as defined by standard criteria. Total plasma antioxidant status (TPAS), and serum concentrations of vitamin E were measured pre-TEST, and 0, 1, 2, 4, 8 and 24 h following the treadmill test. Mean serum vitamin E concentrations fell by 33% in the group as a whole (from 9.53 ± 0.92 mg/L pre-TEST to 6.39 ± 1.06 mg/L immediately post stress test, P < 0.02) and rose to baseline over the subsequent 24 h. The levels of serum vitamin E fell by 34% in the group of patients who had a positive TEST, and 32% in those who did not develop ischaemia during the TEST. Serum cholesterol concentrations also fell significantly during the TEST. In the total group serum cholesterol fell by 6.5% (P = 0.0052), and in the subgroup who were positive for ischaemia the fall in serum cholesterol was 10.3% (P = 0.004). The reduction in serum cholesterol was 4.1% in the subgroup who did not develop ischaemia (P > 0.05). Mean total plasma antioxidant status showed no significant temporal change for the group as a whole, although there was a nonsignificant decrease immediately post‐TEST in the ischaemic group and a slight rise at 8 h in the group negative for ischaemia.
Keywords: total plasma antioxidant status, vitamin E, treadmill exercise test
Introduction
There is considerable evidence supporting a role for the antioxidant vitamins (e.g. vitamins E, C and β-carotene) in the maintenance of health and protection from cardiovascular disease (Halliwell & Gutteridge 1999). Antioxidants appear to be important physiological regulators of vascular function and long-term vitamin E intake has been related to a lowered incidence of coronary heart disease in men and women (Rimm et al. 1993; Stampfer et al. 1993). More recent studies have suggested that dietary supplementation with antioxidant vitamins for patients with established coronary heart disease may be beneficial (Stephens et al. 1996).
Pincemail et al. (1988) and Camus et al. (1990) have reported that tocopherol is mobilized from tissues, and plasma tocopherol levels rise in young, healthy male subjects during exercise. However there have been few studies examining the effects of acute exercise on plasma antioxidant status, particularly in those with circulatory disorders. Child et al. (1998) have reported that after exercise, levels of total antioxidant capacity and malondialdehyde rose in trained healthy male runners. Changes in antioxidant status and free radical release have also been reported in response to short-term maximal exercise, and appear to be different in trained vs. untrained individuals (Ortenblad et al. 1997; Ashton et al. 1998). Furthermore, treatment with antioxidant supplements reduced the release of muscle enzymes under exercise stress (Rokitzki et al. 1994). During exercise stress testing there is an increase in free radical production and oxidative stress (Fontana et al. 1995). Fontana et al. (1995) have also previously reported that plasma concentrations of coenzyme Q and the reduced form of glutathione fall following exercise testing in patients with ischaemic heart disease. This decrease could be attenuated by treatment with vitamin E. More recently Thomas et al. (1998) examined the effects of exercise on levels of vitamin E in healthy men and women, and in patients in cardiac rehabilitation. They reported that vitamin E concentrations were not affected by habitual levels of activity or by a single session of exercise in healthy subjects, whilst levels of vitamin E were normal or elevated in the patients undergoing cardiac rehabilitation.
As an alternative to the measurement of individual components in the assessment of antioxidant status, the total antioxidant capacity of plasma (TPAS) may be measured (Miller et al. 1993). Uric acid, bilirubin, albumin and the antioxidant vitamins are all known to contribute to varying degrees to the TPAS value (Gopinathan et al. 1994). Albumin may constitute up to 49% of total plasma antioxidant status (TPAS) (Gopinathan et al. 1994), dependent on methodology, and acts as an antioxidant by binding iron, bilirubin and lipids, including lipid hydroperoxide. It is an effective scavenger of free radicals (Emerson 1989) by virtue of its sulphydryl groups and peroxidase-like activity (Pirisino et al. 1988) although it requires the presence of other antioxidants to prevent peroxidative damage (Kouoh et al. 1999).
The treadmill exercise stress test has been used to improve the sensitivity of the ECG in the diagnosis of coronary ischaemia for the past 50 years (Selwyn & Fox 1983). The increased workload imposed by exercise increases cardiac output and oxygen consumption by the heart and skeletal muscle. It may therefore be associated with increased consumption of antioxidants. No studies to date have examined the effects of formal exercise stress testing on the antioxidant status of patients with suspected ischaemic heart disease.
In the current study serum vitamin E and TPAS have been measured sequentially to examine the effects of exercise stress testing on antioxidant status.
Materials and methods
Subjects
All subjects were recruited from the Prince Sultan Cardiac Centre, Riyadh, Kingdom of Saudi Arabia. Written consent was obtained from each subject, and ethical approval was granted by the Local Ethics Committee. The demographic data for the patients are summarized in Table 1. Subjects were on their usual diet and medication up until the night preceding the treadmill exercise stress test. They fasted from this time until after the completion of the exercise test. None were taking vitamin supplements.
Table 1.
Demographic and baseline biochemical data on patients undergoing a treadmill exercise stress test
| Total group | Positive for ischaemia | Negative for ischaemia | P-value# | |
|---|---|---|---|---|
| Number of subjects | 24 | 9 | 15 | |
| Female, n (%) | 2 (8) | 1 (11) | 1 (7) | ns |
| Mean age (year) | 53.1 ± 3.3 | 59.7 ± 3 | 46.6 ± 3.5 | 0.02 |
| Mean BMI (Kg/m2) | 27.3 ± 3.6 | 26.4 ± 3.4 | 28.1 ± 3.7 | ns |
| Hypertensive,n (%) | 8(33) | 4(44) | 4(27) | ns |
| Diabetic, n (%) | 12(50) | 7(78) | 5(33) | ns |
| Smoking, n (%) | 14(58) | 6(67) | 8(53) | ns |
| Previous MI, n (%) | 13(54) | 6(67) | 7(47) | ns |
| Lipid concentrations | ||||
| Total cholesterol (mmol/L) | 4.95 ± 0.8 | 4.8 ± 0.7 | 5.1 ± 0.9 | ns |
| HDL cholesterol (mmol/L) | 0.9 ± 0.3 | 0.9 ± 0.2 | 0.9 ± 0.3 | ns |
| LDL cholesterol (mmol/L) | 3.2 ± 0.7 | 3.1 ± 0.7 | 3.3 ± 0.7 | ns |
| Triglycerides (mmol/L) | 1.70 (0.71–3.03) | 1.43 (0.71–2.13) | 1.79 (0.74–3.03) | ns |
| Fasting glucose (mmol/L) | 7.4 ± 3.3 | 8.9 ± 4.9 | 5.8 ± 1.6 | 0.03 |
| Antioxidant concentrations | ||||
| Vitamin E (mg/L) | 9.5 ± 0.9 | 9.0 ± 0.8 | 10.0 ± 1.0 | ns |
| TPAS (mmol/L) | 1.30 ± 0.10 | 1.31 ± 0.13 | 1.29 ± 0.06 | ns |
for comparison between patients positive and negative for ischaemia using unpaired t-tests for normally distributed data, Mann–Whitney tests for non-normally distributed data, and chi-square tests for categorical data. Data represent mean ± SEM, or median and range for triglycerides.
Blood collection
Venous blood (approximately 10 mL) was collected from the antecubital fossa of each subject at each time point (pre-stress test), and within 5 min of completion of the exercise stress test (at 0 h), and 1, 2, 4, 8 and 24 h post-test), via an indwelling catheter. Aliquots were transferred to lithium-heparin, potassium EDTA, fluoride-oxalate and plain tubes. Plasma urea and electrolytes, glucose and full-blood count were measured immediately. Total cholesterol was also measured pre-TEST and at 0 h. Lithium-heparin samples for vitamin E and total plasma antioxidant status (TPAS) were centrifuged immediately at 10 000 g for 10 min at room temperature and stored at −70 °C until analysis.
Biochemical analyses
Serum lipids (total cholesterol, triglycerides and HDL cholesterol) and blood glucose were determined by routine enzymatic methods on a Hitachi 917 (Roche Diagnostics, Riyadh, Saudi Arabia). Total plasma antioxidant status was measured by a commercially available method (Randox Laboratories, County Antrim, UK) using a Cobas Bio analyser (Roche Diagnostics, Lewes, UK).
Serum vitamin E was determined by HPLC as previously described (Ferns et al. 2000a). Briefly, 200 µL internal standard (10 µg/mL d-tocopherol in isopropyl alcohol) was added to 200 µL serum and vortex mixed. Aqueous ammonium sulphate (3.9 m) was added (200 µL) and the solution was again vortex mixed. After centrifugation (1000 g for 5 min), 50 µL of supernatant was used for analysis using a Prodigy 5 µm ODS2 (50 × 4.6 mm) column (Phenomenex Ltd, Macclesfield, Cheshire, UK) with methanol as mobile phase, and detection at 294 nm. At a flow rate of 1.4 mL/min, the retention time for internal standard and vitamin E were 5.2, and 6.6 min, respectively. Vitamin E standard and quality control material were obtained from BioRad Laboratories Ltd, Hemel Hempstead, UK. Inter-assay precision data gave a coefficients of variation (CV) of 5.1% and 3.9% at α-tocopherol concentrations of 3.1 and 10.8 mg/L, respectively, for vitamin E.
Treadmill exercise stress test criteria for ischaemia
A standard Bruce treadmill exercise stress test protocol was used (Bruce & Hornsten 1969). The duration of exercise was for a maximum period of 15 min, but was terminated earlier if signs of ischaemia were evident. Ischaemia was diagnosed on the basis of development of one or more of the following:
ST segment depression of more than 2 mm
ST segment elevation
arterial blood pressure decrease of more than 15 mmHg
new bundle branch blocks (left or right)
prolonged chest pain
exercise-induced dysrhythmias, particularly ventricular tachycardia.
All patients positive by these criteria (n = 9) subsequently underwent angiography. Of these, eight showed evidence of coronary disease of at least one major coronary artery, and one was equivocal.
Statistical methods
Significance was assessed by Analysis of Variance (anova), paired or unpaired t-tests, and Mann–Whitney or Chi-square tests, as appropriate, using an Instat statistical package (Instat Corporation, USA).
Results
Demographic and biochemical data
Of the 24 subjects referred for treadmill exercise stress test, eight were hypertensive, and 12 were diabetic (of whom six were insulin dependent). Nine of the patients were positive and 15 were negative for ischaemia on stress testing. Table 1 summarizes the demographic and baseline biochemical data. Urea and electrolyte results were in the normal range for all subjects. Baseline concentrations of plasma vitamin E were approximately 25% lower than for a group of Caucasian subjects with chest pain, and of comparable age (Ferns et al. 2000b), however, a greater proportion of subjects in the present study were current smokers, or diabetic.
Effects of the treadmill exercise stress test on TPAS, vitamin E, cholesterol and cholesterol-corrected vitamin E
Serum markers of antioxidant status measured in pre-TEST and at 0, 1, 2, 4, 8 and 24 h post-TEST demonstrated the following:
TPAS showed no significant change in the total study group (1.30 ± 0.10 mmol/L pre-TEST to 1.29 ± 0.06 mmol/L post-TEST). There was a small nonsignificant decrease in the subgroup positive for coronary ischaemia (1.31 ± 0.13 pre-TEST to 1.28 ± 0.10 post-TEST), and a slight increase at 8 h in the group negative for coronary ischaemia (1.29 ± 0.06 pre-TEST to 1.42 ± 0.06 8 h post-TEST, P < 0.06).
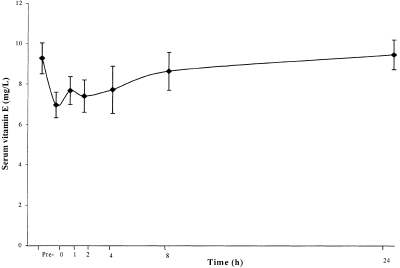
The mean serum vitamin E level fell in the total group by 33% (P < 0.02) immediately after the TEST (Figure 1). The mean decrease was 34% in the group positive for coronary ischaemia (P < 0.06) and 32% in the group negative for coronary ischaemia (P < 0.08). The difference between the groups was only marginally significant (P < 0.06), however, the power of the present study was inadequate to examine differences between subgroups.
Figure 1.
Changes in mean serum vitamin E concentration during a treadmill exercise stress test in 24 subjects with suspected coronary ischaemia.
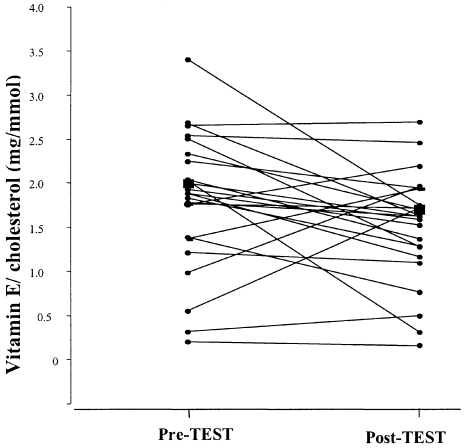
The mean total cholesterol decreased by 6.5%, from 4.6 ± 0.13 pre-TEST to 4.3 ± 0.14 mmol/L immediately post-TEST, in the group as a whole (P = 0.0052). The mean serum cholesterol fell from 4.5 ± 0.14–4.0 ± 0.18 mmol/L (10.3%) in the group positive for coronary ischaemia (P < 0.004), whilst the change observed in the group negative for coronary ischaemia was from 4.6 ± 0.19–4.4 ± 0.19 mmol/L (4.1%). The latter change was not significant (P > 0.05) (Figure 2).
Figure 2.
Changes in serum cholesterol-standarized vitamin E concentrations for 24 individuals pre- and post-treadmill exercise stress test. The large symbol (▪) represents the mean value for the whole group.
When vitamin E was standardized for cholesterol, a decrease was still observed in 66% of the patients in the ischaemic group and 73% of the nonischaemic group, however, the decrease after the TEST for the group as a whole was no longer statistically significant (P < 0.06) (Figure 2).
Discussion
Free radicals are potentially harmful molecules that are usually rapidly removed by cellular antioxidant scavenging enzymes systems, such as superoxide dismutase (SOD), catalase, glutathione peroxidase, and methionine sulphoxide reductase. Free radicals that reach the circulation are also neutralized by extracellular antioxidants (McColl et al. 1998) such as vitamins A, E, and C, bilirubin, uric acid, and albumin. All these species contribute to the TPAS (Gopinathan et al. 1994).
In our study, TPAS showed no statistically significant changes for the group as a whole following the TEST. The small, nonsignificant decrease observed in the group positive for coronary ischaemia suggests that the amount of free radicals produced during exercise-induced ischaemia was too small to produce a significant change in the total antioxidative capacity of the plasma. However, changes in specific components of the TPAS cannot be excluded and it is possible that free radical formation was matched by a rise in antioxidant mobilization from tissues and entering the plasma, as proposed by Camus et al. (1990). For the group that was negative for coronary ischaemia, the marginal increase in TPAS occurring 8 h post‐TEST (P < 0.06), is difficult to explain. Nevertheless, McColl et al. demonstrated a similar increase in TPAS following coronary artery bypass graft (CABG), which they attributed to regeneration or redistribution of antioxidants (McColl et al. 1998).
The decreased serum concentration of vitamin E immediately post-TEST in the group as a whole and in the subgroup positive for coronary ischaemia suggests that vitamin E may have an important first line antioxidant role in protection against free-radical stress associated with exercise. Surmen-Gur et al. (1999) have similarly reported an acute fall in serum vitamin E concentrations following exhaustive exercise in smokers, although they made no correction for lipid concentrations. Vitamin E is lipid soluble and is therefore intimately associated with lipoprotein moieties in plasma. We found that serum cholesterol fell significantly following TEST in the group as a whole. Another recent study has also demonstrated that plasma-volume adjusted total cholesterol concentrations fall acutely following aerobic exercise, and similarly found that they rise to baseline by 24 h (Grandjean et al. 2000). However, the acute effects of exercise on serum cholesterol appear to vary, dependent on the level of fitness of the group under study and the degree of exercise they undertake (Davis et al. 1992; Krum et al. 2000). Standardization of serum vitamin E for serum cholesterol would be expected to correct for changes associated with lipoprotein redistribution. However the percentage change in serum cholesterol concentration occurring post-TEST was approximately 6.5%, whereas vitamin E concentrations fell by 33%. These data therefore suggest that the rapid fall in serum vitamin E is due in part to its utilization as a free radical scavenger. For the group negative for coronary ischaemia, vitamin E showed a smaller change during the time course, suggesting that in patients without demonstrable coronary ischaemia, the production of free radicals following the TEST is insufficient to cause a significant change in steady state serum concentrations of vitamin E and other antioxidants, although there may be an increase in vitamin E recycling. The return of serum vitamin E concentration to the baseline value within 24 h may also be due to the regeneration or redistribution of vitamin E from tissues. Our findings contrast with those of Pincemail et al. (1988) and Camus et al. (1990), who have reported significant increases in plasma tocopherol following exhaustive exercise. However these studies differed from ours in a number of important respects. They were undertaken in young, healthy, presumably Caucasian subjects, who were exercised maximally for 15 min. The mean age of our subjects was substantially older. A large proportion of this group had established coronary artery disease and hence it was not possible to maximally exercise these individuals.
In conclusion, this study has demonstrated changes in antioxidant status induced by treadmill exercise stress test in middle-aged Saudi Arabian subjects with chest pain and suspected coronary artery disease. The changes appear to be related, in part, to lipoprotein redistribution and also to the consumption of antioxidants by the free radicals generated during exercise. Our inability to demonstrate a similar change in TPAS probably relates to the fact that the contribution of vitamin E to TPAS is small, so that any change in this constituent would be masked by the lack of significant change in other lipophilic and hydrophilic constituents.
References
- Ashton T, Rowlands CC, Jones E, et al. Electron spin resonance detection of oxygen-centred radicals in human serum following exhaustive exercise. Eur J. Appl. Physiol. Occup Physiol. 1998;77:498–502. doi: 10.1007/s004210050366. [DOI] [PubMed] [Google Scholar]
- Bruce RA, Hornsten TR. Exercise stress testing in evaluation of patients with ischaemic heart disease. Prog. Cardiovasc Dis. 1969;11:371–390. doi: 10.1016/0033-0620(69)90027-9. [DOI] [PubMed] [Google Scholar]
- Camus G, Pincemail J, Roesgen A, Dreezen E, Sluse FE, Deby C. Tocopherol mobilization during dynamic exercise after beta-adrenergic blockade. Arch. Internatl. Physiol. Biochim. Biophys. 1990;98:121–126. doi: 10.3109/13813459009115745. [DOI] [PubMed] [Google Scholar]
- Child RB, Wilkinson DM, Fallowfield JL, Donnelly AE. Elevated serum antioxidant capacity and plasma malondialdehyde concentration in response to a simulated half-marathon run. Med. Sci. Sport Exercise. 1998;30:1603–1607. doi: 10.1097/00005768-199811000-00008. [DOI] [PubMed] [Google Scholar]
- Davis PG, Bartoli WP, Durstine JL. Effects of acute exercise intensity on plasma lipids and apolipoproteins in trained runners. J. Appl. Physiol. 1992;72:914–919. doi: 10.1152/jappl.1992.72.3.914. [DOI] [PubMed] [Google Scholar]
- Emerson TE., Jr Unique features of albumin: a brief review. Crit. Care Med. 1989;17:690–694. doi: 10.1097/00003246-198907000-00020. [DOI] [PubMed] [Google Scholar]
- Ferns G, Williams J, Forster L, Tull S, Starkey B, Gershlick A. Cholesterol-standardized plasma vitamin E levels are reduced in patients with severe angina pectoris. Int. J. Exp. Path. 2000a;81:57–62. doi: 10.1111/j.1365-2613.2000.00141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferns G, Williams J, Forster L, Tull S, Starkey B, Gershlick A. Effects of vitamin E supplementation on circulating adhesion molecules pre- and post-coronary angioplasty. Ann. Clin. Biochem. 2000b;37:649–654. doi: 10.1258/0004563001899924. [DOI] [PubMed] [Google Scholar]
- Fontana L, Rossi MA, Baccelliere L, Papagna D, Cottalasso D. CoQ10 blood levels and erythrocyte concentrations of GSH in ischaemic heart patients during exercise test. Minerva Cardioang. 1995;43:39–46. [PubMed] [Google Scholar]
- Gopinathan V, Miller NJ, Milner AD, Rice-Evans CA. Bilirubin and ascorbate antioxidant activity in neonatal plasma. FEBS Lett. 1994;349:197–200. doi: 10.1016/0014-5793(94)00666-0. [DOI] [PubMed] [Google Scholar]
- Grandjean PW, Crouse SF, Rohack JJ. Influence of cholesterol status on blood lipid and lipoprotein enzyme responses to aerobic exercise. J. Appl. Physiol. 2000;89:472–480. doi: 10.1152/jappl.2000.89.2.472. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Antioxidant protection by low-molecular-mass agents: compounds derived from the diet. 3. Oxford: Oxford University Press; 1999. Free Radicals in Biology and Medicine; pp. 200–245. [Google Scholar]
- Kouoh F, Gressier B, Luyckx M, et al. Antioxidant properties of albumin: effect on oxidative metabolism of human neutrophil granulocytes. Farmaco. 1999;54:695–699. doi: 10.1016/s0014-827x(99)00082-8. 10.1016/s0014-827x(99)00082-8. [DOI] [PubMed] [Google Scholar]
- Krum H, Conway EL, Howes LG. Acute effects of exercise on plasma lipids, noradrenaline levels and Plasma Volume. Clin. Exp Pharmacol. Physiol. 2000;18:697–701. doi: 10.1111/j.1440-1681.1991.tb01383.x. [DOI] [PubMed] [Google Scholar]
- McColl AJ, Keeble T, Hadjinikolaou L, et al. Plasma antioxidants: evidence for a protective role against reactive oxygen species following cardiac surgery. Ann. Clin. Biochem. 1998;35:616–623. doi: 10.1177/000456329803500504. [DOI] [PubMed] [Google Scholar]
- Miller NJ, Rice-Evans C, Davies MJ, Gopinathan V, Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993;84:407–412. doi: 10.1042/cs0840407. [DOI] [PubMed] [Google Scholar]
- Ortenblad N, Madsen K, Djurhuus MS. Antioxidant status and lipid peroxidation after short-term maximal exercise in trained and untrained humans. Am. J. Physiol. 1997;272:1258–1263. doi: 10.1152/ajpregu.1997.272.4.R1258. [DOI] [PubMed] [Google Scholar]
- Pirisino R, Di Simplicio P, Ignesti G, Bianchi G, Barbera P. Sulfhydryl groups and peroxidase-like activity of albumin as scavenger of organic peroxides. Pharmacol. Res. Commun. 1988;20:545–552. doi: 10.1016/s0031-6989(88)80081-x. [DOI] [PubMed] [Google Scholar]
- Pincemail J, Camus G, Roesgen A, Dreezen E, Sluse F, Deby C. Tocopherol mobilization during heavy exercise does not depend on lipolysis. Pflugers Arch Europ. J. Physiol. 1988;412:S51–S51. [Google Scholar]
- Rimm EB, Stampfer MJ, Ascherio A, Giovannucci E, Colditz GA, Willett WC. Vitamin E consumption and the risk of coronary heart disease in men. N. Eng. J. Med. 1993;328:1450–1456. doi: 10.1056/NEJM199305203282004. [DOI] [PubMed] [Google Scholar]
- Rokitzki L, Logemann E, Sagredos AN, Murphy M, Wetzel-Roth W, Keul J. Lipid peroxidation and antioxidative vitamins under extreme endurance stress. Acta Physiol. Scand. 1994;151:149–158. doi: 10.1111/j.1748-1716.1994.tb09732.x. [DOI] [PubMed] [Google Scholar]
- Selwyn AP, Fox KM. Exercise Electrocardiographs for the detection of myocardial ischaemia: Progress using precordial mapping. In: Sleight P, Vann Jones J, editors. Scientific Foundations of Cardiology. London: Heinmann; 1983. pp. 501–506. [Google Scholar]
- Stampfer MJ, Hennekens CH, Manson JE, Colditz GA, Rosner B, Willett WC. Vitamin E consumption and the risk of coronary disease in women. N. Eng. J. Med. 1993;328:1444–1449. doi: 10.1056/NEJM199305203282003. [DOI] [PubMed] [Google Scholar]
- Stephens NG, Parsons A, Schofield PM, et al. Randomised control trial of vitamin E in patients with coronary disease. Cambridge Heart Antioxidant Study (CHAOS) Lancet. 1996;347:781–786. doi: 10.1016/s0140-6736(96)90866-1. [DOI] [PubMed] [Google Scholar]
- Surmen-Gur E, Ozturk E, Gur H, Punduk Z, Tuncel P. Effect of vitamin E supplementation on post-exercise plasma lipid peroxidation and blood antioxidant status in smokers: special reference to haemoconcentration effect. Eur. J. Appl. Physiol. 1999;79:472–478. doi: 10.1007/s004210050539. 10.1007/s004210050539. [DOI] [PubMed] [Google Scholar]
- Thomas TR, Ziogas G, Yan P, Schmitz D, Lafontaine T. Influence of activity level on vitamin E status in healthy men and women and cardiac patients. J. Cardiopulm. Rehab. 1998;18:52–59. doi: 10.1097/00008483-199801000-00007. [DOI] [PubMed] [Google Scholar]