Abstract
While the origin of tumours, whether from one cell or many, has been a source of fascination for experimental oncologists for some time, in recent years there has been a veritable explosion of information about the clonal architecture of tumours and their antecedents, stimulated, in the main, by the ready accessibility of new molecular techniques. While most of these new results have apparently confirmed the monoclonal origin of human epithelial (and other) tumours, there are a significant number of studies in which this conclusion just cannot be made. Moreover, analysis of many articles show that the potential impact of such considerations as patch size and clonal evolution on determinations of clonality have largely been ignored, with the result that a number of these studies are confounded. However, the clonal architecture of preneoplastic lesions provide some interesting insights — many lesions which might have been hitherto regarded as hyperplasias are apparently clonal in derivation. If this is indeed true, it calls into some question our hopeful corollary that a monoclonal origin presages a neoplastic habitus. Finally, it is clear, for many reasons, that methods of analysis which involve the disaggregation of tissues, albeit microdissected, are far from ideal and we should be putting more effort into techniques where the clonal architecture of normal tissues, preneoplastic and preinvasive lesions and their derivative tumours can be directly visualized in situ.
Keywords: tumours, clonality, clonal evolution, carcinogenesis
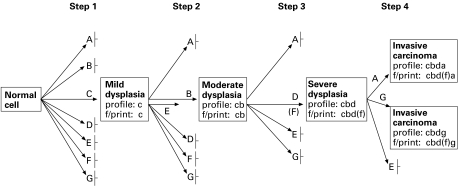
One of the shibboleths which has arisen in modern tumour biology, possibly stimulated by the rarity of malignant transformation in vivo, has been that neoplastic proliferations, perforce, take origin in a single cell. Our ideas about the nature of the neoplastic process are heavily predicated by our concepts of its origin: it is widely accepted that tumours arise from a mutation or series of mutations in this single cell. This view has been largely influenced by numerous observations indicating that not only are chemically induced neoplasms in rodents clonal, but that the preneoplastic lesions from which they arise are also clonal in origin (Iannaccone et al. 1987). Thus, using the paradigm of colorectal cancer, Fearon (1994) has remarked ‘most interesting are those mutations which arise in early tumours which are not found in the germ-line; these mutations are responsible for the clonal development of the adenoma’. The model of development of colorectal carcinoma subscribed to by many (Figure 1) involves a series of sequential mutations in single cells, which leads to the evolution of the definitive malignant phenotype.
Figure 1.
A diagrammatic representation of the process of mutation and selection, starting in a single cell and progressing through a sequence of steps through dysplasia to carcinoma. While based on the well-known Vogelstein model for colorectal cancer, the diagram is generally representative of neoplastic progression in a variety of epithelial tissues. The diagram represents genetic pathways in the development of a tumour, where A, B, C, D, E, F and G indicate the loci of mutations which, when selected, give survival advantage to the cells. Profile, mutationprofile; f/print, mutation fingerprint; │– not selected (from Ilyas & Tomlinson 1996, with permission).
So, largely accepted theories for carcinogenesis maintain, explicitly or implicitly, that tumours are derived from a single mutated cell and it's progeny. Neoplastic cells would then be capable of growth under what would otherwise be limiting conditions, and some accrue genetic changes that allow them to outcompete their neighbouring cells to eventually become the predominant subclone (Kern 1993). Tumours would then be the result of clonal expansion of such unusual cells surviving transformation (Chang 1982): indeed, much earlier work by Fialkow and colleagues (reviewed by Fialkow 1976), using electrophoretic studies of X-linked glucose-6-phosphate variants in homogenized tissues from heterozygous women, has shown monoclonality in a wide range of human tumours. The possible exceptions are hereditary tumours such as familial neurofibromas (Fialkow et al. 1971).
There is also the interesting corollary of this belief: if a lesion can be shown to be the result of a clonal proliferation, then, ipso facto, it is neoplastic, and this concept also seems widely accepted (e.g. Noguchi et al. 1992; Nilbert et al. 1995; Vogrincic et al. 1997; Niho et al. 1998; Walsh et al. 1998). In hyperplasias, where multiple cells respond to a stimulus and therefore we might anticipated a polyclonal expansion (Walsh et al. 1998), we shall see that hyperplastic lesions such as regenerative nodules in the liver, thyroid nodules in hyperplastic goitre and atheromatous plaques in coronary arteries, unlikely candidates for ‘neoplastic’ habitus, are also regarded by many as clonal proliferations.
Until comparatively recently, any departure from this concept (Alexander 1985; Rubin 1985) was indeed regarded as iconoclastic. But as Teixeira et al. (1994) have noted, support for the general conclusion that tumours are clonal in the human situation, at least from the cytogenetic viewpoint, comes from the study of mesenchymal and haematological neoplasms. The increasing karyotypic evidence of polyclonality in carcinomas might indicate a fundamental difference in this respect between epithelial tumours and other neoplasms. In fact, evidence is now accruing, at least for a subset of epithelial tumours, that by no means all of these lesions are clonal, with the result that we probably have to come to terms with the concept that tumourigenesis in epithelial systems demands the co-operation of several distinct clones, and somehow account for this in any global theory of carcinogenesis.
Methods for investigating the clonal composition of tumours
Because this is such an important baseline in our attempts to understand malignant transformation in molecular terms, it is always worthwhile reminding ourselves of the experimental basis of this proposal. Why do we believe that epithelial tumours, such as the early adenoma in the colonic mucosa, are clonal proliferations? The earlier literature has been reviewed by several authors (Fialkow 1976; Iannaccone et al. 1987), but here we should note that the main methods which have been used for the analysis of clonality in human epithelial tumours have been based on X-chromosome inactivation analysis in females and the detection of somatic mutations. Studies of viral integration, for example by Southern blotting in EBV-associated tumours (Wainscoat & Fey 1990; Fukuyama et al. 1994; Leung et al. 1995; McClain et al. 1995; Shin et al. 1996) or in hepatitis B or C-associated liver tumours (Hsu et al. 1991) are also useful; Jiang & Yao (1996) record the excellent agreement between X-inactivation and EBV integration in nasopharyngeal carcinoma. To date, attempts to find methods of assessing the clonal architecture of male tissues and tumours in the human have proved unsuccessful (Zvejnieks et al. 1998).
Classically, in X-chromosome inactivation studies the haplotypes of glucose-6-phosphate dehydrogenase (G6PD) have been exploited (Fialkow 1973), replaced more recently by methods based on restriction length polymorphisms of X chromosome-linked genes such as glycerophosphate kinase (PGK), the androgen receptor gene (HUMARA) (Vogelstein et al. 1987), hypoxanthine phosphoribosyltransferase (HPRT) (Sternlicht et al. 1994), the M27β probe for DXS285 (Fraser et al. 1989; Li et al. 1993), and p55 together with glucose-6-phosphate dehydrogenase (Liu et al. 1997). Such methods are based on the principle that, early in embryogenesis, genes on either of the two X chromosomes are randomly inactivated by methylation of cytosine residues within promoter regions (Lyon 1972); once methylated, such CpG islands are functionally and heritably inactive (Jones 1996) and it is widely believed that this inactivation is stable, even during the neoplastic change. Thus in approximately half of the cells the paternal X chromosome is active, and in the rest, the X chromosome from the mother is active. Consequently the pattern of fragments produced by DNA digestion with a methylation-sensitive enzyme such as SnaB1 and a further endonuclease corresponding to a restriction fragment length polymorphism, in for example PGK — BstCX1 — can be used to investigate the apparent clonality of any tissue specimen. The percentage of informative cases in women using these markers are reported to vary from 45% with PGK and HUMARA (Vogelstein et al. 1987), to over 90% with M27β/DXS255 (Fey et al. 1992).
The methylation pattern of DNA can be abnormal in malignancy, with both increases and reductions in methylation (Jones & Buckley 1990), and the possibility exists that X-chromosome inactivation may not be valid as an indicator of clonality because of these abnormalities in DNA methylation (Jacobs et al. 1992). In fact, a cell is not distinguished by a specific sequence of methylated or nonmethylated sites: the methylation of different cell types is distinguished by the proportion of cells methylated, i.e. the overall methylation frequency, for each site in the population (Silva et al. 1993).
Moreover, it is possible that X-inactivation might be nonrandom, either constitutive (Tihy et al. 1994) or cell-type specific (Wengler et al. 1993). Some studies in normal haemopoietic and lymphoid tissue have shown skewed X-inactivation, possibly favouring the paternal or the maternal X-chromosome (Mutter & Boynton 1995), which could presage nonrandom X-chromosome inactivation patterns. While some studies claim that extremely unbalanced inactivation of the X chromosome is an uncommon phenomenon (Racchi et al. 1998), others report skewed inactivation in some 23% of women with HPRT and PGK, and 22–33% with M27β (Fey et al. 1992; Gale et al. 1992), present in peripheral blood leucocytes (Boyd & Fraser 1990; Fey et al. 1992) and in bone marrow and skin (Lo Coco et al. 1993; Tsuge et al. 1993), suggesting tissue-specificity, and possibly related to the number of stem cells in the tissue at the time of X chromosome inactivation. If this number is small, it will result in skewing, with increased probability with diminishing stem cell pool size (Fialkow 1973). In some embryonic tumours such as retinoblastoma and Wilm's tumour, loss of heterozygosity (LOH) on 13q and 11p, respectively, show the preferential loss of maternal and paternal alleles (Drya et al. 1989; Pal et al. 1990), also seen in sporadic osteosarcoma (Toguchida et al. 1989).
Finding the same mutation in key genes, such as k-ras (Dijkhuizen et al. 1997) or p53 (Franklin et al. 1997) in multiple tissue samples from the same tumour, or from apparently unconnected tumours, has also been proposed as indicating a clonal origin, notwithstanding several perceived problems, notably the possibility of the contemporaneous occurrence of the same mutation in separate precursor cells by a carcinogen — aflatoxin causes specific p53 mutations in hepatocellular carcinoma (Bressac et al. 1991; Hollstein et al. 1991; Hsu et al. 1991; Ozturk 1991). We should also note that DNA fingerprinting has also been proposed as a method for assessing clonal status (Fey et al. 1988).
Demonstration of heterogeneity of microsatellite instabilities, for example in multiple gastric carcinomas (Nakashima et al. 1995) has been used as a marker for polyclonality, but if genome alterations continue to occur at microsatellite loci, with resulting genetic diversity within the same clone, microsatellite instability will not be an appropriate molecular marker of clonality. Others have looked to the presence of cytogenetically unrelated abnormal clones demonstrated by karyotypic analysis as evidence of polyclonality, but the existence of such clones might be due to chromosomal rearrangement in non-neoplastic epithelial or stromal cells. For example, it is now recognized that cytogenetically abnormal clones are present in apparently non-neoplastic breast lesions such as fibrocystic disease (from which breast carcinoma probably arises)(Petersson et al. 1994; Dietrich et al. 1995). Mutated clones have been reported even in histologically normal breast epithelium (Larson et al. 1998), several at potential tumour-suppressor gene sites, indicating that genetic abnormalities accumulate before pathological changes can be detected. There also exists the possibility, however unlikely, that the disparate clones could arise from a single transformed ‘mother cell’ (Teixeira et al. 1995). Or of course, such clones could indeed presage a polyclonal proliferation; indeed, because such karyotypic analysis is perforce carried out in vitro, there are reasons why karyotypic heterogeneity maybe underestimated in human tumours (Teixeira et al. 1995).
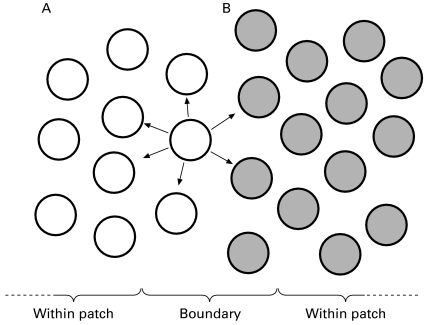
We should note that these observations, in the main, involve the biochemical or molecular examination of homogenized tissues, and in human epithelial tissues, there have been few opportunities indeed to directly observe the clonal development of very early tumours. But why should direct observation be so important? This importance is well illustrated for techniques using X-chromosome inactivation by the thoughtful article from Schmidt & Mead (1990), which introduces the concept of patch size into the argument. It is usual to define a clone as a family of cells which derive from a common progenitor, sometimes adding the caveat, for normal tissues, that these remain contiguous throughout the growth of the embryo (Nesbitt 1974). This definition thus refers to cell lineage. A patch, on the other hand, is a group of cells which share a common genotype, contiguous at the moment of consideration (Nesbitt 1974). Thus clone size and patch size are not strictly equivalent, since multiple clones of the same genotype could contribute to a single patch; similarly, a single clone could be anatomically separated into different patches.
Thus in Figure 2, which again uses the paradigm of the colon, an adenoma arizing from the centre of a patch will, by definition, be of clonal origin when assessed by the pattern of X inactivation. The only chance of detecting a polyclonal, or pleoclonal (Woodruff 1988) proliferation would be when such a lesion arises from the margin of a patch boundary. This is seen in patch boundaries in normal mouse epidermis, where hair follicles appear polyclonal, but of course are monophenotypic within patches (Winton et al. 1989). At X-inactivation, at about the time of implantation, the pool of stem cells is quite small, approximately 15 cells for the skin (Fialkow 1973). Skewing towards one parental allele is thus very possible, and indeed this is seen in human skin specimens (Tate et al. 1997), potentially indicating a large patch size. Such polyclonal tumours will therefore be commonest at patch boundaries, the opportunity for this occurring being dependent on the size of the patch (Schmidt & Mead 1990), and thus the incidence of polyclonal tumours might indeed be small in any one analysis. And if rare polyclonal lesions are found, then the explanation that the lesion consists of more than one single tumour, each of clonal origin, that have mixed or collided, is often proffered (Farber 1997). But the rarity of such lesions would indeed be expected, it as seems likely, X-linked patches are quite large (Schmidt & Mead 1990; Chung et al. 1998).
Figure 2.
Showing the influence of patch size on the determination of clonality, here in colorectal epithelium. using X-linked markers; tumours arising within patches will perforce be clonal, and only lesions arising at the margins of patches (i.e. between A and B) will be detectable as a polyclonal proliferation. The concept can of course be extrapolated to any epithelial sheet, such as the epidermis or urothelium (from Schmidt & Mead 1990, with permission).
Interestingly, the concept of patch size in respect of these arguments is rarely mentioned (Thomas et al. 1988), and, even when adjacent normal tissue is used as a control, and found to be polyclonal, the sample size, a critical factor, is rarely mentioned (e.g. Sawada et al. 1994; Mutter et al. 1995; Niho et al. 1998; Walsh et al. 1998). Indeed the finding of polyclonality in normal tissues, such as the normal liver adjacent to carcinomas, without mention of size, has been regarded as a validation of the PGK (Kawai et al. 1995), HUMARA (Mutter et al. 1995) or HPRT technology (Barsky et al. 1994), and even taken as evidence that the patch size is small (Apel et al. 1995). A recent paper by Chung et al. (1998) has brought this issue sharply into focus: a long-term belief has been that smooth muscle cells in atheromatous plaques are monoclonal (Benditt & Benditt 1973; Pearson et al. 1978, 1979, 1987; Murry et al. 1997) with the clonality in this case dependent upon the smooth muscle component of the plaque (Murry et al. 1997), and such plaques have even been regarded as some sort of benign neoplasm. This proposal has been based on X-chromosome inactivation studies with glucose-6-phosphate dehydrogenase and HUMARA analysis (Murry et al. 1997), supported by karyotypic abnormalities reflecting monoclonality (Casalone et al. 1991) and mutations in a microsatellite in the TGFB1 receptor gene (McCaffrey et al. 1997). Clonal proliferation is thus explained by a somatic mutation, induced perhaps by genotoxic chemicals, or an infection, giving a hit in a single cell, which then develops into an atheromatous plaque. Alternatively, since smooth muscle cells are now known to consist of multiple cell lineages (Lemire et al. 1994) a rare progenitor cell could be selected and clonally expanded to form the plaque (Schwartz & Murry 1998).
While plaques are indeed monoclonal based on such analyses, Murry et al. (1997) reported skewed X-chromosome inactivation patterns in normal media and intima, and Chung et al. (1998) found patch sizes in aortic media and intima exceeding 4 mm in diameter. Although plaques may indeed arise through a mutagenic event, X-chromosome inactivation analysis cannot be relied on to distinguish between a monoclonal and a polyclonal origin for such atheromatous plaques, because of the very large patch size. This underlines the significance of Schmidt and Mead's proposals, shown in Figure 2, and the importance of knowledge of the patch size. It will be interesting to see how many other investigations of clonality, which ignore or side-step this constraint, will be similarly confounded. Sometimes the clone size is preferable — thus relating the number of apparently mixed epidermal tumours in chimaeric mice (6/50) to the epidermal clone size (600 cells) allowed the conclusion that each tumour arises from 8 or fewer cells — the target size (Iannaccone 1980) (but see below for further caveats).
The study of multiple lesions in the same patient does provide a way of circumventing the problem of patch size, especially if more than one clonal marker is used (Sidransky et al. 1992a). Thus the probability that all the tumours examined would have the same X-chromosome inactivation pattern is 0.5n−1, where n equals the tumour number. If say, allele loss on 9q, an independent event, was also sought, then the probability that the same pattern in each tumour is due to chance is (0.5n−1)(0.5n−1), and so on for each independent marker used.
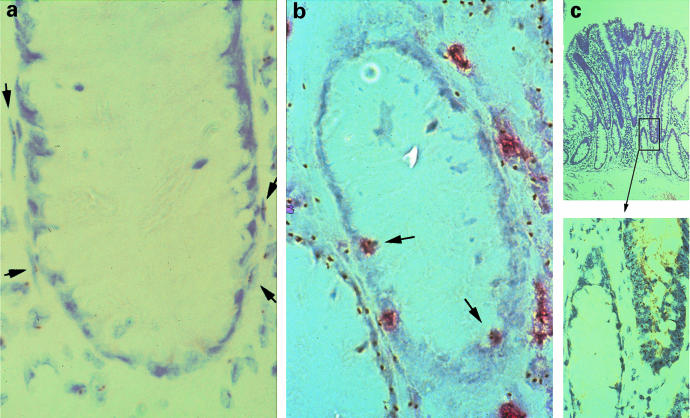
On the other hand, when methods based on the analysis of homogenized tissues have given rise to results suggesting polyclonality, this is often attributed to contamination with underlying stromal cells of different clonal derivation. Even so, some investigators insist on normal tissue contamination, even when the incidence of polyclonal tumours amounts to 40% of the total (Walsh et al. 1998). Having said this, it is clear that even the fibroblasts or myofibroblasts very closely applied to the epithelium in the colonic crypt are of different clonal derivation (Figure 3a). Moreover, there may be invading inflammatory cells (Figure 3b), which, if numerous enough, can give rise to disparate results in clonality analyses involving PCR techniques (Shroyer & Gudlaugsson 1994; Kawai et al. 1995). Figure 3(c) shows how normal tissue can be trapped and enclosed by surrounding neoplastic tissue, and again give discordant results unless microdissection procedures are stringent. The problem of poor sampling may give a false impression of monoclonality in a polyclonal tumour (Williams & Wynford-Thomas (1994); Fey & Tobler 1996; Piao et al. 1997). Enomoto et al. (1994) using the HUMARA method, has estimated that the bands generated by mixing monoclonal tumour tissue with peripheral blood leucocytes are distinguishable in the proportion 8:2, indicating that clonality can be assessed when clonally derived cells comprise 20% or more of the population; Krohn et al. (1998) quoted 40% and Noguchi et al. (1992) 50%.
Figure 3.
The problems of using tissue disaggregation techniques or less than stringent microdissection techniques in the determination of tissue clonality: (a) human colon from an XO/XY mosaic, with NISH demonstration of nuclei containing the Y chromosome. Myofibroblasts which are very closely applied to the epithelium show a different clonal origin; (b) white blood cells, shown red by immunohistochemistry with an antibody against leukocyte common antigen, are present between crypt epithelial cells, and are the source of Y-positive nuclei in an otherwise negative crypt, and (c) a tubular adenoma from the same individual showing a normal, negative crypt in a Y-chromosome positive adenoma, showing how easy it is to include normal tissue in a neoplastic lesion (from Novelli et al. 1996, with permission).
In animals, extensive use of aggregation chimeras, or tetraparental allophenic mice has been made to explore both the clonal architecture of epithelial tissues and their derivative tumours, but it was not until recently that similar advantage was taken of human mosaics to investigate the clonal architecture of human tissues and their tumours (Novelli et al. 1996).
A further caveat of particular relevance to clonal mutation analysis is the point, during the life history of the development of the tumour, at which it is examined. The analysis of mature lesions is inappropriate, since tumours of polyclonal origin may become clonal because of clonal evolution (Nowell 1976, 1986). All clones, except one, are eliminated or reduced to the point of being undetectable, apparently seen in chemically induced mouse fibroasarcomas, initially polyclonal, which evolve to a clonally derived population with time (Woodruff et al. 1982), because of the later selection of a dominant clone (Winton et al. 1989). Thus polyclonality gives rise to ‘pseudomonoclonality’ (Teixeira et al. 1994), when selection forces in the tumour change, as for example after invasion has occurred (Heim et al. 1989). This is possibly exemplified in colorectal adenomas, where heterogeneity of k-ras mutations is seen — which heterogeneity is lost after the lesion has evolved to become an invasive carcinoma (Shibata et al. 1993). Conversely, genetic heterogeneity in a tumour does not necessarily indicate a polyclonal origin, since genetic instability is a major feature of malignant tissues and many new clones may arise during tumour development (Nowell 1976, 1986). We return to this issue at the end of this article.
The clonal architecture of normal tissues
Most authors have drawn a direct comparison between genotype/phenotype determination and clonality: while apparently reasonable, this begs the patch size question, and some have used the terms monophenotypic and polyphenotypic (Thomas et al. 1988) or homotypic and heterotypic (Nomura et al. 1996) to avoid this problem. Thomas et al. (1988) cogently point out that a monophenotypic tumour arising in the colon, where intestinal crypts are themselves clonal populations ( vide infra), can only be described as monocryptal and not monoclonal in derivation. Conversely, in the thyroid, where follicles are mainly (in the mouse) polyclonal, a monophenotypic tumour is much more likely to be monoclonal.
Investigations of clonality in many normal tissues has been aided by the use of tetraparental allophenic or chimaeric mice, coupled with suitable strain-specific markers to distinguish the chimaeric tissues in situ. Thus in the neonatal mouse, intestinal crypts are polyclonal, but, through a process known as ‘purification’, crypts become monoclonal by two weeks of age (Schmidt et al. 1988) and remain so throughout adult life (Ponder et al. 1985; Tatematsu et al. 1996b). This is similar to the retinal epithelium, where a random arrangement is seen in the embryo, but a pattern of small clones is present in the adult (West 1976a). In X-chromosome mosaics, adult mouse thyroid follicles are polyclonal (Thomas et al. 1988). The liver in the adult mouse chimaera apparently contains > 106 small clones, indicating that considerable mixing occurs during development (West 1976b), although direct observations in rat chimaeras shows many large patches, though some small ones (< 50 cells) are seen. Moreover, patch size does not follow anatomical boundaries such a lobules, unlike the intestinal crypt or adrenal cortex, which show clear evidence of clonal development (Weinberg et al. 1985). In chimaeric mouse epidermis, the average clone size was thought to be 600 cells, and the patch size approximately 15000 cells (Iannaccone 1980); in X-chromosome mosaics, the patch size was approximately 6000 cells (Deamant & Iannoconne 1987), and up to 3000 cells in human scalp (Gartler et al. 1971). At least in the mouse epidermis, this concept of fine-grained patchiness, based on the biochemical analysis of small fragments, is not seen when chimaeric epidermis is visualized in situ, when nonhomogeneous transversely orientated stripes are seen (Schmidt et al. 1990).
There is little data concerning the patch size in human tissues, even where the pattern of X-inactivation is concerned, but we have seen above that in human arteries, the smooth muscle cells are a diverse population composed of multiple lineages, and growth is by expanding smooth muscle cell clones, with little or no mixing of adjacent clones (Chung et al. 1998); the evidence indicates a very large patch size. In the human breast, earlier findings suggested that normal breast tissue was polyclonal in origin, with random distribution of X-chromosome inactivation (Noguchi et al. 1992). However, microdissection of normal breast epithelium shows that epithelial patch size is quite large, exceeding 0.45 mm2, and that the larger terminal ductulo-lobular units represent the progeny of a single precursor or stem cell (Deng et al. 1996; Tsai et al. 1996). In the mouse mammary gland, where ducts and acini are apparently polyclonal, while a large patch size is seen due to coherent clonal growth during development (Thomas et al. 1988), there appears to be three multipotent but distinct epithelial stem cells in clonally derived mammary epithelial cell populations. When serially passaged, one stem cell could readily produce an entire mammary gland (Kordon & Smith 1998). Since these stem cells can be mutated during organogenesis, and these mutations conserved in all progeny, such self-renewing, organ-specific stem cells may represent the major potential risk for carcinogenesis in mammary epithelium. As we shall see, this is an important point, since apparently normal breast can contain genetic abnormalities at potentially important sites (Larson et al. 1998). These observations have implications for claims that human breast cancers, and their precursors, are clonal proliferations ( vide infra).
Further evidence for a large X-linked human patch size comes from the bladder epithelium — 1 cm2 (Tsai et al. 1995) hair follicles −0.2–1.0 cm2 (Gartler et al. 1971) and gastric epithelium — 1.0 cm2 (Linder & Gartler 1965a), but other tissues, such as myometrium, may have a smaller patch size, probably less than 0.3 cm2 (Linder & Gartler 1965b). The mature human ovarian follicle is composed of oligoclonally derived — not more than three — groups of granulosa cell progenitors (Deerlin et al. 1997).
Thompson et al. (1990) were the first to show that gastric glands are also clonal populations, using XO/XY mice and localizing the male nuclei with a Y chromosome-specific probe, and also to show that neuroendocrine cells shared the same clonal derivation as the glands. In the human stomach, the situation may be more complex than this: antral gastric glands are clonal, but in the body glands the foveolae and bases of the glands appear homotypic, whereas the isthmus and neck, the area which contains the gastric stem cell population, are heterotypic on HUMARA analysis (Nomura et al. 1996). These glands may be of polyclonal structure, possibly reflecting complex stem cell organization, unless differences in the timing of methylation are important, possibly occurring after the formation of gastric gland primordia in man (Nomura et al. 1996): the methylation of the inactive X chromosome is complete at 8 days in the foregut of the mouse (Tan et al. 1993). Additionally, when mice in which multiple copies of the lacZ gene were placed in tandem close to the hprt locus on the X-chromosome, Lyonization gives gastric glands of different phenotype for analysis (Nomura et al. 1998a). At birth, 75% of glands in the pylorus and 85% in the body are of mixed phenotype, but over the first six weeks of life most glands undergo monoclonal conversion to become monophenotypic. However, even in adult life there is a small fraction, approximately 5–10%, which remain mixed, and given that some monophenotypic glands can also be polyclonal, the total of polyclonal crypts might be as many as 15–26% in the body and 10–15% in the pyloric mucosa.
In the normal human intestine, investigation has been limited by the lack of suitable markers of clonality, although in adults, Fuller et al. (1990) have shown crypt-restricted loss of mucin O-acylation. This may be due to somatic mutation at the O-acylation transferase (OAT) locus and suggests that human crypts may be clonally derived — indeed Endo et al. (1995), using the HUMARA assay, have shown that individual, microdissected human colonic crypts are homotypic and clonal in origin. In human XO/XY mosaics, it is possible to differentiate between XO and XY cells using in situ hybridization with Y chromosome-specific probes (Novelli et al. 1996). In such an individual, intestinal crypts were composed almost exclusively of either XY or XO cells, including the crypt neuroendocrine cells. Thus, intestinal crypts are clonal populations, each derived ultimately from a single stem cell; neuroendocrine cells share this derivation, thus conclusively disproving Pearse's hypothesis of the neuroectodermal derivation of such cells in humans, as part of the global APUD cell theory (Pearse 1987). The patches of XO crypts were irregular in shape and patch size varied widely (mean 11.25 crypts, range 1–14 crypts).
There have been several statements about the polyclonal structure of human endometrium (cf. Mutter et al. 1995), without further refinement, but it is of interest that the clonal architecture of the colonic crypt is also reflected on the endometrium, where microdissection studies show that approximately 80% of glands are clonal in origin (Jiang et al. 1996).
The clonal architecture of non-neoplastic, preneoplastic and preinvasive proliferations
Non-neoplastic and preneoplastic lesions
We now move on to a consideration of the lesions which are thought to precede neoplastic development. Many tumours arise from preneoplastic lesions, not themselves neoplastic, which were previously regarded as hyperplasias, involving changes in many cells and therefore by definition polyclonal. However, it is becoming increasingly clear that such lesions often represent clonal proliferations. Thus in gastric mucosa three separate hyperplastic polyps of the fundus in a single patient which harboured foci of dysplasia showed the same k-ras codon 12 point mutation — glycine:aspartate, present in both hyperplastic and dysplastic areas (Dijkhuizen et al. 1997). Chance was discounted, as was a specific genetic lesion, and the explanation preferred was that the progeny of a single transformed cell has spread through the mucosa. It is difficult to envision how such spread could occur, although the unlikely possibility of surface spreading and surface implantation was considered possible. It is of course possible that a single cell could populate a gastric gland, which could then spread through the mucosa by gland fission (Garcia et al. 1999).
In the female genital tract, endometrioid carcinomas are considered to arise from foci of endometriosis (Jimbo et al. 1997). Recent studies of informative individuals has shown the majority of such endometriotic foci to be clonal (Nilbert et al. 1995; Jiang et al. 1996; Jimbo et al. 1997; Tamura et al. 1998). Endometriosis arises either from implantation of shed endometrial cells, or from metaplasia of the pelvic peritoneum: if patch size considerations can be excluded, this means from a single endometrial or mesothelial cell. It is clear that these investigations have concentrated on the epithelial component of the lesion, although the formal definition does include the presence of glands and stroma, and the clonal relationships of the two tissues would be of interest. It is singular that Nilbert et al. (1995) and Jiang et al. (1996) ignored the 20–40% of cases which were polyclonal (Schmidt & Mead 1990) and also that Jiang et al. (1996) showed that 80% of normal endometrial glands are clonally derived. The patch size in the endometrium thus extends to the size of the gland (many hundreds of cells), and thus if implantation is the mechanism, this certainly increases the chance of an endometriotic focus being polyclonal (Thomas et al. 1988). Moreover, endometriotic foci can show aneuploidy (Ballouk et al. 1994) and loss of heterozygosity at candidate tumour suppressor loci in 9p, 11q and 22q (Jiang et al. 1996). Thus it is not entirely surprising that the derivative tumours, the endometrioid carcinomas, are clonally derived (Shroyer & Gudlaugsson 1994): they evidently arise from a monoclonal proliferation that itself can show genetic defects. These findings led Nilbert et al. (1995) to label endometriotic cysts as neoplasms, and while we might disagree with this proposal, there is a view that some of these lesions could well be endometrioid cystadenomas (Fox 1993). In the endometrium itself, carcinomas arise from areas of atypical hyperplasia (Tavassoli & Kraus 1978); such hyperplasias appear clonal (Sawada et al. 1994; Mutter et al. 1995; Esteller et al. 1997).
Interestingly, the preneoplastic lesions from which breast cancers develop — proliferative breast disease and similar lesions — show cytogenetic abnormalities suggesting the presence of multiple clones (Petersson et al. 1994; Noguchi et al. 1994b; Diettrich et al. 1995; Lakhani et al. 1995, 1996; Petersson et al. 1996; Chaquai et al. 1997; Kasami et al. 1997). X-chromosome inactivation studies shows that atypical duct hyperplasias and intraduct papillomas appear clonal proliferations (Noguchi et al. 1994c), as do detection of monoclonal microsatellite alterations (Rosenberg et al. 1997) and consequently there is no reason not to believe that cytogenetic alterations have not already occurred at this stage, remembering the caveat about the patch size in normal breast, and noting a further report which claims that intraduct papillomas are polyclonal (Malamou-Mitsu et al. 1996).
Naevocellular or melanocytic naevi (thought to be, in some cases the precursor of malignant melanomas), are either congenital or acquired, and indeed studies with HUMARA only show that some 80% of acquired naevi are apparently clonal lesions (Robinson et al. 1998). However, in other work, also using X-chromosome inactivation analysis but here combining HUMARA and PGK, such naevi were polyclonal; malignant melanomas were clonal (Harada et al. 1997). If melanocytic naevi are indeed polyclonal proliferations. it might imply, as is widely held that such naevi are hamartomas — an abnormal proliferation of cells in an organ or tissue where these cell types would normally be found — here melanocytes. However, hamartomas in tuberous sclerosis, where multiple cell types are seen, show clonal 9q34 or 16p13.3 LOH and clonal X-inactivation patterns (Green et al. 1996), while pulmonary chondroid hamartomas also contain clonal cytogenetic abnormalities only in the chondroid component (Fletcher et al. 1993).
Extremely relevant to the concept of field change is the growing recognition that mutations in important genes such as p53 are found in squamous epithelium preceding any dysplastic change (Ahomadegbe et al. 1995; Brennan et al. 1995; Gallo & Bianchi 1995; Nakanishi et al. 1995; Chung et al. 1996; Mandard et al. 1997; Ogden & Hall 1997). Such mutations are seen even in normal mucosa (Nees et al. 1993; Brennan et al. 1995) and in sun-exposed normal epidermis (Ponten et al. 1997). In this respect, it is important to distinguish between field change adjacent to a tumour and in the absence of tumour. Clones of keratinocytes with immunoreactive p53 and p53 mutations (exons 5–8) are seen in sun-exposed but otherwise normal skin (Jonason et al. 1996; Ren et al. 1996; 1997a, b; Ponten et al. 1997), and in morphologically normal mucosa from individuals with upper aerodigestive tract tumours (Nakanishi et al. 1995; Mandard et al. 1997). Moreoever, microsatellite instability has been found in normal mucosa from patients with ulcerative colitis (Brentnall et al. 1996), reflecting the increased risk of malignancy in these patients, since microallelotyping shows no allelic loss in transitional mucosa adjacent to colorectal neoplasms (Boland et al. 1995). However, such losses have been reported in normal breast tissue (Larson et al. 1998).
There is good evidence from rat chimaeras that the preneoplastic nodules which give rise to hepatocellular carcinomas are clonal (Weinberg & Iannoccone 1988). Such experimental mouse hepatic tumours are also monoclonal, as assessed by the X-linked tissue-specific gene ornithine carbomyl transferase (Howell et al. 1985; Tsuji et al. 1988), In the human liver however, opinion is sharply divided concerning the clonality of lesions often regarded as preneoplastic: several studies have shown that while lesions such as benign adenomatous hyperplasia and focal nodular hyperplasia are polyclonal, hepatic adenomas and small (< 25 mm) hepatocellular carcinomas are clonal (Kawai et al. 1995; Paradis et al. 1997b). But Tsuda et al. (1988), examining the integration patterns of hepatitis B virus, claimed that atypical adenomatous hyperplasia was clonal, and similarly, Gaffey et al. (1996) considered that most focal nodular hyperplasias also appear clonal, as do genuine hepatic adenomas. Hepatocellular carcinoma is frequently multifocal (Sheu 1997) and whether these arise from a single clone or independently is controversial: Esumi et al. (1986, 1989) and Govindarajan et al. (1988) proposed a clonal origin for multiple tumours, but most authors agree that an independent origin is more likely (Aoki & Robinson 1989; Chen et al. 1989; Shimoda et al. 1990; Hsu et al. 1991; Ding et al. 1992; Tsuda et al. 1992; Sheu et al. 1993), including recurrences (Chen et al. 1989). But Yamamoto et al. (1999) have proposed that the finding of monoclonality in multiple hepatitis B-associated hepatocellular carcinomas indicates intrahepatic metastases from a primary tumour, while a polyclonal pattern suggests independent multicentric origins.
Thus the strategy of tumour development in the liver consists of two major sequences: (a) after induced liver damage, clonal selection occurs during regeneration (Wada et al. 1990). leading to the genesis of persistent benign focal proliferations, shown to be clonal nodules in the rat (Weinberg & Iannoconne 1988) and (b) the development of clonal hepatocellular cancer from one or more such nodules (Farber 1984, 1990; Weinberg et al. 1987; Aihara et al. 1994; Kawai et al. 1995) — in animals, such induced hepatocellular carcinomas are mainly clonal (Howell et al. 1985; Lee et al. 1991; Tsunashima et al. 1996). Thus in man there is substantial evidence from hepatitis B (Aoki & Robinson 1989; Yasui et al. 1992) and hepatitis C (Aihara et al. 1994) virus integration that between 0.5 and 43% of regenerative nodules in cirrhosis are monoclonal. This has recently been confirmed using X-inactivation analysis (Paradis et al. 1997a), where 54% of macronodules in cirrhotic livers were monoclonal: neither the size of the nodule, its histology or the aetiology of the process were correlated with its clonal status. This underlines the finding of loss of heterozygosity for the mannose-6-phosphate/IGF-II receptor locus in histologically normal nodules adjacent to hepatocellular carcinomas, again suggesting a clonal habitus (Yamada et al. 1997). This might suggest that large regenerative liver nodules showing a polyclonal pattern evolve into a clonal population, developing into hepatocellular carcinomas which are also clonal by X-inactivation, and differ from the nodules by 18q loss (Sakamoto et al. 1989; Kawai et al. 1995; Piao et al. 1997).
Pre-invasive lesions
It is usually held that the field cancerization hypothesis (Slaughter et al. 1953) predicts that multiple cells form independent epithelial tumours. Carcinogenic exposure affects multiple cells in the field, and second primary or synchronous tumours arise from independent genetic events. This concept originated from the observation that 11% of individuals with oral cancer had multiple upper aerodigestive tract tumours, and the finding of multiple invasive foci associated with overlying areas of in situ squamous carcinoma in such lesions. Indeed there is genetic evidence for such an independent origin: in the upper aerodigestive tract, multiple synchronous squamous tumours appear independent and multicentric in origin (Chung et al. 1993; Yang et al. 1995). This search for commonality in genetic lesions in multiple preinvasive or invasive lesions in the same patient has been a popular pursuit in those interested in trying to verify or falsify the field cancerization hypothesis. However, it has been proposed that the concept of clonal origin and expansion is problematic in organs where several metachronous tumours appear (Sidransky et al. 1992a), and a synchronous or second primary tumour may reflect recurrence or indeed lateral spread from a single tumour. Thus, although the field cancerization hypothesis would predict a multicentric, polyclonal origin, if indeed such tumours could be shown to be clonal, then lateral migration from the original clone would be a distinct possibility.
In fact, conflicting evidence for the nature of field cancerization comes from the study of tumours of the upper aerodigestive tract and adjacent mucosa (Bedi et al. 1996). In laryngeal and pharyngeal tumours, multiple samples taken at tumour-distant sites show different and independent mutations in the p53 gene, assessed by SSCP and direct sequencing (Chung et al. 1993; Yang et al. 1995), favouring a discontinuous, multifocal and polyclonal process of field cancerization, rather than migration of premalignant basal keratinocytes giving a clonal development of multiple primary, secondary or recurrent tumours (Gusterson et al. 1991). That this is not the whole story is shown by an exhaustive karyotypic and FISH analysis of two synchronous but anatomically distinct oral squamous carcinomas, and their recurrences, which all contained a Y chromosome rearrangement, acting as a unique marker (Worsham et al. 1995). These lesions also contained the same aneuploid patterns in a number of chromosomes, indicating that the synchronous primary and recurrent tumours were of clonal origin.
But Sozzi et al. (1995) examining 3p deletions, p53 and k-ras mutations, also concluded that preneoplastic lesions, as well as the ensuing tumours of the bronchus, were genetically distinguishable and therefore independent in origin. In the stomach, discordant mutation patterns of APC, MCC and p53 were found in 12 out of 13 cases of multiple gastric carcinomas (Kang et al. 1997), again in accord with a multicentric origin, also seen in multiple colorectal tumours (Fearon et al. 1987), parathyroid adenomas (Arnold et al. 1988) and in separate prostatic intraepithelial neoplastic lesions, which show different clonal patterns of allele loss at 8p12–21, suggesting an independent origin (Emmert Buch et al. 1995; Bostwick et al. 1998).
However, while the field hypothesis has also been raised to account for multifocal ovarian and urothelial cancers (e.g. Mills et al. 1988), multiple synchronous carcinomas in the bladder and other pelvic organs apparently show a common clonal origin — X-chromosome inactivation and allele loss at 9q and 17p are identical, as are c-erB2 and p53 mutations in multiple synchronous urothelial tumours (Lunec et al. 1992). Diffuse carcinomas of the stomach, often apparently arizing multifocally and spreading diffusely through the gastric wall, are predominantly polyclonal (Bamba et al. 1998). In such a tissue as the peritoneum, where implantation is commonly seen, it is not possible to say whether the lesion spreads from a single site or started contemporaneously at multiple sites. The presence of multiple sites of occurrence of sporadic ovarian cancer on the ovarian surface and pelvic peritoneum has been advanced as a possible example of field cancerization change, although in fact most cases are clonal, as assessed by LOH, p53 mutations and X-chromosome inactivation analysis in the same cases (Jacobs et al. 1992; Li et al. 1993), or in a few cases, of dual primary origin. Multiple serous adenocarcinomas in the ovaries, peritoneum and endometrium showed the same p53 mutation (Mok et al. 1992; Kupryjanezyk et al. 1996) and clonal cytogenetic abnormalities (Pejovic et al. 1991), findings also confirmed by X-chromosome inactivation (Sawada et al. 1994).
In multifocal breast carcinomas, an increasingly common finding (Dawson 1993), karyotypically identical clones were detected (Teixeira et al. 1994; Pandis et al. 1995), indicating intramammary spread from a single carcinoma either by focal lymphatic spread or by intraductal spread. However, intra- and inter-focal karyotypic heterogeneity was also found, some of which were related clones, possibly evidence of clonal evolution from a single mutated cell, but other clones were unrelated. This finding was interpreted by Teixeira et al. (1994) as indicating a polyclonal derivation for at least a subset of breast carcinomas, and that clonal synergy might be necessary for tumour progression. But in rats, multiple methyl-nitrosourea-induced mammary carcinomas emerge from independently initiated cells and are each of clonal origin (Lu et al. 1998). However, in phyllodes tumours, widely separated deposits show the same monoclonal stromal component (Noguchi et al. 1993).
But if lesions some distance away from each other are clonal, how can this be explained? Of course there is always the possibility, always very difficult to exclude, that the disease process which causes the tumour to develop has a characteristic genetic fingerprint, which is seen in all examples of the tumour. The other possibility is that of a common mutated progenitor cell, and the spread of this mutant clone in some way, throughout the epithelium at an early stage in tumour development, How can this occur? An interesting link between the clonal origin of cancer and the field cancerization hypothesis is provided by the work of Franklin et al. (1997): examining p53 mutations in the nonmalignant but dysplastic tracheobronchial mucosa of a smoker (Sundaresan et al. 1992; Sozzi et al. 1995) by SSCP and direct sequencing, they found an G:C to T:A transversion in codon 245 in 7/10 sites in both lungs. The results were interpreted in terms of the expression and dispersion of a single mutant progenitor bronchial epithelial cell clone throughout the airways, a ‘novel mechanism for field cancerization’.
What are we to make of these disparate p53 studies? Clonal p53 mutations could of course be explained by the same carcinogen leaving a fingerprint mutation, and polyclonal p53 mutations by the mutation being a late event in aerodigestive tract tumourigenesis with migration of tumour cells before the development of the p53 mutations. But Sundaresan et al. (1992) and Chung et al. (1993) have argued that p53 mutations are early events in upper aerodigestive tract carcinogenesis, prior to the development of invasive lesions (cf. Sozzi et al. 1995), being found in premalignant lesions of the head and neck, lung and oesophagus. p53 mutations apparently do not show an increased incidence with cancer progression (Dolcetti et al. 1992), but do show clonal fidelity in a variety of tumours (e.g. Sameshima et al. 1992; Sidransky et al. 1992b). Against this Boyle et al. (1993) report that p53 mutations increase with progression of head and neck cancer. Importantly, Chung et al. (1995) show that allele loss on 3p precedes p53 mutation in squamous metaplastic, dysplastic bronchial mucosa. Thus an early event, prior to p53 mutation, might establish a mutated clone, which migrates laterally, possibly aided by a mutation in a cell adhesion molecule (Worsham et al. 1995). In multiple bladder cancer, all tumours were found to have lost the same 9q allele as an early event, possibly a growth control or adhesion molecule, enabling cells to repopulate the urothelium by lateral migration or mucosal seeding (Sidransky et al. 1992a). Moreover, in most discontinuous foci of CIN3 in the cervix, individual lesions show the same X-chromosome inactivation pattern, suggesting intraepithelial spread (Enomoto et al. 1997). In the skin, it is not uncommon to see migration of morphologically abnormal cells laterally from a lesion such as Bowen's disease — the so-called Borst phenomenon (Pinkus & Mehregan 1986). But the multifocality of the bronchioloalveolar lung carcinoma, long thought to be due to intra-alveolar spread (Liebow 1960) has been shown by X-chromosome inactivation to be multiclonal, arguing for multiple independent tumours (Barsky et al. 1994). In fact, atypical adenomatous hyperplasias of the lung, postulated as a possible precursor lesion for bronchioloalveolar carcinoma, are individually monoclonal proliferations, and when multiple, also appear to arise from separate events (Niho et al. 1999). Multiple uterine leiomyomas also appear to arise independently, though individually clonal in origin (Mashal et al. 1994; Hashimoto et al. 1995). But the multiple lesions of disseminated peritoneal leiomyomatosis appear to derive from a clonal, unicentric origin (Quade et al. 1997).
Interestingly, it is claimed that the multiple deposits of Kaposi sarcoma, a widely disseminated malignancy, appear clonal in any one patient (Rabkin et al. 1995, 1997), interpreted as indicating a clonal origin and wide intravascular dissemination; other interpretations include an initial vascular hyperplasia, with later clonal evolution (Delabesse et al. 1997; Gill et al. 1997, 1998). Diffusely infiltrating gliomas are also clonal (Kattar et al. 1997), and multiple discrete meningiomas share clonal neurofibromatosis 2 (NF2) mutations (Stangl et al. 1997); most individual meningiomas appear clonally derived (Zhu et al. 1995). In the urothelium, identical point mutations in multiple synchronous and metachronous carcinomas were interpreted as being due to intraluminal seeding rather than intraepithelial spread (Habuchi et al. 1993). Additionally, in situations where migration might be considered unlikely (cf. pelvic peritoneum with multiple ovarian tumours), somatic genetic mosaicism has been proposed, where postzygotic mutations in the embryo are present at multiple sites — and possibly multiple organ systems (Kupryjanezyk et al. 1996).
Nodules, and nodular hyperplasia, in endocrine organs such as the thyroid, parathyroid, pancreas and adrenal glands present a special problem in clonality studies. In several endocrine organs, such as the thyroid and the parathyroid, the distinction between a nodule and a neoplasm is difficult to make on purely histological grounds. However, we have seen that, in mice, the majority of thyroid follicles are polyclonal in origin (Thomas et al. 1989). In humans, sporadic, multinodular goitre contain nodules which are generally regarded as being hyperplastic; consequently, we might expect that these lesions would be predominantly polyclonal (Harrer et al. 1998a, b), but there is substantial X-chromosome inactivation evidence that many of these nodules comprise clonal populations (Hicks et al. 1990; Namba et al. 1990; Aeschimann et al. 1992; Apel et al. 1995; Chung et al. 1999). However, it does appear that some nodules, including hyperfunctioning or toxic nodules, can be polyclonal (Namba et al. 1991). In these lesions, the presence of a TSH receptor mutation may be important (Krohn et al. 1998): 10/11 cases showed monoclonality on HUMARA analysis, but only 6/12 toxic nodules with no TSH receptor mutation are clonal, raising the possibility that during thyroid hyperplasia a cell with a mutation at this locus leads to the initiation of autonomous growth. In recurrent goitres, nodules are predominantly polyclonal (Harrer et al. 1998a). In several studies of clonality in multinodular goitre, the proportion of clonal nodules varies, and it is clear that clonal and polyclonal nodules can coexist in the same gland (Namba et al. 1990; Bamburger et al. 1993; Kim et al. 1998), with no apparent correlation with morphology, although clonal nodules might be larger (Kim et al. 1998). Multiple nodules in the same patient are mostly clonal, with activation of the same allele, possibly indicating intraglandular spread by follicular budding, although clonal nodules with different X-chromosome inactivation patterns can be seen in the same multinodular gland (Kopp et al. 1994). This could mean a different pathogenetic mechanism for clonal and polyclonal nodules, or indeed evolution of clonal from polyclonal nodules (Kopp et al. 1994), as generally proposed by Woodruff et al. (1982) and Alexander (1985). In animals, goitrogen-induced ‘monomorphic adenomas’ are clonal, but ‘nodules’ are polyclonal in X-chromosome mosaics (Thomas et al. 1989). In man, most follicular, papillary and anaplastic carcinomas are clonal (Fialkow 1976; Hicks et al. 1990; Namba et al. 1990; Fey et al. 1992). But while earlier glucose-6-phosphate dehydrogenase allelotype studies showed a clonal origin of medullary carcinoma in MEN2 (Baylin et al. 1976, 1978), later X-chromosome inactivation studies have shown that such tumours, all of which showed mutations in exon 11 of ret, were polyclonal, whereas a case with no family history was monoclonal (Ferraris et al. 1997).
A similar pattern appears to exist where adrenocortical hyperplasias and neoplasms are concerned: ACTH-dependent hyperplasia with associated Cushing's syndrome shows a polyclonal pattern, while adenomas and carcinomas are clonal (Beuschlein et al. 1994). Glands in ACTH-independent adrenocortical hyperplasia contain polyclonal nodules, but a larger monoclonal nodule can emerge, suggesting again clonal evolution.
Parathyroid adenomas are monoclonal (Arnold et al. 1988; Noguchi et al. 1997), as indicated by X-chromosome inactivation and the identification of clonal abnormalities of the parathyroid hormone gene. This is against previous beliefs, based on studies with G6PD allelotypes (Fialkow et al. 1977; Jackson et al. 1982). In MEN 1, where all four parathyroids are enlarged and appear hyperplastic, allelic loss on 11q indicates that proliferation is clonal (Friedman et al. 1989), although when LOH on 11q was combined with X-chromosome inactivation studies, the parathyroid lesions in MEN1 were shown to be polyclonal, suggesting that multiple neoplastic clones grow, coalesce and replace the parathyroid gland (Lubensky et al. 1996). On the other hand, parathyroid hyperplasias, which either occur as a primary phenomenon or secondary to such conditions as chronic renal failure, were previously regarded as polyclonal proliferations (Arnold et al. 1988), but X-inactivation and allelic loss of 11q shows that 38% of primary parathyroid hyperplasias and 64% of hyperplasias secondary to renal failure are clonal, interpreted as neoplastic evolution from a pre-existing polyclonal hyperplasia (Falchetti et al. 1993; Arnold et al. 1995; Shan et al. 1997). However, Tominaga et al. (1996) reported that diffuse parathyroid hyperplasias in uraemia were polyclonal, but the individual nodules in nodular hyperplasia were clonal, indeed showing different clonal patterns of X-inactivation in the same gland, once more favouring the hypothesis that monoclonal proliferations evolve from hyperplasias. Parathyroid grafts, all taken from nodular glands, retained clonality. These findings favour the view that hyperplasia begins in endocrine glands as a polyclonal process, but then becomes a clonal hyperplasia, and the borderline between this phase and that of a benign clonal neoplasm is difficult to delineate. In benign pancreatic endocrine neoplasms, some 50% of lesions appeared polyclonal, and 60% of malignant tumours were shown to be clonal in derivation; these results were again interpreted in terms of such tumours being poly/oligoclonal neoplastic lesions which are eventually outgrown by a single, more aggressive clone with invasive and metastatic potential (Perren et al. 1998).
Invasive carcinomas of the cervix, which are clonal in origin (Enomoto et al. 1994; Park et al. 1996), are considered to originate in areas of cervical intraepithelial neoplasia (CIN). Severe dysplasia or CIN3 is also a clonal proliferation, although lesser degrees of dysplasia (CIN2) appear more commonly polyclonal in X-inactivation studies (Park et al. 1995, 1996; Enomoto et al. 1994), with a strong correlation between HPV subtype and clonality. In this respect, some vulvar hyperplasias, considered to be preneoplastic lesions in this tissue, appear clonal: the derivative VIN and invasive carcinomas are also clonal proliferations (Tate et al. 1997), but in a smaller series, Kim et al. (1996) showed that, while invasive carcinomas are indeed clonal, adjacent squamous hyperplasias were polyclonal and did not show p53 mutations in instances where p53 in the tumour was mutated. While VIN itself was not apparently examined, Kim et al. were doubtful about the provenance of VIN as a preneoplastic lesion in a proportion of cases. The squamous hyperplasias associated with HPV-negative carcinomas may also not be direct precursor lesions, based on clonal analysis (Young-Tak et al. 1996). In nasophayngeal carcinoma (NPC), a combination of X-inactivation studies (PGK), X-linked RPLPs, and EBV integration shows that carcinomas were mainly clonal (Jiang & Yao 1996). However, hyperplastic epithelia, and early atypical hyperplastic epithelia are polyclonal, and clonality emerges only at the moderate/severe dysplastic stage; only NPCs were EBV-positive. At this stage, i.e. severe atypia, p53 (Sheu et al. 1995) and bcl-2 (Lu et al. 1993) mutations appear, and clonality is established.
A relationship between intestinal metaplasia and carcinoma of the stomach has long been suspected, possibly as a direct precancerous lesion, as an epiphenomon caused by the same mutation(s) in tumourigenesis, or possibly the sharing of a carcinogenic milieu. Since intestinal crypts are clonal in both animals (Ponder et al. 1985) and man (Endo et al. 1995; Novelli et al. 1996), it would be anticipated that intestinal metaplastic crypts would also be clonal (Slack 1985). They are not, even in human pyloric mucosa, where HUMARA analysis indicates a clonal origin for gastric glands (Nomura et al. 1996). About 50% of intestinal metaplastic glands are heterotypic or polyclonal (Nomura et al. 1998b); if we can disregard stromal contamination or white cell insudation, or X chromosome inactivation not being determined in stem cells until after the formation of gastric glands, then intestinal metaplastic glands are polyclonal and contain multiple, independent, clonally derived cell lineages. If body-type glands are indeed polyclonal (Nomura et al. 1996), then a metaplastic change in such glands could well be polyclonal, but in the antrum, where gastric glands are clonal in origin? Such polyclonal crypts were explained by the presence of dormant stem cells, while the other possibility entertained, of gastric and intestinal cells in a single gland, which does occur (Silva et al. 1990), would not explain the phenomenon, since, in the antrum, both lineages would be of the same clonal origin. In addition, 11/13 metaplastic areas, each 6 mm in diameter, contained glands of different clonal derivation, suggesting that clonal expansion, at least in intestinal metaplasia, does not occur by the gland fission process proposed for the colonic mucosa (Garcia et al. 1999). But several groups have reported mutations in oncogenes and tumour suppressor genes in intestinal metaplasia including evidence for clonal expansion of cells showing the same p53 mutation (Ochiai et al. 1996). It is possible that the process of field cancerization in the gastric mucosa is in fact intestinal metaplasia.
Carcinoma in Barrett's oesophagus arises from dysplastic changes in metaplastic epithelium that replaces the stratified squamous epithelium of the lower oesophagus. While the nature of this metaplastic change is disputed — whether it is a unique, specific change or related to intestinal metaplasia — the nondysplastic metaplasia adjacent to carcinomas is clonal on X-inactivation analysis (HUMARA), and also shows LOH for APC, changes also seen in the dysplastic and neoplastic tissues of Barrett's oesophagus (Zhuang et al. 1996). In the context of a field change, it is not yet clear if the APC change is clonal, but microsatellite analysis shows allelic imbalance not only on 5q but at multiple other sites, in both premalignant and malignant Barrett's epithelium, supporting the concept of clonal expansion from metaplasia through dysplasia to carcinoma (Gleeson et al. 1998).
In conclusion, it is true to say that there are preneoplastic changes which appear to be clonal but may not be neoplastic and that clonality in many instances is derived quite early on, before the emergence of severe dysplastic changes.
Clonality in established tumours — conflicting evidence
Tumours are clonal in origin
Earlier reviews (Iannaccone et al. 1987) of experimental models of chemically induced neoplasms in mouse chimaeras showed that benign and malignant neoplasms, as well as precursors of these neoplasms, arise from the clonal expansion of a single transformed cell, and very few studies demur (Woodruff 1988). But we should note here the constraints of Williams & Wynford-Thomas (1994), who point to the considerable variation in patch size in these models and the possible differences in susceptibility to carcinogens between the two strains; possibly models where mice are heterozygous for a defective X-linked gene (Howell et al. 1985) or X-linked PGK polymorphisms (Tsunashima et al. 1996) are more reliable. More recently, Tatematsu et al. (1996a), using C3H/Balb/c chimaeras and a C3H strain-specific antigen (CSA) claim that the preneoplastic hyperplastic changes in the squamous forestomach of chimeric mice treated with methylnitrosourea (MNU) or diethylnitrosamine are polyclonal aggregates from which monoclonal squamous cell carcinomas are derived. Polyclonal carcinomas, which occurred at low incidence (2/62) during progression, were dismissed as being due to two or more lesions coalescing. The patch size is considerable, not measured, but up to 5 rete pegs in diameter (see Figure 2). But in focal epidermal hyperplasias initiated with DMBA or acetone in CBA/C57Bl chimaeric mice, and promoted with TPA or DMBA, some 20% were polyphenotypic, using anti-Hsk and H2b antibodies as discriminating markers (Winton et al. 1989). Of the early papillomas which arose from these preneoplastic lesions, some 25% appeared polyclonal: this would be a minimum value, recalling the caveat about patch margins and patch size, which was about 100 basal cells in diameter.
Using the same marker, Tatematsu et al. (1994) also showed that experimentally induced adenomatous hyperplasias and carcinomas in the mouse stomach were apparently clonal proliferations, although later, clones emerged in the carcinomas which displayed different differentiation features. Furthermore, Tatematsu et al. (1996b) found only one polyclonal adenoma in 91 adenomas and 12 polyclonal adenocarcinomas in a total of 119 colon tumours induced by dimethylhydrazine in chimaeric mice, results interpreted in terms of a clonal origin of colonic adenomas: the mixed tumours were all explained by coalescence. Previous results with azoxymethane (Ponder & Wilkinson 1986) indicated that small, carcinogen-induced dysplastic foci in chimaeric mice were monoclonal, although 5/17 larger lesions were polyclonal, explained as collision tumours. These observations led Farber (1997) to conclude that individual cancers are derived initially or early on from single cells showing monoclonal growth.
In the human, there are many articles claiming a clonal origin for tumours (see review by Fialkow 1976). In more recent years, X-linked restriction fragment length polymorphisms showed 50 human colorectal tumours, adenomas and carcinomas, of both familial and sporadic type to be monoclonal (Vogelstein et al. 1985, 1987; Fearon et al. 1987). However, because the tumours were of sufficient size to allow microdissection, these results are consistent either with a true clonal origin, or a polyclonal proliferation with later outgrowth of a dominant tumour clone. The ability to use X-linked polymorphic markers for the HUMARA or PGK genes in microdissected, paraffin-embedded tissues has again shown apparent clonality in many tumours and tumour-like lesions: sclerosing haemangioma of the lung (Niho et al. 1998), with both pale and cuboidal cells sharing the same clonal origin; most gynaecological cancers — cervix, endometrium, fallopian tube and ovary appear clonal (Jacobs et al. 1992; Enomoto et al. 1994), including pseudomyxoma peritonei, which however, appears usually associated with a mucinous adenoma of the appendix rather than the ovary (Szych et al. 1999). Leiomyomas of the uterus (Nilbert & Strombeck 1992; Mashal et al. 1994; Hashimoto et al. 1995) and Wilm's tumour (Vogelstein et al. 1987; Wilimas et al. 1991) also appear to be clonal proliferations. Most pituitary adenomas are clonal in X-inactivation studies (Alexander et al. 1990; Herman et al. 1990), and gastric carcinomas are also reported as clonal (Cho et al. 1994). Immature teratomas are clonal by X-inactivation analysis (Sinnock et al. 1996), while malignant teratoma in men contains the i(12p) clonal marker in all derived malignant lineages (Motzer et al. 1998); combined seminomatous/nonseminomatous germ cell tumours in males are also clonal (Gillis et al. 1994). Basal cell carcinomas are clonal by X-inactivation (Walsh et al. 1996, 1998) and by the demonstration of a ‘founder’ p53 mutation in multiple samples from the same tumour (Ponten et al. 1997).
Mixed tumours, which consist of both epithelial and stromal components, have for some time generated considerable debate about their histogenesis. Carcinosarcomas pose a special and interesting problem in clonality (Wick & Swanson 1993). The available evidence, from commonality of p53 protein immunohistochemistry in both malignant lineages, would support the same clonal origin for both (Mayall et al. 1994), providing objections, such as the possible unreliability of immunohistochemistry in detecting mutated p53, or the presence of different mutations, or indeed no mutations at all being present, can be met (Williams & Wynford-Thomas 1994). However, cell disaggregation of carcinosarcomas, with consequent cell sorting and clonality determination by the X-linked HPRT gene also showed the same clonal origin for both lineages (Thompson et al. 1996). Further X-inactivation and cytogenetic studies on carcinosarcomas from multiple sites (Thompson et al. 1996), from the pancreas (Millis et al. 1994) and from the breast (Zhuang et al. 1997; Teixeira et al. 1998) confirm the same clonal origin for both components. An oesophageal carcinosarcoma showed the unusual production of granulocyte colony stimulating factor in both components, suggesting a common origin (Ota et al. 1998). Zhuang et al. (1997) noted co-ordinated LOH for int2 and NM23 in the epithelial and stromal components of a breast carcinosarcoma, and the finding of the same clonal pattern in the in situ epithelial component favours the view that the epithelial component contains the progenitor cell. Finally, we should note that mixed Mullerian tumours in the female genital tract show the same clonal p53 mutation in all components (Kounelis et al. 1998).
In other apparently mixed tumours, such as pulmonary chondromatous hamartomas, combined chromosome banding and immunohistochemistry on disaggregated tissues revealed that only the stroma possessed a clonal cytogenetic abnormality, and thus was possibly neoplastic (Fletcher et al. 1993). Paraganglionomas, a biphasic tumour which often shows a familial element and allelic imbalance on 11q, appear polyclonal on X-inactivation analysis (Devilee et al. 1994). We have seen that gliomas are themselves clonal (Kattar et al. 1997), and furthermore, biphasic gliomas are clonal (Kattar et al. 1997) as are gangliogliomas (Zhu et al. 1997).
Mixed tumours of the breast such as fibroadenomas, have presented special problems: several studies have shown karyotypic abnormalities (e.g. Calabrese et al. 1991; Dietrich et al. 1995), but opinion is divided as to where these occur — either in the epithelial (Dietrich et al. 1995) or stromal component (Belda et al. 1993; Fletcher et al. 1993). In the related phyllodes tumour, where the stromal component is cellular and can be atypical in appearance, the stroma and not the epithelium shows clonal expansion (Noguchi et al. 1993). The same PGK technique applied to fibroadenomas showed that both epithelium and stroma were polyclonal, which is really inconsistent with the claim that such lesions develop from a single lobule if patch size in the breast comprises the terminal ductal-lobular unit (Tsai et al. 1996). However, others (Dietrich et al. 1994; Petersson et al. 1997) have demonstrated deletion of 3p and gain of 1q in the epithelial portion of phyllodes tumours, and multiple cytogenetically abnormal and unrelated clones have been identified in breast fibroadenomas (Stephenson et al. 1992; Diettrich et al. 1995).
Tumours are polyclonal in origin
Not everyone has accepted uncritically the clonal origin of neoplasms, insisting instead that carcinogenesis requires the interactions of multiple cells (Rubin 1985; Alexander 1985), with outgrowth of a dominant clone during subsequent development. Others have pointed to the marked heterogeneity of tumour cell populations in regard to both their morphology and metastatic behaviour (Poste et al. 1981), notwithstanding the view that such phenotypic diversity could develop within a clonal population (Marsh et al. 1993). Indeed we have seen that some recent studies using aggregation chimeras support the concept of a polyclonal origin: the earliest morphologically recognizable stages of development of mouse skin papillomas induced by a chemical initiation-promotion regime are polyclonal, suggesting that interaction between cells of more than one clone is involved (Winton et al. 1989).
Mammary tumours and hyperplasias arising in mice appear to be clonal populations (Cardiff & Aguilar-Cordova 1988; Kordon et al. 1995; Kordon & Smith 1998), and we have seen above that the preneoplastic lesions in the breast and breast cancer itself in man also appear to be clonally derived (Noguchi et al. 1992). However, cytogenetic analysis of multifocal and bilateral breast carcinomas showed independent origins (Shibata et al. 1996a), and indeed unrelated clones, prompting the suggestion that breast carcinoma may be polyclonal (Teixeira et al. 1994; Pandis et al. 1995). It is surprising how often the pattern of chromosomal aberrations in breast cancer is nonrandom: in fact, cytogenetically unrelated clones are found in some 50% of breast cancers (Heim 1992; Pandis et al. 1993, 1995; Teixeira et al. 1994, 1995) and include all the three most common breast cancer deletions — del(1:16, i(1q) and del(3p). Most breast cancers are cytogenetically complex when this is related to anatomical site (Heim et al. 1997), and because proliferative and preneoplastic breast lesions also contain similar unrelated clones, it maybe that such clonal divergence is an early phenomenon (Heim et al. 1997).
Now, does the finding of multiple unrelated clones in breast cancer imply a polyclonal origin of the tumour? Well, Heim et al. (1997) and Teixeira et al. (1996) point to the result that when no histological evidence of tumour is visible, then no chromosomal abnormalities are present in mammary epithelia, but we have seen that histologically normal breast tissue can show LOH at potential tumour suppressor gene loci (Larson et al. 1998). Thus somatic cell mosaicism is present in normal breast and could, of course, be the origin of the independent clones by clonal divergence. Are the clones really unrelated? Heim et al. (1997) emphasize the evidence that breast cancers show a far higher incidence of cytogenetic polyclonality than haematological (Heim & Mittelman 1989) or mesenchymal neoplasms (Orndal et al. 1994). X-inactivation demonstrations of clonality (Noguchi et al. 1992) with skewing towards one X chromosome was regarded as being ‘eminently compatible with multiple clones of various size’, and clonal proliferation of cells as shown by X-inactivation could occur with different oncogene or tumour suppressor gene mutations, even in premalignant cells as part of field cancerization (Chung et al. 1993). Thus if X-inactivation is an early marker of clonal relationships, later genetic events may then show disparate patterns in a tumour(s) which are still clonally related (Fey & Tobler 1996). Heterogeneity in a marker (cf. clonal LOH demonstrated by microsatellite analysis) is no unequivocal demonstration of independent clonal origin, since such genetic heterogeneity could mark clonal relationships as subclones (Sidransky et al. 1992a; Fey & Tobler 1996). Thus molecular heterogeneity within and between lesions, as a result of clonal evolution, does not predict clonal independence.
However, an interesting development is the recent study of an acinic cell tumour, a low grade salivary carcinoma, which might provide at least a partial answer to this problem. Subcultures containing two cytogenetically independent clones were shown to have different X-chromosome inactivation patterns, showing that the clones must have originated from different cells (Jin et al. 1998): true demonstration of a polyclonal origin? But in gastrointestinal cancers showing a uniform X-inactivation pattern, LOH at 18q and clonal microsatellite mutations which differed markedly between defined areas would be expected on the basis of clonal evolution, urging a note of caution, at least where these markers are concerned (Nagel et al. 1995). Heterogeneity in c-k-ras mutations in multiple regions of the same tumour occurs in human adenomas in that substantial areas do not show mutated k-ras, but interestingly, this is not seen in carcinomas, which are uniformly positive, and also show mutated p53 (Shibata et al. 1993). Adenoma formation may include a stage in which multiple and genetically distinct neoplastic clones are present, while most carcinomas appear to have a homogeneous composition that results from the successful progression of one clone. This concept has been recently reinforced by the observation that clonal divergence and DNA aneuploidy seems to take place during the transition from adenoma to carcinoma (Tollenaar et al. 1997).
MMR deficient mice have been generated by targeted gene disruption (De Wind et al. 1998) of the Msh2 gene: introduction of the Apcmin allele leads to the development of intestinal tumours with loss of the wild type Msh2 allele in a significant fraction of the Apc+/min/ Msh+/− mice, and some areas of the same tumours showing loss of the Msh+/+allele but not of the Apc+ allele (De Wind et al. 1998). Other areas were Msh2 positive and showed LOH for Apc. These findings indicate a biclonal origin for these tumours, arguing against speculation (Jass et al. 1994), with loss of Apc and Msh2 in separate ancestor cells. Since only three tumours were found per animal, coalescence is an unlikely event, and suggests that RER-and RER + tumour areas develop interdependently and have a different clonal origin, rather than loss of MMR being a late event in the genesis of HNPCC. Again this biclonality was interpreted as showing a requirement for collaboration between independent tumour clones during carcinogenesis. In this respect, Palacios (1984) has interestingly hypothesized that bi-clonal interaction, analogous to that underlying embryogenesis, could be the origin of the earliest malignant phenotype.
These results have recently been confirmed (Merrit et al. 1997) using a model in which a chimaera was made from a Min mouse, which has an Apc mutation, and a Min/ROSA mouse, allowing the clonality of the resulting intestinal tumours to be assessed; 79% of adenomas were polyclonal. Moreover, there was evidence that the neoplastic proliferations lost the wild-type Apc allele in both lineages: both quantitative PCR for the Min region of APC and immunohistochemistry for the APC gene product showed that all cell lineages within the polyclonal adenomas had lost APC expression. This is in contrast to the findings of Bjerknes et al. (1997) who reported that adenomatous crypts from FAP patients contained a mixture of APC + and APC-cells. Thus in these mouse models, adenomas appear distinctly polyclonal, unlike chemically induced lesions.
In the patient reported by Novelli et al. (1996) the colon contained hundreds of adenomas, ranging in size from monocryptal adenomas to microadenomas of 2.5 mm in diameter (Figure 4a,b); no larger adenomatous polyps were seen. If an adenoma were of clonal origin, all dysplastic crypts within it would be expected to be entirely XO or entirely XY. NISH showed that monocryptal adenomas were entirely XO or XY, with no mixed pattern (Figure 4a); this is perhaps not surprising, given the clonal nature of normal crypts. However, many adenomas — 76% — were polyclonal. Isoenzyme studies of G6PD in black females have shown that colonic adenomas from three patients with Gardner syndrome were polyclonal (Hsu et al. 1983), while X-linked RFLPs showed that both spontaneous and familial adenomas were clonal (Fearon et al. 1987); the minimum size of the adenomas in this latter study was 3–4 mm, much larger than the very early lesions examined by Novelli et al. (1996), which might explain the discrepancy, through monoclonal conversion.
Figure 4.
(a) A monocryptal adenoma from an XO/XY individual with FAP. These lesions are always monophenotypic, and (b) a microadenoma from the same individual, showing adenomatous crypts of different clonal derivation (from Novelli et al. 1996, with permission).
Novelli et al. (1996) and Merritt et al. (1997) have speculated on possible mechanisms of polyclonality in these lesions: ‘field’ effects may cause adenomas to cluster (nonrandom collision), a passive process involving fusion of two or more APC-negative clones early in tumour development. The high frequency of mixed adenomas found in both these studies is inconsistent with a random appearance of APC-negative clones in the mucosa, and suggests instead that some regions of the intestine have an increased potential for initiation. Multiple adenomatous clones may be required for (or strongly favour) early adenoma growth; or perhaps more likely, early adenomas may induce adenomatous growth in surrounding crypts (this may apply especially in FAP since all cells already have a single APC mutation and perhaps some derangement of APC function). These latter scenarios imply active co-operation between clones.
The importance of these results may have basic implications for more generalized models of tumorigenesis (Merritt et al. 1997). If in excess of one crypt must be involved to produce an adenoma, this would predict that not one but two hits would be required in FAP and four hits in sporadic adenomas. We should perhaps note that adenomas in FAP do not make their appearance until several years after birth, even in an environment where dietary carcinogens are common, and a two or three-hit model does not fit the age incidence for colorectal cancers (Moolgavkar & Luebeck 1992). This hypothesis would suggest that at least four hits were required. In respect of FAP, it is of note that desmoid tumours, which contain an APC mutation (Sen Gupta et al. 1993) are clonal (Lucas et al. 1997) as are desmoid fibromatoses (Li et al. 1995).
The concept of interactions between different neoplastic clones as a prerequisite for tumour development has been underlined by Muller et al. (1988), in an analysis of multiple preneoplastic or neoplastic lesions in transgenic mice, to assess the stepwise progression of carcinogenesis in mammary epithelium. Unlike the stochastic occurrence of solitary mammary tumours in transgenic mice bearing the MMTV/c-myc or the MMTV/v-Ha- ras oncogenes, transgenes uniformly expressing the MMTV/c-neu gene, an activated c-neu oncogene driven by a mouse mammary tumour virus (MMTV), develop mammary adenocarcinomas that involve the entire epithelium in each gland. These tumours arise synchronously and are polyclonal in origin. In contrast, expression of c-neu transgene in the parotid gland or epididymis leads to benign, bilateral hypertrophy and hyperplasia which does not progress to full malignant transformation during the observation period. These results indicate that the combination of activated oncogene and tissue context are major determinants of malignant progression and that expression of the activated form of c-neu in the mammary epithelium has particularly deleterious consequences.
Clonal evolution of epithelial tumours and its relationship to determination of tumour clonality
The concept of sequential mutations arizing in tumour cells as neoplastic progression proceeds is now well-engrained in our consciousness (Vogelstein et al. 1987; Jen et al. 1994), This concept of clonal evolution (Nowell 1976, 1986) has profound implications for our perceptions of tumour clonality, both from the viewpoint of possible regression to monoclonality through clonal proliferative advantage (Woodruff et al. 1982) and for clonal divergence (Symmans et al. 1995). Thus single tumours studied during sequential recurrence, while retaining the changes seen ab initio, develop multiple karyotypic abnormal clones. We have already noted how this genetic heterogeneity does not predict clonal independence, and the coexistence of these clones of sequentially mutated cells (Figure 1) give insights into the mechanisms of tumour heterogeneity (Heppner 1984; Fey & Tobler 1996) particularly where the distribution of subclones is concerned. A corollary of this is that clonality assessment is constrained to the time of analysis, and the clonal composition may of course change in the course of clonal evolution (Van Elsas et al. 1995; Balaban et al. 1986; Orndal et al. 1993; Fey & Tobler 1996; Jin et al. 1998).
It is very clear that clonal evolution occurs in tumours as they become invasive and metastasize (Gotoh et al. 1998; Hovey et al. 1998; Pandis et al. 1998). At its simplest level, the incidence of aneuploid stem cells increase from 30% in small colorectal adenomas to 82% after invasion (Tollenaar et al. 1997), while larger adenomas also show the development of cytogenetically distinct subclones (Bomme et al. 1998). This probably reflects the distribution of p53 mutations in these lesions, i.e. in high grade dysplastic and invasive lesions, since immunohistochemically detectable p53 is correlated with an increased frequency of aneuploid stem lines (Carder et al. 1995), indicating that clonal p53 mutation precedes clonal divergence in colorectal tumour progression. Microsatellite LOH analysis for 5q, 17p and 18q also indicates a rapid clonal expansion at this stage (Boland et al. 1995): most early adenomas with mild dysplasia show LOH for 5q, reflecting APC allele loss, with a few showing 18q allele loss, but it is not until the development of high grade dysplasia that 17p allele loss, indicating p53 allele loss, was detected. This reflects abrupt waves of clonal expansion during adenoma development, with clonal homogeneity (for 5q in early adenomas, but clonal heterogeneity with widespread allelic instability in lesions with high grade dysplasia, Young et al. 1993). However, where the mutator phenotype is concerned, greater genetic diversity can be expected in older regions of the tumour (Shibata et al. 1994, 1996b; Shibata 1998). Rapid clonal expansion is thus associated with relatively homogeneous microsatellite alleles, whereas older lesions should show a higher number of alleles — while considerable diversity was seen in a colorectal carcinoma, the antecedent adenoma showed greatest variation.
Allele loss seen in the clonal ductal carcinoma in situ (DCIS) in the breast is shared by the derivative and clonal invasive and metastatic component (Noguchi et al. 1994b; Radford et al. 1995), strongly suggesting a clonal relationship between these lesions, but other changes, cf. LOH on 11p, can be restricted to the invasive component; such observations obviously should not be interpreted in terms of a polyclonal process.
While early mutations might be expected to be present in all alleles, mutations confined to subclones might therefore be restricted to particular geographical areas: implicit is the concept that regional (in the tumour that is) separation of subclones occurs (Ajiki et al. 1994), a situation which does not occur in prostatic carcinoma, where cytogenetic heterogeneity is a prominent feature but clonal cytogenetic aberrations are apparently confined to advanced prostatic carcinomas (Micale et al. 1992; Henke et al. 1994). Both the overall LOH rate and the mean fractional allele loss are lower in grade 1 compared with grade 2 and 3 areas in prostatic cancers, with increased frequency of loss of Rb, DCC, BBC1, and p53 in grade 2 and 3 areas, suggesting clonal development (Hugel & Wernert 1999). Using chromosome 1 probes and FISH, Alers et al. (1995) showed not only intraregional but intraglandular cytogenetic heterogeneity, reflecting prominent interspersion of these subclones.
Any one colorectal tumour (Carder et al. 1995) shows a clonal p53 mutation, and this may favour the emergence of aneuploid subclones in carcinomas. The overgrowth of low-grade astrocytomas by cells with acquired p53 mutations, already present in such low grade lesions, is associated with movement to a more aggressive glioblastoma habitus (Fults et al. 1992; Leenstra et al. 1992; Sidransky et al. 1992b; Tada et al. 1996). The pattern of p53 mutations in any one advanced gastric carcinoma is also constant (Cho et al. 1994), although in basal cell carcinomas, subclones arise with a second or even a third p53 mutation (Ponten et al. 1997), explained again by clonal evolution rather than polyclonality, since all samples from the same tumour possessed a unique ‘founder’ mutation in p53. Clonal evolution in squamous carcinoma of the lung shows additional and different p53 mutations arising over a 9-month period (Chung et al. 1996), also seen in some colorectal carcinomas (Kuwabara et al. 1998). The distribution of k-ras mutations in colorectal adenomas is heterogeneous, but in invasive lesions becomes homogeneous, reflecting clonal evolution during growth (Shibata et al. 1993, 1994; Dix et al. 1995), but variation is also seen in k-ras mutations in adenomas and the resulting carcinoma (Ohmura & Hattori 1996). Thus a serious implication for clonal studies in tumours is the possibility of mutation in a marker gene, changing its nature or expression in a subclone, and indicating polyclonality in a clonally derived population, or indeed leading to apparent monoclonal regression, both seen in experimental (Griffiths et al. 1988) and human studies (Beutler et al. 1967; Williams & Wynford-Thomas 1994).
Conclusions
It is difficult to come to a firm general conclusion. While most evidence does favour the single cell origin of human tumours, many investigators have been less than critical in their experimental analysis, have ignored aspects of basic tissue architecture, and have often over-interpreted the data. On the other hand, protagonists of polyclonality have often ignored clonal evolution as a concept. The relationship between the clonal architecture of normal tissues, preneoplastic and preinvasive lesions, and their ensuing tumours is complex and difficult to analyse. This review underlines the need for the development of more general markers that can be used to visualize these relationships directly in space and time.
Acknowledgments
SB Garcia was funded by CNPq, Brazil. This work was supported by the Imperial Cancer Research Fund.
References
- Aeschimann S, Kopp P, Kimura ET, et al. Morphological and functional polymorphism within clonal thyroid nodules. J. Clin. Endocrinol. Metab. 1992;77:846–851. doi: 10.1210/jcem.77.3.8370709. [DOI] [PubMed] [Google Scholar]
- Ahomadegbe JC, Barrois M, Fogel S. High incidence of p53 alterations (mutation, deletion, overexpression) in head and neck primary tumours and metasteses; absence of correlation with clinical outcome, frequent protein overexpression in normal epithelium and in early non-invasive lesions. Oncogene. 1995;10:1217–1227. [PubMed] [Google Scholar]
- Aihara T, Hoguchi S, Sasaki Y, Hakano H, Imoaka S. Clonal analysis of regenerative nodules in hepatitis C virus-induced liver cirrhosis. Gastroenterol. 1994;107:1805–1811. doi: 10.1016/0016-5085(94)90824-9. [DOI] [PubMed] [Google Scholar]
- Ajiki T, Fujimori T, Ikehara H, Siatoh Y, Maeda S. k-ras gene mutation related to histological atypias in human colorectal adenomas. Biotechnic. Histochem. 1994;70:90–94. doi: 10.3109/10520299509108323. [DOI] [PubMed] [Google Scholar]
- Alers JC, Ktijtenburg PJ, Voissers CJ, Bosman FT, Van Der Kwast TK, Van Dekken H. Cytogenetic hetrogeneity and histologic tumour growth patterns in prostatic cancer. Cytometry. 1995;21:84–91. doi: 10.1002/cyto.990210116. [DOI] [PubMed] [Google Scholar]
- Alexander P. Do cancers arise from a single transformed cell or is monoclonality of tumours a late event in carcinogenesis? Brit. J. Cancer. 1985;51:453–457. doi: 10.1038/bjc.1985.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JM, Biller BMK, Bikkal H, Arnold A, Klibanski A. Clinically non-functioning pituitary tumours are monoclonal in origin. J. Clin. Invest. 1990;86:336–340. doi: 10.1172/JCI114705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki N, Robinson WS. State of hepatitis viral genomes in cirrhotic and hepatocellular carcinoma nodules. Mol. Biol. Med. 1989;6:395–408. [PubMed] [Google Scholar]
- Apel RL, Ezzat S, Bapat B, Pan N, Livolsi VA, Asa S. Clonality of thyroid nodules in sporadic goitre. Diagnostic Molec. Pathol. 1995;4:113–121. doi: 10.1097/00019606-199506000-00007. [DOI] [PubMed] [Google Scholar]
- Arnold A, Staunton CE, Kim HJG, Gaz RD, Kronenberg HM. Monoclonality and abnormal parathyroid hormone genes in parathyroid adenoma. New Eng. J. Med. 1988;318:658–662. doi: 10.1056/NEJM198803173181102. [DOI] [PubMed] [Google Scholar]
- Arnold A, Brown MF, Urena P, Gaz RD, Sarfati E, Drueke TB. Monoclonality of parathyroid tumors in chronic renal failure and in primary parathryroid hyperplasia. J. Clin. Invest. 1995;95:2047–2053. doi: 10.1172/JCI117890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban GB, Herlyn M, Clark WH, Nowell PC. Karyotypic evolution in human malignant melanoma. Cancer Genet. Cytogenet. 1986;19:113–122. doi: 10.1016/0165-4608(86)90378-x. [DOI] [PubMed] [Google Scholar]
- Ballouk F, Ross JS, Wolf BC. Ovarian endometriotic cysts: an analysis of cytlogical atypia and DNA ploidy. Am. J. Clin. Pathol. 1994;102:415–419. doi: 10.1093/ajcp/102.4.415. [DOI] [PubMed] [Google Scholar]
- Bamba M, Sugihara H, Okada K, Bamba T, Hattori T. Clonal analysis of superficial depressed-type gastric carcinoma in humans. Cancer. 1998;83:867–875. doi: 10.1002/(sici)1097-0142(19980901)83:5<867::aid-cncr10>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Bamburger AM, Bamburger CM, Barth J, Heidorn K, Kreipe H, Schulte HM. Clonal composition of thyroid nodules from patients with mutlinodular goiters: determination by X-chromosome inactivation analysis with M27beta. Exp. Clin. Endocrinol. 1993;101(Suppl.):73–90. [Google Scholar]
- Barsky SH, Grossman DA, Ho J, Holmes EC. The multifocality of brochioloalveolar lung carcinoma: evidence and implications of a multiclonal origin. Mod. Pathol. 1994;7:633–640. [PubMed] [Google Scholar]
- Baylin SB, Gann DS, Hsu SH. Clonal origin of inherited medullary thyroid carcinoma and phaeochromocytoma. Science. 1976;193:321–323. doi: 10.1126/science.935869. [DOI] [PubMed] [Google Scholar]
- Baylin SB, Hsu SH, Gann DS, Smallbridge RC, Wells SA. Inherited medullary thyroid carcinoma: a final monoclonal mutation in multiple clones of susceptible cells. Science. 1978;100:429–431. doi: 10.1126/science.619463. [DOI] [PubMed] [Google Scholar]
- Bedi GC, Westra WH, Gabrielson E, Koch W, Sidransky D. Multiple head and neck tumors: evidence for a common clonal origin. Cancer Res. 1996;56:2484–2487. [PubMed] [Google Scholar]
- Belda F, Lester SC, Pinkus JI, Pinkus GS. Lineage-restricted chromosome translocation in a benign fibrous tumour of the breast. Human Pathol. 1993;24:923–927. doi: 10.1016/0046-8177(93)90145-7. [DOI] [PubMed] [Google Scholar]
- Benditt EP, Benditt JM. Evidence for a monoclonal origin of human atherosclerotic plaques. Proc. Natl. Acad. Sci. USA. 1973;70:1753–1756. doi: 10.1073/pnas.70.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuschlein F, Reincke M, Karl M, et al. Clonal composition of human adrenocortical neoplasms. Cancer Res. 1994;54:4927–4932. [PubMed] [Google Scholar]
- Beutler E, Collins Z, Lowell E, Irwin MD. Value of genetic variants of glucose-6-phosphate dehydrogenase in tracing the origin of malignant tumours. New Engl. J. Med. 1967;276:389–391. doi: 10.1056/NEJM196702162760706. [DOI] [PubMed] [Google Scholar]
- Bjerknes M, Cheng H, Kim H. Clonality of dysplastic epithelium in colorectal adenomas from familial adenomatous polyposis patients. Cancer Res. 1997;57:355–361. [PubMed] [Google Scholar]
- Boland CR, Sato J, Appelman H, Bresalier RS, Feinberg AP. Microallelotyping defines the sequence and tempo of allelic losses at tumour suppressor gene loci during colorectal cancer progression. Nature (Medicine) 1995;9:902–909. doi: 10.1038/nm0995-902. [DOI] [PubMed] [Google Scholar]
- Bomme L, Bardi G, Pandis N, Fenger C, Kronberg O, Heim S. Cytogenetic analysis of colorectal adenomas: karyotypc comparisons of synchronous tumours. Cancer Genet. Cytogenet. 1998;106:66–71. doi: 10.1016/s0165-4608(98)00047-8. [DOI] [PubMed] [Google Scholar]
- Bostwick DG, Shan A, Qian J, et al. Independent origin of multiple foci of prostatic intrepithelial neoplasia. Cancer. 1998;83:1995–2002. doi: 10.1002/(sici)1097-0142(19981101)83:9<1995::aid-cncr16>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Boyd Y, Fraser NJ. Methylation patterns of the hypervariable X-chromosome locus DXS255 (M27β): correlation with X-inactivation status. Genomics. 1990;7:182–187. doi: 10.1016/0888-7543(90)90539-7. [DOI] [PubMed] [Google Scholar]
- Boyle JO, Hakim J, Koch W. The incidence of p53 mutations increases with progression of head and neck cancer. Cancer Res. 1993;53:4477–4480. [PubMed] [Google Scholar]
- Brennan JA, Mao I, Hrudan RH. Molecular assessment of histopathological staging in squamous cell carcinoma of the head and neck. New Engl. J. Med. 1995;332:429–435. doi: 10.1056/NEJM199502163320704. [DOI] [PubMed] [Google Scholar]
- Brentnall TA, Crispin DA, Bronner MP, et al. Microsatellite instability in non-neoplastic mucosa from patients with chronic ulcerative colitis. Cancer Res. 1996;56:1237–1240. [PubMed] [Google Scholar]
- Bressac B, Kew M, Wands J, Ozturk M. Selective G to T mutations of p53 gene in hepatocellular carcinoma from Southern Africa. Nature. 1991;350:429–433. doi: 10.1038/350429a0. [DOI] [PubMed] [Google Scholar]
- Calabrese G, Di Virgilio C, Cianchetti E, et al. Chromosome abnormalities in breast fibroadenomas. Genes Chromosomes Cancer. 1991;3:202–204. doi: 10.1002/gcc.2870030305. [DOI] [PubMed] [Google Scholar]
- Carder PJ, Cripps KJ, Morris R, White S, Bird CC, Wyllie AH. Mutation of the p53 gene precedes aneuploid clonal divergence in colorectal carcinoma. Br. J. Cancer. 1995;71:215–218. doi: 10.1038/bjc.1995.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardiff RD, Aguilar-Cordova E. Proto-neoplasia revisited: the molecular biology of mouse mammary hyperplasia. Anticancer Res. 1988;8:925–934. [PubMed] [Google Scholar]
- Casalone R, Granata P, Minelli E, et al. Cytogenetic analysis reveals clonal proliferation of smooth muscle cells in atherosclerotic lesions. Human Genet. 1991;87:139–143. doi: 10.1007/BF00204169. [DOI] [PubMed] [Google Scholar]
- Chaquai RF, Zhuang Z, Emmert-Buck MR, Liotta L, Merino M. Analysis of loss of heterozygosity on chromosome 11q13 in atypical duct hyperplasia and in situ carcinoma of the breast. Am. Pathol. 1997;150:297–303. [PMC free article] [PubMed] [Google Scholar]
- Chang WW. Morphological basis of multistep process in experimental colonic carcinogenesis. Virch Arch. B Cell Pathol. 1982;41:17–37. doi: 10.1007/BF02890268. [DOI] [PubMed] [Google Scholar]
- Chen D-S, Lai M-Y, Chang M-H, et al. Clonal origin of recurrent hepatocellular carcinomas. Gastroenterol. 1989;96:527–529. doi: 10.1016/0016-5085(89)91581-3. [DOI] [PubMed] [Google Scholar]
- Cho JH, Noguchi M, Ochiai A, Uchino S, Hirohashi S. Analysis of regional differences of p53 mutation in advanced gastric carcinoma: relation to heterogenous differentiation and invasiveness. Mod Pathol. 1994;7:205–211. [PubMed] [Google Scholar]
- Chung DH, Kang GH, Kim WH, Ro JY. Clonal analysis of a solitary follicular nodule of the thyroid with the polymerase chain reaction method. Mod. Pathol. 1999;12:265–271. [PubMed] [Google Scholar]
- Chung GTY, Sunderasan V, Hasleton P, Rudd R, Taylor R, Rabbitts P. Sequential molecular changes in lung cancer development. Oncogene. 1995;11:2591–2598. [PubMed] [Google Scholar]
- Chung GTY, Sundaresan V, Hasleton P, Rudd RT, Taylor R, Rabbitts PH. Clonal evolution of lung tumours. Cancer Res. 1996;56:1609–1614. [PubMed] [Google Scholar]
- Chung I-M, Schwartz SM, Murry CE. Clonal architecture of normal and atherosclerotic aorta. Implications for atherogenesis and vascular development. Amer. J. Pathol. 1998;152:913–923. [PMC free article] [PubMed] [Google Scholar]
- Chung NY, Mukhopahhyay T, Kim J, et al. Discordant p53 gene mutations in primary head and neck cancers and corresponding second primary cancers of the upper aerodigestive tract. Cancer Res. 1993;53:1676–1683. [PubMed] [Google Scholar]
- Dawson PJ. What is new in our understanding of multifocal breast cancer. Pathol., Res. Pract. 1993;189:111–116. doi: 10.1016/S0344-0338(11)80130-5. [DOI] [PubMed] [Google Scholar]
- Deamant RD, Iannoconne PM. Clonal origin of chemically-induced papillomas: separate analysis of epidermis and dermal components. J. Cell Sci. 1987;88:305–314. doi: 10.1242/jcs.88.3.305. [DOI] [PubMed] [Google Scholar]
- Deerlin PGV, Cekleniak N, Coutifaris C, Boyd J, Strauss JF. Evidence for the oligoclonal origin of the granulosa cell population of the mature human follicle. J. Clin. Endocrinol. Metab. 1997;82:3019–3024. doi: 10.1210/jcem.82.9.4208. [DOI] [PubMed] [Google Scholar]
- Deng G, Lu Y, Zlotnikov G, Thor AD, Smith HS. Loss of heterozygosity in normal tissue adjacent to breast carcinomas. Science. 1996;274:2057–2059. doi: 10.1126/science.274.5295.2057. [DOI] [PubMed] [Google Scholar]
- Delabesse E, Oksenhendler E, Lebbe C, Verola O, Varet B, Turhan AG. Molecular analysis of clonality in Kaposi's sarcoma. J. Clin. Pathol. 1997;50:664–668. doi: 10.1136/jcp.50.8.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilee P, Van Schothurst EM, Bardoel AFJ, et al. Allelotype of head and neck paraganglionomas: allelic imbalance is confined to the long arm of chromosome 11, the site of the predisposing locus PGL. Genes Chromosomes Cancer. 1994;11:71–78. doi: 10.1002/gcc.2870110202. [DOI] [PubMed] [Google Scholar]
- De Wind N, Dekker M, Berns A, Radman M, Te Riele H. Inactivation of the mouse MSH2 gene results in mismatch repair deficiency, methylation tolerance, hyper-recombination and predisposition to cancer. Cell. 1995;82:321–330. doi: 10.1016/0092-8674(95)90319-4. [DOI] [PubMed] [Google Scholar]
- De Wind N, Dekker M, Rossun Van A, Valk Van Der M, Riele HT. Mouse models for hereditary nonpolyposis colorectal cancer. Cancer Res. 1998;58:248–255. [PubMed] [Google Scholar]
- Dietrich CU, Pandis N, Bardi G, et al. Karyotypic changes in phyllodes tumors of the breast. Cancer Genet. Cytogenet. 1994;78:200–206. doi: 10.1016/0165-4608(94)90090-6. [DOI] [PubMed] [Google Scholar]
- Dietrich CU, Pandis N, Teixeeira MR, Bardi G, Gerdes A-M, Andersen J. Chromosome abnormalities in benign hyperproliferative disorders of epithelial and stromal breast tissue. Int. J. Cancer. 1995;60:49–53. doi: 10.1002/ijc.2910600107. [DOI] [PubMed] [Google Scholar]
- Dijkhuizen SMM, Entius MM, Clement MJ, et al. Multiple hyperplastic polyps in the stomach: evidence for clonality and neoplastic potential. Gastroenterol. 1997;112:561–566. doi: 10.1053/gast.1997.v112.pm9024310. [DOI] [PubMed] [Google Scholar]
- Ding SF, Jalleh RP, Wood CB, et al. Different DNA changes in primary and recurrent hepatocellular carcinoma. Gut. 1992;33:1433–1435. doi: 10.1136/gut.33.10.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix BR, Robbins PR, Spagnolo DV, Padovan GI, House AK, Iacopatte BJ. Clonal analysis of colorectal tumours using k-ras and p53 gene mutations as markers. Diag. Molec. Pathol. 1995;4:261–265. doi: 10.1097/00019606-199512000-00006. [DOI] [PubMed] [Google Scholar]
- Dolcetti R, Doglioni C, Maestro R, et al. p53 overexpression is an early event in the development of human squamous cell carcinoma of the larynx — genetic and prognostic implications. Int. J. Cancer Res. 1992;52:178–182. doi: 10.1002/ijc.2910520204. [DOI] [PubMed] [Google Scholar]
- Drya PT, Mukai S, Petersen R, Rapaport JM, Walton D, Yandell DW. Paternal origin of mutations in the retinoblastoma gene. Nature. 1989;339:556–558. doi: 10.1038/339556a0. [DOI] [PubMed] [Google Scholar]
- Emmert Buch MR, Vocke CD, Pozzatti RO, Duray PH, et al. Allelic loss on chromosome 8p12–21 in microdissected prostatic intraepithelial neoplasia. Cancer Res. 1995;55:2959–2962. [PubMed] [Google Scholar]
- Endo Y, Sugimara H, Kino I. Monoclonality of normal human colonic crypts. Pathol. Int. 1995;45:602–604. doi: 10.1111/j.1440-1827.1995.tb03509.x. [DOI] [PubMed] [Google Scholar]
- Enomoto T, Fujita M, Inoue M, Tanizawa O, Nomura T, Shroyer K. Analysis of clonality by amplification of short tandem repeats. Carcinomas of the female reproductive tract. Diag. Molec. Pathol. 1994;3:292–297. doi: 10.1097/00019606-199412000-00013. [DOI] [PubMed] [Google Scholar]
- Enomoto T, Haba T, Fujita M, et al. Clonal analysis of high-grade squamous intraepithelial lesions of the uterine cervix. Int J. Cancer. 1997;73:339–344. doi: 10.1002/(sici)1097-0215(19971104)73:3<339::aid-ijc6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Esteller M, Garcia A, Martinez-Palonesa JM, Xercavins J, Reventos J. Detection of clonality and genetic alterations in endometrial pipelle biopsy and its surgical specimen counterpart. Lab. Invest. 1997;76:109–116. [PubMed] [Google Scholar]
- Esumi M, Aritaka T, Arii M. Clonal origin of human hepatoma determined by integration of hepatitis B virus DNA. Cancer Res. 1986;46:5767–5771. [PubMed] [Google Scholar]
- Esumi M, Tanaka Y, Tozuka S, Shikata T. Clonal state of human hepatocellular carcinoma and non-tumorous hepatocytes. Cancer Chemother. Pharmacol. 1989;23(Suppl.):1–3. doi: 10.1007/BF00647228. [DOI] [PubMed] [Google Scholar]
- Falchetti A, Bale LE, Amorosi C, et al. Progression of uraemic hyperparathyroidism involves allelic loss on chromosome 11. J. Clin. Endocrinol. Metab. 1993;76:139–144. doi: 10.1210/jcem.76.1.8421078. [DOI] [PubMed] [Google Scholar]
- Farber E. The multistep mature of cancer development. Cancer Res. 1984;44:4217–4223. [PubMed] [Google Scholar]
- Farber E. Clonal adaptation during carcinogenesis. Biochem. Pharmacol. 1990;39:1837–1846. doi: 10.1016/0006-2952(90)90599-g. [DOI] [PubMed] [Google Scholar]
- Farber E. Monoclonal and polyclonal development of digestive tract tumors in chimeric mice. Jap. J. Cancer Res. 1997;88:663. [PubMed] [Google Scholar]
- Fearon ER, Hamilton SR, Vogelstein B. Clonal analysis of human colorectal tumours. Science. 1987;238:193–197. doi: 10.1126/science.2889267. [DOI] [PubMed] [Google Scholar]
- Fearon ER. Molecular genetic studies of the adenoma-carcinoma sequence. Adv. Int. Med. 1994;39:123–147. [PubMed] [Google Scholar]
- Ferraris AM, Mangerrini R, Gaetani GF, Romei C, Pinchera A, Pacini F. Polyclonal origin of medullary carcinoma of the thyroid in multiple endocrine neoplasia type 2. Hum. Genet. 1997;99:202–205. doi: 10.1007/s004390050339. [DOI] [PubMed] [Google Scholar]
- Fey MF, Wells RA, Wainscoat JS. Assessment of clonality in gastrointestinal cancer by DNA fingerprinting. J. Clin. Invest. 1988;82:1532–1537. doi: 10.1172/JCI113762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey MF, Peter HJ, Hinds HL. Clonal analysis of human tumours with M27β, a highly informative polymorphic X-chromosome probe. J. Clin. Invest. 1992;89:1438–1444. doi: 10.1172/JCI115733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey MF, Tobler A. Tumour heterogeneity and clonality — an old theme revisited. Ann. Oncol. 1996;7:121–128. doi: 10.1093/oxfordjournals.annonc.a010537. [DOI] [PubMed] [Google Scholar]
- Fialkow PJ, Sagebiel RW, Gartler SM, Rimoin DL. Multiple cell origin of hereditary neurofibromas. N. Engl. J. Med. 1971;284:298–300. doi: 10.1056/NEJM197102112840604. [DOI] [PubMed] [Google Scholar]
- Fialkow P. Primordial cell pool size and lineage relationships of five human cell types. Ann. Hum. Genet. 1973;37:39–48. doi: 10.1111/j.1469-1809.1973.tb01813.x. [DOI] [PubMed] [Google Scholar]
- Fialkow PJ. Clonal origin of human tumours. Biochem. Biophys. Acta. 1976;458:283–321. doi: 10.1016/0304-419x(76)90003-2. [DOI] [PubMed] [Google Scholar]
- Fialkow PJ, Jackson CE, Block MA, Greenawald KA. Multicellular origin of parathyroid ‘adenomas’. New Engl. J. Med. 1977;297:696–698. doi: 10.1056/NEJM197709292971304. [DOI] [PubMed] [Google Scholar]
- Fletcher JA, Pinkus GS, Weidner N, Morton CC. Lineage-restricted clonality in biphasic solid tumours. Am. J. Pathol. 1993;138:1199–1207. [PMC free article] [PubMed] [Google Scholar]
- Fox H. Pathology of early malignant change in the ovary. Int. J. Gynec. Pathol. 1993;12:153–155. doi: 10.1097/00004347-199304000-00011. [DOI] [PubMed] [Google Scholar]
- Franklin WA, Gazdar AF, Haney J, et al. Widely dispersed p53 mutation in respiratory epithelium. J. Clin. Invest. 1997;100:2133–2137. doi: 10.1172/JCI119748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser NJ, Boyd Y, Craig I. Isolation and characterisation of a human variable copy number tandem repeat at Xcen-p11.22. Genomics. 1989;5:144–148. doi: 10.1016/0888-7543(89)90099-2. [DOI] [PubMed] [Google Scholar]
- Friedman E, Sakaguchi K, Bale AE, et al. Clonality of parathyroid tumours in familial multiple endocrine neoplasia type 1. N. Engl. J. Med. 1989;321:213–218. doi: 10.1056/NEJM198907273210402. [DOI] [PubMed] [Google Scholar]
- Fukuyama M, Hayashi Y, Iwasaki Y, et al. Epstein-Barr virus-associated gastric carcinoma and Epstein-Barr virus infection of the stomach. Lab. Invest. 1994;73:73–81. [PubMed] [Google Scholar]
- Fuller CE, Davies RP, Willaims GT, Willaims ED. Crypt-restricted heterogeneity of goblet cell mucus glycoprotein in histologically-normal human colonic mucosa: a potential marker of somatic mutation. Brit. J. Cancer. 1990;61:382–384. doi: 10.1038/bjc.1990.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fults D, Brockmeyer D, Tullous MW. p53 mutation and loss of heterozygosity on chromosomes 17 and 10 during human astrocytoma progression. Cancer Res. 1992;52:674–679. [PubMed] [Google Scholar]
- Gaffey MJ, Iezzoni JC, Weiss JM. Clonal analysis of focal nodular hyperplasia of the liver. Am. J. Pathol. 1996;148:1089–1096. [PMC free article] [PubMed] [Google Scholar]
- Gale RE, Wheadon H, Linch DC. Assessment of X-chromosome inactivation patterns using the hypervariable probe M27β in normal hemopoietic cells and acute myeloid leukaemia blasts. Leukemia. 1992;6:649–655. [PubMed] [Google Scholar]
- Gallo O, Bianchi S. p53 expression: a potential biomarker for risk of multiple primary malignancies in the upper aerodigestive tract. Eur. J. Cancer. 1995;31:53–57. doi: 10.1016/0964-1955(94)00031-x. [DOI] [PubMed] [Google Scholar]
- Garcia S, Park H-S, Novello M, Wright NA. Field cancerisation, clonality and epithelial stem cells; the spread of mutated clones in epithelial sheets. J. Pathol. 1999;187:43–61. doi: 10.1002/(SICI)1096-9896(199901)187:1<61::AID-PATH247>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gartler SM, Gandini E, Hutchinson HT, Campbell B, Zechhi G. Glucose-6-phosphate dehydrogenase mosaicism: utilisation of the study of hair follicles. Ann. Human Genet. 1971;35:1–7. [PubMed] [Google Scholar]
- Gill P, Tsai Y, Rao AP, Jones P. Clonality in Kaposi's sarcoma. N. Eng. J. Med. 1997;8:571–572. doi: 10.1056/NEJM199708213370813. [DOI] [PubMed] [Google Scholar]
- Gill PS, Tsai YC, Pao AP, et al. Evidence for multiclonality in multicentric Kaposi's sarcoma. Proc. Natl. Acad. Sci. USA. 1998;95:8257–8261. doi: 10.1073/pnas.95.14.8257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis AJM, Looijenga LHB, De Jong B, Oosterhuis JW. Clonality of combined testicular germ cell tumors of adults. Lab. Invest. 1994;71:874–878. [PubMed] [Google Scholar]
- Gleeson C, Sloan JM, McGuigan JA, Ritchie AJ, Weber JL, Russell SEH. Barrett's oesophagus: microsatellite analysis provides evidence to support the proposed metaplasia-dysplasia-carcinoma sequence. Genes Chromosomes Cancer. 1998;21:49–60. [PubMed] [Google Scholar]
- Gotoh T, Sugihara H, Matsumara T, Katsura K, Takamatsu T, Sawada T. Human neuroblastoma demonstrating clonal evolution in vivo. Genes Chromosomes Cancer. 1998;22:42–49. doi: 10.1002/(sici)1098-2264(199805)22:1<42::aid-gcc6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Govindarajan S, Craig JR, Valinluck B. Clonal origin of hepatitis B-associated hepatocellular carcinoma. Human Pathol. 1988;19:403–405. doi: 10.1016/s0046-8177(88)80488-x. [DOI] [PubMed] [Google Scholar]
- Green AJ, Sepp T, Yates JR. Clonality of tuberous sclerosis hamartomas shown by non-random X-chromosome inactivation. Human Genet. 1996;97:240–243. doi: 10.1007/BF02265273. [DOI] [PubMed] [Google Scholar]
- Griffiths DFR, Davies SJ, Williams D, Williams GT, Williams ED. Demonstration of somatic mutation and colonic crypt clonality by X-linked enzyme histochemistry. Nature. 1988;333:461–463. doi: 10.1038/333461a0. [DOI] [PubMed] [Google Scholar]
- Gusterson BA, Anbazhagan R, Warren W, et al. Expression of p53 in premaliganant and malignant squamous epithelium. Oncogene. 1991;6:1785–1789. [PubMed] [Google Scholar]
- Habuchi T, Takahashi R, Yamada H, Kakehi Y, Sugiyama T, Yoshida O. Metachronous, multifocal development of urothelial cancers by intraluminal seeding. Lancet. 1993;342:1087–1088. doi: 10.1016/0140-6736(93)92066-3. [DOI] [PubMed] [Google Scholar]
- Harada M, Suzuki M, Ikeda T, Kaneko T, Harad S, Fukayama M. Clonality in nevocellular nevus and melanoma: an expression-based clonality analysis at the X-linked genes by polymerase chain reaction. J. Invest. Dermatol. 1997;109:656–660. doi: 10.1111/1523-1747.ep12337678. [DOI] [PubMed] [Google Scholar]
- Harrer P, Brocker M, Zint A, Derwahlm Barbera L, Zumtobel V. The clonality of recurrent goiters at second surgery. Langenbecks Arch. Surg. 1998a;383:453–355. doi: 10.1007/s004230050159. [DOI] [PubMed] [Google Scholar]
- Harrer P, Broeker M, Zint A, Schatz H, Zumtobel V, Derwahl M. Thyroid nodules in recurrent multinodular goitres are predominantly polyclonal. J. Endocrinol. Invest. 1998b;21:380–385. doi: 10.1007/BF03350774. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Azuma C, Kamiura S, et al. Clonal determination of uterine leiomyomas by analysing differential inactivation of the X-chromosome-linked phosphoglycerokinase gene. Gynec. Obstet. Invest. 1995;40:204–208. doi: 10.1159/000292336. [DOI] [PubMed] [Google Scholar]
- Henke R-P, Kruger E, Ayhan N, Hubner D, Hammerer P. Frequency and distribution of numerical chromosomal aberrations in bladder cancer by in situ hybridisation. Human Pathol. 1994;25:476–484. doi: 10.1016/0046-8177(94)90119-8. [DOI] [PubMed] [Google Scholar]
- Heim S, Mittelman F. Cytogenetically-unrelated clones in hematological neoplasms. Leukaemia. 1989;3:6–8. [PubMed] [Google Scholar]
- Heim S, Caron M, Jin Y, Mandahl N, Mitelman F. Genetic convergence during serial in vitro passage of a polyclonal squamous carcinoma. Cytogenet. Cell Genet. 1989;52:133–135. doi: 10.1159/000132862. [DOI] [PubMed] [Google Scholar]
- Heim S. Is cytogenetics reducible to the molecular genetics of cancer cells? Genes Chromosomes Cancer. 1992;5:188–196. doi: 10.1002/gcc.2870050303. [DOI] [PubMed] [Google Scholar]
- Heim S, Teixiera MR, Dietrich CU, Pandis N. Cytogenetic polyclonality in tumors of the breast. Cancer Genet. Cytogenet. 1997;95:16–19. doi: 10.1016/s0165-4608(96)00322-6. [DOI] [PubMed] [Google Scholar]
- Heppner GH. Tumor heterogeneity. Cancer Res. 1984;44:2259–2265. [PubMed] [Google Scholar]
- Herman V, Fagin J, Gonsky R, Kovacs K, Melmed S. Clonal origin of pituitary adenomas. J. Clin. Endocrinol. Met. 1990;71:1427–1433. doi: 10.1210/jcem-71-6-1427. [DOI] [PubMed] [Google Scholar]
- Hicks DG, Livolsi VA, Neidich JA, Puck JM, Kant JA. Clonal analysis of solitary follicular nodules in the thyroid. Am. J. Pathol. 1990;137:553–562. [PMC free article] [PubMed] [Google Scholar]
- Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Hovey RM, Chu L, Balazs M, et al. Genetic alterations in primary bladder cancers and their metasteses. Cancer. 1998;58:3555–3560. [PubMed] [Google Scholar]
- Howell S, Wareham KA, Williams ED. Clonal origin of mouse liver cell tumours. Am. J. Pathol. 1985;121:426–432. [PMC free article] [PubMed] [Google Scholar]
- Hsu SH, Luk GD, Krish AJ, Hamilton SR, Hoover HH. Multiclonal origin of polyps in Gardner syndrome. Science. 1983;251:951–953. doi: 10.1126/science.6879192. [DOI] [PubMed] [Google Scholar]
- Hsu H-C, Chou T-Z, Chen J-Y, Lee C-S, Lee P-H, Peng S-Y. Clonality and clonal evolution of hepatocellular carcinoma with multiple nodules. Hepatology. 1991;13:923–928. [PubMed] [Google Scholar]
- Hugel A, Wernert N. Loss of heterozygosity (LOH), malignancy grade and clonality in microdissected prostate cancer. Br. J. Cancer. 1999;79:551–557. doi: 10.1038/sj.bjc.6690087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannaccone PM. Clone and patch size in chimaeric mouse skin. Math. Biosciences. 1980;51:117–123. [Google Scholar]
- Iannaccone PM, Weinberg WC, Deamant FD. On the clonal origin of tumors: a review of experimental models. Int. J. Cancer. 1987;39:778–784. doi: 10.1002/ijc.2910390621. [DOI] [PubMed] [Google Scholar]
- Ilyas M, Tomlinson IPM. Genetic pathways in colorectal cancer. Histopathology. 1996;28:389–399. doi: 10.1046/j.1365-2559.1996.339381.x. [DOI] [PubMed] [Google Scholar]
- Jacobs IJ, Kohler MF, Wiseman RW, et al. Clonal origin of epithelial ovarian carcinoma; analysis by loss of heterozygosity, p53 mutation, and X-chromosome inactivation. J. Natl. Cancer Inst. 1992;84:1793–1798. doi: 10.1093/jnci/84.23.1793. [DOI] [PubMed] [Google Scholar]
- Jackson CE, Cerny JC, Block MAS, Fialkow PJ. Probable clonal origin of aldosteronomas versus multicellular origin of parathyroid ‘adenomas’. Surgery. 1982;92:875–879. [PubMed] [Google Scholar]
- Jass JR, Stewart SM, Stewart J, Lane MR. Hereditary non-polyposis colorectal cancer — morphologies, genes and mutations. Mut. Res. 1994;310:125–133. doi: 10.1016/0027-5107(94)90016-7. [DOI] [PubMed] [Google Scholar]
- Jen J, Powell S, Papadopoulos N, et al. Molecular determinants of dysplasia in colorectal lesions. Cancer Res. 1994;54:5523–5526. [PubMed] [Google Scholar]
- Jiang X, Hitchcock A, Bryan EJ, et al. Microsatellite analysis of endometriosis reveals loss of heterozygosity at candidate ovarian tumour suppressor gene loci. Cancer Res. 1996;56:3534–3539. [PubMed] [Google Scholar]
- Jiang X, Yao KT. The clonal progression in the neoplastic process of nasopharygeal carcinoma. Biochem. Biophys. Res. Commun. 1996;221:122–128. doi: 10.1006/bbrc.1996.0556. [DOI] [PubMed] [Google Scholar]
- Jimbo H, Hitomi Y, Yoshikawa H, et al. Evidence for monoclonal expansion of epithelial cells in ovarian endometrial cysts. Am. J. Pathol. 1997;150:1173–1178. [PMC free article] [PubMed] [Google Scholar]
- Jin CY, Hoglund M, Wennerberg J, et al. Cytogenetic and molecular genetic demonstration of polyclonality in an acinic cell carcinoma. Brit. J. Cancer. 1998;78:292–295. doi: 10.1038/bjc.1998.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonason AS, Kunala S, Price GJ, et al. Frequent clones of p53-mutated keratinocytes in normal skin. Proc. Natl. Acad. Sci. USA. 1996;93:14025–14029. doi: 10.1073/pnas.93.24.14025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PA, Buckley JD. The role of DNA methylation in cancer. Adv. Cancer Res. 1990;54:1–23. doi: 10.1016/s0065-230x(08)60806-4. [DOI] [PubMed] [Google Scholar]
- Jones PA. DNA methylation errors and cancer. Cancer Res. 1996;56:2463–2467. [PubMed] [Google Scholar]
- Kang GH, Kim CJ, Kim WH, Kang YK, Kim HO, Kim YI. Genetic evidence for the multicentric origin of synchronous gastric carcinoma. Lab. Invest. 1997;76:407–417. [PubMed] [Google Scholar]
- Kasami M, Vnencak-Jones CL, Manning S, Dupont WD, Page DL. Loss of hetreozygosity and microsatellite instability in breast hyperplasia: no obligate correlation of these alterations with subsequent malignancy. Am. J. Pathol. 1997;150:1925–1932. [PMC free article] [PubMed] [Google Scholar]
- Kattar MM, Kupsky WJ, Shimoyama RK, et al. Clonal analysis of gliomas. Human Pathol. 1997;28:1166–1179. doi: 10.1016/s0046-8177(97)90255-0. [DOI] [PubMed] [Google Scholar]
- Kawai S, Imazeki F, Yokosuka O, et al. Clonality in hepatocellular carcinoma; analysis of methylation pattern of polymorphic X-chromosome-linked phosphoglycerate kinase gene in females. Hepatology. 1995;22:112–117. [PubMed] [Google Scholar]
- Kern SE. Clonality: more than just a tumor-progression model. J. Natl. Cancer Inst. 1993;85:1020–1021. doi: 10.1093/jnci/85.13.1020. [DOI] [PubMed] [Google Scholar]
- Kim Y-T, Thomas NF, Kessis TD, Wilkinson EJ, Hedrick L, Cho KR. p53 mutations and clonality in vulvar carcinoma and squamous hyperplasias; evidence suggesting that squamous hyperplasias do not serve as direct precursors of human papillomavirus-negative vulvar carcinomas. Human Pathol. 1996;27:389–395. doi: 10.1016/s0046-8177(96)90113-6. [DOI] [PubMed] [Google Scholar]
- Kim H, Piao Z, Park C, Chung WY, Park CS. Clinical significance of clonality in thyroid nodules. Brit. J. Surg. 1998;85:1125–1128. doi: 10.1046/j.1365-2168.1998.00783.x. [DOI] [PubMed] [Google Scholar]
- Kordon EC, Smith GH, Callahan R, Callahan D. A novel mouse non-mammary tumor virus activation of the Int-3 gene in a spontaneous mouse mammary tumor. J. Virol. 1995;69:8066–8069. doi: 10.1128/jvi.69.12.8066-8069.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordon EC, Smith GH. An entire functional mammary gland may comprise the progeny from a single cell. Development. 1998;125:1921–1940. doi: 10.1242/dev.125.10.1921. [DOI] [PubMed] [Google Scholar]
- Kopp P, Kimura ET, Aeschimann S, et al. Polyclonal and monoclonal thyroid nodules coexist within human mutlinodular goiters. J. Clin. Endocrinol. Metab. 1994;79:134–139. doi: 10.1210/jcem.79.1.7517946. [DOI] [PubMed] [Google Scholar]
- Kounelis S, Jones MW, Papdaki H, Bakker A, Swalsky P, Finkelstein SD. Carcinosarcomas (malignant mixed Mullerian tumors) of the femlae genital tract. Comparative molecular analysis of epithelial and mesenchymal components. Human Pathol. 1998;29:82–87. doi: 10.1016/s0046-8177(98)90394-x. [DOI] [PubMed] [Google Scholar]
- Krohn K, Fuhrer D, Holzappel Paschke R. Clonal origin of toxic thyroid nodules with constitutively-activating thyrotropin receptor mutations. J. Clin. Endocrinol. Metab. 1998;83:130–134. doi: 10.1210/jcem.83.1.4477. [DOI] [PubMed] [Google Scholar]
- Kupryjanezyk. J, Beuachamp R, Poremba C, Scully RE, Yandell DW. Ovarian, peritoneal and endometrial serous carcinoma: clonal orgin of multifocal disease. Mod. Pathol. 1996;9:31–49. [PubMed] [Google Scholar]
- Kuwabara A, Wanatabe H, Ajioka Y, et al. Alteration of p53 clonality accompanying colorectal cancer progression. Jpn. J. Cancer Res. 1998;89:40–46. doi: 10.1111/j.1349-7006.1998.tb00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhani SR, Collins N, Stratton MR, Sloane JP. Atypical ductal hyperplasia of the breast: clonal proliferation with loss of heterozygosity on chromosomes 16q and 17p. J. Clin. Pathol. 1995;48:611–615. doi: 10.1136/jcp.48.7.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhani SR, Slack DN, Hamoudi RA, Collins N, Stratton MR, Sloane JP. Detection of allelic imbalance indicates that a proportion of mammary hyperplasias of usual type are clonal neoplastic proliferations. Lab. Invest. 1996;74:129–135. [PubMed] [Google Scholar]
- Larson PS, Morenas A, Cupples LA, Huang K, Rosenberg CL. Genetically abnormal clones in histologically normal breast tissue. Am. J. Pathol. 1998;152:1591–1598. [PMC free article] [PubMed] [Google Scholar]
- Lee G-H, Nomura K, Kanda H, et al. Strain-specific sensitivity to diethylnitrosoamine-induced carcinogenesis is maintained in hepatocytes of C3H/HeN↔C57Bl/6N chimeric mice. Cancer Res. 1991;51:3257–3263. [PubMed] [Google Scholar]
- Leenstra S, Troost D, Westerwald A. Molecular characterisation of areas with low grade tumour or satellitosis in human astrocytomas. Cancer Res. 1992;52:1568–1572. [PubMed] [Google Scholar]
- Lemire JM, Covin CW, White S, Giachelli CM, Schwartz SM. Characterization of cloned aortic smooth muscle cells from young rats. Am. J. Pathol. 1994;144:1068–1081. [PMC free article] [PubMed] [Google Scholar]
- Leung SY, Chung LP, Yuen ST, Ho CM, Wong MP, Chan SY. Lymphoepithelial carcinoma of the salivary gland: in situ detection of Epstein-barr virus. J. Clin. Pathol. 1995;48:1022–1027. doi: 10.1136/jcp.48.11.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Cordon-Cardo C, Gerald WL, Rosai J. Desmoid fibromatosis is a clonal process. Human Pathol. 1996;27:939–943. doi: 10.1016/s0046-8177(96)90221-x. [DOI] [PubMed] [Google Scholar]
- Li S, Han H, Resnik E, Carcangiu ML, Schwartz PE, Yang-Feng TL. Advanced ovarian carcinoma: molecular evidence of unifocal origin. Gynec. Oncol. 1993;51:21–25. doi: 10.1006/gyno.1993.1240. [DOI] [PubMed] [Google Scholar]
- Liebow A. Brochiolo-alveolar carcinoma. Adv. Intern. Med. 1960;10:358–367. [PubMed] [Google Scholar]
- Linder G, Gartler SM. Distribution of glucose-6-phosphate dehydrogenase electrophoretic variants in different tissues of heterozygotes. Am. J. Hum. Genet. 1965a;45:212–220. [PMC free article] [PubMed] [Google Scholar]
- Linder G, Gartler SM. Glucose-6-phosphate dehydrogenase mosaicism: utilisation as a cell marker for the study of leiomyomas. Science. 1965b;150:67–69. doi: 10.1126/science.150.3692.67. [DOI] [PubMed] [Google Scholar]
- Liu Y, Phelan J, Go RCP, Prchal J, Prchal J. Rapid determination of clonality by detection of two closely-linked X chromosome exonic polymorphisms using allele-specific PCR. J. Clin. Invest. 1997;99:1984–1990. doi: 10.1172/JCI119366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Coco F, Pelicci PG, D'adamo F. Polyclonal hemopoietic reconstitution in leukemia patients at remission after suppression of specific gene rearrangements. Blood. 1993;82:606–612. [PubMed] [Google Scholar]
- Lu J, Jiang C, Mitrenga T, Cutter G, Thompson HJ. Pathogenetic characterisation of 1-methyl-1-nitrososurea-induced mammary carcinomas in the rat. Carcinogenesis. 1998;19:223–227. doi: 10.1093/carcin/19.1.223. [DOI] [PubMed] [Google Scholar]
- Lu QL, Elia G, Thomas JA. Bcl-2 proto-oncogene expression in Epstein-Barr virus associated nasopharyngeal carcinoma. Int. J. Cancer. 1993;53:29–35. doi: 10.1002/ijc.2910530107. [DOI] [PubMed] [Google Scholar]
- Lubensky IA, Debelanko LV, Zhuang Z, et al. Allelic deletions on chromosome 11q13 in multiple tumors from individual MEN1 patients. Cancer Res. 1996;56:5272–5278. [PubMed] [Google Scholar]
- Lucas DR, Shroyer K, McCarthy PJ, Markham NE, Fujita M, Enomoto TE. Desmoid tumour is a clonal cellular proliferation: PCR amplification of HUMARA for analysis of patterns of X-chromosome inactivation. Am. J. Surg. Pathol. 1997;21:306–311. doi: 10.1097/00000478-199703000-00006. [DOI] [PubMed] [Google Scholar]
- Lunec J, Challen C, Wright C, Mellon K, Neal D. c-erB2 amplification and identical p53 mutations in concomitant transitional carcinomas of renal pelvis and urinary bladder. Lancet. 1992;339:439–440. doi: 10.1016/0140-6736(92)90135-p. [DOI] [PubMed] [Google Scholar]
- Lyon MF. X-chromosome inactivation and developmental patterns in mammals. Biol. Rev. 1972;47:1–35. doi: 10.1111/j.1469-185x.1972.tb00969.x. [DOI] [PubMed] [Google Scholar]
- McCaffrey TA, Du B, Consigli S, et al. Genomic instability in the type II TGFβ1 receptor gene in atherosclerotic and restenotic vascular cells. J. Clin. Invest. 1997;100:2182–2188. doi: 10.1172/JCI119754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain KL, Jenson HB, Joshi VV, et al. Association of Epstein-Barr virus with leiomyosarcomas in young people with AIDS. N. Engl. J. Med. 1995;332:12–18. doi: 10.1056/NEJM199501053320103. [DOI] [PubMed] [Google Scholar]
- Malamou-Mitsu VD, Krikoni OC, Agnantis NJ. Clonal analysis by PCR and RFLP in breast cancer and precancerous lesions. Preliminary data. Anticancer Res. 1996;16:3943–3948. [PubMed] [Google Scholar]
- Mandard AM, Marnay J, Lebeau C, Banard S, Mandard JC. Expression of p53 in oesophageal epithelium from surgical specimens resected for squamous carcinoma of the oesophagus, with special reference to uninvolved mucosa. J. Pathol. 1997;181:153–157. doi: 10.1002/(SICI)1096-9896(199702)181:2<153::AID-PATH743>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Marsh KA, Stamp GW, Kirkland SC. Isolation and characterisation of multiple cell types from a single human colonic carcinoma cell; tumourigenicity of these cell types in a xenograft system. J. Pathol. 1993;170:441–450. doi: 10.1002/path.1711700407. [DOI] [PubMed] [Google Scholar]
- Mashal RD, Fezjo MLS, Friedman AJ, et al. Analysis of androgen receptor DNA reveals independent clonal origins of uterine leiomyomata and the secondary nature of cytogenetic aberrations in the development of leiomyomata. Genes Chromosomes Cancer. 1994;11:11–16. doi: 10.1002/gcc.2870110102. [DOI] [PubMed] [Google Scholar]
- Mayall F, Rutty K, Campbell F, Goddard H. p53 immunostaining suggests that uterine carcinosarcomas are monoclonal. Histopathology. 1994;24:211–214. doi: 10.1111/j.1365-2559.1994.tb00512.x. [DOI] [PubMed] [Google Scholar]
- Merritt AJ, Gould KA, Dove WF. Polyclonal structure of intestinal adenomas in ApcMin/+mice with concomitant loss of Apc+ from all tumor lineages. Proc. Natl. Acad. Sci. USA. 1997;94:13927–13931. doi: 10.1073/pnas.94.25.13927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micale MA, Mohamed A, Sakr W, Powell IJ, Wollman SR. Cytogenetics of primary prostatic adenocarcinoma. Clonality and chromosome instability. Cancer Genet. Cytoegenet. 1992;61:165–173. doi: 10.1016/0165-4608(92)90082-j. [DOI] [PubMed] [Google Scholar]
- Millis JM, Chang B, Zinner MJ, Barsky SH. Malignant mixed tumour (carcinosarcoma) of the pancreas: a case report supporting organ-induced differentiation of malignancy. Surgery. 1994;115:132–137. [PubMed] [Google Scholar]
- Mills SE, Andersen WA, Fechner RE, Austin MB. Serous papillary carcinoma. A clinicopathological study of 10 cases and comparison with stage II-IV ovarian serous carcinoma. Am. J. Surg. Pathol. 1988;12:827–838. [PubMed] [Google Scholar]
- Mok CH, Tsao SW, Knapp RC, Fishbaugh PM, Lau CC. Unifocal origin of advanced human epithelial ovarian cancers. Cancer Res. 1992;52:5119–5122. [PubMed] [Google Scholar]
- Moolgavkar SH, Luebeck EG. Multistage carcinogenesis: population-based model for colon cancer. J. Natl. Cancer Inst. 1992;84:610–618. doi: 10.1093/jnci/84.8.610. [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Amsterdam A, Prieto V, et al. Teratoma with malignant transformation: diverse malignant histologies arising in men with germ cell tumours. J. Urol. 1998;159:133–138. doi: 10.1016/s0022-5347(01)64035-7. [DOI] [PubMed] [Google Scholar]
- Muller WJ, Sinn E, Pattengale PK, Wallace R, Leder P. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell. 1988;54:105–115. doi: 10.1016/0092-8674(88)90184-5. [DOI] [PubMed] [Google Scholar]
- Murry CE, Gipaya CT, Bartosek T, Benditt EP, Schwatz SM. Monoclonality of smooth muscle cells in human atherosclerosis. Am. J. Pathol. 1997;151:697–706. [PMC free article] [PubMed] [Google Scholar]
- Mutter G, Boynton K. PCR bias in amplification of androgen receptor alleles, a trinucleotide repeat marker used in clonality studies. Nucl. Acid Res. 1995;23:1411–1418. doi: 10.1093/nar/23.8.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutter GL, Chaponot M, Fletcher JA. A polymerase chain reaction assay for non-random chromosome inactivation identifies monoclonal endometrial cancers and precancers. Am. J. Pathol. 1995;146:501–508. [PMC free article] [PubMed] [Google Scholar]
- Nagel S, Borisch B, Thein SL. Somatic mutations detected by mini- and microsatellite DNA markers reveal clonal intratumoral heterogeneity in gastrointestinal cancers. Cancer Res. 1995;55:2966–2870. [PubMed] [Google Scholar]
- Nakashima H, Honda M, Inoue H, et al. Microsatellite instability in multiple gastric cancers. Int. J. Pathol. 1995;64:239–242. doi: 10.1002/ijc.2910640405. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y, Noguchi M, Matsuno Y. p53 expression in multicentric squamous cell carcinoma and surrounding squamous epithelium of the upper aerodigestive tract. Cancer. 1995;75:1657–1662. doi: 10.1002/1097-0142(19950401)75:7<1657::aid-cncr2820750716>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Namba H, Matsuo K, Fagin JA. Clonal composition of benign and malignant human thyroid tumours. J. Clin. Invest. 1990;86:120–125. doi: 10.1172/JCI114673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba H, Ross JL, Goodman D, Fagin IA. Solitary polyclonal autonomous thyroid nodule: a rare case of childhood hyperthyroidism. J. Clin. Endocrinol. Metab. 1991;72:1108–1112. doi: 10.1210/jcem-72-5-1108. [DOI] [PubMed] [Google Scholar]
- Nees M, Homann N, Discher H, et al. Expression of mutated p53 occurs in tumor-distant epithelia of head and neck cancer patients: a possible molecular basis for the development of multiple tumors. Cancer Res. 1993;53:4189–4196. [PubMed] [Google Scholar]
- Nesbitt M. Chimaeras v. X-inactivation mosaics: significance of pigment distribution. Dev. Biol. 1974;38:202–207. doi: 10.1016/0012-1606(74)90272-3. [DOI] [PubMed] [Google Scholar]
- Niho S, Suzuki K, Yokose T, Kodama T, Nishiwaka Y, Esumi H. Monoclonality of both pale cells and cuboidal cells of sclerosing hemangioma of the lung. Am. J. Pathol. 1998;152:1065–1069. [PMC free article] [PubMed] [Google Scholar]
- Niho S, Yokose T, Suzuki K, Kodama Y, Mukai K. Monoclonality of atypical adenomatous hyperplasia of the lung. Am. J. Pathol. 1999;154:249–254. doi: 10.1016/S0002-9440(10)65271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilbert M, Strombeck B. Independent origin of uterine leiomyomas with karyotypically identical alterations. Gynecol. Obstet. Invest. 1992;33:246–248. doi: 10.1159/000294895. [DOI] [PubMed] [Google Scholar]
- Nilbert M, Pejovic T, Mandahl N, Iosif S, Willen H, Mitelman F. Monoclonal origin of endometriotic cysts. Int. J. Gynec. Cancer. 1995;5:61–63. doi: 10.1046/j.1525-1438.1995.05010061.x. [DOI] [PubMed] [Google Scholar]
- Noguchi S, Motomura K, Inaji H, Imoaka S, Koyama H. Clonal analysis of human breast cancer by means of the polymerase chain reaction. Cancer Res. 1992;52:6594–6597. [PubMed] [Google Scholar]
- Noguchi S, Motomura K, Inaji H, Imoaka S, Koyama H. Clonal analysis of fibroadenoma and phyllodes tumor of the breast. Cancer Res. 1993;53:4071–4074. [PubMed] [Google Scholar]
- Noguchi S, Aihara T, Koyama H. Discrimination between multicentric and multifocal carcinomas of the breast through clonal analysis. Cancer. 1994a;74:872–877. doi: 10.1002/1097-0142(19940801)74:3<872::aid-cncr2820740313>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Noguchi S, Motomura K, Inaji H, Imoaka S, Koyama H. Clonal analysis of predominantly intraductal carcinoma and precancerous lesions of the breast by means of polymerase chain reaction. Cancer Res. 1994b;54:1849–1853. [PubMed] [Google Scholar]
- Noguchi S, Motomura K, Inaji H, Imoaka S, Koyama H. Clonal analysis of solitary intraductal papilloma of the breast by means of the polymerase chain reaction. Am. J. Pathol. 1994c;144:1320–1325. [PMC free article] [PubMed] [Google Scholar]
- Noguchi S, Motomura K, Inaji H, Imoaka S, Koyama H. Clonal analysis of parathyroid adenomas by means of the polymerase chain reaction. Cancer Lett. 1997;78:93–97. doi: 10.1016/0304-3835(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Nomura S, Kaminishi M, Sugiyama K, Oohara T, Esumi H. Clonal analysis of isolated single fundic and pyloric glands of stomach using X-linked polymorphisms. Biochem. Biophys. Res. Commun. 1996;226:385–390. doi: 10.1006/bbrc.1996.1365. [DOI] [PubMed] [Google Scholar]
- Nomura S, Esumi H, Job C, Tan S-S. Lineage and clonal development of gastric glands. Dev Biol. 1998a;204:124–135. doi: 10.1006/dbio.1998.9055. [DOI] [PubMed] [Google Scholar]
- Nomura S, Kaminishi M, Sugiyama K, Oohara T, Esumi E. Clonal analylsis of isolated intestinal metaplastic glands of stomach using X-linked polymorphism. Gut. 1998b;42:663–668. doi: 10.1136/gut.42.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelli MR, Williamson JA, Tomlinson IPM, et al. Polyclonal origim of colonic adenomas in an XO/XY patient with FAP. Science. 1996;272:1187–1190. doi: 10.1126/science.272.5265.1187. [DOI] [PubMed] [Google Scholar]
- Nowell PC. The clonal evolution of tumour cell populations. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Nowell PC. Mechanisms of tumor progression. Cancer Res. 1986;46:2203–2207. [PubMed] [Google Scholar]
- Ochiai A, Yamauchi Y, Hirohashi S. p53 mutations in the non-neoplastic mucosa of the human stomach showing intestinal metaplasia. Int. J. Cancer. 1996;69:28–33. doi: 10.1002/(SICI)1097-0215(19960220)69:1<28::AID-IJC6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Ogden GR, Hall PA. Field change, clonality and early epithelial cancer: possible lessons from p53. J. Pathol. 1997;181:127–129. doi: 10.1002/(SICI)1096-9896(199702)181:2<127::AID-PATH737>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Ohmura M, Hattori T. A possible multiclonal development of human colonic carcinomas. J. Cancer Res. Clin. Oncol. 1996;121:321–326. doi: 10.1007/BF01225683. [DOI] [PubMed] [Google Scholar]
- Orndal C, Mandahl F, Rydholm A, et al. Cytogenetic heterogeneity and clonal evolution in a recurrent fibrosarcoma. J. Exp. Clin. Cancer Res. 1993;12:23–31. [Google Scholar]
- Orndal C, Rydholm A, Willen H, Mitelman F, Mandahl N. Cytogenetic heterogeneity in soft tissue tumors. Cancer Genet. Cytogenet. 1994;78:127–138. doi: 10.1016/0165-4608(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Ota S, Kato AS, Kobayashi H, et al. Monoclonal origin of an esophageal carcinosarcoma producing granulocyte-colony stimulating factor. Cancer. 1998;82:2102–2111. doi: 10.1002/(sici)1097-0142(19980601)82:11<2102::aid-cncr4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Ozturk M. p53 mutation in p53 after aflotoxin exposure. Lancet. 1991;338:1356–1359. doi: 10.1016/0140-6736(91)92236-u. [DOI] [PubMed] [Google Scholar]
- Pal N, Wadey RB, Buckle B, Yeomans E, Pricthard J, Cowell JK. Preferential loss of maternal alleles in Wilm's tumour. Oncogene. 1990;5:1665–1668. [PubMed] [Google Scholar]
- Palacios Llopis S. Is a bi–clonal interaction, analogous to that underlying embryogenesis the origin of the earliest malignant phenotype? Med. Hypotheses. 1984;13:175–188. doi: 10.1016/0306-9877(84)90030-6. [DOI] [PubMed] [Google Scholar]
- Pandis N, Heim S, Bardi G, Idvall I, Mandahl N, Mitelman F. Chromosome analysis of 20 breast carcinomas: cytogenetic polyclonality and karyotypic-pathologic correlations. Genes Chromosomes Cancer. 1993;6:51–57. doi: 10.1002/gcc.2870060110. [DOI] [PubMed] [Google Scholar]
- Pandis N, Teixeira MR, Gerdes AM, et al. Chromosome abnormalities in bilateral breast carcinomas. Cytogenetic evaluation of the clonal origin of multiple primary tumors. Cancer. 1995;76:250–258. doi: 10.1002/1097-0142(19950715)76:2<250::aid-cncr2820760215>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Pandis N, Teixeira MR, Adeyinka A, et al. Cytogenetic comparison of primary tumors and lymph node metastases in breast cancer patients. Genes Chromosome Cancer. 1998;22:122–129. [PubMed] [Google Scholar]
- Paradis V, Laurendeau I, Viraud M, Bedossa P. Clonal analysis of macronodules in cirrhosis. Hepatology. 1997a;28:953–958. doi: 10.1002/hep.510280409. [DOI] [PubMed] [Google Scholar]
- Paradis V, Laurent A, Flejou J-F, Vidaud M, Bedossa P. Evidence for the polyclonal nature of focal nodular hyperplasia of the liver by the study of X-chromosome inactivation. Hepatology. 1997b;26:891–895. doi: 10.1002/hep.510260414. [DOI] [PubMed] [Google Scholar]
- Park TW, Fujiwara H, Wright TC. Molecular biology of cervical cancer and its precursors. Cancer. 1995;76:1902–1913. doi: 10.1002/1097-0142(19951115)76:10+<1902::aid-cncr2820761306>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Park TW, Richart RM, Sun XW, Wright TC. Association between human papilloma virus type and clonal status of cervical intraepithelial lesions. J. Natl. Cancer Inst. 1996;88:355–388. doi: 10.1093/jnci/88.6.355. [DOI] [PubMed] [Google Scholar]
- Pearse AGE. Clonality and endocrine (APUD) cell tumours. In: Polak JM, Bloom SL, editors. In: Endocrine Tumours. Churchill Livingstone: Edinburgh; pp. 82–94. [Google Scholar]
- Pearson TA, Dillman JM, Solez K, Heptinstall RH. Clonal markers in the study of the origin and growth of human atheroscleotic lesions. Circ. Res. 1978;43:10–18. doi: 10.1161/01.res.43.1.10. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Solez K, Dillman J, Heptinstall R. Monoclonal characteristics of organising arterial thrombi: significance in the origin and growth of human atherosclerotic plaques. Lancet. 1979;1:7–11. doi: 10.1016/s0140-6736(79)90453-7. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Dillman JM, Heptinstall RH. Clonal mapping of the human aorta: relationship of monoclonal characteristics, lesion thickness, and age in normal intima and atherosclerotic lesions. Am. J. Pathol. 1987;126:33–39. [PMC free article] [PubMed] [Google Scholar]
- Pejovic T, Heim S, Mandahl N, et al. Bilateral ovarian carcinomas: cytogenetic evidence of unicentric origin. Int. J. Cancer. 1991;47:358–361. doi: 10.1002/ijc.2910470308. [DOI] [PubMed] [Google Scholar]
- Perren A, Roth J, Muletta-Feurer S, et al. Clonal analysis of sporadic endocrine tumours. J. Pathol. 1998;186:363–371. doi: 10.1002/(SICI)1096-9896(199812)186:4<363::AID-PATH197>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Petersson C, Johansson B, Pandis N, Gorunova L, Ingvar C, Idvall I. Clonal chromosome aberrations in fibrocystic disease associated with increased risk of cancer. Int. J. Oncol. 1994;5:1207–1210. doi: 10.3892/ijo.5.6.1207. [DOI] [PubMed] [Google Scholar]
- Petersson C, Pandis N, Mertens F, et al. Chromosome aberrations in prophylactic mastectomies in women belonging to breast cancer families. Genes Chromosomes Cancer. 1996;16:185–188. doi: 10.1002/(SICI)1098-2264(199607)16:3<185::AID-GCC5>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Petersson C, Pandis N, Rizou H, Ingvar C, Heim S, Mitelman F. Karyotypic abnormalities in fibroadenomas of the breast. Int. J. Cancer. 1997;70:282–286. doi: 10.1002/(sici)1097-0215(19970127)70:3<282::aid-ijc6>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Piao Z, Park YN, Kim H, Park C. Clonality of large regenerative nodules in liver cirrhosis. Liver. 1997;17:251–256. doi: 10.1111/j.1600-0676.1997.tb01026.x. [DOI] [PubMed] [Google Scholar]
- Pinkus H, Mehregan C. A Guide to Dermatopathology. St Louis: Mosby; 1986. p. 378. [Google Scholar]
- Ponder BAJ, Schimidt GM, Wilkinson MM, Wood MJ, Monk M, Reid A. Derivation of mouse intestinal crypts from single progenitor cells. Nature. 1985;313:689–691. doi: 10.1038/313689a0. [DOI] [PubMed] [Google Scholar]
- Ponder BAJ, Wilkinson MM. Direct examination of the clonality of carcinogen-induced colonic epithelial dysplasia in chimeric mice. J. Natl. Cancer Inst. 1986;77:967–976. [PubMed] [Google Scholar]
- Poste G, Doll J, Fidler IJ. Interactions among clonal populations affect stability of the metastatic phenotype in polyclonal populations of B16 melanoma cells. Proc. Natl. Acad. Sci. USA. 1981;78:6226–6230. doi: 10.1073/pnas.78.10.6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponten F, Berg C, Ahmadian A, et al. Molecular pathology in basal cell cancer with p53 as a genetic marker. Oncogene. 1997;15:1059–1067. doi: 10.1038/sj.onc.1201435. [DOI] [PubMed] [Google Scholar]
- Quade BJ, McLachlin C, Soto-Wright V, Zuckerman J, Mutter GL, Morton CC. Disseminated peritoneal leiomyomatosis. Clonality analysis by X chromosome inactivation and cytogenetics of a clinically benigh smooth muscle proliferation. Am. J. Pathol. 1997;150:2153–2166. [PMC free article] [PubMed] [Google Scholar]
- Rabkin CS, Bedi G, Musaba E. AIDS-related Kaposi's sarcoma is a monoclonal neoplasm. Clin. Cancer Res. 1995;1:257–260. [PubMed] [Google Scholar]
- Rabkin CS, Janz S, Lash A. Monoclonal origin of multicentric Kaposi's sarcoma lesions. N. Engl. J. Med. 1997;336:988–993. doi: 10.1056/NEJM199704033361403. [DOI] [PubMed] [Google Scholar]
- Racchi O, Mangerini R, Rapezzi D, Rolfo M, Gaetani GF, Ferraris AM. X chromosome inactivation patterns in normal females. Blood Cells Mol. Dis. 1998;24:439–447. doi: 10.1006/bcmd.1998.0213. [DOI] [PubMed] [Google Scholar]
- Radford DM, Phillips NJ, Fairk L, Ritter JH, Holt M, Donis-Keller H. Allelic loss and the progression of breast cancer. Cancer Res. 1995;55:5180–5183. [PubMed] [Google Scholar]
- Ren Z-P, Ponten F, Nister M, Ponten J. Two distinct p53 immunohistochemical patterns in human squamous skin cancer, precursors and normal epidermis. Int. J. Cancer. 1996;69:174–179. doi: 10.1002/(SICI)1097-0215(19960621)69:3<174::AID-IJC4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Ren Z-P, Ahmadian A, Ponten F. Benign clonal keratinocyte patches wth p53 mutations show no genetic link to synchronous squamous cell precancer or cancer in human skin. Am. J. Pathol. 1997a;150:1791–1803. [PMC free article] [PubMed] [Google Scholar]
- Ren Z-P, Ponten F, Nister M, Ponten J. Reconstruction of the two-dimensional distribution of p53-positive staining patches in sun-exposed, morphologically-normal skin. Int. J. Oncol. 1997b;11:111–115. doi: 10.3892/ijo.11.1.111. [DOI] [PubMed] [Google Scholar]
- Robinson WA, Lemon M, Elefanty A, Harrison-Smith M, Markham Norris D. Human acquired naevi are clonal. Melanoma Res. 1998;8:499–503. doi: 10.1097/00008390-199812000-00004. [DOI] [PubMed] [Google Scholar]
- Rosenberg CL, Larson PS, Romo JD, De Las Morenas A, Faller DV. Microsatellite alterations indicating monoclonality in atypical hyperplasia associated with breast cancer. Human Pathol. 1997;28:214–218. doi: 10.1016/s0046-8177(97)90109-x. [DOI] [PubMed] [Google Scholar]
- Rubin H. Cancer as a dynamic developmental disorder. Cancer Res. 1985;45:2935–2942. [PubMed] [Google Scholar]
- Sakamoto M, Hirohashi S, Tsuda H, Shimosato Y, Makuuchi M, Hosaoda Y. Multicentric independent development of hepatocellular carcinoma revealed by analysis of hepatitis B virus integration pattern. Am. J. Surg. Pathol. 1989;13:1064–1067. doi: 10.1097/00000478-198912000-00009. [DOI] [PubMed] [Google Scholar]
- Sameshima Y, Matsumo Y, Hirohashi S, et al. Alterations of the p53 gene are common and critical events for the maintenance of the malignant phenotypes in small cell lung carcinoma. Oncogene. 1992;7:451–457. [PubMed] [Google Scholar]
- Sawada M, Azuma C, Hashimoto K, et al. Clonal analysis of human gynecologic cancers by means of the polymerase chain reaction. Int. J. Cancer. 1994;58:492–496. doi: 10.1002/ijc.2910580406. [DOI] [PubMed] [Google Scholar]
- Schimidt GH, Wilton DJ, Ponder BAJ. Development of the pattern of cell renewal in the crypt-villus unit of the mouse small intestine. Development. 1988;103:785–790. doi: 10.1242/dev.103.4.785. [DOI] [PubMed] [Google Scholar]
- Schmidt GH, Mead R. On the clonal origin of tumours — lessons from studies of intestinal epithelium. Bioessays. 1990;12:37–40. doi: 10.1002/bies.950120109. [DOI] [PubMed] [Google Scholar]
- Schmidt GH, Blount MA, Ponder BAJ. Immunochemical demonstration of the clonal organisation of chimaeric mouse epidermis. Development. 1990;100:535–541. doi: 10.1242/dev.100.3.535. [DOI] [PubMed] [Google Scholar]
- Schwartz SM, Murry CE. Proliferation and monoclonal origins of atherosclerotic lesions. Ann. Rev. Med. 1998;49:437–460. doi: 10.1146/annurev.med.49.1.437. [DOI] [PubMed] [Google Scholar]
- Sen Gupta S, Van Der Luijt RB, Bowles LV, Meera-Khan P, Delhanty JD. Somatic mutation of APC gene in desmoid tumour in familial adenomatous polyposis. Lancet. 1993;342:552–553. doi: 10.1016/0140-6736(93)91677-e. [DOI] [PubMed] [Google Scholar]
- Shan L, Nakamura M, Nakamura Y, et al. Comparative analysis of clonality and pathology in primary and secondary hyperparathyroidism. Virch. Arch. 1997;430:247–251. doi: 10.1007/BF01324809. [DOI] [PubMed] [Google Scholar]
- Sheu JC, Huang GT, Chou HC. Multiple hepatocellular carcinomas have different clonality. Gastroenterol. 1993;105:1471–1476. doi: 10.1016/0016-5085(93)90153-4. [DOI] [PubMed] [Google Scholar]
- Sheu J-C. Molecular mechanism of hepatocarcinogenesis. J. Gastroenterol. Hepatol. 1997;12(Suppl.):S09–S313. doi: 10.1111/j.1440-1746.1997.tb00514.x. [DOI] [PubMed] [Google Scholar]
- Sheu LF, Chen A, Tseng HH, et al. Assessment of p53 expression in nasopharyngeal carcinoma. Hum Pathol. 1995;26:380–386. doi: 10.1016/0046-8177(95)90137-x. [DOI] [PubMed] [Google Scholar]
- Shibata A, Tsai YC, Press MF, Henderson BE, Jones PA, Ross RK. Clonal analysis of bilateral breast cancer. Clin. Cancer Res. 1996a;2:743–748. [PubMed] [Google Scholar]
- Shibata D, Schaeffer J, Li ZH, Capella G, Perucho M. Genetic heterogeneity of the c-K -ras locus in colorectal adenomas but not in adenocarcinomas. J. Natl. Cancer Inst. 1993;85:1058–1063. doi: 10.1093/jnci/85.13.1058. [DOI] [PubMed] [Google Scholar]
- Shibata D, Peinado MA, Ionov Y, Malkhosyan S, Perucho M. Genomic instability in repeated sequences is an early somatic event in colorectal tumorigenesis that persists after transformation. Nature Genet. 1994;6:273–281. doi: 10.1038/ng0394-273. [DOI] [PubMed] [Google Scholar]
- Shibata D, Navidi W, Salovaara R, Li Z-H, Aaltonen LA. Somatic microsatellite mutations as molecular tumor clocks. Nature Med. 1996b;2:676–681. doi: 10.1038/nm0696-676. [DOI] [PubMed] [Google Scholar]
- Shibata D. Ponten J. In Precancer: Biology, Importance and Possible Prevention. Vol. 32. 1998. The dynamics of early intestinal tumour proliferation: to be or not to be; pp. 181–200. Cancer Surveys. [PubMed] [Google Scholar]
- Shimoda A, Kaneko S, Uchijama M. Clonal origin of of mammalian hepatitis B virus related hepatocellular carcinoma. J. Med. Virol. 1990;30:282–286. doi: 10.1002/jmv.1890300410. [DOI] [PubMed] [Google Scholar]
- Shin WS, Kang MW, Kang JH, et al. Epstein-Barr virus-associated gastric adenocarcinomas among Koreans. Am. J. Clin. Pathol. 1996;105:174–181. doi: 10.1093/ajcp/105.2.174. [DOI] [PubMed] [Google Scholar]
- Shroyer KR, Gudlaugsson EG. Analysis of clonality in archival tissues by polymerase chain reaction amplification. Human Pathol. 1994;25:287–292. doi: 10.1016/0046-8177(94)90201-1. [DOI] [PubMed] [Google Scholar]
- Sidransky D, Frost P, Von Eschenbach A, Oyasu R, Preisinger AC, Vogelstein B. Clonal origin of bladder cancer. N. Engl. J. Med. 1992a;326:737–740. doi: 10.1056/NEJM199203123261104. [DOI] [PubMed] [Google Scholar]
- Sidransky D, Mikkelsen T, Schwechheimer K. Clonal expansion of p53 mutant cells is associated with brain tumour progression. Nature. 1992b;355:846–847. doi: 10.1038/355846a0. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Ward K, White R. Mosaic methylation in clonal tissue. Dev. Biol. 1993;156:391–398. doi: 10.1006/dbio.1993.1086. [DOI] [PubMed] [Google Scholar]
- Silva S, Filipe MI, Pinho A. Variants of intestinal metaplasia in the evolution of chronic atrophic gastritis and gastric ulcer. A follow-up study. Gut. 1990;31:1097–2004. doi: 10.1136/gut.31.10.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnock KL, Perz-Atayde AR, Boynton KA, Mutter GL. Clonal analysis of sacrococcygeal teratomas. Paed. Pathol. Lab. Med. 1996;16:865–875. doi: 10.1080/15513819609168710. [DOI] [PubMed] [Google Scholar]
- Slack JM. Homeotic transformations in man: implications for the mechanism of embryonic development and for the organisation of epithelia. J. Theor. Biol. 1985;114:463–490. doi: 10.1016/s0022-5193(85)80179-x. [DOI] [PubMed] [Google Scholar]
- Slaughter DP, Southwick HW, Smejkal W. ‘Field cancerisation’ in oral stratified squamous epithelium. Cancer. 1953;6:963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Sozzi G, Miozzo M, Pastorino U, et al. Genetic evidence for an independent origin of multiple pre-neoplastic and neoplastic lung lesions. Cancer Res. 1995;55:135–140. [PubMed] [Google Scholar]
- Stangl AP, Wellenreuther R, Lenartz D, et al. Clonality of multiple meningiomas. J. Neurosurg. 1997;86:853–858. doi: 10.3171/jns.1997.86.5.0853. [DOI] [PubMed] [Google Scholar]
- Stephenson CF, Davis RL, Moore GE, Sandberg AA. Cytogenetic and fluorescence in situ hybridisation analysis of breast fibroadenomas. Cancer Genet. Cytogenet. 1992;63:32–36. doi: 10.1016/0165-4608(92)90060-l. [DOI] [PubMed] [Google Scholar]
- Sternlicht M, Mirell C, Safarians S, Barsky S. A novel strategy for the investigation of clonality in precancerous disease states and early stages of tumor progression. Biochem. Biophys. Res. Commun. 1994;199:511–518. doi: 10.1006/bbrc.1994.1258. [DOI] [PubMed] [Google Scholar]
- Sundaresan V, Ganly P, Hasleton P, et al. p53 and chromosome 3 abnormalities characteristic of malignant lung tumours, are detectable in preinvasive lesions of the bronchus. Oncogene. 1992;7:1989–1997. [PubMed] [Google Scholar]
- Symmans FW, Liu J, Knowles DM, Inghirami G. Breast cancer heterogeneity: evaluation of clonality in primary and metastatic lesions. Hum Pathol. 1995;26:210–216. doi: 10.1016/0046-8177(95)90039-x. [DOI] [PubMed] [Google Scholar]
- Szych C, Sraebler A, Connolly DC, Wu R, Cho KR, Ronnett BM. Molecular genetic evidence supporting the clonality and appendiceal origin of pseudomyxoma peritonei in women. Am. J. Pathol. 1999;154:1849–1855. doi: 10.1016/S0002-9440(10)65442-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M, Iggo RD, Ishi N, et al. Clonality and stability of the p63 gene in human astrocytic tumor cells: quantitative analysis of p53 gene mutations by yeast functional assay. Int. J. Cancer. 1996;67:447–450. doi: 10.1002/(SICI)1097-0215(19960729)67:3<447::AID-IJC22>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Tamura M, Fukaya T, Murakami T, Uehara S, Yajima A. Analysis of clonality in human endometriotic cysts based on evaluation of X-chromosome inactivation in archival formalin-fixed, paraffin-embedded tissue. Lab. Invest. 1998;78:213–218. [PubMed] [Google Scholar]
- Tan S, Williams E, Tam P. X-inactivation occurs at different times in different tissues of the post-implantation mouse embryo. Nature Genet. 1993;3:170–174. doi: 10.1038/ng0293-170. [DOI] [PubMed] [Google Scholar]
- Tate JE, Mutter GL, Boynton KA, Crum CP. Monoclonal origin of vulvar intraepithelial neoplasia and some vulvar hyperplasias. Am. J. Pathol. 1997;150:315–322. [PMC free article] [PubMed] [Google Scholar]
- Tatematsu M, Fukami H, Yamamoto M, et al. Clonal analysis of glandular somach carcinogenesis in C3H/HeN↔BalB/c chimeric mice treated with N-methyl- N-nitrosurea. Cancer Lett. 1994;37:42–51. doi: 10.1016/0304-3835(94)90296-8. [DOI] [PubMed] [Google Scholar]
- Tatematsu M, Masui T, Fukami. H, et al. Monoclonal development of squamous cell carcinomas from polyclonal papillary or nodular hyperplasias in the forestomach of CEH/Hen↔BALB/c chimeric mice treated with N-methyl-n-nitrosourea or diethylnitrosamine. Carcinogenesis. 1996a;17:1365–1371. doi: 10.1093/carcin/17.6.1365. [DOI] [PubMed] [Google Scholar]
- Tatematsu M, Masui T, Fukami H, et al. Primary monoclonal and secondary polyclonal growth of colon neoplastic lesions in CEH/Hen↔BALB/c chimeric mice treated with 1,2-dimethylhydrazine: immunohistochemical detection of C3H strain-specific antigen and simple sequence length polymorphism analysis of DNA. Int. J.Cancer. 1996b;66:234–238. doi: 10.1002/(SICI)1097-0215(19960410)66:2<234::AID-IJC16>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Tavassoli F, Kraus F. Endometrial lesions in uteri resected for atypical endometrial hyperplasia. Am. J. Clin. Pathol. 1978;70:770–779. doi: 10.1093/ajcp/70.5.770. [DOI] [PubMed] [Google Scholar]
- Teixeira MR, Pandis N, Bardi G, et al. Cytogenetic analysis of multifocal breast carcinomas: detection of karyotypically unrelated clones as well as clonal similarities between tumour foci. Br. J.Cancer. 1994;70:922–927. doi: 10.1038/bjc.1994.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira MR, Pandis N, Bardi G, Andersen JA, Mitelman F, Heim S. Clonal heterogeneity in breast cancer: karyotypic comparisons of multiple intra and extra-tumorous samples from 3 patients. Int. J. Cancer. 1995;63:63–68. doi: 10.1002/ijc.2910630113. [DOI] [PubMed] [Google Scholar]
- Teixeira MR, Pandis N, Bardi G, Andersen JA, Heim S. Karyotypic comparisons of multiple tumorous and macrosopically normal surrounding tissue samples from patients with breast cancer. Cancer Res. 1996;56:855–859. [PubMed] [Google Scholar]
- Teixeira MR, Qvist H, Bohier PJ, Pandis N, Heim S. Cytogenetic analysis shows that carcinosarcomas of the breast are of monoclonal origin. Genes Chromosomes Cancer. 1998;22:145–151. [PubMed] [Google Scholar]
- Thomas GA, Williams D, Williams ED. The demonstration of tissue clonality by X-linked enzyme histochemistry. J. Pathol. 1988;155:101–108. doi: 10.1002/path.1711550205. [DOI] [PubMed] [Google Scholar]
- Thomas GA, Williams D, Williams ED. The clonal origin of thyroid nodules and adenomas. Am. J. Pathol. 1989;134:141–147. [PMC free article] [PubMed] [Google Scholar]
- Thompson EM, Fleming KA, Evans DJ, Fundele R, Surani MA, Wright NA. Gastric endocrine cells share a clonal origin with other gut cell lineages. Development. 1990;10:477–481. doi: 10.1242/dev.110.2.477. [DOI] [PubMed] [Google Scholar]
- Thompson L, Chang B, Barsky SH. Monoclonal origins of malignant mixed tumors (carcinosarcomas) Am. J. Surg. Pathol. 1996;20:277–285. doi: 10.1097/00000478-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Tihy F, Vogt N, Recan D, et al. Skewed inactivation of an X chromosome deleted at the dystrophin gene in an asymptomatic mother and her affected daughter. Hum. Genet. 1994;93:563–567. doi: 10.1007/BF00202824. [DOI] [PubMed] [Google Scholar]
- Toguchida J, Ishizaki KA, Sasaki MS, et al. Preferential mutation of paternally-derived RB gene as the initial event in sporadic osteosarcoma. Nature. 1989;338:156–158. doi: 10.1038/338156a0. [DOI] [PubMed] [Google Scholar]
- Tominaga T, Kohara S, Namil Y, et al. Clonal analysis of nodular parathyroid hyperplasia in renal hyperparathyroidism. World J. Surg. 1996;20:744–752. doi: 10.1007/s002689900113. [DOI] [PubMed] [Google Scholar]
- Tollenaar RA.E.M, Bonsing BA, Kuipers-Dijkshoorn NJ, et al. Evidence of clonal divergence in colorectal carcinoma. Cancer. 1997;79:1304–1314. [PubMed] [Google Scholar]
- Tsai YC, Simoneau AR, Spruck CH, Nicholas PW, Steven K, Jones PA. Mosaicism in human epithelium: macroscopic mosiac patches cover the urothelium. J. Urol. 1995;153:1697–1700. doi: 10.1016/s0022-5347(01)67507-4. [DOI] [PubMed] [Google Scholar]
- Tsai YC, Lu Y, Nichols PW. Contiguous patches of normal human mammary epithelium derived from a single stem cell: implications for breast carcinogenesis. Cancer Res. 1996;56:402–404. [PubMed] [Google Scholar]
- Tsuda H, Hirohashi S, Shimasato Y, Terada M, Hasegawa H. Clonal origin of atypical adenomatous hyperplasia of the liver and clonal identity with hepatocellular carcinoma. Gastroenterol. 1988;95:1664–1666. doi: 10.1016/s0016-5085(88)80093-3. [DOI] [PubMed] [Google Scholar]
- Tsuda H, Oda T, Sakaomoto M, Hirohashi S. Different pattern of chromosomal allele loss in multiple hepatocellular carcinomas as evidence of their multifocal origin. Cancer Res. 1992;52:1504–1509. [PubMed] [Google Scholar]
- Tsuge I, Kojima S, Matsuoka H, et al. Clonal haemopoiesis in children with acquired aplastic anaemia. Br. J. Haematol. 1993;84:137–143. doi: 10.1111/j.1365-2141.1993.tb03036.x. [DOI] [PubMed] [Google Scholar]
- Tsuji S, Ogawa K, Tsakasaka H, Sonoda T, Mori M. Clonal origin of gamma-glutanyl transpeptidase-positive hepatic lesions induced by initiation-promotion in ornithine carbamoyltransferase mosaic mice. Jpn. J. Cancer. Res. 1988;79:148–151. doi: 10.1111/j.1349-7006.1988.tb01569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunashima K, Endo Y, Asakuura H, et al. A novel clonality assay for the mouse: application to hepatocellular carcinomas induced with diethylnitrosamine. Mol. Carcinogenesis. 1996;15:33–37. doi: 10.1002/(SICI)1098-2744(199601)15:1<33::AID-MC5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Van Elsas A, Zerp S, Van Der Flier S, et al. Analysis of n- ras mutations in human cutaneous melanoma: tumour heterogeneity detected by polymerase chain reaction/single stranded conformation polymorphism analysis. Rec. Results Cancer Res. 1995;139:58–67. doi: 10.1007/978-3-642-78771-3_5. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Fearon ER, Hamilton SR, Feinberg AP. Use of restriction fragment length polymorphisms to determine the clonal origin of human tumours. Science. 1985;227:624–645. doi: 10.1126/science.2982210. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Fearon ER, Hamilton SR, et al. Clonal analysis using recombinant DNA probes from the X-chromosome. Cancer Res. 1987;47:4806–4813. [PubMed] [Google Scholar]
- Vogrincic GS, O'connel JX, Gilks CB. Giant cell tumour of tendon sheath is a polyclonal cellular proliferation. Hum. Pathol. 1997;28:815–819. doi: 10.1016/s0046-8177(97)90155-6. [DOI] [PubMed] [Google Scholar]
- Wada Y, Tsukada S, Kamiyama S, Koizumi A. Evidence on clonal proliferation of hepatocytes after carbon tetrachloride-induced hepatic injury in PGK-1 mosaic mice. Toxicol. Lett. 1990;52:81–90. doi: 10.1016/0378-4274(90)90168-l. [DOI] [PubMed] [Google Scholar]
- Wainscoat JS, Fey MF. Assessment of clonality in human tumours: a review. Cancer Res. 1990;50:1355–1360. [PubMed] [Google Scholar]
- Walsh DS, Tsou HC, Harrington A, James WD, Peacocke M. Clonality of basal cell carcinoma — molecular analysis of an interesting case. J. Invest. Dermatol. 1996;106:579–582. doi: 10.1111/1523-1747.ep12344982. [DOI] [PubMed] [Google Scholar]
- Walsh DS, Peacocke M, Harrington A, Jamres WD, Tsou HC. Patterns of X chromosome inactivation in sporadic basal cell carcinomas. Evidence for clonality. J. Am. Acad. Dermatol. 1998;38:49–55. doi: 10.1016/s0190-9622(98)70538-9. [DOI] [PubMed] [Google Scholar]
- Weinberg WC, Howard JC, Iannaconne PM. Histological demonstration of mosaicism in a series of chimeric rats produced between congenic strains. Science. 1985;227:524–527. doi: 10.1126/science.3966159. [DOI] [PubMed] [Google Scholar]
- Weinberg WC, Berkwitz I, Iannoconne PM. The clonal nature of carcinogen-induced altered foci of γ-glutamyl transpeptidase expression in rat liver. Carcinogenesis. 1987;8:565–570. doi: 10.1093/carcin/8.4.565. [DOI] [PubMed] [Google Scholar]
- Weinberg WC, Iannoccone PM. Clonality of preneoplastic liver lesions: histological analysis in chimaeric rats. J. Cell Sci. 1988;89:423–431. doi: 10.1242/jcs.89.3.423. [DOI] [PubMed] [Google Scholar]
- Wengler GS, Allen RC, Parolini O, Smith H, Conley ME. Non-random X-chromosome inactivation in natural killer cells from obligate carriers of X-linked severe combined immunodeficiency. J. Immunol. 1993;150:700–704. [PubMed] [Google Scholar]
- West JD. Clonal development of the retinal epithlium in mouse chimaeras and X-inactivation mosaics. J. Embryol. Exp. Morphol. 1976a;35:445–446. [Google Scholar]
- West JD. Patches in the livers of chimaeric mice. J. Embryol. Exp. Morphol. 1976b;36:151–161. [PubMed] [Google Scholar]
- Wick MR, Swanson PE. Carcinosarcomas: current perspectives and historical review of nosological concepts. Sem. Diagn. Pathol. 1993;10:118–127. [PubMed] [Google Scholar]
- Williams GT, Wynford-Thomas D. How may clonality be studied in human tumours? Histopathology. 1994;24:287–292. doi: 10.1111/j.1365-2559.1994.tb00526.x. [DOI] [PubMed] [Google Scholar]
- Wilimas JA, Dow LW, Douglass EC, et al. Evidence for clonal development of Wilm's tumour. Am. J. Pediat. Oncol. 1991;13:26–28. doi: 10.1097/00043426-199121000-00006. [DOI] [PubMed] [Google Scholar]
- Winton DJ, Blount MA, Ponder BA. Polyclonal origin of mouse skin papillomas. Brit. J. Cancer. 1989;60:59–63. doi: 10.1038/bjc.1989.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff MFA. Tumour clonality: biological significance. Adv. Cancer Res. 1988;50:197–229. doi: 10.1016/s0065-230x(08)60438-8. [DOI] [PubMed] [Google Scholar]
- Woodruff MFA, Ansell JD, Forbes GM, Gordon JC, Burton DI, Micklem HS. Clonal interaction in tumours. Nature. 1982;299:822–824. doi: 10.1038/299822a0. [DOI] [PubMed] [Google Scholar]
- Worsham MJ, Wolam SR, Carey TE, Zarbo RJ, Benninger MS, Van Dyke DL. Common clonal origin of synchronous head and neck squamous cell carcinoma: analysis by tumor karyotypes and fluorescence in situ hybridisation. Human Pathol. 1995;26:251–261. doi: 10.1016/0046-8177(95)90054-3. [DOI] [PubMed] [Google Scholar]
- Yamada T, De Souza AT, Finkelstein S, Jirtle RL. Loss of the gene encoding mannose 6-phoshate/insulin-like growth factor II receptor is an early event in liver carcinogenesis. Proc. Natl. Acad. Sci. USA. 1997;94:10351–10355. doi: 10.1073/pnas.94.19.10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Kajino K, Kudo M, Sasaki Y, Arakawa Y, Hino O. Determination of the clonal origin of multiple human hepatocellular carcinomas by cloning and polymerase chain reaction of the integrated hepatitis B virus DNA. Hepatology. 1999;29:1446–1452. doi: 10.1002/hep.510290523. [DOI] [PubMed] [Google Scholar]
- Yang HK, Linnola RI, Conrad NK, et al. TP53 and RAS mutations in metachronous tumors from patients with cancer of the upper aerodigestive tract. Int. J. Cancer. 1995;64:229–233. doi: 10.1002/ijc.2910640403. [DOI] [PubMed] [Google Scholar]
- Yasui H, Hino O, Ohtake H, MacHinami R, Kitagawa T. Clonal growth of hepatitis B virus-integrated hepatocytes in cirrhotic liver nodules. Cancer Res. 1992;52:6810–6814. [PubMed] [Google Scholar]
- Young J, Leggett Gustafson G, Ward M, et al. Genomic instability occurs in colorectal carcinomas but not in adenomas. Hum. Mut. 1993;2:351–354. doi: 10.1002/humu.1380020505. [DOI] [PubMed] [Google Scholar]
- Young-Tak K, Neena F, Thomas BA, et al. p53 mutations and clonality in vulvar carcinomas and squamous hyperplasias: evidence suggesting that squamous hyperplasias do not serve as direct prescursors of human papillomavirus-negative vulvar carcinomas. Human Pathol. 1996;27:389–395. doi: 10.1016/s0046-8177(96)90113-6. [DOI] [PubMed] [Google Scholar]
- Zhu J, Frosch MPO, Bisque L, et al. Analysis of meningiomas by methylation and transcription-based clonality assays. Cancer Res. 1995;55:3865–3872. [PubMed] [Google Scholar]
- Zhu JJ, Leon SP, Folkerth RD, Guo S-Z, Wu JK, Black PM. Evidence for clonal origin of neoplastic neuronal and glial cells in gangliogliomas. Am. J. Pathol. 1997;151:565–571. [PMC free article] [PubMed] [Google Scholar]
- Zhuang S, Lininger RA, Man Y-G, Albuquerque A, Merino MJ, Tavassoli FA. Identical clonality of both components of mammary carcinosarcoma with differential loss of heterozygosity. Mod. Pathol. 1997;10:354–362. [PubMed] [Google Scholar]
- Zhuang Z, Vortmeyer A, Mark E, Odze R, Emmert-Buck M, Merino M. Barrett's esophagus: metaplastic cells with loss of hetrozygosity at the APC gene locus are clonal precursors to invasive adenocarcinoma. Cancer Res. 1996;56:1961–1964. [PubMed] [Google Scholar]
- Zvejnieks PA, Tellschow SR, Gudlaugsson EG, Markham N, Shroyer KR. Amelogenin dosage composition in carcinoma of colon, lung, liver and kidney is not a marker of clonality in males. Mol. Cell Probes. 1998;12:185–190. doi: 10.1006/mcpr.1998.0172. [DOI] [PubMed] [Google Scholar]