Abstract
The ability of Japanese encephalitis virus (JEV) and JEV-induced macrophage derived neutrophil chemotactic factor (MDF) to produce nitric oxide (NO), and the possible antiviral effect of NO during JEV infection, was investigated. Splenic macrophages of JEV infected mice produced maximum NO in vivo at day 7 post infection, and in vitro at 24 h after JEV stimulation. MDF-induced NO production was dose dependent and maximal at 60 min after MDF treatment. The response was sensitive to anti-MDF antibody treatment and the nitric oxide synthase inhibitor NG-monomethyl-l-arginine (L-NMMA). Pretreatment of mice with L-NMMA increased the mortality to 100% in JEV infected mice in vivo and inhibited NO production in vitro, while MDF stimulated macrophages inhibited virus replication with high levels of NO production. MDF treatment increased the survival rate of JEV infected mice. The findings thus demonstrate that MDF induces production of NO during JEV infection, which has an antiviral effect. This may be one of the important mechanisms of natural immunity in controlling the initial stages of JEV infection.
Keywords: Japanese encephalitis, nitric oxide, host defence
Nitric oxide [NO] has emerged as an important intra and intercellular regulatory molecule, which is now recognized to play important roles in immunological pathways, in the mediation of central and peripheral nervous system functions, the regulation of blood pressure and flow, and in the regulation of cell activation. Many cell types are able to produce NO through the enzymatic conversion of l-arginine to l-citrulline by nitric oxide synthase (NOS) in presence of NADPH (Hibbs et al. 1988). Macrophages, neutrophils, neurones, endothelial cells, hepatocytes, pancreatic cells, mast cells and smooth muscle cells are the best characterized sources of NO. The synthesis of NO is inhibited by NG-monomethyl-l-arginine (L-NMMA), a competitive inhibitor of NOS (Palmer & Moncada 1989). Recently NO has also been implicated as a mediator of antiviral host defence. NO has an inhibitory effect on ectromelia virus, vaccinia virus, herpes simplex virus type-1, influenza virus and vesicular stomatitis virus replication (Bi & Reiss 1995).
Japanese encephalitis virus (JEV), an arthropod borne flavivirus is one of the major causes of epidemic encephalitis in Asia. After haematogenous spread in the host, JEV replicates in number of organs as well as in local and regional lymph nodes and generates a rapid inflammatory response with mononuclear and polymorphonuclear cell infiltration (Mathur et al. 1988; Lin et al. 1996). Virus invasion of the nervous system occurs via blood. Early host defence against JEV infection is mediated by phagocytic cells (Srivastava et al. 1999) and later on by a complex mechanism involving B and T effector cells (Mathur et al. 1983). The primary infection is followed by establishment of persistent infection in mice (Mathur et al. 1989), in humans (Sharma et al. 1991; Ravi et al. 1993) and in tissue culture (Chen et al. 1996). The role of the immune response in recovery from infection with JEV is poorly understood.
Previous observations in our laboratory have revealed the production of a unique, low molecular weight (10 kD) macrophage derived neutrophil chemotactic factor (MDF) following JEV infection in mice (Khanna et al. 1991). MDF induces neutrophil leukocytosis, regulates granulocytosis, breaches the blood brain barrier (Mathur et al. 1992), controls iron metabolism (Bharadwaj et al. 1991), and regulates vascular permeability (Khanna et al. 1994). MDF primes the neutrophils to enhance superoxide anion generation, stimulate the respiratory burst in response to JEV via activation of cytosolic NADPH and subsequent formation of hydrogen peroxide, and help in degradation of the phagocytosed virus (Srivastava et al. 1999). Through its inhibitory effect on viral replication NO has been demonstrated to play an important role in host defence. The present study was planned to investigate the ability of JEV and JEV induced MDF to produce NO and the inhibitory effect of NO on JEV replication.
Materials and methods
Animals
Inbred Swiss albino mice, aged 6 weeks, obtained from a mouse colony maintained in this Department were used throughout the study.
Virus
Japanese encephalitis virus (JEV strain 78668 A), isolated from human brain (Mathur et al. 1983) was propagated in suckling mouse brain and was used as 10% (w/v) suspension in Earle's minimum essential medium. The infectivity titre of virus was 104.7 LD50/0.025 ml. It produced 100% mortality by day six following intracerebral (i.c.) inoculation while intraperitoneal (i.p.) inoculation induced no clinically evident disease. Mice simultaneously injected with normal brain suspension were used as controls.
Preparation of macrophage derived factor (MDF)
Macrophage derived factor (MDF) is produced by splenic macrophages of JEV infected mice (Khanna et al. 1991). Briefly, mice were inoculated (i.p.) with 300 μl of 10 LD50 of JEV. On day 7 p.i. the spleens were collected aseptically, and splenic cells (1 × 107/ml) were cultured in minimum essential medium with 25 mm N-hydroxyethyl piperazine N′ ethane sulphonic acid (MEM-HEPES) containing 10% fetal calf serum (FCS) for 2 h at 37 °C in 5% CO2 — air. Glass adherent cells were washed with phosphate buffered saline (PBS) and kept in saline at 37 °C for 24 h. The supernatant was collected, centrifuged and assayed for neutrophil chemotactic activity by a modified Boyden chamber technique as described previously (Khanna et al. 1991). The MDF was purified by high pressure liquid chromatography (HPLC) (Pharmacia, Uppsala, Sweden) on a Superose-12 column using 0.1 m PBS as elution buffer (Khanna et al. 1991). The chemotactic fractions were collected, protein content was measured by the technique of Lowry et al. (1951), and stored at − 70 °C. Normal mouse spleen macrophage culture supernatant was used as control.
Preparation of anti-MDF antisera
Anti-MDF antisera were prepared as described by Khanna et al. (1997). Briefly MDF (100 μg) emulsified in Freund's complete adjuvant (Sigma Chemical Co., St Louis, MO) was injected intramuscularly at the inner side of the flank and this dose in Freund's incomplete adjuvant (Sigma) was repeated after three weeks intramuscularly in Swiss albino mice. This was followed by three intradermal injections (60 μg protein/100 μl) in the peritoneal cavity at weekly intervals without any adjuvant at four to five places and an intravenous injection (40 μg/mouse) 3–4 days before bleeding. Mice were bled and serum separated and inactivated at 56 °C for 30 min. The optimal dilution of antibody which abrogated MDF-induced chemotactic activity was measured and antibody was stored at this dilution at −70 °C.
Preparation of spleen cell culture
Mouse spleen cells were teased out in chilled minimum essential medium with 25 mm HEPES containing 10% FCS, penicillin G (100 U/ml) and streptomycin (100 μg/ml). A single cell suspension was prepared and viable nucleated cells were counted using the trypan blue exclusion test. The viable cell count was then adjusted to 10 × 106 cells/ml. The cells were cultured by layering 4 ml of the cell suspension in 5 cm glass Petri dishes and were incubated at 37 °C in the presence of 5% CO2-air.
Preparation of enriched cell populations
Spleen cells (10 × 106 cells/ml) were seeded in glass Petri dishes with MEM-HEPES (25 mm) for 2 h at 37 °C. The nonadherent cells were removed and Petri dishes were thoroughly washed with phosphate-buffered saline (PBS). More than 90% of these cells were macrophages as judged by morphology and phagocytosis of latex particles (Mathur et al. 1988). T and B lymphocyte-enriched populations were obtained by successive filtration of nonadherent cells through a nylon wool column as described previously (Khanna et al. 1991). Pure T and B lymphocyte populations (95% and 94%, respectively) were obtained by treating the cells with anti-Thy1.2 antisera or antimouse IgG antisera (New England Nuclear, Cambridge, MA) and complement.
Determination of NO concentration
The concentration of NO was determined by assaying its stable end product nitrite (NO2−) (Bi & Reiss 1995). Phenol red free medium (Earle's solution containing Earle's 1X, dextrose, 5% NaHCO3, 5% FCS, penicillin G (100 U/ml) and streptomycin (100 μg/ml) antibiotics) was used in all NO assays. Briefly, equal volumes of test sample and Greiss reagent (1% sulphanilamide, 0.1% naphthylethylene diamine dihydrochloride, 5% H3PO4; LOBA Chemie Ltd, India) were incubated at room temperature for 10 min in a flat-bottomed microtitre plate. The absorbance was measured at 540 nm in a microplate reader (Titertek Multiskan PLUS version 1.4, Bio-Tek Instruments, Inc., USA). A range of dilutions of sodium nitrite was used to generate a standard curve with each assay.
Indirect immunofluorescence (IIF)
Cells were fixed with chilled acetone and examined by IIF as described previously (Mathur et al. 1988). Briefly, cells were incubated at 37 °C with anti-JEV monoclonal antibody designated 98.9.5i (kindly provided by Dr E.A. Gould, Institute of Virology, Oxford, U.K.) diluted 1 : 100, of mouse ascitic fluid in PBS. This antibody reacts exclusively with JEV and not with any other flavivirus. The monoclonal antibody has been checked for absence of nonspecific reaction with normal mouse embryo fibroblast cells. The cells were washed thrice with PBS and incubated at 37 °C for 30 min with FITC labelled goat antimouse IgG antibodies (Wellcome, Beckenham, U.K.). The cells were screened with a Leitz Dialux-20 fluorescent microscope. The results were expressed as antigen positive cells/100 high power fields (h.p.f.). The data are expressed as the arithmetic mean (A.M.) ± SD of 8–10 values and the experiment was repeated five times.
Experimental protocol
NO production by spleen cells was investigated in vitro and in vivo. The experimental groups consisted of a test group inoculated with JEV or reacted with MDF or both. Control (mock) groups were inoculated with normal splenic macrophage culture supernatant (in place of MDF) or normal mouse brain suspension (in place of JEV) or a blank group inoculated with the diluents alone. Each experiment was repeated at least 6 times. Data have been presented after deduction of the background value as A.M. ± SD
Statistical analysis
The data were analysed using student's t-test and P < 0.05 was considered significant.
Results
Effect of JEV on nitric oxide (NO) production
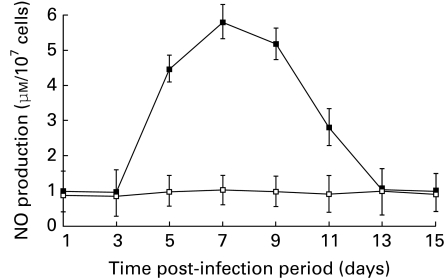
Spleen cells of mice inoculated i.p. with JEV were collected at different time periods post infection, and screened for NO. The findings (Figure 1) show maximum production of NO at day 7 p.i. followed by a gradual decline. To delineate the spleen cell type responsible for production of NO, splenic cell suspensions were prepared post JEV infection, enriched for macrophages, T and B lymphocytes. The individual cell populations were cultured at 37 °C for 24 h. NO production was observed in the JEV primed macrophage culture supernatant only, while T and B lymphocyte cultures failed to produce significant NO in supernatent.
Figure 1.
Production of NO by spleen cells of JEV-inoculated mice. Groups of mice were inoculated with JEV intraperitonially (i.p.). Spleens were harvested at different time periods, a single cell suspension was prepared (10 × 106cells/ml) and cultured for 24 h at 37 °C. NO production was assayed in the cell free culture supernatents (▪) as described in Materials and methods. Control (□) mice were inoculated with normal mouse brain suspension. Values are presented as A. M. ± SD from 10 cultures.
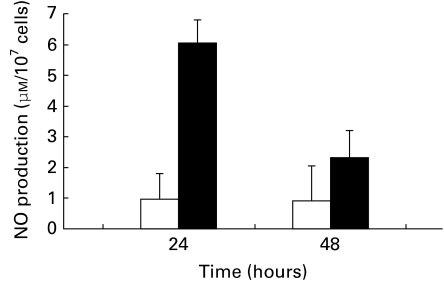
The production of NO by normal mouse spleen cell cultures exposed to JEV in vitro for different time periods was investigated. The data summarized in Figure 2 show that JEV triggered significant production of NO at 24 h post stimulation which was 6.02 ± 0.04 μm after deducting the background value (0.92 ± 0.03 μm).
Figure 2.
Production of NO by JEV stimulated spleen cells in vitro. Normal mouse spleen cell (10 × 106 cells/ml) cultures were inoculated with 104 TCID50 JEV and incubated at 37 °C. At different time periods NO production was assayed (▪) as described in Materials and methods. The control cells (□) were inoculated with normal mouse brain suspension. Each point represents A.M. ± SD from 10 cultures.
MDF as a inducer of NO production
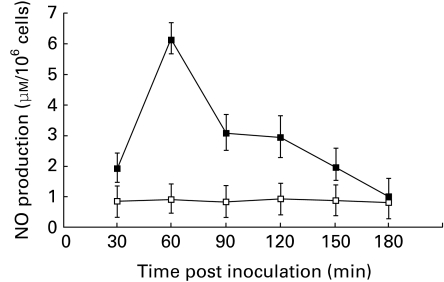
We have previously shown that the degradation of JEV in neutrophils is mediated by macrophage derived neutrophil chemotactic factor (MDF), a low molecular weight polypeptide secreted during JEV infection (Srivastava et al. 1999). Therefore we sought to ascertain the regulatory role of MDF in NO production. The time course of NO production in normal mouse splenic macrophages stimulated in vitro with 5 μg of MDF for different time periods was studied. The data summarized in Figure 3 show significant NO production (P < 0.001) at 60 min after MDF addition, which returned to normal by 3 h. Therefore in further experiments observations were recorded at 60 min after MDF inoculation. In studying the effect of different concentrations of MDF induced alterations in NO production, we observed a linear relationship between increasing dose of MDF and NO production.
Figure 3.
MDF induced NO production in vitro, after stimulation of macrophage cultures with 5 μg of MDF (▪). Controls were stimulated with normal mouse splenic macrophage culture supernatent (□). At different time periods NO production was assayed as described in Materials and methods. Values presented as A.M. ± SD from eight cultures.
Effect of anti-MDF antiserum on NO production
This experiment was carried out to ascertain the effect of anti-MDF antibodies on NO production by MDF treated macrophages. Equal volumes of different dilutions (1 : 10 to 1 : 100) of anti-MDF antibodies and MDF (5 μg) were incubated at 37 °C for 60 min and added to normal mouse macrophage (1 × 106/ml) cultures. Control macrophages were treated with normal mouse serum in place of anti-MDF antibodies or MDF only. The different culture supernatants were assayed for NO production. A significant dose dependent reduction in MDF-induced NO production (0.09 ± 0.003 μm to 2.05 ± 0.02 μm) was observed in cells treated with various dilutions of anti-MDF antiserum as compared to MDF treated cells (6.06 ± 0.18 μm).
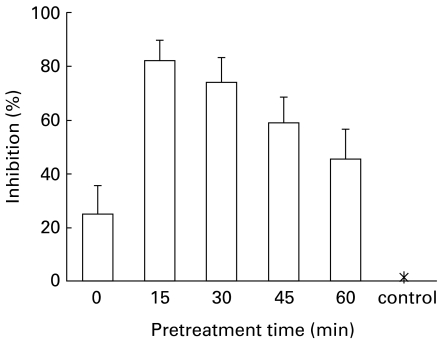
Effect of NG-monomethyl-l-arginine on MDF induced NO production
The arginine analogue NG-monomethyl-l-arginine (L-NMMA) is a potent inhibitor of macrophage NO2−/NO3− synthesis in cultured cells (Hibbs et al. 1987). Experiments were carried out to determine whether L-NMMA was capable of inhibiting NO synthesis induced by MDF. Normal mouse macrophage cultures were pretreated with L-NMMA (100 μm) for different time periods followed by MDF (5 μg) inoculation and then assayed for NO production as described above. NO production was inhibited in cells preteated with L-NMMA. The data summarized in Figure 4 show maximum inhibition of MDF induced NO production (1.2 ± 0.03 μm) in cell cultures pretreated with L-NMMA for 15 min as compared to MDF treated cells (6.8 ± 0.19 μm). The cells pretreated with L-NMMA alone had a background value of 0.71 ± 0.02 μm. Treatment with L-NMMA (100 μm) had no effect on the viability of normal mouse macrophages.
Figure 4.
Inhibition of NO production by treatment with NG-monomethyl-l-arginine (L-NMMA). Normal mouse macrophage cultures were pretreated with 100 μm L-NMMA for indicated time periods followed by inoculation of 5 μg MDF for 60 min at 37 °C. Control group of cells (*) were treated with MDF only. Cells treated with L-NMMA for different time periods were used for background values. NO production was assayed as described in Materials and methods. Results are presented after deduction of background values as A.M. ± SD from eight cultures.
Effect of NG-monomethyl-l-arginine on mortality rate in JEV infection
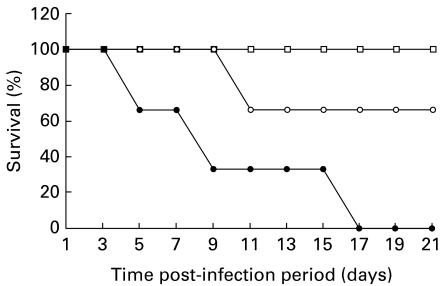
We determined the role of NO on the outcome of JEV infection. Swiss albino mice were inoculated with a sublethal dose (105 LD50) of JEV i.c., To inhibit NOS activity in vivo, the mice were treated with L-NMMA from the beginning of the virus infection. Controls were infected with JEV (sublethal) or L-NMMA only. Mortality was observed daily for three weeks. The data summarized in Figure 5 show L-NMMA treatment significantly increased the mortality in JEV infected Swiss albino mice as compared to JEV controls (P < 0.05). Mock infected mice treated with L-NMMA alone showed no mortality. These results suggest an important role of NO in restricting JEV infection in vivo.
Figure 5.
Effect of L-NMMA treatment on JEV infection. Mice were inoculated with the sublethal dose (105LD50) of JEV (i.c.) with (•) or without (○) treatment of L-NMMA. Mice were treated with L-NMMA (100 μm) daily. As control (□) the mock infected mice were treated with L-NMMA alone. The mortality rate of the mice was monitored daily for three weeks. Each group consisted of 20 mice.
Further, in order to know the effect of L-NMMA on JEV induced NO production in vitro, normal mouse macrophages (1 × 106/ml) were either pretreated with 100 μm L-NMMA for 15 min followed by inoculation of JEV or treated with JEV alone for 2 h. After washing of cells and further incubation for 24 h at 37 °C, NO production was assayed as described. The L-NMMA treated JEV-induced cells show significant reduction in NO production (2.62 ± 0.83 μm) as compared to JEV stimulated cells (5.82 ± 0.91 μm) at 24 h (P < 0.05). The NO production observed in macrophages stimulated with normal mouse brain suspension (0.89 ± 0.06 μm) in place of JEV, and L-NMMA (0.85 ± 0.04 μm) were included as controls.
Effect of MDF on JEV replication
We have earlier demonstrated that MDF induces an increase in intracellular oxidative signals with generation of superoxide anions and degradation of phagocytosed JE viral protein and nucleic acid (Srivastava et al. 1999). Further, to study the effect of MDF on JEV replication, the murine macrophages were stimulated with MDF (5 μg) followed by JEV 1 h later. Control cells were treated with normal mouse splenic culture supernatant or JEV alone and screened for the presence of viral antigen by indirect immunofluorescence after 24 h. A significant decrease in virus specific antigen positive cells in MDF and JEV costimulated macrophages (9 ± 0.4/100hpf) as compared to JEV alone (32 ± 3/100hpf) was observed (P < 0.001).
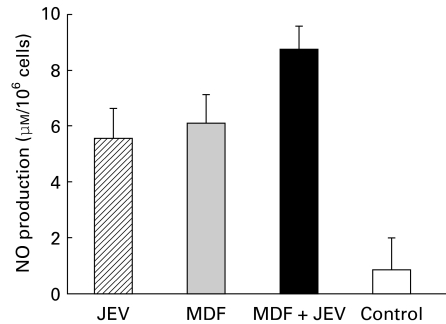
To see whether the anti-JEV effect in MDF treated macrophages was correlated with their NO production, the NO release in MDF and JEV treated and JEV control macrophage cultures was assayed. The findings summarized in Figure 6 show high level of NO production in MDF treated cells as compared to controls.
Figure 6.
Effect of MDF on JEV induced NO production by macrophages. NO production was assayed in MDF activated (▪) or nonactivated ( ) macrophages after 24 h of in vitro JEV stimulation, as described in Materials and methods. Controls consisted of cells treated with MDF alone (
) macrophages after 24 h of in vitro JEV stimulation, as described in Materials and methods. Controls consisted of cells treated with MDF alone ( ) or unstimulated (□). Values are presented as A.M. ± SD of six cultures.
) or unstimulated (□). Values are presented as A.M. ± SD of six cultures.
Effect of MDF on survival rate in JEV infected mice
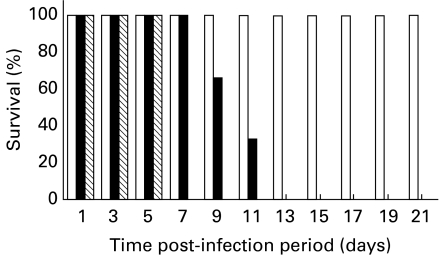
The effect of MDF, an NO inducer, in conferring protection against JEV was studied, in vivo. Mice were injected with purified MDF (5 μg) i.v. followed by i.c. challenge with JEV. Control mice were given normal mouse splenic macrophage culture supernatant in place of MDF with or without JEV. The findings show an increase in survival time of MDF treated JEV infected mice as compared to JEV controls while no mortality was observed in mice treated with MDF alone (Figure 7).
Figure 7.
Survival of JEV-infected Swiss albino mice treated with MDF or left untreated. Twenty mice per group were infected with the 102LD50 of JEV mouse brain suspension(i.c.). Starting from the begining of inoculation, the mice were treated with MDF (5 μg) intravenously daily till the animal died (▪) or left untreated ( ). As control (□) mock infected mice were also treated with MDF. The survival rate of the mice was monitored daily for three weeks.
). As control (□) mock infected mice were also treated with MDF. The survival rate of the mice was monitored daily for three weeks.
Discussion
Japanese encephalitis virus (JEV) infection in mice resulted in production of NO by activated macrophages via secretion of macrophage derived chemotactic factor (MDF). This response was inhibited by pretreatment of cells with anti-MDF antiserum confirming the importance of MDF in NO production during JEV infection. NO production also correlated with the amount of MDF. Our earlier studies have revealed that MDF is a low MW protein (10 kD), which attracts neutrophils. The maximum activity of MDF appears at day 7 post JEV infection in vivo and at 24 h postinoculation (p.i.) in cell cultures (Khanna et al. 1991). A number of proinflammatory cytokines viz. IFN-γ, IL-1, IL-8, IL-10 and TNF and lipopolysaccharides like LPS, upregulate the production of NO by macrophages (Sunyer et al. 1996; Corriveau et al. 1998) while others like IL-1β, IL-2, IL-4, IFN-α, IFN-β, CSF-M and transforming growth factor-β1 downregulate it (Ding et al. 1988). Our previous studies have shown that MDF acts as a proinflammatory cytokine. It regulates granulocytosis, modulates the activation of neutrophils (Mathur et al. 1992) and results in accumulation of neutrophils at the site of intradermal inoculation (Khanna et al. 1994).
Nitric oxide is a free radical, gaseous molecule, synthesized by a variety of cells and tissues including neurones, endothelial cells and macrophages from l-arginine by cellular nitric oxide synthase (NOS) in the presence of NADPH. Besides being an important neurotransmittor (Snyder 1992) it modulates the host defence (Tucker et al. 1996). NO and other nitrogen intermediates have direct microbicidal or microbiostatic effects against a variety of bacterial, parasitic and viral infections (Nathan & Hibbs 1991). In the present study we have demonstrated that JEV induced MDF generates NO which protects the mice from the lethal effects of JEV in vivo. This anti JEV effect was changed completely if mice infected with a sublethal dose of JEV were treated with L-NMMA, a competitive inhibitor of NOS, subsequently developing fatal disease. The exact mechanism involved in protection requires further elucidation. These results suggest that NO produced during JEV infection via MDF protects mice from fatal encephalitis. Tucker et al. (1996) have made the observation that NO produced during Sindbis virus infection, induces a protein, important for cell suvival.
The anti-JEV effect is mediated by the cooperative activity of various components of phagocytic cells and T and B effector cells (Mathur et al. 1983; Srivastava et al. 1999). Our recent data revealed that the anti-JEV effect is mediated by JEV induced MDF via triggering of oxidative signals with generation of superoxide anion and subsequent formation of toxic radicals. Toxic oxygen species had an adverse effect on JEV replication as they inhibited viral protein synthesis (Srivastava et al. 1999). This study demonstrates the anti-JEV effect of NO generated either by JEV or JEV induced MDF. NO may be neuroprotective or neurotoxic. The macrophage/microglial enzyme NOS is induced by stimulation with LPS, IFN-γ, IL-1, IL-8, IL-10 and TNF, etc., and has an important role in modulating inflammation (Sunyer et al. 1996) and inhibiting virus growth (Lin et al. 1996). For this, the oxidation-reduction state of NO is important. The reduced state of the molecule [NOo] is neurotoxic, while the oxidized state [NO+] is neuroprotective. NOo reacts with superoxide ions generating peroxynitrite, a neurotoxic molecule, while when NO+ reacts with the thiol groups of the N-methyl-d-aspartate receptor it is neuroprotective (Tucker et al. 1996). Perhaps the redox state favours NO+ in JEV infection.
It appears that the anti-JEV effect in initial stages of infection is controlled by an increase in intracellular oxidative signals with subsequent cellular NO production and release of superoxide anions and toxic radicals. An anti-JEV effect of NO in gamma interferon activated RAW 264.7 murine macrophages has been demonstrated via inhibition of viral RNA synthesis, viral protein accumulation, and virus release. JEV inhibition was observed only when gamma-interferon activated RAW264.7 cells were used, but not by their culture supernatents (Lin et al. 1997). The exact mechanism of the anti-JEV effect of activated macrophages is not known. The present study suggests that macrophages can mediate antiviral effects through a soluble mediator.
Our earlier studies identified depression of serum iron following JEV infection in mice and in JE patients. The hypoferraemia is associated with large accumulation of iron in the spleen (Bharadwaj et al. 1991). The control of iron concentration has been attributed partly to the production of NO probably by modulating the transferrin receptor and ferritin mRNA translation (Drapier et al. 1993). The role of NO in hypoferraemia during JEV infection and the modulating effect of MDF is currently being investigated.
Collectively, our data indicate that MDF induces production of NO during JEV infection and has an antiviral effect which may be one of the important factors of natural immunity in controlling the initial stages of JEV infection.
References
- 1.Bharadwaj M, Prakash V, Mathur A, Chaturvedi UC. Prognostic significance of serum iron levels in cases of Japanese encephalitis. Postgrad. Med. J. 1991;67:247–249. doi: 10.1136/pgmj.67.785.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bi Z, Reiss CS. Inhibition of vesicular stomatitis virus infection by nitric oxide. J. Virol. 1995;69:2208–2213. doi: 10.1128/jvi.69.4.2208-2213.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen L-K, Liao C-L, Lin C-G, et al. Persistence of Japanese encephalitis virus is assosiated with abnormal expression of the nonstructural protein NS1 in host cell. Virology. 1996;217:220–229. doi: 10.1006/viro.1996.0109. [DOI] [PubMed] [Google Scholar]
- 4.Corriveau CC, Madara PJ, Van Dervort AL, Tropea MM, Wesley RA, Danner RL. Effect of nitric oxide on chemotaxis and endotoxin-induced interleukin-8 production in human neutrophils. J. Infect. Dis. 1998;177:116–126. doi: 10.1086/513829. [DOI] [PubMed] [Google Scholar]
- 5.Ding AH, Nathan CF, Stuehr DJ. Release of reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J. Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 6.Drapier JC, Hirling H, Wietzerbin J, Kaldy P, Kuhn LC. Biosynthesis of nitric oxide activates iron regulatory factor in macrophages. EMBO J. 1993;12:3643–3649. doi: 10.1002/j.1460-2075.1993.tb06038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hibbs JB, JR, Taintor RR, Vavrin Z. Macrophage cytotoxicity, role for L-arginine deiminase and iminase nitrogen oxidation to nitrite. Science. 1987;235:473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- 8.Hibbs JB, Taintor R, Vavrin Z, Rachlin EM. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem. Biophys. Res. Commun. 1988;157:87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- 9.Khanna N, Agnihotri M, Mathur A, Chaturvedi UC. Neutrophil chemotactic factor produced by Japanese encephalitis virus stimulated macrophages. Clin. Exp. Immunol. 1991;83:299–303. doi: 10.1111/j.1365-2249.1991.tb05813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khanna N, Mathur A, Chaturvedi UC. Regulation of vascular permeability by macrophage derived chemotactic factor produced in Japanese encephalitis. Immunol. Cell Biol. 1994;72:200–204. doi: 10.1038/icb.1994.30. [DOI] [PubMed] [Google Scholar]
- 11.Khanna N, Mathur A, Bharadwaj M, Chaturvedi UC. Induction of hypoglycaemia in Japanese encephalitis virus infection: the role of T-lymphocytes. Clin. Exp. Immunol. 1997;107:282–287. doi: 10.1111/j.1365-2249.1997.281-ce1173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin Y-L, Liao CL, Yeh CI, et al. A highly attenuated strain of Japanese encephalitis virus induces a protective immune response in mice. Virus Res. 1996;44:45–56. doi: 10.1016/0168-1702(96)01343-3. [DOI] [PubMed] [Google Scholar]
- 13.Lin Y-L, Huang Y-L, Ma SH, et al. Inhibition of Japanese encephalitis virus infection by nitric oxide: antiviral effect of nitric oxide on RNA virus replication. J. Virol. 1997;71:5227–5235. doi: 10.1128/jvi.71.7.5227-5235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurements with Folin-phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 15.Mathur A, Arora KL, Chaturvedi UC. Host defence mechanisms against Japanese encephalitis virus infection in mice. J. Gen. Virol. 1983;64:805–811. doi: 10.1099/0022-1317-64-4-805. [DOI] [PubMed] [Google Scholar]
- 16.Mathur A, Bharadwaj M, Kulshreshtha R, Rawat S, Jain A, Chaturvedi UC. Immunopathological study of spleen during Japanese encephalitis virus infection in mice. Brit. J. Exp. Path. 1988;69:423–432. [PMC free article] [PubMed] [Google Scholar]
- 17.Mathur A, Kulshreshtha R, Chaturvedi UC. Evidence of latency of Japanese encephalitis virus in T lymphocytes. J. Gen. Virol. 1989;70:461–465. doi: 10.1099/0022-1317-70-2-461. [DOI] [PubMed] [Google Scholar]
- 18.Mathur A, Khanna N, Chaturvedi UC. Breakdown of blood brain barrier in Japanese encephalitis by a virus induced cytokine. Int. J. Exp. Path. 1992;73:603–611. [PMC free article] [PubMed] [Google Scholar]
- 19.Nathan CF, Hibbs JB. Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr. Opin. Immunol. 1991;3:65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- 20.Palmer RMJ, Moncada S. A novel citrulline — forming enzyme implicated in the formation of nitric oxide by vascular endothelial cells. Biochem. Biophys. Res. Commun. 1989;158:348–352. doi: 10.1016/s0006-291x(89)80219-0. [DOI] [PubMed] [Google Scholar]
- 21.Ravi V, Desai AS, Shenoy PK, Satishchandra P, Chandramukhi A, Gourie-Devi M. Persistence of Japanese encephalitis virus in the human nervous system. J. Med. Virol. 1993;40:326–329. doi: 10.1002/jmv.1890400412. [DOI] [PubMed] [Google Scholar]
- 22.Sharma S, Mathur A, Prakash V, Kulshreshtha R, Kumar R, Chaturvedi UC. Japanese encephalitis virus latency in peripheral blood lymphocytes and recurrence of infection in children. Clin. Exp. Immunol. 1991;85:85–89. doi: 10.1111/j.1365-2249.1991.tb05687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snyder SH. Nitric oxide: first in a new class of neurotransmitters? Science. 1992;257:494–496. doi: 10.1126/science.1353273. [DOI] [PubMed] [Google Scholar]
- 24.Srivastava S, Khanna N, Saxena SK, Singh A, Mathur A, Dhole TN. Degradation of Japanese encephalitis virus by neutrophils. Int. J. Exp. Path. 1999;80:17–24. doi: 10.1046/j.1365-2613.1999.00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sunyer T, Rothe L, Jiang X, Osdoby P, Collin-Osdoby P. Proinflammatory agents, IL-8 and IL-10, upregulate inducible nitric oxide synthase expression and nitric oxide production in avian osteoclast-like cells. J. Cell. Biochem. 1996;60:469–483. doi: 10.1002/(sici)1097-4644(19960315)60:4<469::aid-jcb4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 26.Tucker PC, Griffin DE, Choi S, Bui N, Wesselingh S. Inhibition of nitric oxide synthesis increases mortality in Sindbis virus encephalitis. J. Virol. 1996;70:3972–3977. doi: 10.1128/jvi.70.6.3972-3977.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]