Abstract
Primary synovial chondromatosis (PSC) is a rare disorder of the synovium typified by cartilaginous nodule formation within the synovial membrane. Fibroblast growth factor receptor 3 (FGFR3) is a recently described specific marker of mesenchymal precartilaginous stem cells. Expression patterns of FGFR3 and its specific ligand, fibroblast growth factor 9 (FGF 9), were evaluated both in situ and in cell cultures. Histologically, cells at the periphery of the cartilage nodules express FGFR3 and PCNA (proliferating cell nuclear antigen). Elevated levels of FGF 9, its specific ligand, have been found in synovial fluids of patients with synovial chondromatosis. Synoviocytes but not chondrocytes from affected patients express FGF9 in culture. This pattern is absent in normal synovium and cartilage. Downregulation of FGF9 may provide a possible nonoperative therapy for PSC.
Keywords: Synovial chondromatosis, Synovia, fibroblast growth factor 9, fibroblast growth factor receptor 3, FGF9, FGFR3, cartilage, mesenchymal cells
Primary synovial chondromatosis (PSC) is a disorder typified by the production of cartilage nodules by synovial cells; some of the nodules eventually ossify. Classification of this disorder is uncertain. Some authors have found that the cartilage cells do not express cell proliferation-related antigens and thus have suggested that the process is a reactive one (Apte & Athanasou 1992). The synovial cells appear to be reactive as they express leucocyte common antigen, CD68 and HLA-DR (Apte & Athanasou 1992). The cells in PSC have normal diploid chromosomes in contrast to cells of malignant lesions such as chondrosarcomas (Coughlan et al. 1995). On the other hand, other authors have suggested that while no proliferation activity takes place in PSC, expression of C-erb B-2 occurs in about half of the cells (Davis et al. 1996). Cell proliferation studies of PSC specimens indicate that the disorder is most probably a metaplasia of synovium. However, mean DNA content, the percentage of hyperploid cells and the percentage of aneuploid cells are all significantly higher in PSC than in enchondromas. This implies that a component of the cells in PSC must undergo a proliferative process. Therefore PSC appears to occupy an intermediate position between proliferating malignant lesions such as chondrosarcomas and resting benign lesions such as enchondromas (Davis et al. 1998a).
The current study explores possible factors responsible for metaplasia of synovial cells in cases of PSC. Recently, fibroblast growth factor receptor 3 (FGFR3) was described as a marker of mesenchymal stem cells (Robinson et al. 1999a,b). FGFR3 allows the isolation of precartilaginous cells. These residual stem cells are present in minute amounts in all normal mesenchymal tissues. They are capable of responding to mitogenic factors and differentiating into multiple types of mesenchymal tissue. The current study examines their role in PSC pathogenesis.
Materials and Methods
Samples
Five specimens of each of the following diagnoses were used for immunohistochemical studies: PSC, secondary synovial chondromatosis in osteoarthritis (SSC), foetal cartilage and normal articular cartilage.
Surgical discard material from five cases of PSC was used for cell culture experiments. Both synovia and cartilage were grown separately as explant cultures. In previous studies, we have demonstrated that this method of cell culture of mesenchymal-originated tissue encourages the migration of mesenchymal progenitor cells with spindle-shaped fibroblast-like morphology, onto the culture dish and yields a uniform culture of mesenchymal progenitor cells expressing FGFR3 (Nevo et al. 1998). During the last decade, reconstruction of articular surfaces has been performed using autologous chondrocytes cultured in vitro (Brittberg et al. 1994). During this procedure biopsies of synovia and articular cartilage were performed. The cells were cultured in vitro and their numbers expanded. At the second stage the cells were transplanted into cartilage defects. Cells from such biopsies taken from joints with traumatic articular cartilage defects were used as controls.
Synovial fluid samples from PSC patients and from joints with traumatic chondral fractures were collected and compared using western blotting analysis for the presence of fibroblast growth factor 9 (FGF 9), the specific ligand of FGFR3 (Hecht et al. 1995).
Histochemical evaluation
Alcian blue staining at pH 1.0 and pH 2.5 were performed to assess proteoglycan content in both cell cultures and surgical tissue specimens. Staining was carried out as previously described (Robinson et al. 1993).
Immunohistochemical evaluation
Immunohistochemical studies were performed on tissue blocks from patients afflicted by PSC. SSC (loose bodies removed during total knee replacement) specimens were used as a control group.
The following antibodies were evaluated: anti-FGFR3 (Santa Cruz, Santa Cruz, CA, USA), FGF 9 (Cytolab, Rehovot, Israel), collagen type II (Developmental Hybridoma Bank, Iowa City, IA, USA) and proliferative cell nuclear antigen (PCNA) (Dako, Glostrup, Denmark).
Detailed immunohistochemical procedures used in our laboratory were previously described (Robinson et al. 1994). This technique prevents deterioration of antigens during decalcification. In brief, samples are placed in formalin solution (4%, pH 7.4) containing 0.5% cetylpyridinium chloride for 24 h. They are later transferred to 4% formalin solution for another 24 h. The samples measuring up to 1.5 cm in thickness underwent decalcification in ethylene-diamine tetra-acetic acid (EDTA, which was previously shown to minimize antigen alteration during decalcification (Mori et al. 1988)) for 10 days, dehydrated with alcohol and embedded in paraffin. The blocks were sectioned by a standard microtome into 5-micron thick slices. Sections were glued to polylysine-coated glass slides. The paraffin was removed with xylol. The slides were later rehydrated in serial alcohol and stained with Mayer's haematoxylin and eosin. Separation of stained vs. unstained cells is easy to perform.
Cell cultures
Methodology of obtaining cultures from explants of skeletal tissues was previously described (Nevo et al. 1993). In short, samples removed from the joint including synovia and cartilage nodules were placed in Dulbecco's minimal essential medium (DMEM) growth medium under sterile conditions. The samples were immediately shipped to our tissue culture laboratory at Tel Aviv University. Tissue fragments were minced into 1 mm cubes. These cubes were partially dried on the surface of 25 cm2 tissue culture flasks, to promote attachment. The flasks were later filled with minimal amounts of DMEM (to which 10% foetal calf serum — FCS was added). Cells started to migrate out of the cubes after 7 days. The cells were later trypsinized and collected. Manipulation of the cellular phenotypes was performed in FAD medium as previously described (Grigeriadis et al. 1989) by the addition of FGF2 (basic FGF, 25 nanogram per ml), ascorbic acid (50 microgram per ml) and dexamethasone (0.4 microgram per ml). In prior studies, this cell culture protocol has been shown to stimulate a cell population of mesenchymal stem cells present in bone marrow. The culture is greatly enriched with precartilaginous mesenchymal progenitor cells. These are later able to undergo chondrogenic differentiation (Butnariu et al. 1996).
Immunohistochemistry of cell cultures
Immunohistochemical stains of the cells were performed using the same antibodies as used for the tissue sections. Experiments were carried out in 24 well plates. 5000 cells were seeded in a well. Cultures were kept for 96 h (till about 50% confluent, approximately 10 000 cells per well). Total cell numbers per culture were determined using a Coulter counter (Model Industrial D, Coulter, UK).
Image analysis of cell cultures
Image analysis was used to quantify the numbers of cells positively stained by antibodies. The following protocol was followed: cell cultures were photographed using a video camera mounted on a microscope. The images were digitized using a video card (Capture-to-Go, MARGI systems inc., Fremont, CA, USA). The images were analysed using the Scion image analysis software (Scion Corp., Frederic, MD, USA). In confluent cultures the number of stained cells per × 400 magnification field was measured in 10 random fields. Total number of cell nuclei was counted as well. The ratio was represented as percentage of cells stained in the culture. This protocol applied to the quantification of FGFR3 and collagen type II staining in cell cultures. Alcian blue at pH 1 stains preferentially sulphated proteoglycans such as aggrecan. Cultures stained by it yield a diffuse staining in the cultures. Thus in this case a more representative measure of the amount of aggrecan present is a quantification of the average staining intensity expressed in grey scale levels of the culture. Scion image analysis software employs a scale with 256 grey scale levels. 0 represents absolute white and 255 represents absolute black. The images are converted to 256 levels grey scale image for analysis.
Isolation of FGF9 from synovial fluids
Specimens of 1–2 ml of synovial fluids were collected from patients suffering from PSC, SSC, osteoarthritis, rheumatoid arthritis, and cartilage fracture, torn meniscus and tear of the anterior cruciate ligament. Samples were removed during arthroscopy or arthrotomy, prior to any joint lavage to avoid dilution. Samples were immediately placed in sterile culture flasks and kept frozen at −20 °C until analysis.
For the FGF 9 isolation procedure the thawed synovial fluid samples were diluted (1 : 1, volume per volume) with phosphate buffered saline (PBS) and centrifuged for 3 min at 960 g, to discard the formed precipitate. The precipitate is composed mainly of floating synovial cells and cryoprecipitate. Fifty μL of dense heparin-Sepharose beads (Pharmacia, Uppsala, Sweden) were added to the supernatant of each sample. The suspensions were constantly mixed on a rotary shaker overnight at 4 °C. The beads with the absorbed fibroblast growth factors were collected by centrifugation. The beads were washed once with 0.5 M NaCl and then resuspended in small volume of × 5 sample buffer containing: glycerol, 10% SDS (sodium dodacyl(lannyl) sulfate), β-mercapto-ethanol (2 : 1 : 1, volume per volume) with a few drops of bromo-phenyl-blue, and boiled for 5 min. The supernatants were collected by centrifugation. They were separated on 12% SDS-polyacrylamide (SDS-PAGE) gels for 60–90 min, at 110 mV under reducing conditions and further processed by Western immunoblotting.
Western immunoblotting of FGF 9
Proteins separated on SDS-PAGE gels were electrophoretically transferred to nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany), 120 min at 200 mA. The nitrocellulose membranes were blocked for 30 min with 10% milk in PBS. Then anti-FGF 9 (polyclonal, dilution 1 : 2000, Cytolab. Rehovot, Israel) was added. Solutions were incubated overnight at 4 °C. After 24 h the immunocomplexes were washed three times with 1% Tween 20 in PBS (PBS-T). A secondary immunological reagent incubated for 45 min at room temperature was used for tracing. Protein A (dilution 1 : 10 000) or goat antirabbit antibodies (dilution 1 : 20 000) conjugated with horseradish peroxidase (Amersham, Buckinghamshire, UK), further interacted with the ECL Kit (Amersham, Buckinghamshire, UK) producing a light reaction, measured on a Kodak X-Omat AR film (Eastman Kodak, Rochester, NY, USA) to be visualized within minutes.
The identification of the proper molecular weight bands FGF 9 (27 kDa) was compared to authentic marker proteins.
Results
Histological immunohistochemical staining of tissue sections
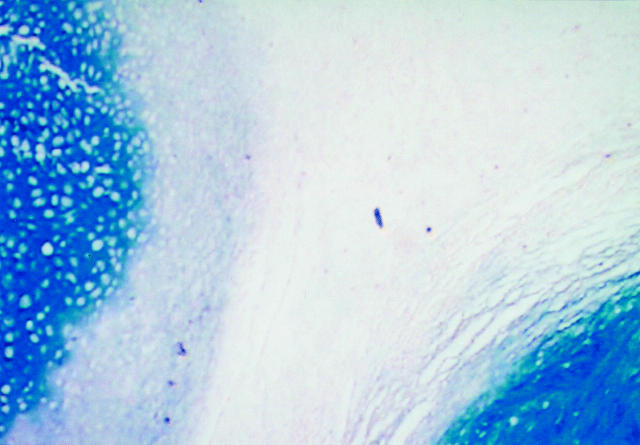
It is well known that synovial chondromatosis is typified by direct metaplasia of synovial cells into cartilage (Figure 1). Staining by anti-FGFR3 occurs at the margins of the cartilage nodules. The margins contain tissues whose features are intermediate between cartilage and synovium. The border areas contain FGFR3 positive cells apparently undergoing differentiation into cartilage (Figure 2). Positive staining by PCNA (Figure 3), an indicator of cellular proliferation, is limited to these same cells. These cells occupy less than 1% of the specimen area. FGF 9 is expressed in a patchy manner within the synovium (not shown) and in a specific and limited layer of cells in the superficial part of the cartilage nodule (Figure 4). Specimens of secondary chondromatosis contain few if any PCNA-positive and FGFR3-positive cells and no staining by anti-FGF 9 antibodies are observed in the synovium. As expected the cartilage nodules in PSC contain type II collagen (Figure 5).
Figure 1.
The boundary of synovium and cartilage contains a layer that secretes a small amount of proteoglycans as evidenced by slight staining by alcian blue. In this layer direct metaplasia of synovial cells into cartilage occurs (alcian blue pH 1, Original magnification × 100).
Figure 2.
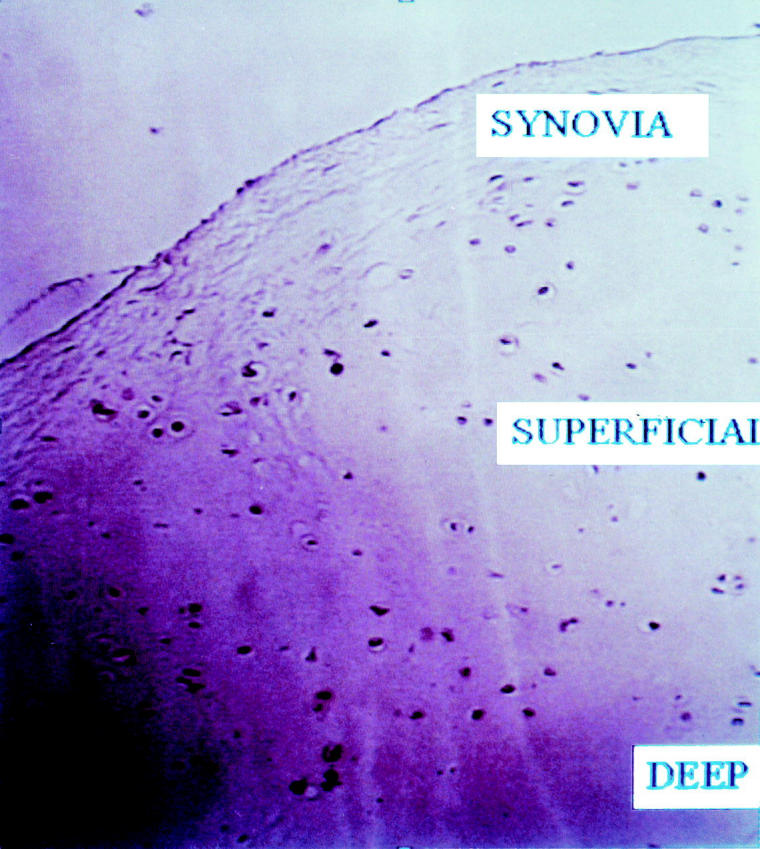
Cells in the superficial layer (SUPERFICIAL) of cartilage are uniformly stained by antibodies to FGF receptor 3. Cells in the deep layer (DEEP) that are typically cartilaginous do not stain. Neither do cells in the synovium (synovia) stain by these antibodies (diaminobenzidine, anti-FGFR3 antibody, 1 : 100 dilution, Original magnification × 400).
Figure 3.
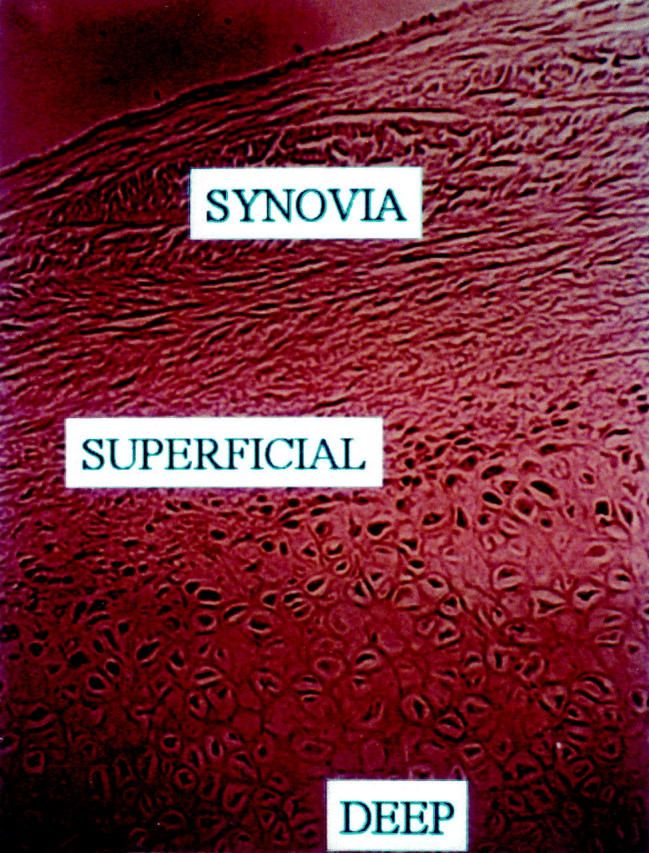
Cells in the superficial-transitional zone of the cartilaginous nodules express PCNA as a marker of cell proliferation. This antigen is not expressed in either the synovia or the deeper areas of typical cartilage (diaminobenzidine, anti-PCNA, Original magnification × 400).
Figure 4.

Cells in the superficial layer of the cartilaginous layer express FGF 9, the specific ligand of FGF receptor 3 (diaminobenzidine, anti-FGF 9 antibodies, Original magnification × 200).
Figure 5.
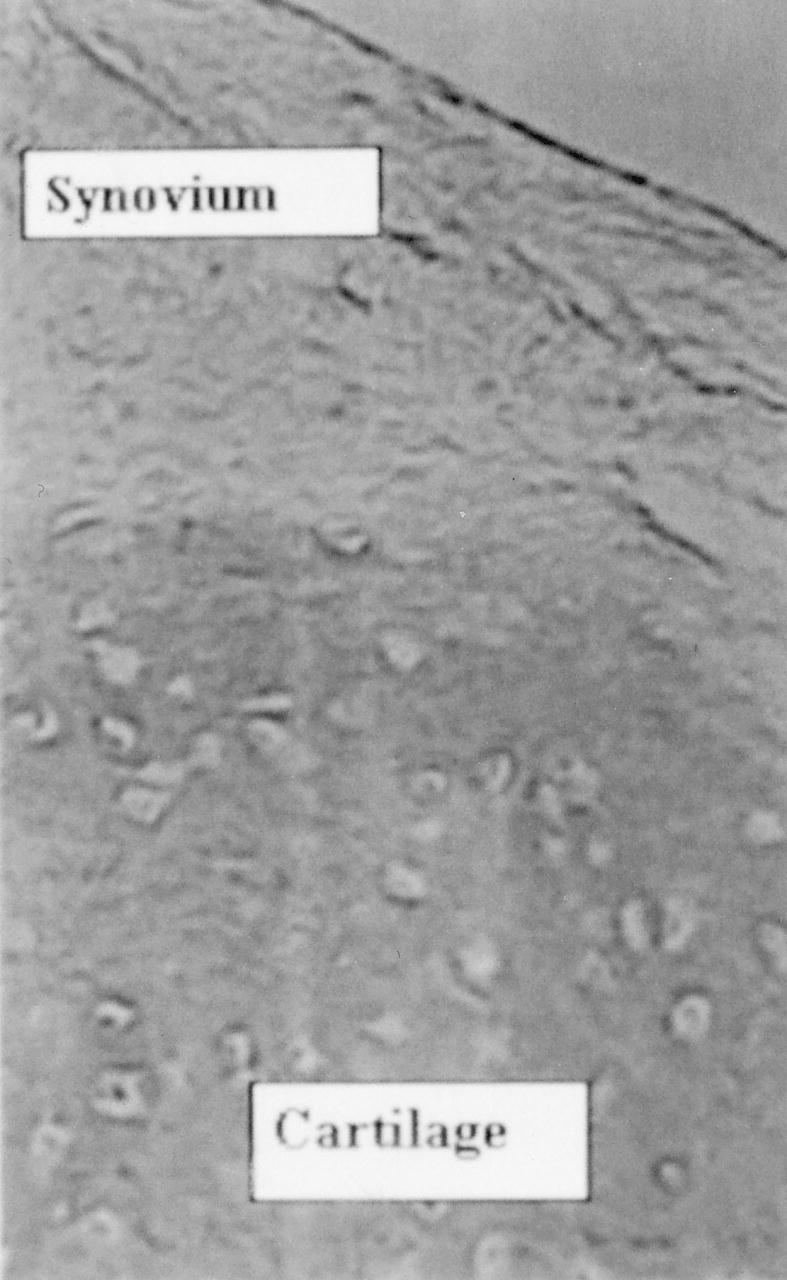
Deep layer matrix contains collagen type II, the synovia and superficial zone do not contain collagen type II (diaminobenzidine, anticollagen type II, Original magnification × 200).
Immunohistochemical studies of cell cultures
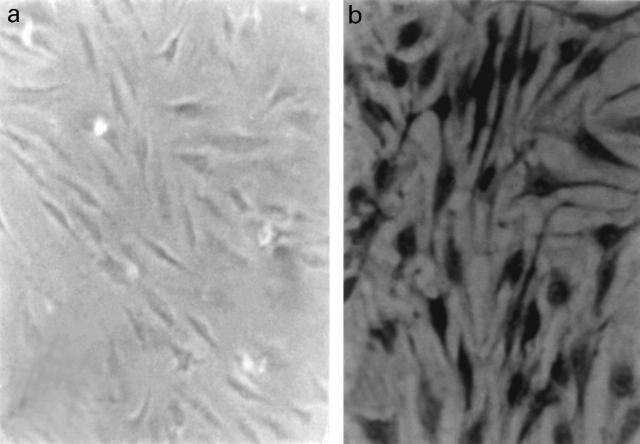
Cell cultures of synovial fibroblast-like cells from primary synovial chondromatosis but not from normal synovia demonstrate FGFR3 expression. In synovial cells from PSC, only 43 ± 6% of the cells were FGFR3 positive. All cultures were negative for collagen type II. PSC-derived cultures were positively stained by anti-FGF9 antibodies. Staining by anti-FGF 9 antibodies occurs only in PSC cells and not in other cultures of synoviocytes (Figure 6).
Figure 6.
Cell cultures of synovia from patients without PSC do not express FGF 9 (a). In cell cultures from joints afflicted by PSC, many of the cells express FGF 9 (b) (diaminobenzidine, anti-FGFR3 antibody, 1 : 100 dilution, Original magnification × 400).
Chondrocytes derived from cartilage nodules undergo de-differentiation in monolayer culture. Collagen type II expression does not occur. Synthesis of aggrecan, a high molecular weight proteoglycan as assessed by staining intensity by alcian blue pH 1, is minimal (23 ± 8 grey levels). FGFR3 expression is intense at first. However, longer term cultures (over 2 weeks) form nodules composed of multilayers. These cells have regained the chondrogenic phenotype, expressing collagen type II (38 ± 4%) as well as producing aggrecan (alcian blue staining at pH 1, 126 ± 5 grey levels). In these areas, expression of FGFR3 is absent.
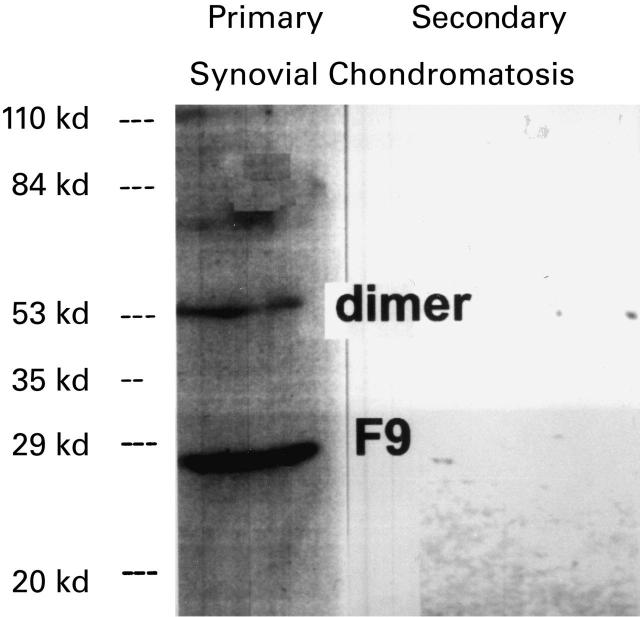
Synovial fluids of PSC patients contain significant levels of FGF 9 as demonstrated by Western blotting. On the other hand, synovial fluids from other joint pathologies do not contain FGF 9 (Figure 7).
Figure 7.
Western blot analysis of synovial fluids from patients (secondary synovial chondromatosis (secondary), PSC (primary)). Only synovial fluid from PSC contains FGF 9 (F9). Part of the FGF 9 is in a dimeric form (Dimer).
Discussion
In both PSC and SSC, loose bodies are formed within the joint cavity. The bodies are composed of cartilage and bone and are quite similar histologically. The principal difference is that in PSC the bodies are embedded within synovial tissue, which appears to undergo a direct metaplasia into cartilage (Abraham & Canoso 1993; Crotty et al. 1996). PSC is a benign lesion, and the cells are diploid (Coughlan et al. 1995). However, 15% recurrence rate seems to indicate an aggressive potential which is also indicated by the relatively frequent (5%) transformation rate (Davis et al. 1998a). Davis et al. have shown that while synovial chondromatosis does not show intensive proliferative activity, the cells do express c-erb B-2. This antigen is commonly expressed in chondrosarcomas but is not found in other benign cartilage lesions or in articular cartilage (Davis et al. 1996). Some PSC lesions are not diploid, and contain a higher DNA content than enchondromas, which are benign cartilage lesions (Davis et al. 1998a). Cytogenetic analysis appears to indicate that PSC is the result of clonal proliferation (Mertens et al. 1996). Our results are consistent with these observations. A few cells in the periphery of the cartilage nodules are PCNA-positive as well as FGFR3-positive. Apparently in culture these cells rapidly proliferate and become dominant in cultures of patients with PSC. Studies such as Davis's that analyse DNA profile of the lesions (Davis et al. 1998a) show relatively normal DNA content, as most cells of the lesions do not proliferate. In previous studies we have demonstrated that mesenchymal stem cells are typified by FGFR3 expression as well as by their capacity in vitro to undergo a chondrogenic differentiation into mature cartilage (Davis et al. 1998a; Nevo et al. 1998). Such cells are found in bunions and appear to be responsible for growth of the lesions (Robinson et al. 1999a). These cells are known to occur within bone marrow and synovia and are capable of induction of cartilage and bone (Kadiyala et al. 1997).
In synovial fluid from PSC, in contrast to other synovial fluids, high levels of FGF 9 are expressed. FGF 9 is the primary ligand of FGFR3 and is capable of maintaining mesenchymal stem cells in a proliferative status prior to their final differentiation (Hecht et al. 1995; Nevo et al. 1998). The existence of excess FGF 9 in synovial fluid of PSC patients can explain the induction of cartilage formation by residual mesenchymal stem cells of the synovium. The lack of FGF 9 in other pathologies involving cartilage injuries and loose body formation explains why in these situations there is no proliferation in the loose bodies. The existence of these activated mesenchymal stem cells within the synovium explains why removal of loose bodies alone is not sufficient in PSC (Ogilvie & Saleh 1994) while it is adequate in secondary synovial chondromatosis.
Our results demonstrate that the majority of the cartilaginous cells in the specimen do not proliferate, as has been found by most previous authors (Apte & Athanasou 1992; Davis et al. 1998b). Only a subpopulation of cells actively proliferates. This subpopulation expresses FGFR3, indicating a mesenchymal stem cell origin. These mesenchymal stem cells undergo rapid proliferation and become dominant in the cultures. As these cells stain positively by FGF 9 it is assumed that they secrete it as well. Thus an autocrine feedback loop is formed. Such a loop is typical for malignant cells.
In conclusion, it appears that, similar to the situation in bunions, cartilage nodule formation in PSC involves recruitment of FGFR3 positive mesenchymal stem cells. Apparently, this recruitment is due to the secretion of FGF 9 by the synovia and some of the recruited mesenchymal cells. Inhibition of FGF 9 might offer a nonoperative method for treating PSC.
References
- 1.Abraham JH, Canoso JJ. Tumors of soft tissues and bone. Curr. Opin. Rheumatol. 1993;5:193–198. doi: 10.1097/00002281-199305020-00012. [DOI] [PubMed] [Google Scholar]
- 2.Apte SS, Athanasou NA. An immunohistological study of cartilage and synovium in primary synovial chondromatosis. J. Pathol. 1992;166:277–281. doi: 10.1002/path.1711660310. [DOI] [PubMed] [Google Scholar]
- 3.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 4.Butnariu EM, Robinson D, Mendes DG, Halperin N, Nevo Z. Resurfacing of goat articular cartilage by chondrocytes derived from bone marrow. Clin. Orthop. 1996;330:234–243. doi: 10.1097/00003086-199609000-00031. [DOI] [PubMed] [Google Scholar]
- 5.Coughlan B, Feliz A, Ishida T, Czerniak B, Dorfman HD. p53 expression and DNA ploidy of cartilage lesions. Hum. Pathol. 1995;26:620–624. doi: 10.1016/0046-8177(95)90166-3. [DOI] [PubMed] [Google Scholar]
- 6.Crotty JM, Monu JU, Pope TLJ. Synovial osteochondromatosis. Radiol. Clin. North Am. 1996;34:327–342. [PubMed] [Google Scholar]
- 7.Davis RI, Foster H, Biggart DJ. C-erb B-2 staining in primary synovial chondromatosis: a comparison with other cartilaginous tumours. J. Pathol. 1996;179:392–395. doi: 10.1002/(SICI)1096-9896(199608)179:4<392::AID-PATH600>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 8.Davis RI, Foster H, Arthur K, Trewin S, Hamilton PW, Biggart DJ. Cell proliferation studies in primary synovial chondromatosis. J. Pathol. 1998a;184:18–23. doi: 10.1002/(SICI)1096-9896(199801)184:1<18::AID-PATH956>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 9.Davis RI, Hamilton A, Biggart JD. Primary synovial chondromatosis: a clinicopathologic review and assessment of malignant potential. Hum. Pathol. 1998b;29:683–688. doi: 10.1016/s0046-8177(98)90276-3. [DOI] [PubMed] [Google Scholar]
- 10.Grigeriadis AE, Heersch JNM, Aubin JE. Differentiation of muscle, fat, cartilage and bone from progenitor cells present in bone derived clonal cell population: effect of dexamethasone. J. Cell Biology. 1989;106:2139–2151. doi: 10.1083/jcb.106.6.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hecht D, Zimmerman N, Bedford M, Avivi A, Yayon A. Identification of fibroblast growth factor 9 (FGF9) as a high affinity, heparin dependent ligand for FGF receptors 3 and 2 but not for FGF receptors 1 and 4. Growth Factors. 1995;12:223–233. doi: 10.3109/08977199509036882. [DOI] [PubMed] [Google Scholar]
- 12.Kadiyala S, Young RG, Thiede MA, Bruder SP. Culture expanded canine mesenchymal stem cells possess osteochondrogenic potential in vivo and in vitro. Cell Transplant. 1997;6:125–134. doi: 10.1177/096368979700600206. [DOI] [PubMed] [Google Scholar]
- 13.Mertens F, Jonsson K, Willen H, et al. Chromosome rearrangements in synovial chondromatous lesions. Br. J. Cancer. 1996;74:251–254. doi: 10.1038/bjc.1996.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mori S, Sawai T, Teshima T, Kyogoku M. A new decalcifying technique for immunohistochemical studies of calcified tissue, especially applicable to cell surface marker demonstration. J. Histochem. Cytochem. 1988;36:111–114. doi: 10.1177/36.1.3275709. [DOI] [PubMed] [Google Scholar]
- 15.Nevo Z, Silver J, Chorev Y, Riklis I, Robinson D, Yosipovitch Z. Adhesion characteristics of chondrocytes cultured separately and in co-cultures with synovial fibroblasts. Cell Biol. Int. 1993;17:255–273. doi: 10.1006/cbir.1993.1062. [DOI] [PubMed] [Google Scholar]
- 16.Nevo Z, Robinson D, Horowitz S, Hasharoni A, Yayon A. The manipulated mesenchymal stem cells in regenerated skeletal tissues. Cell Transplant. 1998;7:63–70. doi: 10.1177/096368979800700109. [DOI] [PubMed] [Google Scholar]
- 17.Ogilvie HD, Saleh K. Generalized synovial chondromatosis of the knee: a comparison of removal of the loose bodies alone with arthroscopic synovectomy. Arthroscopy. 1994;10:166–170. doi: 10.1016/s0749-8063(05)80088-x. [DOI] [PubMed] [Google Scholar]
- 18.Robinson D, Halperin N, Nevo Z. Bone density in old chickens' metaphyses, as affected by local trauma and chondrocyte implantation. Bull. Hosp. Jt. Dis. 1993;53:83–87. [PubMed] [Google Scholar]
- 19.Robinson D, Tieder M, Halperin N, Burshtein D, Nevo Z. Maffucci's syndrome — the result of neural abnormalities? Evidence of mitogenic neurotransmitters present in enchondromas and soft tissue hemangiomas. Cancer. 1994;74:949–957. doi: 10.1002/1097-0142(19940801)74:3<949::aid-cncr2820740325>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 20.Robinson D, Halperin N, Yayon A, Nevo Z. Mesenchymal cells and growth factors in bunions. Foot Ankle. Int. 1999a;20:727–732. doi: 10.1177/107110079902001109. [DOI] [PubMed] [Google Scholar]
- 21.Robinson D, Hasharoni A, Cohen N, Yayon A, Moskowitz RM, Nevo Z. Fibroblast growth factor receptor-3 as a marker for precartilaginous stem cells. Clin. Orthop. 1999b;367:S163–S175. doi: 10.1097/00003086-199910001-00018. [DOI] [PubMed] [Google Scholar]