Abstract
In this study of the seminomatous human testis the composition, activity and apoptosis of lymphocytes infiltrating the immune-privileged seminiferous tubules with in situ seminoma were studied by immunohistochemistry and DNA fragmentation detection. Likewise the lymphocytes infiltrating the invasive seminomas were studied. The study showed equal numbers of CD4+ and CD8+ T cells and B cells, about 30% of the cells. Very few T γ/δ and NK cells were present. The activity in terms of IL-2-R, FasL and perforin expression was low. Apoptosis of the lymphocytic cells was limited. No differences were observed between the lymphocytes in seminiferous tubules with in situ seminoma and the lymphocytes in invasive tumours. The study suggests that either specifically committed lymphocytes are not present or, if present, immune-suppressing mechanisms in addition to FasL may be working.
Keywords: in situ seminoma, immune privilege, immune surveillance
Concepts of immune surveillance of cancer maintain that tumour-infiltrating lymphocytes (TIL) more or less successfully eliminate tumour cells (Klein 1980; Kradin & Bhan 1993). Cytotoxic T cells are believed to play a major role by recognising tumour-specific peptides presented by major histocompatibility complex (MHC) class I molecules on the surface of tumour cells (Elliot et al. 1989; Townsend & Bodmer 1989).
In immune-privileged areas such as the testis where immune reactions are suppressed (Wayne Streilein 1995) the role of immune surveillance of cancer is obscure. In the testis the seminiferous tubules are protected from immune attack by FasL molecules expressed by Sertoli cells, inducing apoptosis in Fas-expressing lymphocytes (Bellgrau et al. 1995). The FasL molecules form a layer at the periphery of the tubules located at the foot processes of the Sertoli cells in rodents and humans (French et al. 1996; Lee et al. 1997; Brændstrup et al. 1999).
In seminiferous tubules with in situ seminoma (cis) Sertoli cells are eventually displaced to central areas of the cis tubules by the neoplastic cells (Brændstrup 1996). The FasL layer then becomes discontinuous and only sporadic FasL expression can be recognized on the displaced Sertoli cells. This may allow T lymphocytes to infiltrate the cis tubules (Brændstrup et al. 1999). Evidently in invasive seminomas Sertoli-cell FasL has no significance. Invasive seminomas are characteristically infiltrated by numerous TIL including many CD8+ T cells (Bell et al. 1987; Nakanoma et al. 1992; Wei et al. 1992; Nouri et al. 1993; Torres et al. 1997).
However, since the seminoma cells in cis and invasive tumour do not express MHC class I and ICAM-1 molecules which mediate cellular interaction between CD8+ T cells and their targets, the role of the CD8+ T cells in seminomas is not clear (Bell et al. 1987; Vanky et al. 1990; Nouri et al. 1993; Brændstrup 1996; Brændstrup et al. 1996). Sertoli cells of cis tubules as opposed to those of normal tubules, on the other hand, express both MHC class I and ICAM-1 molecules and therefore one may speculate that some of the lymphocytes infiltrating cis tubules are autoreactive CD8+ T cells with specificity against the antigens presented by the modified Sertoli cells (Brændstrup 1996; Brændstrup et al. 1996). If so, immune activity might be observed in these lymphocytes.
The present study was therefore undertaken to determine the composition, activity and apoptosis of the infiltrating lymphocytes in in situ seminomas and to compare these lymphocytes with those of the invasive tumours.
Materials and methods
The material derives from the files of the Department of Pathology, Glostrup Hospital. It consists of 22 cases of seminomas collected from 1988 to 1996 and selected on the basis of technical quality and the presence of cis seminiferous tubules. Blocks containing invasive tumour and surrounding tissue with normal and cis seminiferous tubules were chosen. These specimens had been fixed in formaldehyde and stored as paraffin-embedded blocks. In addition the material consists of frozen tissue stored at −80°C from another 12 consecutive cases of seminomas collected from 1996 to 1999. Specimens from these cases were selected on the basis of technical quality only. Some of these samples contain only tumour or surrounding tissue. Sections from the material were cut and stained with hematoxylin and eosin (H & E) for conventional histology to identify relevant areas for immunohistochemical studies.
Immunohistochemistry
The antibodies used in the study are described in Table 1. As secondary antibodies were used biotinylated rabbit antimouse Immunoglobulins (DAKO A/S, Copenhagen, Denmark; code E 0354, lot 067) and a swine antirabbit Immunoglobulin (DAKO; code E 353, lot 053).
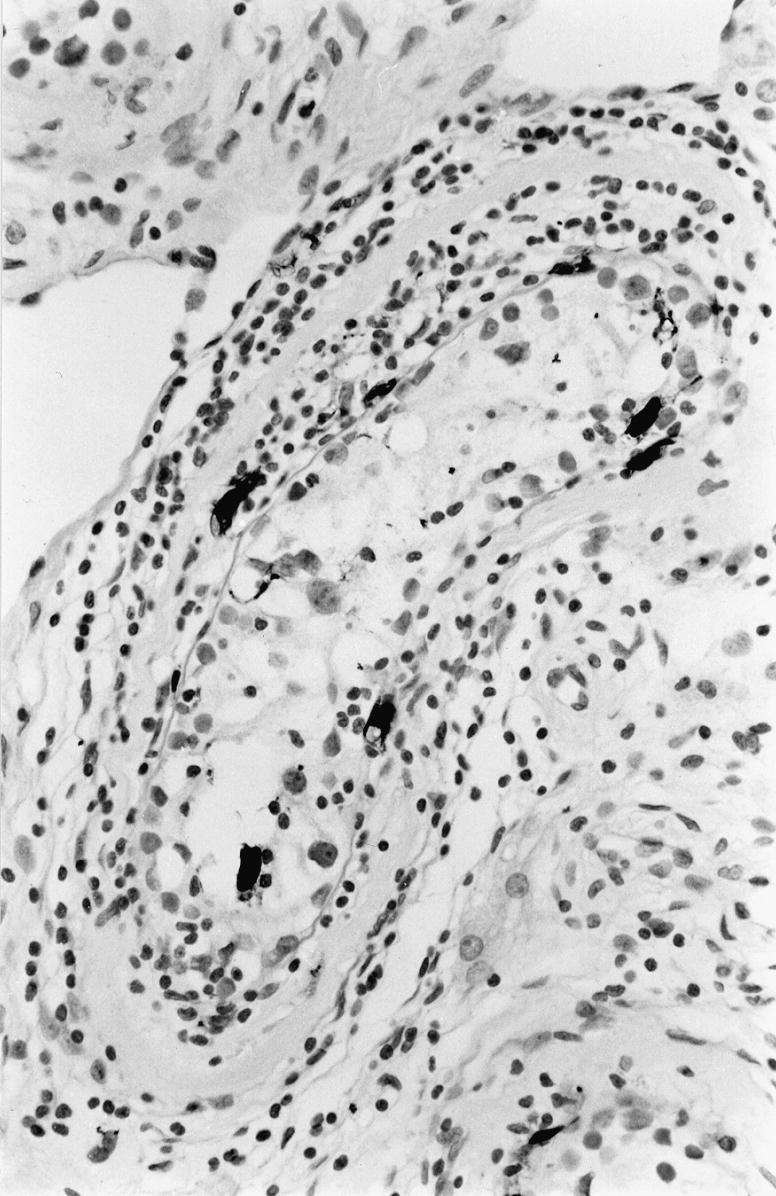
Table 1.
Primary antibodies used for immunohistochemistry
Antibodies were monoclonal except for those marked (p), *anticow S-100 crossreacts with human S-100.
DAKO EnVision + System (DAKO; code 4004) was used as secondary antibody binding to anti-Fas. This includes a horseradish peroxidase-labelled polymer conjugated with the secondary antimouse antibody. Biotinylated rabbit antirat immunoglobulin (DAKO; code E 0467, lot 097) was used as secondary antibody to anti-FasL.
Antibodies to CD3, CD8, CD20, CD79, CD68, CD56, CD57 and S100 were used on formalin-fixed tissue. Antibodies to CD4, perforin, IL-2-R, T γ/δ receptor, Fas and Fas-L were used on frozen tissue.
The avidin–biotin/peroxydase immunohistochemical procedure for formalin-fixed and fresh frozen tissue used has been described previously (Brændstrup 1996; Brændstrup et al. 1999). Mayers haemalun was used as counter stain. Endogenous peroxydase was initially blocked by H2O2.
Controls
As positive controls were used formalin-fixed and frozen tissue from various organs containing lymphoid tissue such as tonsils, small intestine and spleen. For negative controls the specific antibodies were omitted.
Apoptosis
A DNA fragmentation kit was used, Klenow-FragELTM (Oncogene Research Products, Cambridge, MA, USA/Calbiochem, cat QIA21). In this kit DNA polymerase I adds biotin-labelled and unlabeled deoxynucleotides to the ends of DNA fragments generated during apoptosis. Streptavidin-horseradish peroxidase and diaminobenzidine were used as a detection system. Methyl green was used as a counterstain. Endogenous peroxidase was blocked by H2O2. This system was used on the paraffin-embedded material and deparaffinization and rehydration were initially performed. Intestinal mucosa was used as a positive control. For negative controls the labelling step was omitted and the slides kept in reaction buffer.
Scoring of stained cells
Each slide was labelled at random with ink dots using a metal grid. In each slide cis tubules with and without lymphocytic infiltrates were identified and enumerated. In the invasive tumours, areas which happened to be located on one side of the ink dots inside a square in an ocular grid were selected for counting. In the cis tubules with lymphocytic infiltrates all lymphocytes and all labelled lymphocytes were counted. In the selected tumour areas approximately 500 lymphocytes including labelled and unlabelled lymphocytes were counted. The percentages of labelled lymphocytes were calculated. Each of the two observers (BB and LJ) examined half of the material.
The inter- and intra-observer variations were estimated separately. In each of 25 randomly chosen slides a given area (square) was counted twice by both observers. The percentages of labelled lymphocytes were determined. The Spearman rank–order correlation coefficient θ was used to analyse inter- and intra-observer variation. The Mann–Whitney rank sum test was used to examine differences in percentages of labelled cells between in situ and invasive seminomas.
Results
Varying numbers of cis tubules were infiltrated by lymphocytes, the average being 15% of the cis tubules. The degree of inflammation in these tubules varied from a few lymphocytes to heavy concentric infiltrates invading the epithelium (Figures 1–4). Normal seminiferous tubules were devoid of inflammatory cells.
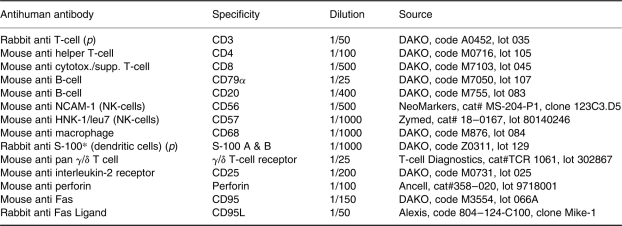
Figure 1.
Immunohistochemical staining for CD8+ T cells in and around cis seminiferous tubule. Positive cells have dark granules along the cell membrane. × 400.
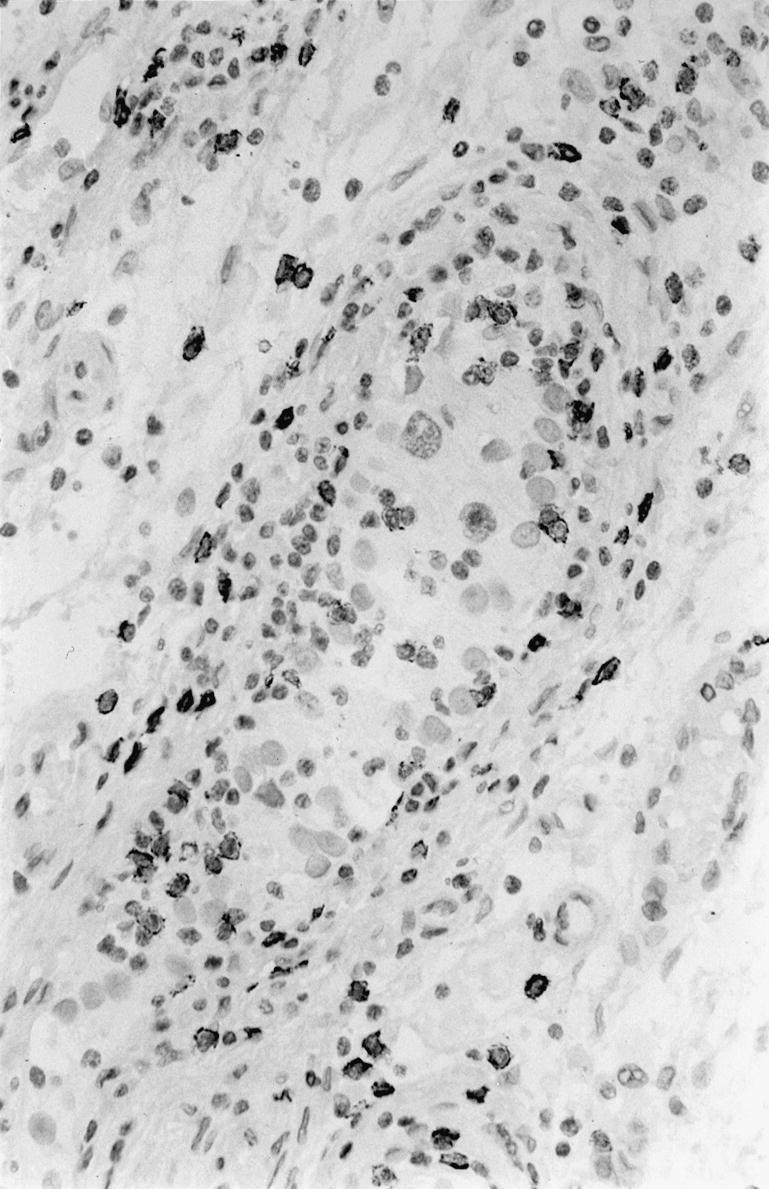
Figure 4.
Immunohistochemical staining for S-100 positive dendritic cells seen as strongly stained cells in and around a cis seminiferous tubule. × 400.
The results of the immunohistochemistry are presented in Table 2. As will be seen, great variation in the percentages of labelled cells was observed, as illustrated by the ranges. Only minor differences between the mean percentages of stained cells in the cis tubules and the tumours were seen. These differences were significant only for T γ/δ cells, CD 68 and FasL. CD8+ T cells (Figure 1), CD4+ T cells and B cells (Figure 2) were present in equal numbers around one-third of the cells. As a ‘control’ of T cells the number of CD3-expressing T cells corresponded to the sum of CD8 and CD4 cells. B cells were demonstrated by CD20 as well as CD79, the latter showing more positive cells in accordance with the broader range of B cells expressing this marker. Some of these cells had a plasmocytoid appearance.
Table 2.
Immunohistochemistry and apoptosis of lymphocytes in carcinoma in situ and seminoma
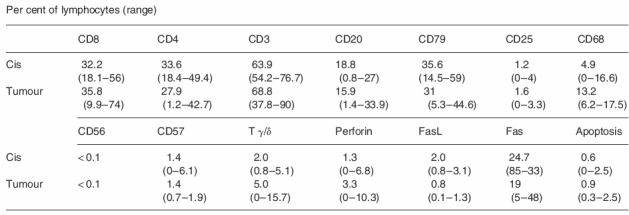
The Mann–Whitney rank sum test showed significant difference for CD68 and Tγ/δ (P < 0.05) and for FasL (P < 0.01).
Figure 2.
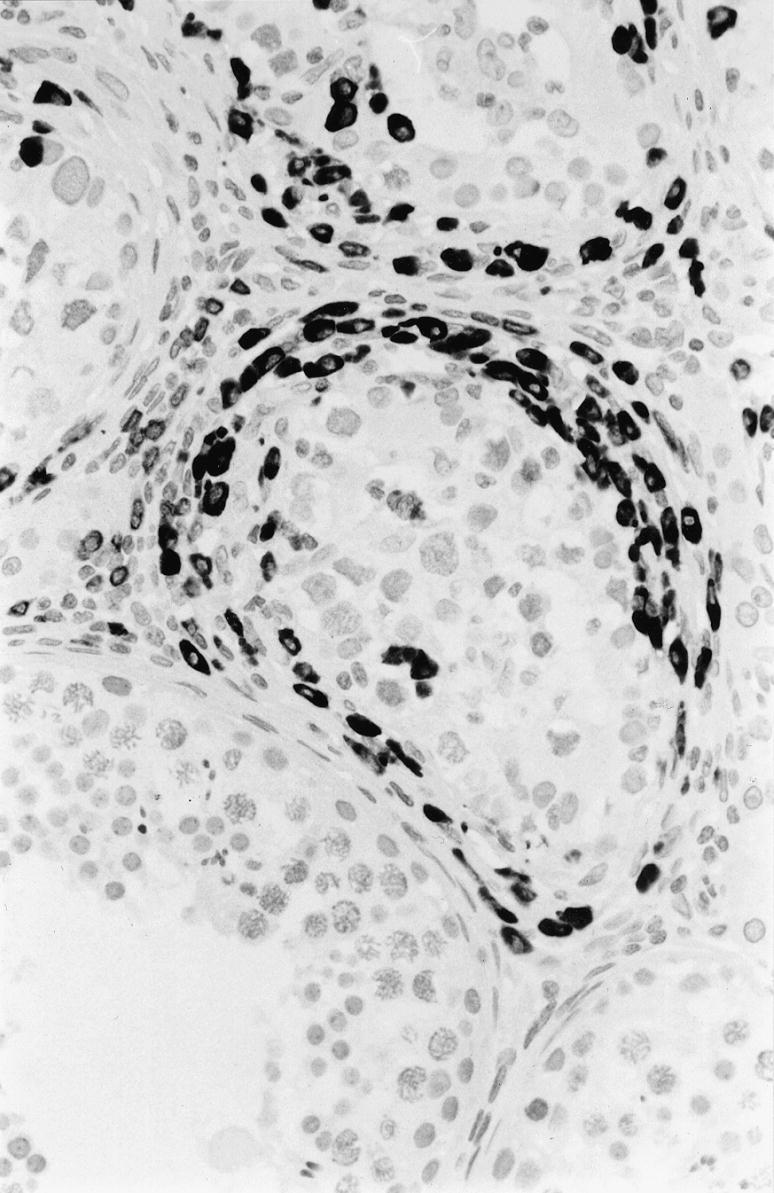
Immunohistochemical staining for CD79+ B cells in and around three cis seminiferous tubules. One normal seminiferous tubule is present, lower part. × 400.
CD56-, CD57-, IL-2-R-, Perforin- and FasL-expressing cells each comprised a few per cent of the inflammatory cells.
About 25% of the cells expressed Fas. CD68 expressing cells, i.e. macrophages, were seen in substantial numbers (Figure 3) with relatively more positive cells inside the tumours.
Figure 3.
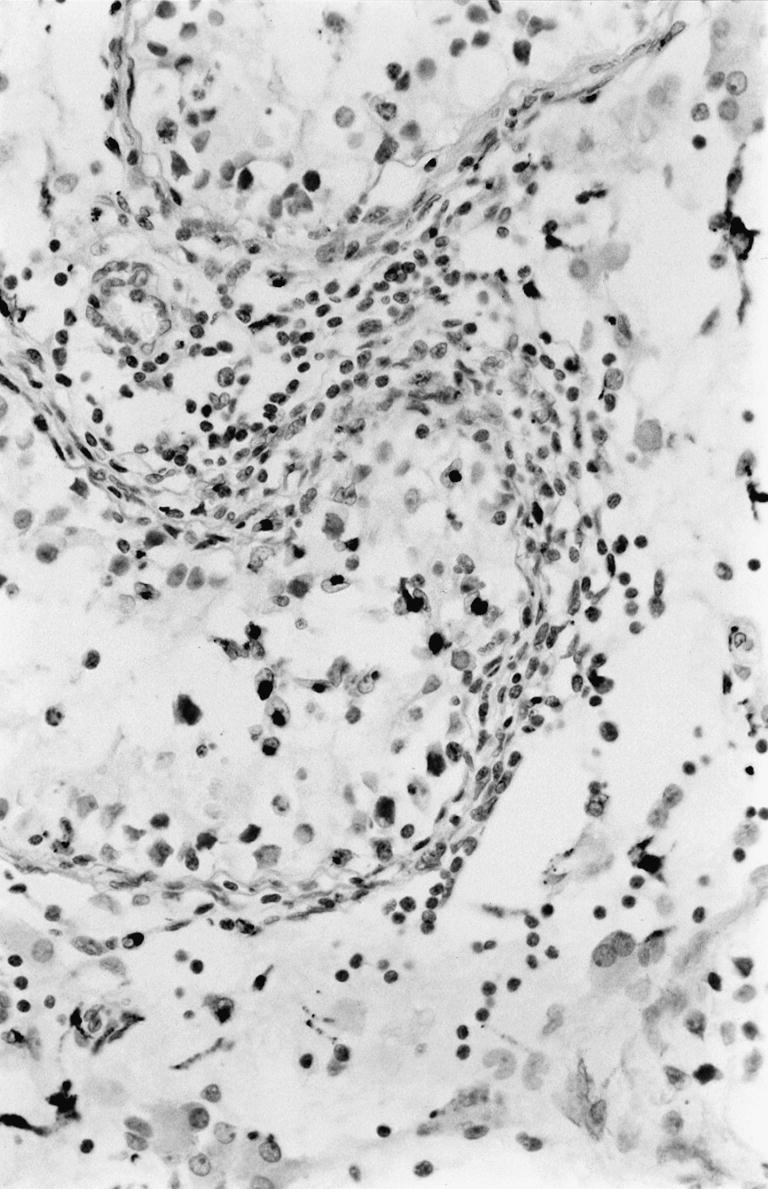
Immunohistochemical staining for CD68 positive macrophages seen as dark cells in and around a cis seminiferous tubules. × 400.
S-100 positive dendritic cells were observed in the cis tubules with lymphocytic infiltrates (Figure 4), the number of cells per cross-section was 0.7. Dendritic cells were present among lymphocytes in the tumours, the average number per high power field (× 400) was 5.2 cells.
The number of apoptotic lymphocytes in and around cis tubules was 0.61% and inside tumours 0.91%. Apoptosis was observed in only a few tumour cells in the seminomas and no identifiable Sertoli cells were observed in the tubules studied.
The inter- and intra-observer variations were low, Spearman's θ: BB/LJ: 0.8853–0.9269, BB/BB: 0.9746 and LJ/LJ: 0.9396.
Discussion
In seminomas TIL and tumour cells are separated creating an almost morphologically biphasic pattern. In contrast the peri- and intra-tubular lymphocytes of a number of cis tubules gives an impression of an inflammatory process targeted at the content of these cis tubules (Figures 1–4).
The present study showed that CD8+ T cells, CD4+ T cells and B cells each constituted about one-third of the lymphocytic infiltrates whether attacking cis tubules or being TIL in the seminomas.
In previous studies which have focused on lymphocytes inside the infiltrating tumour only, CD8+ T cells were the dominating cells (Bell et al. 1987; Nakanoma et al. 1992; Wei et al. 1992; Nouri et al. 1993; Torres et al. 1997). Only one additional study found as many B cells in the tumours as shown here (Nakanoma et al. 1992). Autoimmune antibodies towards the germinative epithelium of cis tubules has been reported (Lehmann & Müller 1987) and a humoral immune response is induced in the mouse testis used for induction of experimental teratoma (Sundström et al. 1999). These observations may be pertinent to the many B cells in the cis tubules in the present study.
The few IL-2-R-expressing lymphocytes in cis tubules and the tumours and the only occasional presence of lymphocytes expressing perforin and FasL molecules used by cytotoxic T cells to kill target cells (Lowin et al. 1994; Lynch et al. 1995) do not support substantial immune activity although more FasL positive cells were observed in cis tubules. Furthermore very little apoptosis of the lymphocytes was observed.
Previous studies of TIL inside seminomas have shown activity of TIL in terms of in vitro responsiveness to IL-2 and MHC class II expression (Nouri et al. 1993) and also a considerable number of Fas-expressing lymphocytes (Brændstrup et al. 1999). This may merely reflect the propensity of such activated lymphocytes to randomly pass vessel walls expressing relevant adhesion molecules (Carlos & Harlan 1994). ICAM-1, VCAM-1 and ELAM-1 are expressed on endothelial cells in seminomas (Brændstrup et al. 1996).
NK cells and T γ/δ cells, believed to be part of an early response to tumours, seem to play no significant role even though seminoma cells without MHC class I molecules would seem to be ideal targets for NK cells (Ljunggren & Kärre 1986; Mak & Ferrick 1998). A study of 22 seminomas confirms our data of T γ/δ cells (Nouri et al. 1993) while a study of three dysgerminomas and one seminoma found increased numbers of T γ/δ cells compared to peripheral blood (Zhao et al. 1995).
The absence of MHC class I molecules and ICAM-1 on seminoma cells is not compatible with conventional concepts of a direct cytotoxic T-cell attack on tumours and this is consistent with the limited immune activity of the TIL.
Mouse Sertoli cells show adhesion of lymphocytes and expression of ICAM-1 in vitro when exposed to IL-1, TNF-alfa and IF-gamma and it has been suggested that such Sertoli cells, normally being immunosuppressive, could contribute to autoimmunity in the testis (Riccioli et al. 1995).
In the cis tubules the low apoptosis/activity of the infiltrating lymphocytes may be explained by the reduced expression of FasL on the Sertoli cells (Brændstrup et al. 1999), and/or the absence of lymphocytes with specificity to the tubular content and/or absence or insufficient numbers of the relevant stimulatory molecules.
Dendritic cells, present in cis tubules and tumours, can transport phagocytozed apoptotic tumour cells and other cells from the periphery to the draining lymph nodes and give rise to clonal expansion of lymphocytes with specificity to antigens from the apoptotic cells (Grabbe et al. 1995; Albert et al. 1998). In this way the dendritic cells in seminomas and cis could prime CD4+ T cells by conventional MHC class II pathway and CD8+ T cells by cross-presentation of exogenous Sertoli cell and tumour cell peptides by MHC class I molecules on DCs (Kurts et al. 1996; Rock 1996; Albert et al. 1998).
These lymphocytes could be among those in the seminomas unable to recognize the target cells. The absence of an immune reaction with subsequent apoptosis of the lymphocytes may explain the large numbers of lymphocytes which accumulate in seminomas.
In summary, even though the immune privilege of the testis in terms of FasL protection is disturbed during development of neoplasia, only low CD8+ T cell activity in terms of expression of perforin, FasL and apoptosis of the lymphocytes infiltrating the cis tubules can be recognized. The same low activity was observed in TIL in the invasive seminomas. This may indicate that either no lymphocytes with specificity towards tumour cells or Sertoli cells are present or, if present, the lymphocytes are unable to react to or recognize the target cells. In the case of the seminoma cells this can be explained by the negative expression of MHC class I and ICAM-1 molecules. In the case of Sertoli cells in the cis tubules, immune-suppressing mechanisms alternative to FasL may be acting.
References
- 1.Albert ML, Sauter B, Bhardwaj N. Dendritic cells aquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 2.Bell DA, Flotte TJ, Bhan AK. Immunohistochemical characterization of seminoma and its inflammatory infiltrate. Hum. Pathol. 1987;18:511–520. doi: 10.1016/s0046-8177(87)80037-0. [DOI] [PubMed] [Google Scholar]
- 3.Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke RC. A role for CD95 ligand in preventing graft rejection. Nature. 1995;337:630–632. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- 4.Brændstrup O. HLA class I antigens are expressed by Sertoli cells of intratubular germ cell neoplasia. APMIS. 1996;104:579–582. doi: 10.1111/j.1699-0463.1996.tb04914.x. [DOI] [PubMed] [Google Scholar]
- 5.Brændstrup O, Jensen L, Werdelin O. Sertoli cells, but not tumor cells, of seminoma in situ express ICAM-1. APMIS. 1996;104:817–820. [PubMed] [Google Scholar]
- 6.Brændstrup O, Bols B, Jensen L. Fas and Fas- ligand expression in seminomatous testes. APMIS. 1999;107:431–436. doi: 10.1111/j.1699-0463.1999.tb01577.x. [DOI] [PubMed] [Google Scholar]
- 7.Carlos TM, Harlan JM. Leucocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- 8.Elliot BE, Carlow DA, Rodricks A-M, Wade A. Perspectives on the role of MHC antigens in normal and malignant cell development. Adv. Cancer Res. 1989;53:181–245. doi: 10.1016/s0065-230x(08)60282-1. [DOI] [PubMed] [Google Scholar]
- 9.French LE, Hahne M, Viard I, Radlgruber G, Zanone R, Becker K, Et AL. Fas and Fas ligand in embryos and adult mice: ligand expression in several immune-privileged tissues characterized by apoptotic cell turnover. J. Cell. Biol. 1996;133:335–343. doi: 10.1083/jcb.133.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grabbe S, Beissert S, Schwarz T, Granstein RD. Dendritic cells as initiators of tumor immune responses: a possible strategy for tumor immunotherapy? Immunol. Today. 1995;16:117–121. doi: 10.1016/0167-5699(95)80125-1. [DOI] [PubMed] [Google Scholar]
- 11.Klein G. Immune and non-immune control of neoplastic development: contrasting effects of host and tumor evolution. Cancer. 1980;45:2486–2499. doi: 10.1002/1097-0142(19800515)45:10<2486::aid-cncr2820451005>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 12.Kradin RL, Bhan AT. Tumor infiltrating lymphocytes. Lab. Invest. 1993;69:653–658. [PubMed] [Google Scholar]
- 13.Kurts C, Heath WR, Carbone FR, Allison J, Miller JF.A.P, Kosaka H. Constitutive class I-restricted exogenous presentation of self antigens in vivo. J. Exp. Med. 1996;184:923–930. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J, Richburg JH, Younkin SC, Boekelheide K. The Fas system is a key regulator of germ cell apoptosis in the testis. Endocrinology. 1997;138:2081–2088. doi: 10.1210/endo.138.5.5110. [DOI] [PubMed] [Google Scholar]
- 15.Lehmann D, Müller H. Analysis of the autoimmune response in an ‘in situ’ carcinoma of the testis. Int. J. Androl. 1987;10:163–168. doi: 10.1111/j.1365-2605.1987.tb00178.x. [DOI] [PubMed] [Google Scholar]
- 16.Ljunggren H-G, Kärre K. Variations in MHC antigen expression on tumors and its significance. J. Immunogenetics. 1986;13:141–151. doi: 10.1111/j.1744-313x.1986.tb01095.x. [DOI] [PubMed] [Google Scholar]
- 17.Lowin B, Hahne M, Mattmann C, Tschopp J. Cytolytic T cell cytotoxicity is mediated through Fas lytic pathways. Nature. 1994;370:650–652. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- 18.Lynch DH, Ramsdall F, Alderson MR. Fas and FasL in the homeostatic regulation of immune responses. Immunol. Today. 1995;16:569–574. doi: 10.1016/0167-5699(95)80079-4. [DOI] [PubMed] [Google Scholar]
- 19.Mak TW, Ferrick DA. The T γ/δ cell-bridge: linking innate and aquired immunity. Nature Med. 1998;4:764–765. doi: 10.1038/nm0798-764. [DOI] [PubMed] [Google Scholar]
- 20.Nakanoma T, Nakamura K, Deguchi N, Fujimoto J, Tazaki H, Hata J-I. Immunohistochemical analysis of tumour infiltrating lymphocytes in seminoma using monoclonal antibodies. Virch. Arch. A Pathol. Anat. 1992;421:409–413. doi: 10.1007/BF01606913. [DOI] [PubMed] [Google Scholar]
- 21.Nouri AME, Hussain RF, Oliver RTD, Handy AM, Bartkova I, Bodmer JG. Immunological paradox in testicular tumors: The presence of large numbers of activated T cells despite the complete absence of MHC antigens. Eur. J. Cancer. 1993;29A:1895–1899. doi: 10.1016/0959-8049(93)90545-q. [DOI] [PubMed] [Google Scholar]
- 22.Riccioli A, Filippini A, De-Cesaris P, Barbacci E, Stefanini M, Starace G, Ziparo E. Inflammatory mediators increase surface expression of integrin ligands, adhesion of lymphocytess, and secretion of interleukin 6 in mouse Sertoli cells. Proc. Natl. Acad. Sci. USA. 1995;92:5808–5812. doi: 10.1073/pnas.92.13.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rock KL. A new foreign policy: MHC class I molecules monitor the outside world. Immunol. Today. 1996;17:131–137. doi: 10.1016/0167-5699(96)80605-0. [DOI] [PubMed] [Google Scholar]
- 24.Sundström J, Veräjänkorva E, Salminen E, Pelliniemi LJ, Pöllänen P. Experimental testicular teratoma promotes formation of humoral immune responses in the host testis. J. Reprod. Immunol. 1999;42:107–126. doi: 10.1016/s0165-0378(98)00084-9. [DOI] [PubMed] [Google Scholar]
- 25.Torres A, Casanowa JF, Nistal M, Regadera J. Quantification of immunocompetent cells in testicular germ cell tumours. Histopathology. 1997;30:23–30. doi: 10.1046/j.1365-2559.1997.d01-560.x. [DOI] [PubMed] [Google Scholar]
- 26.Townsend A, Bodmer H. Antigen recognition by class I restricted T lymphocytes. Ann. Rev. Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- 27.Vanky F, Wang P, Patarraoyo M, Klein E. Expression of the adhesion molecule ICAM-1 and major histocompatibility complex class I antigens on human tumor cells is required for their interaction with autologous lymphocytes in vitro. Cancer Immunol. Immunother. 1990;31:19–27. doi: 10.1007/BF01742491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wayne Streilein J. Unraveling immune privilege. Science. 1995;270:1158–1159. doi: 10.1126/science.270.5239.1158. [DOI] [PubMed] [Google Scholar]
- 29.Wei Y-Q, Hang Z-B, Liu K-F. In situ observation of inflammatory cell-tumor cell interaction in human seminomas (Germinomas): light, electron microscopic, and immunohistochemical study. Hum. Pathol. 1992;23:421–428. doi: 10.1016/0046-8177(92)90090-p. [DOI] [PubMed] [Google Scholar]
- 30.Zhao X, Wei Y-Q, Kariya Y, Teshigawara K, Uchida A. Accumulation of γ/δ T cells in human dysgerminoma and seminoma: roles in autologous tumor killing and granuloma formation. Immunol. Invest. 1995;24:607–618. doi: 10.3109/08820139509066861. [DOI] [PubMed] [Google Scholar]