Abstract
Amyloid-β protein (Aβ) and its precursor (βPP) play important roles in the pathogenesis of Alzheimer disease and inclusion-body myositis. In humans, Aβ deposits are found in brain, skeletal muscle, and skin. Therefore, we have investigated possible Aβ deposits in multiple tissues of two transgenic mouse lines overexpressing the signal plus Aβ-bearing 99-amino acid carboxyl terminal sequences of βPP under the control of a cytomegalovirus enhancer/β-actin promoter. One of the lines developed Aβ-immunoreactive intracellular deposits consistently in the pancreas and lacrimal gland, and occasionally in gastric, DeSteno's, and lingual glands. Although the Aβ deposits increased during ageing and degenerative changes of the tissues were observed, little or no extracellular Aβ deposits were observed up to the age of 25 months. These lines of transgenic mice are useful for studying the molecular mechanisms of development and clearance of intracellular Aβ deposits.
Keywords: Alzheimer disease, amyloidosis, transgenic mouse, lacrimal gland, immunocytochemistry, biomarker
Pathological hallmarks of Alzheimer disease (AD) are neurofibrillary tangles, mainly composed of hyperphosphorylated tau, and deposits of aggregated amyloid-β protein (Aβ) in neuritic plaques and cerebral vessels in brain. Severe loss of large neurones in the nucleus basalis of Meynert, the hippocampus, and the cortex is also characteristic of AD. Aβ consisting of 39–43 amino acids is derived from a larger transmembrane glycoprotein, β-amyloid protein precursor (βPP). βPP undergoes several proteolytic pathways by several proteases, designated α-, β-, and γ-secretases. α-secretase cleaves βPP between residues 16 and 17 of Aβ releasing a soluble form of βPP (Esch et al. 1990). This pathway is thought to be nonamyloidogenic because intact Aβ is not produced. Generation of Aβ requires cleavage of βPP by β- and γ-secretase at the N-terminal and C-terminal ends of Aβ, respectively. Important roles of Aβ and βPP in the pathogenesis of AD are underscored by discoveries of missense mutations in the gene encoding βPP in a small subset of early onset familial AD (Hardy 1992). Additionally, causative mutations found in the presenilin-1 and -2 genes of most patients with early onset familial AD increase production of highly amyloidogenic Aβ consisting of 42 amino acids (Borchelt et al. 1996; Duff et al. 1996; Scheuner et al. 1996; Citron et al. 1997; Xia et al. 1997). Thus, several distinct mutations found in familial AD seem to cause a common pathological cascade of β-amyloidosis in the human brain. Furthermore, Aβ accumulation in skeletal muscle is thought to play an important role in the development of inclusion-body myosistis and myopathies (IBM) (Askanas & Engel 1998).
Overexpression of the mutant forms of βPP in transgenic mice using neurone specific promoters leads to extracellular β-amyloid deposits in mouse brain (Games et al. 1995; Hsiao et al. 1996), while overexpression of the signal plus Aβ-bearing 99-amino acid carboxyl terminal sequences (SβC) of βPP in transgenic mice using a cytomegalovirus (CMV) enhancer/β-actin promoter causes deposition of intracellular Aβ aggregates in pancreas (Kawarabayashi et al. 1996) and skeletal muscle (Fukuchi et al. 1996, 1998; Jin et al. 1998). The signal sequence was added so that the SβC would be transported to the cell membrane via the endoplasmic reticulum. Since the CMV enhancer/β-actin promoter is expected to be highly active in many tissues, we have further investigated possible accumulation of Aβ in multiple tissues from two lines of transgenic mice overexpressing SβC (mouse lines, 11430 and 13592) (Fukuchi et al. 1996, 1998). We found Aβ-immunoreactive deposits in several exocrine glands of 13592 mice. Particularly, these deposits were consistently observed in lacrimal gland and pancreas of 13592 mice. Although Aβ deposition resulted in degeneration of such glands, Aβ deposits appeared to be exclusively intracellular.
Materials and methods
Transgenic mice
The establishment and propagation of the transgenic mouse lines, 13592 and 11430, has been described previously (Fukuchi et al. 1996, 1998). All of the transgenic mice used in this study had been backcrossed to C57BL/6J mice more than five generations (B6.13592[N6 to N8] and B6.11430 [N8]) (Silver 1995). For simplicity, these two lines are hereafter referred to as 13592 and 11430. Segregation of the transgene was determined by Southern blot analysis using cDNA for SβC as described before (Fukuchi et al. 1996). We previously reported that the 13592 and 11430 mice had approximately five and three copies of the transgene, respectively. Non-transgenic C57BL/6J mice were used as controls. Mice were monitored for the presence of murine pathogens by a comprehensive battery of virus serologies, bacterial cultures, endo- and ectoparasite examinations, and histopathology of all major organs, as described previously (Faulkner et al. 1995). All mice were consistently negative for pathogens by these tests.
Northern blot analysis
Northern blot analysis was used to determine levels of mRNA produced from the SβC transgene. Two mice were sacrificed at 3 months of age from each of the 13592,11430, and nontransgenic C57BL/6J strains at 3 months of age with intraperitoneal sodium pentobarbital injection for the collection of tissues. Total RNA was extracted from mouse tissues using Trizol reagents (Life Technologies, Gaithersburg, MD, USA) according to the manufacturer's protocol. Twenty-five μg of total RNA from each tissue were electrophoresed thorough a 1% agarose-formaldehyde gel, followed by capillary transfer to a nylon membrane. A human βPP cDNA probe (bp 901–2851) (Fukuchi et al. 1992) was radiolabeled with 32P dCTP to a specific activity of 4 or 6 × 108 cpm/μg using a random primed labelling kit (Boehringer Mannheim, Indianapolis, IN, USA). The membranes were hybridized for 20 h at 42 °C in 50% formamide 5 × SSC (1 × SSC = 0.15 m NaCl, 0.015 m sodium citrate, pH 7.0), 0.1% sodium dodecyl sulphate (SDS) and 100 μg/ml denatured herring sperm DNA. After hybridization, the membranes underwent two 30 minute washings at 65 °C in 0.25 × SSC containing 0.25% SDS. The membranes were then exposed to Kodak XAR film with enhancer screens at −80 °C for 4 h. The relative levels of mRNA expression were determined by densitometric scanning (the Bio-Rad Model GS-670 densitometer and Molecular Analyst™/PC software). Equal loading of the RNA samples was confirmed by probing the stripped membranes with cDNAs for β-actin and GAPDH (glyceraldehyde-3-phosphate dehydrogenase).
Western blot analysis
Four mice between 3 and 5 months old from each of the 13592,11430, and nontransgenic C57BL/6J strains were used to determine the steady state levels of SβC expression in the pancreas, muscle, and lacrimal gland by western blot analysis. The tissues were homogenized in 2 × Laemmli buffer (1× = 62.5 mm Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 5% 2-mercaptoethanol, 0.001% bromophenol blue), boiled for 5 min, and sheared with 26-gauge needles. Protein concentration was determined by Protein Assay (Bio-Rad, Hercules, CA, USA). Fifty μg of protein for each sample were applied to a 16.5% Tris-Tricine SDS-polyacrylamide gel electrophoresis (PAGE) and electrotransferred to polyvinylidine difluoride (PVDF) membranes (Immobilon-P, Millipore, Bedford, MA, USA). The membranes were blocked with phosphate-buffered saline (PBS) containing 5% nonfat dried milk (w/v), 0.02% sodium azide, and 0.02% Tween 20, incubated at 4 °C for 16 h with the 994B antibody and immunostained with an enhanced chemiluminescence system (Amersham, Arlington Heights, IL, USA) according to the manufacturer's protocol. The 994B antibody was developed against the 39 carboxyl-terminal residue of βPP (Fukuchi et al. 1998). The relative concentration of the protein was determined by densitometric scanning. Student's t-test was used for statistical analysis.
Immunocytochemistry and thioflavin staining
The following mice were used for immunocytochemical and histochemical analyses: (1) 10 3- to 6-month-old, 21 7- to 12-month-old, six 13- to 18-month-old, and eight 19- to 24-month-old 13592 mice; (2) two 15-month-old, two 22-month-old, two 24-month-old, and one 25-month-old 11430 mice; (3) Six 15-month-old nontransgenic C57BL/6J mice. Mice were killed by intraperitoneal injection of sodium pentobarbital. Tissues were removed, fixed in 10% formaldehyde: 90% alcohol, embedded in paraffin and sectioned at 5 μm for immunocytochemistry and 10 μm for thioflavin S and T staining. Sections were then subjected to the avidin–biotin immunoperoxidase method to detect SβC and its derivatives using Vectastain ABC kit (Vector, Burlingame, CA, USA). Endogenous peroxidase was eliminated by treatment with 3% H2O2 for 30 min after deparaffinization of the sections. After washing with distilled water, sections were treated with 88% formic acid and rinsed with water and 0.1 m Tris-saline (TBS) (pH 7.4). Sections were blocked with 5–15% goat serum in TBS for 60 min at room temperature and incubated with primary antibodies in 0.1 m TBS containing 5–15% serum (goat serum for rabbit antibodies, horse serum for mouse monoclonal antibodies) for 16 h at 4 °C. Sections were rinsed in 0.1 m TBS containing 1% serum and incubated with appropriate biotinylated secondary antibodies for 60 min at room temperature. After washing, sections were incubated with Vectastain ABC reagent for 60 min at room temperature. Peroxidase activity was detected by treatment with 3,3′-diaminobenzidine. Sections were counterstained with haematoxylin. Antibodies used for immunocytochemistry were 6E10 (1 μg IgG/ml; a mouse monoclonal antibody raised against amino acid 1–16 of Aβ; Senetek, Maryland Heights, MO, USA), 4G8 (0.5 μg IgG/ml; a mouse monoclonal antibody raised against amino acid resides 17–24 of Aβ; Senetek), two rabbit polyclonal antibodies against Aβ (anti-β-amyloid peptide, 1 : 200 working dilution, Zymed, San Francisco, CA, USA; anti-β-amyloid (1–40), 1 : 250 working dilution, Sigma, St. Louis, MO, USA) and 994B (1 : 1000). Tissues also were stained with haematoxylin and eosin for the evaluation of general histology and with thioflavin S and T for the detection of amyloid. For each immuno- and thioflavin staining, prefrontal cortex tissues from patients with Alzheimer disease were used as positive controls.
Electron microscopy of 13592 pancreas and lacrimal gland
Tissues from the following mice were evaluated by electron microscopy: three 13592 mice aged 17–24 months, two 11430 mice aged 24–25 months, and one C57BL/6 mouse aged 15 months. The pancreas and lacrimal glands (intra- and exorbital) were removed from the mice and fixed with cold 2% paraformaldehyde in PBS. Thin sections (0.1 μm thick, embedded in epon) were prepared and examined in a Hitachi 7000 transmission electron microscope (TEM) at an accelerating voltage of 75 kV.
Results
Northern blot analysis
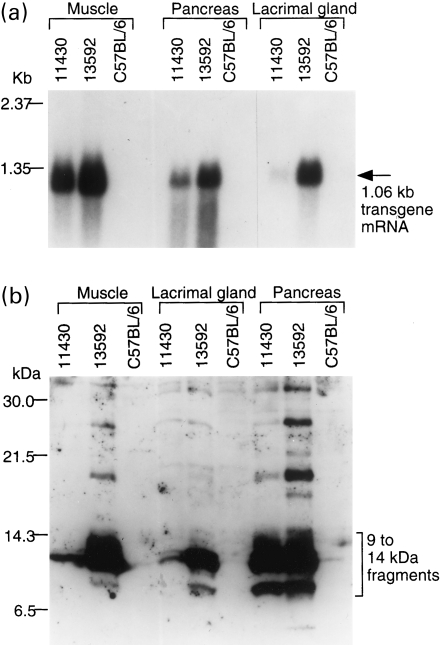
By northern blot analysis, 1.06 kb mRNA from the SβC transgene was detected in the pancreas, muscle, and exorbital lacrimal glands of both 11430 and 13592 transgenic mice but not in the control nontransgenic tissues (Figure 1a). Endogenous βPP mRNA (3.2 kb) was not detected using human βPP cDNA as a probe under the hybridization and washing conditions used. The levels of mRNA in the muscle, pancreas, and exorbital lacrimal glands of the 13592 mice were approximately 3, 2.5, and 12 times higher than those of the 11430 mice, respectively. Among the tissues, the muscle appeared to have the highest level of SβC mRNA expression followed by the lacrimal glands and pancreas for the 13592 mice.
Figure 1.
Northern (a) and western (b) blot analyses demonstrating high levels of SβC transgene expression in the pancreas, lacrimal gland, and muscle of 3-month-old 13592 mice. For comparison, 3-month-old 11430 and C57BL/6J mice were also used. (a) The levels of the 1.06 kb SβC mRNA in the tissues from 13592 mice are much higher than those from 11430 mice; (b) The 9–14 kDa carboxyl-terminal fragments of βPP produced from the SβC transgene were detected by the 994B antibody in the tissues. The amounts of the 9–14 kDa fragments in 13592 muscle and lacrimal gland are much higher than those in 11430 mice while the levels of the 9–14 kDa fragments in 13592 and 11 430 pancreas are similar.
Western blot analysis
Using 16.5% Tris-Tricine SDS-PAGE and the 994B antibody, 9–14 kDa carboxyl-terminal fragments of βPP were observed in the pancreas, muscle, and lacrimal glands of both 11430 and 13592 transgenic mice but not in control nontransgenic mice (Figure 1b). The amounts of the 9–14 kDa fragments in the muscle and lacrimal glands of the 13592 mice were 6 ± 1.0 (mean ± SD) and 10 ± 3.3 times greater than those of the 11430 mice, respectively. The amount of the 9–14 kDa fragments in 13592 pancreas was not significantly higher than that in 11430 pancreas (1.2 ± 0.2 times, P = 0.08). The amount of the 9–14 kDa fragments in 13592 pancreas was higher than that in 13592 lacrimal glands (1.9 ± 0.5 times, P = 0.004) while that in 13592 pancreas was not significantly different from that in 13592 muscle (P = 0.09). The amount of the 9–14 kDa fragments in the 11430 pancreas was 1.6 ± 0.4 times greater than that in the 13592 lacrimal glands (P = 0.009).
The 994B-immunoreactive bands greater than 14 kDa are presumably polymeric forms of the carboxyl-terminal fragments of βPP as previously reported (Ohman et al. 1996).
Immunocytochemistry
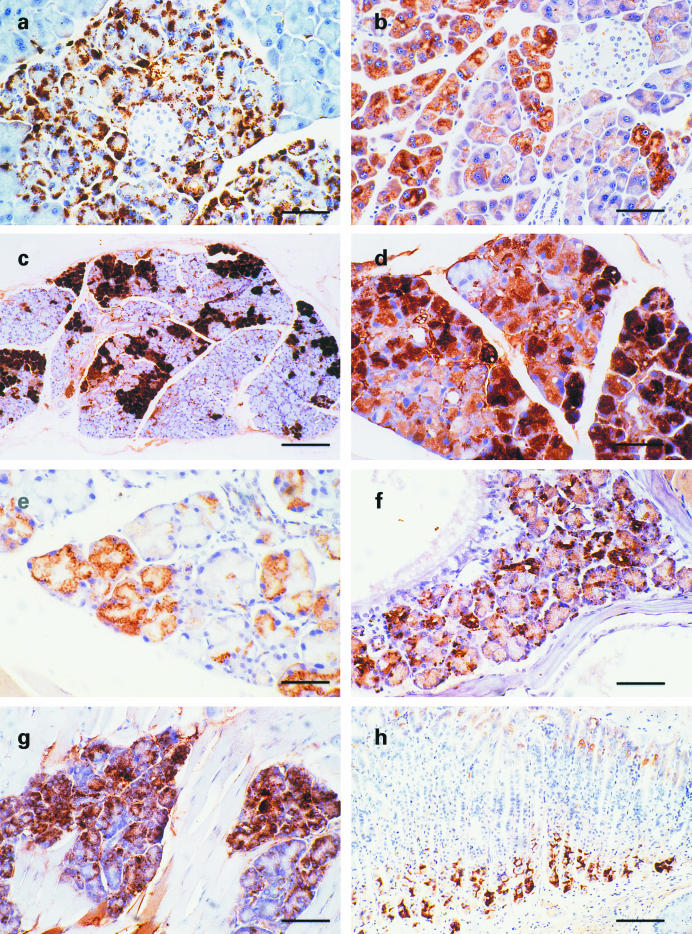
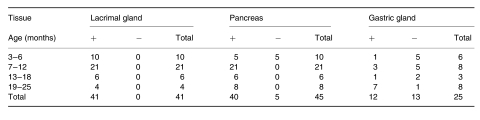
In order to investigate accumulation of Aβ and/or SβC, immunocytochemical analyses were performed using antibodies against Aβ (6E10, 4G8, and rabbit polyclonal antibodies). Aβ-immunoreactive granular to globular deposits consistently occurred within acinar cells in the pancreas of 13592 mice older than 6 months (Figure 2a and Table 1). Fifty percent of 13592 mice younger than 7 months had Aβ-immunoreactive deposits. Although the number of cells containing the deposits and the degree of Aβ-immunoreactivity varied greatly between mice, such deposits appeared to increase and sometimes occupy the whole cytoplasm during ageing. No obvious Aβ-immunoreactive deposit, however, was extracellularly found in 13592 pancreas. C57BL/6J and 11430 mice did not develop Aβ-immunoreactive deposits in pancreas. Such Aβ-immunoreactivity was also found in cytoplasm of both intraorbital and exorbital lacrimal gland cells in all 13592 mice examined (older than 2 months, Figure 2c–d, and Table 1). No Aβ-immunoreactive deposit in lacrimal gland was found in 11430 and C57BL/6 mice except for the intra- and exorbital lacrimal glands of one 24-month-old 11430 mouse, which showed a similar Aβ-immunoreactivity. Similar Aβ-immunoreactivities were also found in DeSteno's gland (lateral nasal gland) (Figure 2f) and tongue mucous gland (Figure 2g) in some of 13592 mice, but not in 11430 and C57BL/6 mice. Most of the cells showing Aβ-immunoreactivity also were stained with the 994B antibody against the carboxyl-terminus of βPP. The staining patterns with the 994B antibody, however, were more dispersed within cytoplasm and less granular compared to those with the antibodies against Aβ (Figure 2b,e). Diffuse, rarely granular, cytoplasmic staining with the antibodies against both Aβ and the carboxyl-terminus of βPP also was found in chief cells deep in the gastric glands of 13592 mice, but not in 11430 or C57BL/6J mice (Figure 2h).
Figure 2.
Aβ-immunoreactive deposits in exocrine glands of 13592 mice. The pancreas and head of 13592 mice were stained with various antibodies using the avidin-biotin immunoperoxidase method. (a) Aβ-immunoreactive granular or globular deposits detected by the 4G8 antibody within acinar cells of a 7-month-old 13592 mouse; (b) A section adjacent to (a) was stained with the 994B antibody. The staining is found in the cytoplasm of acinar cells but is diffuse and less intense compared to (a); (c) Aβ-immunoreactive deposits visualized by the 6E10 antibody are patchy in this exorbital lacrimal gland from a 3-month-old 13592 mouse; (d) Aβ-immunoreactive granular or globular deposits detected by the 6E10 antibody in the intraorbital lacrimal gland of a 11-month-old 13592 mouse; (e) When stained with the 994B antibody, the cytoplasmic staining of a lacrimal gland from a 22-month-old 13592 mouse appears to be diffuse and lighter compared to that stained with antibodies against Aβ, such as 6E10 and 4G8; f-h are Aβ-immunoreactive deposits in (f) the DeSteno's gland of a 18-month-old 13592 mouse stained with the 6E10 antibody (g) the mucus glands in the tongue of a 11-month-old 13592 mouse stained with the 6E10 antibody, and (h) the gastric gland of a 24-month-old 13592 mouse stained with 4G8 antibody. Scale bars, 65 μm (a, b, and d), 205 μm (c), 50 μm (e and f), 75 μm (g) and 120 μm (h).
Table 1.
Aβ-immunoreactivity in several exocrine glands of 13592 mice
Other than the exocrine glands described above and the skeletal muscle reported previously (Fukuchi et al. 1998), Aβ-immunoreactivity with much lesser intensity was occasionally found in the cytoplasm of brain neurones in 13592 mice but not in 11430 mice (not shown). In 13592 mice, the degree of Aβ-immunoreactivity was greatest in the pancreas and skeletal muscle, followed by the other exocrine glands, and slightest in the brain neurones. No other tissues showed Aβ-immunoreactivity in 13592 mice.
Thioflavin staining and electron microscopy
Thioflavin-S and -T staining and TEM were performed to study amyloid fibril formation in the pancreas and lacrimal glands. Clusters of amyloid-like ~10 nm fibrils were found enclosed by membranes in the cytoplasm of pancreatic acinar cells of 13592 mice (Figure 3a) but not in the lacrimal glands. It was not uncommon to find cells having cytoplasm filled with the amyloid fibrils and showing signs of degeneration in 13592 pancreas. Two 24-month-old 13952 mice showed loss of acinar cells and fibrotic changes in the pancreas, but extracellular amyloid deposits were seldom found (Figure 3b). Using TEM, no amyloid-like fibrils were found in the pancreas and lacrimal glands from 11430 and C57BL/6J mice. Consistent with the TEM results, only the pancreas from 13592 mice was positive for thioflavin-S and -T staining (Figure 3c), but positive staining was not found in lacrimal glands from 13592 mice. Neither the lacrimal gland nor the pancreas from 11430 and C57BL/6J mice showed positive thioflavin-T or -S staining.
Figure 3.
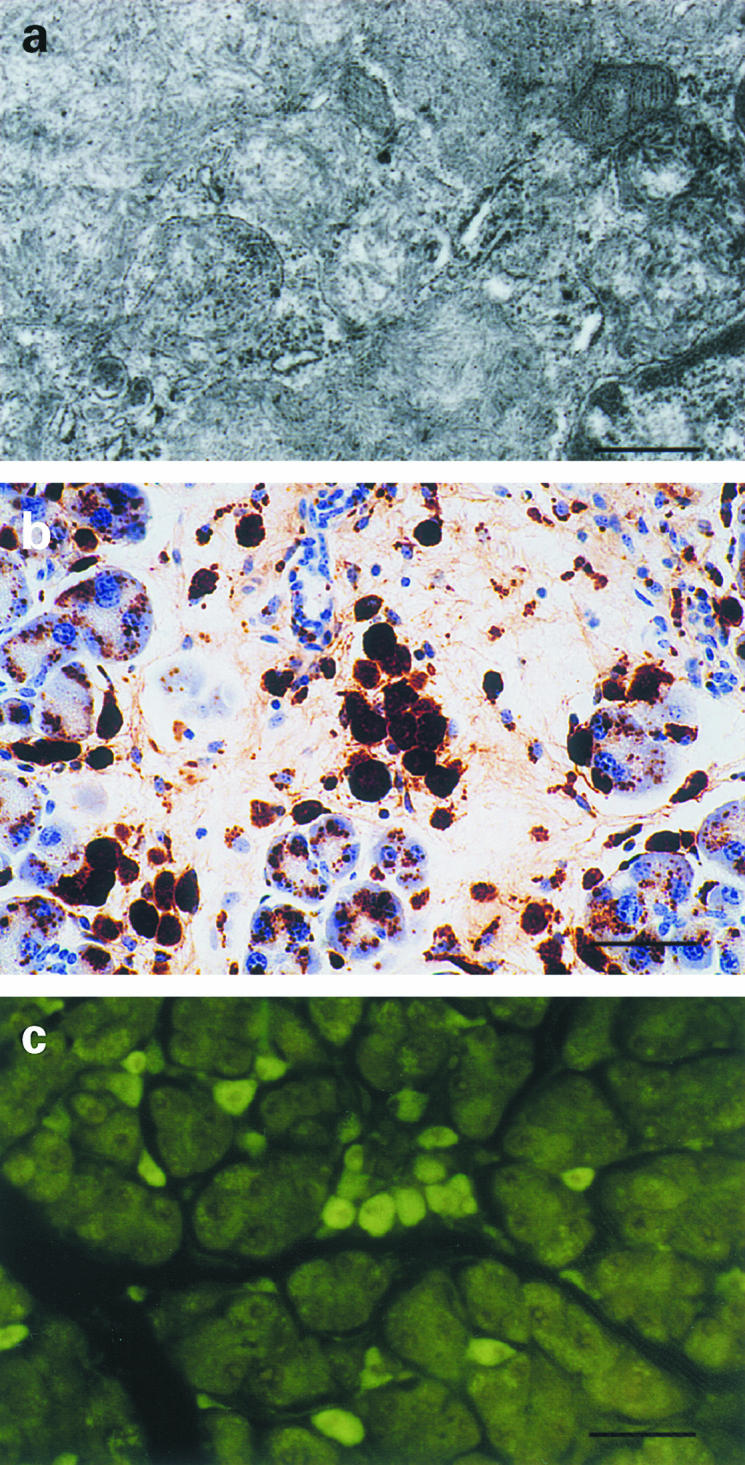
Aβ amyloid fibril formation and degeneration of 13592 pancreas. (a) Electron micrograph of the pancreas of a 24-month-old 13592 mouse, showing clusters of ~10 nm amyloid fibrils enclosed by membranes in cytoplasm of a pancreatic acinar cell. (b) Degeneration of pancreas from a 24-month-old 13592 mouse, showing fibrotic changes. Aβ-immunoreactive deposits detected by the 4G8 antibody remain mostly within cells but hardly detected extracellularly. (c) Thioflavin-T positive cells in the pancreas of a 15-month-old 13 592 mouse. Scale bars, 410 nm (a), 50 μm (b), and 100 μm (c).
Discussion
Lines of transgenic mice bearing the SβC construct under the control of a CMV enhancer/β-actin promoter highly produced 9–14 kDa carboxyl-terminal fragments of βPP in multiple tissues. One of the transgenic lines (13592) developed accumulations of overexpressed protein derivatives in several exocrine glands including lacrimal gland and pancreatic acinar cells. The accumulation was immunocytochemically detectable using antibodies against part of βPP and Aβ. Except for gastric glands, the immunostaining patterns visualized by antibodies against Aβ and a carboxyl-terminus of βPP were distinct: granular or globular staining by two mouse monoclonal and two rabbit polyclonal antibodies against Aβ, and diffuse light staining by one rabbit polyclonal antibody against a carboxyl terminus of βPP. Such distinct staining patterns suggest that Aβ itself accumulated within one or more subcellular compartments after cleavage of the carboxyl terminus of βPP (SβC) by γ-secretase. Although the true identity of the Aβ-immunoreactive deposits remains to be determined, this interpretation is consistent with the previous observation that Aβ-immunoreactive deposits are mostly composed of Aβ (Fukuchi et al. 1998; Shoji et al. 1998). Kawarabayashi et al. (1996) also established transgenic mice bearing a SβC construct similar to ours and reported that Aβ amyloid fibrils accumulated within lysosomes of pancreatic acinar cells in their transgenic mice. It is most likely that the Aβ-immunoreactive deposits in 13592 mice accumulated in lysosomes of pancreatic acinar cells because of close similarities between DNA constructs, as well as immunocytochemical and TEM findings in the two transgenic mice. Other than pancreas, however, no Aβ-immunoreactive deposit was reported to be found in their transgenic mice.
Some of the phenotypic discrepancies between 11430 and 13592 mice such as Aβ-immunoreactive deposits in the lacrimal gland and skeletal muscle can be attributed to differences in levels of SβC expression between the two transgenic lines due to differences in copy numbers of the SβC transgene and their integration sites. The level of SβC expression in 11430 pancreas was much higher than that in 13592 lacrimal gland and was not significantly different from that in 13592 pancreas. Aβ-immunoreactive deposits were readily detectable in 13592 pancreas and lacrimal gland but no Aβ-immunoreactive deposit was found in 11430 pancreas. The reasons for this discrepancy are not clear. However, it is possible that the differences in genetic background between 13592 and 11430 mice influenced development of Aβ-immunoreactive deposits in pancreas. Such deposits in pancreas were positive by thioflavin staining and composed of ~10 nm fibrils. While heavy Aβ-immunoreactive deposits were easily detectable in the lacrimal gland of all 13592 mice even at the age of 3 months, amyloid fibrils were not identified in the lacrimal gland by TEM or thioflavin staining. Tissue specific post translational processing, and/or protein trafficking might have contributed to amyloid fibril formation and Aβ-immunoreactivity, since the level of SβC mRNA in 13592 lacrimal gland was similar to that in 13592 pancreas. It also is possible that tissue specific molecular chaperons which modulate formation and stability of amyloid fibrils led to the phenotypic differences.
Aβ-immunoreactive cells in exocrine glands often clustered (Figure 2a, c, g). The molecular basis of this patchy staining is not clear. The possible mechanisms, however, include mosaicism for the SβC transgene, differences in the transgene expression levels due to different subtypes of exocrine cells, differences in available factors which may accelerate or inhibit the accumulation of Aβ.
A definitive diagnosis of AD depends on brain pathology characterized by abundant neuritic plaques and neurofibrillary tangles (Mckhann et al. 1984). Although low levels of Aβ consisting of 42 amino acids in cerebrospinal fluid (CSF), high levels of tau in CSF, and an apolipoprotein E e4 allele can increase confidence to the clinical diagnosis, at present, there is no satisfactory biomarker for diagnosing late-onset and sporadic AD (The Ronald and Nancy Reagan Research Institute of the Alzheimer's Association and the National Institute on Ageing Working Group 1998). Although Aβ-immunoreactive deposits in skin had been considered as a useful biomarker (Joachim et al. 1989), substantial overlaps with nondemented elderly control subjects were found (Soininen et al. 1992). Since Aβ-immunoreactive deposits were readily detectable in exocrine glands of 13592 mice even at the age of 3 months and since some of the exocrine glands such as lacrimal glands are easily accessible, it is intriguing to determine if possible Aβ-immunoreactive deposits in exocrine glands can be a diagnostic biomarker for AD. Since βPP is expressed in lacrimal gland and secreted into tears in humans (van Setten et al. 1996), levels of secreted βPP and Aβ in tears may also serve as noninvasive biomarkers.
Aβ deposits in AD brain occur extracellularly. Recently, however, intracellular Aβ and its aggregates have emerged as possible causative agents in the pathogenesis of AD, but they are difficult to detect and have not been ascribed a specific role in AD brain (Hartmann et al. 1997; Wild-Bode et al. 1997). On the other hand, accumulation of intracellular Aβ amyloid fibrils in 13592 pancreas was readily detectable and was associated with degeneration and loss of acinar cells. No extracellular amyloid deposits were observed in 13592 pancreas, suggesting that extracellular amyloid released from degenerating cells was quickly removed by macrophages. Thus, the mechanism of clearing Aβ deposits in the pancreas may differ from that in the brain. Since the Aβ-immunoreactive deposits are readily detectable in 13592 lacrimal gland and pancreas, these transgenic mice may serve as a unique model system for the investigation of the mechanisms engendering intracellular Aβ, its deposition and clearance, which may provide a logical basis for prevention and cure of β-amyloidosis in brain (AD) and skeletal muscle (IBM).
References
- 1.Askanas V, Engel WK. Does overexpression of βAPP in aging muscle have a pathogenic role and a relevance to Alzheimer's disease? Clues from inclusion body myositis, cultured human muscle, and transgenic mice. Am. J. Pathol. 1998;153:1673–1677. doi: 10.1016/s0002-9440(10)65680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borchelt DR, Thinakaran G, Eckman CB, et al. Familial Alzheimer's disease-linked presenilin 1 variants elevate Aβ1–42/1–40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- 3.Citron M, Westaway DW, Xia G, et al. Mutant presenilins of Alzheimer's disease increase production of 42-residue amyloid-β protein in both transfected cells and transgenic mice. Nat. Med. 1997;3:67–72. doi: 10.1038/nm0197-67. [DOI] [PubMed] [Google Scholar]
- 4.Duff K, Eckman C, Zehr C, et al. Increased amyloid-β 42 (43) in brains of mice expressing mutant presenilin 1. Nature. 1996;383:710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- 5.Esch FS, Keim PS, Beattie EC, et al. Cleavage of amyloid β peptide during constitutive processing of its precursor. Science. 1990;248:1122–1124. doi: 10.1126/science.2111583. [DOI] [PubMed] [Google Scholar]
- 6.Faulkner CB, Simecka JW, Davidson MK, et al. Gene expression and production of tumor necrosis factor alpha, interleukin 1, interleukin 6, and gamma interferon in C3H/HeN and C57BL/6N mice in acute Mycoplasma pulmonis disease. Infect. Immun. 1995;63:4084–4090. doi: 10.1128/iai.63.10.4084-4090.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukuchi K, Deeb SS, Kamino K, et al. Increased expression of β-amyloid protein precursor and microtubule-associated protein tau during the differentiation of murine embryonal carcinoma cells. J. Neurochem. 1992;58:1863–1873. doi: 10.1111/j.1471-4159.1992.tb10063.x. [DOI] [PubMed] [Google Scholar]
- 8.Fukuchi K, Ho L, Younkin SG, et al. High levels of β-amyloid protein in peripheral blood do not cause cerebral β-amyloidosis in transgenic mice. Am. J. Pathol. 1996;149:219–227. [PMC free article] [PubMed] [Google Scholar]
- 9.Fukuchi K, Pham D, Hart M, Li L, Lindsey JR. β-Amyloid protein deposition in skeletal muscle of transgenic mice: Possible modes of inclusion body myopathy. Am. J. Pathol. 1998;153:1687–1693. doi: 10.1016/s0002-9440(10)65682-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Games D, Adama D, Alessandrini R, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 11.Hardy J. Framing β-amyloid. Nat. Genet. 1992;1:233–234. doi: 10.1038/ng0792-233. [DOI] [PubMed] [Google Scholar]
- 12.Hartmann T, Bieger SC, Bruhl B, et al. Distinct sites of intracellular production for Alzheimer's disease Aβ40/42 amyloid peptides. Nat. Med. 1997;3:1016–1020. doi: 10.1038/nm0997-1016. [DOI] [PubMed] [Google Scholar]
- 13.Hsiao K, Chapman P, Nilsen S, et al. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 14.Jin LW, Hearn MG, Ogburn CE, et al. Transgenic mice over-expressing the C-99 fragment of βPP with an alpha-secretase site mutation develop a myopathy similar to human inclusion body myositis. Am. J. Pathol. 1998;153:1679–1686. doi: 10.1016/s0002-9440(10)65681-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joachim CT, Mori H, Selkoe DL. Amyloid β-protein deposition in tissues other than brain in Alzheimer's disease. Nature. 1989;341:226–230. doi: 10.1038/341226a0. [DOI] [PubMed] [Google Scholar]
- 16.Kawarabayashi T, Shoji M, Sato M, et al. Accumulation of β-amyloid fibrils in pancreas of transgenic mice. Neurobiol. Aging. 1996;17:215–222. doi: 10.1016/0197-4580(95)02061-6. [DOI] [PubMed] [Google Scholar]
- 17.Mckhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 18.Ohman T, Dang N, Leboeuf RC, Furlong CE, Fukuchi K. Expression of apolipoprotein E inhibits aggregation of the C-terminal fragments of β-Amyloid Precursor Protein. Neurosci. Lett. 1996;210:65–68. doi: 10.1016/0304-3940(96)12663-x. [DOI] [PubMed] [Google Scholar]
- 19.Scheuner D, Eckman C, Jensen M, et al. Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat. Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 20.Shoji M, Kawarabayashi T, Sato M, et al. Accumulation of amyloid β protein in transgenic mice. Neurobiol. Aging. 1998;19:S59–S63. doi: 10.1016/s0197-4580(98)00043-8. [DOI] [PubMed] [Google Scholar]
- 21.Silver LM. Oxford: Oxford University Press; 1995. Mouse Genetics; pp. 45–51. [Google Scholar]
- 22.Soininen H, Syrjänen S, Heinonen O, et al. Amyloid β-protein deposition in skin of patients with dementia. Lancet. 1992;339:245. doi: 10.1016/0140-6736(92)90046-6. [DOI] [PubMed] [Google Scholar]
- 23.The Ronald And Nancy Reagan Research Institute Of The Alzheimer's Association And The National Institute On Aging Working Group. Consensus Report of the Working Group on molecular and biochemical markers of Alzheimer's disease. Neurobiol. Aging. 1998;19:109–116. [PubMed] [Google Scholar]
- 24.van Setten GB, Nilsson L, Hahne S, et al. β-amyloid protein precursor expression in lacrimal glands and tear fluid. Invest. Ophthalmol. Vis. Sci. 1996;37:2585–2593. [PubMed] [Google Scholar]
- 25.Wild-Bode C, Yamazaki T, Capell A, et al. Intracellular generation and accumulation of amyloid β-peptide terminating at amino acid 42. J. Biol. Chem. 1997;272:16085–16088. doi: 10.1074/jbc.272.26.16085. [DOI] [PubMed] [Google Scholar]
- 26.Xia W, Zhang J, Kholodenko D, et al. Enhanced production and oligomerization of the 42-residue amyloid β-protein by Chinese hamster ovary cells stably expressing mutant presenilins. J. Biol. Chem. 1997;272:7977–7982. doi: 10.1074/jbc.272.12.7977. [DOI] [PubMed] [Google Scholar]