Abstract
This investigation studied the contribution of antioxidants in delaying healing in excision cutaneous wounds (8 mm) in diabetic, aged and immunocompromised animals. Skin levels of catalase, glutathione (GSH), ascorbic acid (AA) and vitamin E in streptozotocin-induced diabetic rat were lower as compared to nondiabetics. The 7-d wound tissue of diabetic rats showed an increased vitamin E level along with depleted GSH content. In aged rats (18 months old), higher levels of skin superoxide dismutase (SOD), glutathione peroxidase (Gpx) and thiobarbituric acid reactive substances (TBARS) and lower levels of catalase and GSH were found as compared to their values in young rats (3–4 months old). The levels of SOD, GPx, catalase, AA, GSH and vitamin E in 7-d wound tissue of aged rats were significantly lower in comparison to those in young rats. However, TBARS were elevated in these wound tissues. The non-wounded skin of immunocompromised (athymic) mice showed lower levels of SOD, catalase, and TBARS and higher GSH and GPx levels in comparison to those present in normal mouse skin. Surprisingly, the analysis of 7-d wound tissue showed higher levels of SOD, catalase, GPx, and GSH and lower TBARS level in athymic mice compared to the wound tissue of normal mice.
Thus low levels of antioxidants accompanied by raised levels of markers of free radical damage play a significant role in delaying wound healing in aged rats. In diabetic rats reduced glutathione levels may have a contributory role in delaying the healing process. However, in immunocompromised mice the antioxidant status following injury showed an adapted response.
Keywords: antioxidants, wound, diabetes, athymic mice, aged rats
Introduction
Healing is a normal physiological process that proceeds through a series of co-ordinated cellular and cytokine-mediated events, culminating in the restoration of functional integrity of tissues. Under certain circumstances like diabetes, starvation, ageing, and immunocompromised situations, healing is delayed for several reasons. In the clinical setting delay in healing may lead to severe complications requiring extended hospitalization, amputation or even death of a patient. All over the world efforts are being made to identify the mechanisms lying behind delayed healing, so that new therapies can be developed. The literature indicates that in diabetic rat wound, delayed healing could be a consequence of less or delayed expression of some growth factors, and exogenous application of these factors could be helpful in normalizing the healing process (Werner et al. 1994; Doxey et al. 1995; Frank et al. 1995; Tanaka et al. 1996). Elderly patients are known to heal more slowly than young patients, evident clinically as increased rates of wound dehiscence, prolonged recovery time and increased duration of hospital care (Jones & Millman 1990). Ageing affects total collagen production as well as the rate of collagen production in response to injury, and this contributes to slower healing and reduced wound strength (Reiser et al. 1995). Cellular immunity has an important role in regulation of wound repair. Proinflammatory cytokines help to protect against infection, prepare injured tissue for repair and enhance phagocytic recruitment and activation (Lowry 1993). Dysregulation of cytokines can impair wound healing (Barbul 1990). In an immunosuppressed situation inflammatory reactions induced after wounding are largely suppressed and this is probably responsible for delayed healing. Free radicals and their scavenging systems are also known to play a very important role in healing of normal and delayed healing types of wounds (Hallberg et al. 1996; Shukla et al. 1997; McDaniel et al. 1998). The magnitude of free radical generation and their disposal mechanisms are known to be altered in diabetic and aged animals (Harman 1982; Wolff & Dean 1987; Oberley 1988; Wolff et al. 1991; Kakkar et al. 1995; Hallberg et al. 1996). Therefore, it is possible that some kind of correlation exists between altered free radical cascades and delayed wound healing. More experiments are needed in this direction to generate relevant data that should be helpful in developing therapeutic measures for chronic wounds. In the present investigation diabetic, immunocompromised and aged animal wounds were studied for enzymatic and nonenzymatic antioxidants to identify their role in delayed wound healing.
Materials and methods
Diabetic rat wound
Sprague Dawely (S/D) male rats (150–175 g) were made diabetic by a single injection of streptozotocin (STZ, Sigma Chemical Company, St Louis, MO, USA) in citrate buffer, pH 4.5 (50 mg/kg, i.p.) after overnight fasting. In diabetic rats wounds were made seven days after STZ treatment. Wounds were made according to the method described earlier (Shukla et al. 1997). After sterilizing the preshaved dorsal surface of the rat with 70% alcohol, four full thickness skin deep wounds were made using an Acupunch (Acuderm Inc., St Loudero, USA) of 8 mm diameter. All surgical procedures were carried out under thiopentone anaesthesia (25 mg/kg i.p.). Animals were allowed to recover and housed individually in cages kept under standard animal house conditions. The animals had free access to a pellet diet and tap water. All wounding procedures were performed aseptically and animals were maintained on autoclaved paper cuttings to avoid infections. Rats of the control group were treated with citrate buffer (0.5 ml, i.p.) and their wounds were made exactly the same way as described for the treated groups. Blood glucose of these animals was estimated just before wounding and excision of wounds, using a digital glucometer (Ames, India). Rats showing blood glucose higher than 250 mg/dl were considered to be diabetic. Wound tissues were excised after seven days using the same size punch.
Aged rat wounds
Eighteen-month-old male S/D rats were taken and wounds were made on them using the same procedure. For comparison, 3–4-month-old young adult rats were selected. In both groups wounds were made and then excised on day 7 post-wounding. Zero-day wounds (i.e. normal skin) of two groups were also compared for their antioxidant profile.
Immunocompromised mice wounds
Nude (athymic) Swiss mice (8–12 weeks) were selected to represent immunocompromised animals. Wounds were made on these animals under a laminar hood and thereafter they were maintained in an isolator provided with autoclaved food and water throughout the experimental period as described by Shukla et al. (1998). Comparison of antioxidant status was done with normal mice of the same age, sex and strain (Swiss) maintained under similar conditions. Normal skins as well as 7-day-old wounds were studied for enzymatic and nonenzymatic antioxidant profiles.
Appropriate and documented ethical criteria have been met with special care taken to avoid animal suffering. Unnecessary exposure to oxygen was minimized by processing the tissue quickly. Metal contamination was avoided with the use of properly washed equipment.
Biochemical analyses
Superoxide Dismutase (SOD). Total SOD activity was assayed by monitoring the inhibition of coloured osazone formation due to transfer of electrons from nitrobliue tetrazolium to phenazenemethosulphate in the presence of β-Nicotinamide adenine dinucleotide, reduced form (NADH). The reduction in the colour intensity was followed at 560 nm. One unit of SOD activity was defined as the amount of enzyme which inhibited the colour formation by 50% (Kakkar et al. 1984).
Catalase (CAT)
The activity of CAT was measured as disappearance of hydrogen peroxide at 240 nm (Aebi 1984).
Glutathione Peroxidase (GPx)
Total GPx was assayed by coupled enzyme assay method of Flohe & Gunzler (1984).
Reduced Glutathione (GSH)
GSH was measured in whole homogenate using a fluorometric method of Cohn & Lyle (1966).
Vitamin C
Ascorbic acid estimation was done by using the method of Rae (1984) in which the coloured complex formed by the reaction of ascorbic acid with dinitrophenylhydrazine was monitored at 540 nm.
Vitamin E
Vitamin E estimation was done fluorometrically after saponification with KOH and extraction with purified hexane (Desai 1984).
Thiobarbituric Acid Reactive Substance (TBARS)
This is a marker of lipid peroxide and expressed as malondialdehyde equivalent. It was measured as thiobarbituric acid reactive material by the procedure of Ohkawa et al. (1979). The pink coloured adduct formed at 85°C was read at 532 nm.
Statistical analysis
All data were examined by analysis of variance using the Student Newman–Keuls procedure to adjust for multiple pairwise comparisons between groups.
Results
Antioxidant status of skin and wound tissue of diabetic rats
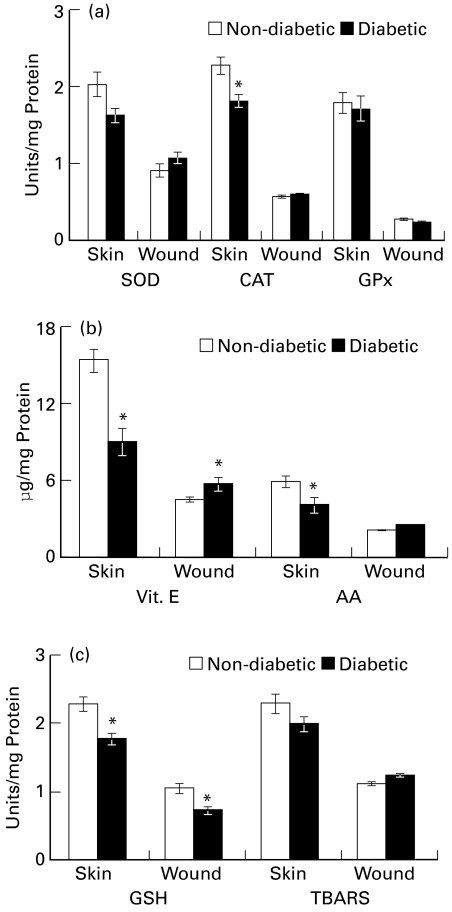
When skin of diabetic animals was compared with the skin of nondiabetic rats, a 22% (P ≤ 0.05) decrease was observed in the catalase activity of diabetic skin. The enzyme activity in 7-day wound tissue was comparable in both groups. A trend of decrease was observed in SOD activity in diabetic skin compared to normal skin. Wound tissues from both groups showed no significant variation in SOD activity. No change in GPx activity was observed in skin or in wound tissue of diabetic rats as compared to control (Figure 1a).
Figure 1.
Antioxidant status of skin and wound tissue of diabetic rats (a) superoxide dismutase (SOD), unit represents the amount of enzyme that inhibited the rate of reaction by 50%; catalase (CAT), unit represents 100 nmol H2O2 utilized/min; and glutathione peroxidase (GPx), unit represents 10 nmol NADPH oxidized/min (b) Vitamin E and ascorbic acid (AA) content (c) reduced glutathione (GSH) (μg/mg protein) and thiobarbituric acid reactive substances (TBARS, nmol/mg protein) in skin and wound tissue of diabetic rats as compared to control rat. Values are represented as mean ± SE (n = 6), *P ≤ 0.05.
Diabetic rat skin showed a 65% (P ≤ 0.05) decrease in ascorbate level. Wound tissues from both groups showed comparable ascorbate levels. Vitamin E content decreased (57%, P ≤ 0.05) significantly in diabetic skin, as compared to control skin. In wound tissues, it was found to increase (28%, P ≤ 0.05) as compared to respective control (Figure 1b). The increased vitamin E content in diabetic wounds could be brought about by inflammatory cells migrating to wound area.
Diabetic rats showed significant decrease in glutathione content (22%, P ≤ 0.05) of their skin as compared to nondiabetic rat skin. The depletion found in the skin of diabetic rats remained maintained in newly formed wound tissues also (33%, P ≤ 0.05). In normal and wound tissues from both the groups TBARS content was comparable (Figure 1c).
Antioxidant status of skin and wound tissue of aged rat
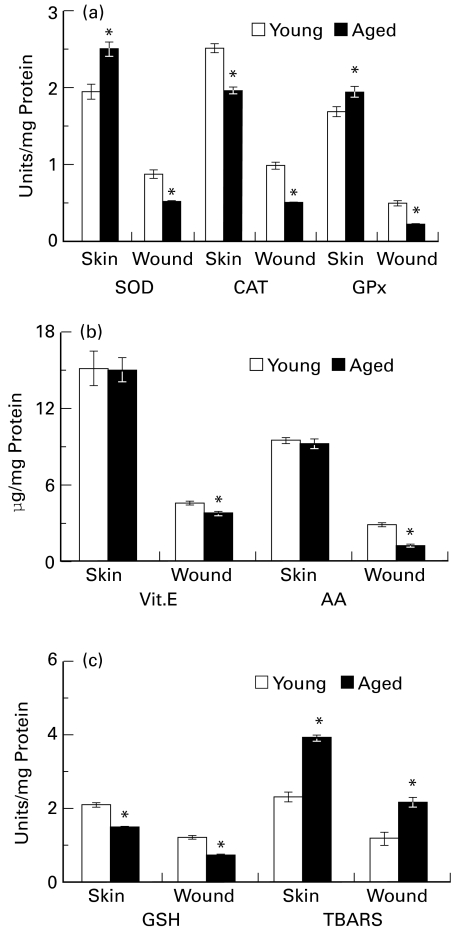
Catalase activity in the skin of aged rats was decreased by 22% (P ≤ 0.05) as compared to that of young rats. The enzyme activity was decreased further in 7-day wound tissue (48%, P ≤ 0.05) from aged rats as compared to similar tissue from young adult rats. SOD activity in the skin of aged rats was significantly higher (26%, P ≤ 0.05) as compared to that of young adult rats. However, the newly formed wound tissues of aged rats showed significantly decreased (45%, P ≤ 0.05) levels of this enzyme in comparison to the wound tissue of young adult rats. A high GPx activity (P ≤ 0.05) was observed in the skin of aged rats as compared to young rats. The wound tissues of aged animals showed 51% (P ≤ 0.05) less activity as compared to young rat wound (Figure 2a).
Figure 2.
Antioxidant status of skin and wound tissue of aged rats (a) superoxide dismutase (SOD), unit represents the amount of enzyme that inhibited the rate of reaction by 50%; catalase (CAT), unit represents 100 nmol H2O2 utilized/min; and glutathione peroxidase (GPx), unit represents 10 nmol NADPH oxidized/min (b) Vitamin E and ascorbic acid (AA) content (c) GSH (μg/mg protein) and thiobarbituric acid reactive substances (TBARS, nmol/mg protein) in skin and wound tissue of aged rats as compared to control rat. Values are represented as mean ± SE (n = 6), *P ≤ 0.05.
Normal skin of aged and young adult rats showed no difference in their ascorbic acid contents. However, a 59% (P ≤ 0.05) decrease in ascorbate content in wound tissues of aged animals was observed, as compared to its content in young adult wounds. Vitamin E content of skin did not show any difference between aged and young animals. However, 7-day wound tissue produced a slight but significant (17%, P ≤ 0.05) depletion in its content in aged animals (Figure 2b).
Aged rats showed 29% (P ≤ 0.05) lower GSH levels in their skin than young rats. A further lower GSH level was observed (43%, P ≤ 0.05) in wound tissues of aged animals in comparison to wound tissues of adult rats. Normal skin as well as wound tissues of aged animals showed about 70% (P ≤ 0.05) increased formation of TBARS compared to young rat skin and wound (Figure 2c).
Antioxidant status of skin and wound of athymic mice
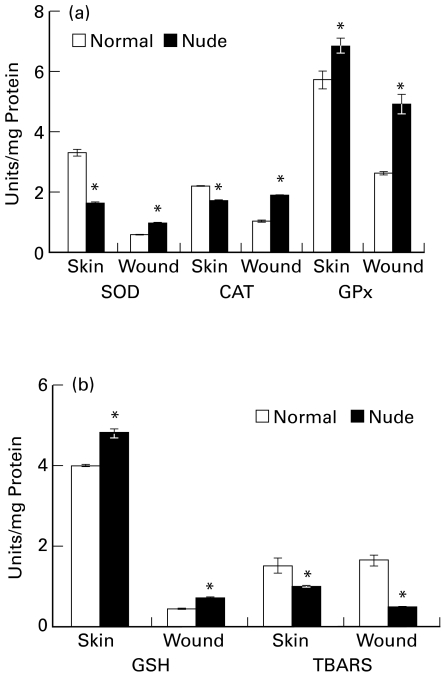
The nude mouse skin showed significantly less activity of catalase (23%, P ≤ 0.05) compared to normal mouse skin. However, the activity was found to be (P ≤ 0.05) higher in 7-day wound tissue in nude mouse as compared to normal mouse wound. SOD activity was also found to be lower (54%, P ≤ 0.05) in the skin of nude mouse. Seven-day wound tissue of nude mouse presented a 59% (P ≤ 0.05) increase in SOD activity over normal mouse wound. Nude mouse skin had 19% (P ≤ 0.05) higher GPx activity as compared to normal mouse skin. Wound tissue of nude mouse showed 88% (P ≤ 0.05) increase in its activity over normal mouse wound (Figure 3a).
Figure 3.
Antioxidant status of skin and wound tissue of immunocompromised mice (a) superoxide dismutase (SOD), unit represents the amount of enzyme that inhibited the rate of reaction by 50%; catalase (CAT), unit represents 100 nmol H2O2 utilized/min; and glutathione peroxidase (GPx), unit represents 100 nmol NADPH oxidized/min (b) GSH (μg/mg protein) and thiobarbituric acid reactive substances (TBARS, nmol/mg protein) in skin and wound tissue of immunocompromised mice as compared to control mice. Values are represented as mean ± SE (n = 6), *P ≤ 0.05.
Reduced glutathione content was found to be higher (21%, P ≤ 0.05) in normal skin as well as 7-day wound tissue (68%, P ≤ 0.05) of nude mouse as compared to respective tissue from normal mouse. TBARS were lowered in both normal skin (35%, P ≤ 0.05) and 7-day wound tissue (68%, P ≤ 0.05) of nude mouse in comparison to respective tissues of normal mouse (Figure 3b).
Discussion
Wound healing is a complicated process that requires several steps, and any alteration at any step may delay the healing process. Factors affecting healing could be either local for example sepsis, prior irradiation, recurrent trauma, poor oxygenation, arterial insufficiency or lymphoedema, or systemic such as hypoxia, collagen disorder, diabetes, uraemia, jaundice, malignancy, malnutrition or autoimmune disorders (e.g. rheumatoid arthritis, scleroderma). Some drugs which are cytotoxic or immunosuppressent, in particular steroids, can also delay healing. Recently we have demonstrated a differential expression of some important proteins in normal and chronic wounds (diabetic and immunocompromised) which may be responsible for delayed healing (Shukla et al. 1998). The ultimate cause for delayed healing could either be single or manifold. Since free radicals and their scavengers play an important role in many of the above discussed disorders, the possibility of altered scavenging systems as a culprit for delayed healing cannot be ignored. Therefore, in the present experiments the levels of certain enzymatic and nonenzymatic antioxidants have been studied in three different delayed healing types of models. During diabetes, persistent hyperglycaemia causes increased production of free radicals via autoxidation of glucose and also via nonenzymatic protein glycation (Wolff & Dean 1987; Oberley 1988; Wolf et al. 1991). It has also been shown that in mammary fibroblasts derived from some patients, proliferation was inhibited by high glucose concentration and these cells were found to be resistant to some growth factors like IGF-1 and EGF. This high glucose-induced growth factor resistance was found to be reversed by many antioxidants (Hehenberger & Hansson 1997). In the present investigation catalase, ascorbate and vitamin E were found to decrease in the skin of diabetic animals. Decreased catalase activity has been reported in other organs like kidney and liver of diabetic animals (Wohaieb & Godin 1987). Reduced glutathione was also found to be decreased in the skin as well as in 7-day wound tissues of diabetic animals. Among all the antioxidants studied only GSH showed a decrease from the very onset of healing and remained low throughout the studied period. Mak et al. (1996) have reported significantly increased glutathione levels in the kidney and heart tissues and slightly decreased levels in the liver of STZ-induced diabetic rats. Loven et al. (1986) have also reported reduced levels of glutathione and SOD activity in STZ-treated rat organs. It has been shown that glutathione plays an important role in diabetes-associated complications and its treatment was partially effective in the prevention of STZ-induced diabetic neuropathy in rats (Bravenboer et al. 1992). It appears from our findings and from the available literature that apart from other antioxidants, depletion of glutathione may play an important role in delayed healing in diabetic animals and its supplementation either orally or topically could be of great help. Recently it has been demonstrated by our group that a plant product, asiaticoside, which is known to enhance healing in normal and diabetic type of wounds (Shukla et al. 1999a), is also known to elevate different antioxidant levels of a healing wound after topical application (Shukla et al. 1999b).
Ageing is also a process associated with increased free radical reaction which may lead to cancer, atherosclerosis, hypertension, myloidosis, senile dementia and immune disorder (Harman 1982). Ageing is a condition known to affect total collagen production in response to injury (Reiser et al. 1995). The rate of cellular proliferation, revascularization, deposition and remodelling of collagen at wound sites are decreased in old rodents compared to young ones. These changes are known to be associated with slower healing and reduced wound strength (Goodson & Hunt 1979; Eaglestein 1989; Quirinia & Viidik 1991). Since an altered free radical system and delayed healing are associated with ageing, a correlation may be drawn showing a possible contribution of altered antioxidant systems in delayed healing. Since studies with old rodents correlate with the clinical observations in humans, this model was selected for studies. In the present investigation when the skin of aged animals was compared with the skin of young rats, SOD and GPx showed increase in their activities in aged rats, while catalase activity and GSH levels were found to be decreased in these rats as compared to young rats. Increased activity of SOD and GPx may indicate the adaptive nature of these enzymes against increasing oxidative stress in old rats. TBARS levels as an index of free radical-induced damage was several fold higher in the skin of old rats indicating that elevated SOD and GPx levels are not sufficient to take care of oxidative damage. In 7-day-old wound tissues of aged rats, there was a significant decrease in levels of SOD, GPx, CAT, GSH, vitamin E and ascorbic acid and increase in TBARS levels as compared to the wounds of young rats. Our studies indicate that wounds of old rats have lower antioxidant status and increased oxidative damage, which may make the healing slower in these animals. Transforming growth factor-beta (TGF-β) which is known to reduce the local circulating superoxide anions also reverses the healing impairment associated with age (Beck et al. 1993), a finding that supports our hypothesis that free radicals may delay healing in aged rats.
Athymic mice were used as an immunocompromised model for wound healing assessment because these mice have a very weak immune system. The immune system plays a very important role in healing (Barbul 1990; Lowry 1993). Skin of athymic mice showed lower activities of SOD, CAT, GPx and increased levels of GSH when compared with skin of normal mice. Seven-day wound tissues of athymic mice showed considerably higher activity of all these enzymes as well as of GSH content. This study indicates that athymic mice have remarkable adaptive capabilities that enable them to induce activities of several antioxidant enzymes following injury.
Based on these results, it can be concluded that depletion in glutathione levels in diabetic rats may have a role in delayed healing. In aged rodents, lowered antioxidants status in wound tissues indicates their possible role in delayed healing. However, in athymic mice an adaptive response was observed in antioxidant enzymes following injury, thus demonstrating that antioxidants may not have a significant role to play in delay of wound healing under an immunocompromised situation.
References
- 1.Aebi H. Catalase. In: BErgmeyer H U, editor. Methods of Enzymatic Analysis. Vol 2. New York and London: Academic Press; 1984. pp. 673–684. [Google Scholar]
- 2.Barbul A. Immune aspects of wound repair. Clin. Plast Surg. 1990;17:433–442. [PubMed] [Google Scholar]
- 3.Beck LS, Deguzman L, Lee WP, Xu Y, Siegel MW, Amento EP. One systemic administration of transforming growth factor B1 reverses age or glucocorticoid impaired wound healing. J. Clin. Inves. 1993;92:2841–2849. doi: 10.1172/JCI116904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bravenboer B, Kappelle AC, Hamers FPT, Vanburen T, Erkelens DW, Gispen WH. Potential use of glutathione for the prevention and treatment of diabetic neuropathy in the streptozotocin induced diabetic rats. Diabetologica. 1992;35:813–817. doi: 10.1007/BF00399926. [DOI] [PubMed] [Google Scholar]
- 5.Cohn VH, Lyle J. A fluorometric assay for glutathione. Analyt. Biochem. 1966;14:434–440. doi: 10.1016/0003-2697(66)90286-7. [DOI] [PubMed] [Google Scholar]
- 6.Desai ID. Vitamin E analysis method for animal tissues. In: PAcker L, editor. Methods of Enzymology. Vol. 105. Orlando: Academic Press; 1984. pp. 138–147. [DOI] [PubMed] [Google Scholar]
- 7.Doxey DL, Ng MC, Dill RE, Lacopino AM. Platelet derived growth factor levels in wounds of diabetic rats. Life Sci. 1995;57:1111–1123. doi: 10.1016/0024-3205(95)02056-o. [DOI] [PubMed] [Google Scholar]
- 8.Eaglestein WH. Wound healing and ageing. Clin. Ger. Med. 1989;5:183–188. [PubMed] [Google Scholar]
- 9.Flohe L, Gunzler WA. Assay of glutathione peroxidase. In: PAcker L, editor. Methods of Enzymology. Vol. 105. Orlando: Academic Press; 1984. pp. 114–121. [DOI] [PubMed] [Google Scholar]
- 10.Frank S, Hubner G, Breier G, Longaker MT, Greenhalgh DG, Werner S. Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implication for normal and impaired wound healing. J. Biol. Chem. 1995;270:12607–12613. doi: 10.1074/jbc.270.21.12607. [DOI] [PubMed] [Google Scholar]
- 11.Goodson WH, Hunt TK. Wound healing and ageing. J. Invest. Dermatol. 1979;73:88–91. doi: 10.1111/1523-1747.ep12532775. [DOI] [PubMed] [Google Scholar]
- 12.Hallberg CK, Trocme SD, Ansari NH. Acceleration of corneal wound healing in diabetic rats by the antioxidant Trolox. Res. Comm Mol Pathol Pharmacol. 1996;93:3–12. [PubMed] [Google Scholar]
- 13.Harman D. Free Radicals in Biology. Vol V. Orlando: Academic Press Inc; 1982. The free radical theory of ageing; pp. 255–273. [Google Scholar]
- 14.Hehenberger K, Hansson A. High glucose-induced growth factor resistance in human fibroblasts can be reversed by antioxidants and protein kinase C inhibitors. Cell Bioch. Func. 1997;15:197–201. doi: 10.1002/(SICI)1099-0844(199709)15:3<197::AID-CBF740>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Jones PL, Millman A. Wound healing and the aged patient. Nurs Clin. North America 25: 263–277. Cell. Biochem. 151:113–119. [PubMed] [Google Scholar]
- 16.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 1984;21:130–132. [PubMed] [Google Scholar]
- 17.Kakkar R, Kalra J, Mantha SV, Prasad K. Lipid peroxidation and activity of antioxidant enzymes in diabetic rats. Mol. Cell. Biochem. 1995;151:113–119. doi: 10.1007/BF01322333. [DOI] [PubMed] [Google Scholar]
- 18.Loven D, Schedl H, Wilson H. Effect of insulin and oral glutathione levels and superoxide dismutase activities in organs of rats with streptozotocin-induced diabetes. Diabetes. 1986;35:503–507. doi: 10.2337/diab.35.5.503. [DOI] [PubMed] [Google Scholar]
- 19.Lowry SF. Cytokine mediators of immunity and inflammation. Arch. Surg. 1993;28:1235–1241. doi: 10.1001/archsurg.1993.01420230063010. [DOI] [PubMed] [Google Scholar]
- 20.Mak DHF, Ip SP, Li PC, Poon MKT, Ko KM. Alterations in tissue glutathione antioxidant system in streptozotocin induced diabetic rats. Mol. Cell. Biochem. 1996;162:153–158. doi: 10.1007/BF00227543. [DOI] [PubMed] [Google Scholar]
- 21.McDaniel DH, Ash K, Lord J, Newman J, Zukowski M. Accelerated laser resurfacing wound healing using a triad of topical antioxidants. Dermatol. Surg. 1998;24:661–664. doi: 10.1111/j.1524-4725.1998.tb04224.x. [DOI] [PubMed] [Google Scholar]
- 22.Oberley LW. Free radicals and diabetes. Free Rad. Biol. Med. 1988;5:113–124. doi: 10.1016/0891-5849(88)90036-6. [DOI] [PubMed] [Google Scholar]
- 23.Ohkawa H, Ohishi N, Yogi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analyt. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 24.Quirinia A, Viidik A. The influence of age on the healing of normal and ischemic incisional skin wounds. Mech. Ageing Dev. 1991;58:221–232. doi: 10.1016/0047-6374(91)90094-g. [DOI] [PubMed] [Google Scholar]
- 25.Rae JH. Chemical determination of ascorbic acid and diketogluconic acid. In: GLiek Q, editor. Methods of Biochemical Analysis. Vol. 1. New York: Interscience Publishers; 1984. pp. 115–139. [Google Scholar]
- 26.Reiser K, McGee C, Rucker R, McDonald R. Effect of ageing and caloric restriction on extracellular matrix biosynthesis in a model of injury repair in rats. J Geront A Biol Sci. Med. Sci. 1995;50 A:B40–47. doi: 10.1093/gerona/50a.1.b40. [DOI] [PubMed] [Google Scholar]
- 27.Shukla A, Rasik AM, Patnaik GK. Depletion of reduced glutathione, ascorbic acid, vitamin E and antioxidant defence enzymes in healing cutaneous wound. Free Rad. Res. 1997;26:93–101. doi: 10.3109/10715769709097788. [DOI] [PubMed] [Google Scholar]
- 28.Shukla A, Dubey MP, Srivastava R, Srivastava BS. Differential expression of proteins during healing of cutaneous wounds in experimental normal and chronic models. Biochem. Biophys. Res. Comm. 1998;244:434–439. doi: 10.1006/bbrc.1998.8286. [DOI] [PubMed] [Google Scholar]
- 29.Shukla A, Rasik AM, Jain GK, Shankar R, Kulshrestha DK, Dhawan BN. In vitro and in vivo wound healing activity of asiaticoside isolated from Centella asiatica. J. Ethnopharmacol. 1999a;65:1–11. doi: 10.1016/s0378-8741(98)00141-x. [DOI] [PubMed] [Google Scholar]
- 30.Shukla A, Rasik AM, Dhawan BN. Asiaticoside-induced elevation of antioxidant levels in healing wound. Phytotherapy Res. 1999b;12:1–5. doi: 10.1002/(SICI)1099-1573(199902)13:1<50::AID-PTR368>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka E, Ase K, Okuda T, Okumura M, Nogimore K. Mechanism of acceleration of wound healing by basic fibroblast growth factor in genetically diabetic mice. Biol. Pharmacol. Bull. 1996;19:1141–1148. doi: 10.1248/bpb.19.1141. [DOI] [PubMed] [Google Scholar]
- 32.Werner S, Breeden M, Hubner G, Greenhalgh DG, Longaker T. Induction of keratinocyte growth factor expression is reduced and delayed during wound healing in the genetically diabetic mouse. J. Invest. Dermatol. 1994;103:469–473. doi: 10.1111/1523-1747.ep12395564. [DOI] [PubMed] [Google Scholar]
- 33.Wohaieb SA, Godin DV. Alteration in free radical tissue defense mechanism in streptozotocin induced diabetes in rats. Effect of insulin treatment. Diabetes. 1987;36:1014–1018. doi: 10.2337/diab.36.9.1014. [DOI] [PubMed] [Google Scholar]
- 34.Wolff SP, Jiang ZY, Hunt JV. Protein glycation and oxidative stress in diabetes mellitus and ageing. Free Rad. Biol. Med. 1991;10:339–352. doi: 10.1016/0891-5849(91)90040-a. [DOI] [PubMed] [Google Scholar]
- 35.Wolff SP, Dean RT. Glucose auto-oxidation and protein modification. The potential role of autoxidative glycosylation in diabetes. Biochem. J. 1987;245:243–250. doi: 10.1042/bj2450243. [DOI] [PMC free article] [PubMed] [Google Scholar]