Abstract
Sexual dimorphism exists in the response of rats to lead nitrate, liver hyperplasia occuring earlier and being more pronounced in males. Excess dietary choline in females shifted the growth pattern towards that of males. To determine whether phosphatidylcholine-induced growth modulations could be related to a derangement of cholesterol metabolism, liver accumulation of cholesterol esters and plasma lipoprotein patterns were investigated. In males, lead-induced liver hyperplasia was associated with increased total cholesterol hepatic content, accumulated cholesterol esters and reduced concentration of plasma High Density Lipoprotein (HDL) cholesterol. Females were less responsive to the liver mitogenic signal of lead nitrate; there was no elevation of cholesterol content nor any marked accumulation of cholesterol esters. This is consistent with the lack of change in the plasma levels of HDL cholesterol. Continuous choline feeding displaced the liver cholesterol ester pattern and plasma HDL cholesterol levels in females, and in parallel that of DNA synthesis, towards those of males. Choline was not observed to have any effect in males. These results suggest that the derangement of phosphatidylcholine metabolism induces growth-related changes in cholesterol turnover; they are consistent with the proposal that the intracellular content of cholesterol esters may have a role in regulating liver growth rates.
Keywords: cholesterol esters, high density lipoprotein, liver hyperplasia, choline
Introduction
The metabolism of phosphatidylcholine (PC), a major component of membrane phospholipids (PL), is involved in the regulation of liver cell proliferation. The cytidine diphosphate (CDP)-choline pathway is required for cell division (Jackowski 1994), while the phosphatidylethanolamine methyltransferase (PEMT)-dependent biosynthetic pathway negatively regulates liver growth (Cui et al. 1994; Sesca et al. 1996; Tessitore et al. 1997; Tessitore et al. 1999a; b). On the other hand, the degradation of PC provides various second messengers potentially involved in the transduction of mitogenic signals (Nakamura & Nishizuka 1994).
We have shown that lead-induced liver hyperplasia as well as its regression by apoptosis occurs earlier and is more pronounced in male than in female rats (Tessitore et al. 1995). We have also found the female proliferative pattern to be associated with a delay in the activation of the CDP-choline pathway and in the suppression of PEMT2 expression (Tessitore et al. 1997), as well as in the downregulation of α and β protein kinase C (PKC) and activation of ε PKC (Tessitore et al. 1994). Dietary choline supplementation shifts the female pattern of DNA synthesis, cytidine triphosphate: phosphocholine cytidylyltransferase (CT) activation, PEMT2 inactivation and PKC isozyme modulation towards those of males (Tessitore et al. 1994; Tessitore et al. 1997).
Liver hyperplasia induced by lead nitrate is characterized not only by changes in phospholipid metabolism but also by increased liver cholesterol (CH) synthesis, CH ester accumulation and low plasma levels of high density lipoprotein (HDL) cholesterol (Dessi et al. 1989; Dessi et al. 1994). These findings raise the question of whether a relationship between PL and CH metabolism during proliferative processes may exist. Recently, Dessi et al. (1997) showed that the intracellular levels of CH esters are critical to progression through the cell cycle and are probably related to changes in the plasma levels of HDL CH. It has been suggested that a larger store of CH esters within the cell would allow for a more rapid mobilization and utilization of cholesterol for new membrane biogenesis. It is well known that the cellular FC(free cholesterol):PL molar ratio is an important aspect of normal cell function, thus it may be supposed that the biological response to mitogenic stimulus includes concomitant changes in both PL and CH metabolism.
To address this question, this study investigates the distribution of CH and PL in liver cells and in the plasma of male and female rats fed or not with excess choline during liver hyperplasia induced by lead nitrate.
Materials and methods
Animals
Six-week-old Wistar rats of both sexes (Charles River, Como, Italy) were maintained on a regular light-dark cycle (light 8.00–20.00 h) and fed a standard basal diet (AIN-76, Piccioni, Brescia, Italy). Some groups of rats received choline chloride (1 g/day per kg of body weight; Sigma Chemical Co., St Louis, MO) in the drinking water for 3 weeks before a single intravenous dose of lead nitrate (30 mg/kg of body weight; Analyticals, Farmitalia Carlo Erba S.p.a., Milano, Italy).
Blood was collected from the aorta and centrifuged at 3500 g for 10 min at 4°C to obtain platelet-poor plasma; it was immediately aliquoted for storage at −80°C.
DNA specific radioactivity
Rats were injected intraperitoneally with 3H thymidine (500 μCi/kg body weight; New England Nuclear, Boston, MA) 1 h before being killed; livers were excised, weighed and frozen at −20°C for determination of radioactivity in DNA, as reported elsewhere (Tessitore et al. 1992a).
Analytic procedures
Hepatic-free and esterified CH as well as triglycerides (TG) and PL contents were determined after extraction of total lipids by the method of Folch et al. (Folch et al. 1957). CH, lipid and protein plasma compositions were estimated using commercially available kits (Boehringer, Mannheim, Germany: protein assay ESL n°1767–283 cat. 1998, PL n°691–844 cat. 1995, total lipids n°124–303 cat. 1992, CH n°1442341 cat. 1997, NEFA quick n°450459E cat. 1995). Very low density lipoprotein (VLDL) and low density lipoprotein (LDL) were isolated by precipitation with a mixture of phosphotungstic acid and magnesium ions. Serum lipoproteins were resolved by HPLC as described by Okazaky et al. (Okazaki et al. 1980). Proteins in VLDL, LDL, HDL2 and HDL3 subfractions were monitored by absorbance at 280 nm and CH, TG and PL were assayed as above. All parameters were measured until day 8 after lead injection, except in male rats receiving choline, because choline does not affect lead nitrate induced liver hyperplasia (Tessitore et al. 1995).
Statistical analysis
Statistical comparison of two means was done with the Student's t-test (GraphPad InStat programme). Multiple comparisons were computed using one-way analysis of variance followed by the Bonferroni corrected t-test.
Results
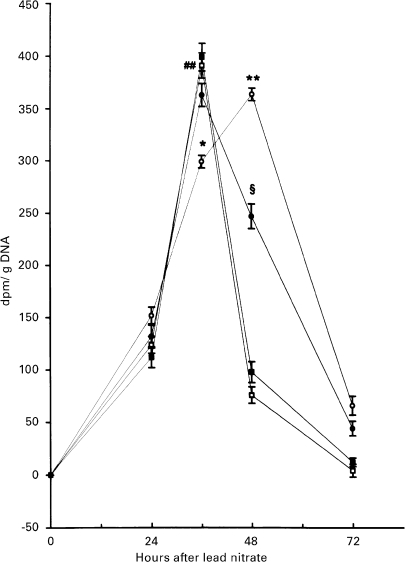
In male rats, lead nitrate induced a transient increase in the liver of incorporation of 3H thymidine in DNA with a peak 36 h after treatment, returning to near normal values at 48 h (Figure 1). When female rats were treated with lead nitrate, the peak of 3H thymidine incorporation in DNA was delayed to 48 h after lead administration and returned to a level close to that of normal liver by 72 h after treatment. Excess dietary choline shifted the proliferative pattern of female rat livers to that of males, while it was without effect in males (Figure 1).
Figure 1.
Effect of lead nitrate on the time-course of 3H-thymidine incorporation into DNA in rats of both sexes. Vertical bars denote S.D.; n = 4. (○) females (•) choline females (□) males (▪) choline-males. ## P < 0. 001 male vs. h 0 after lead nitrate administration *P < 0.01 and **P < 0.001 female vs. male; §P < 0.01 choline-female vs. female.
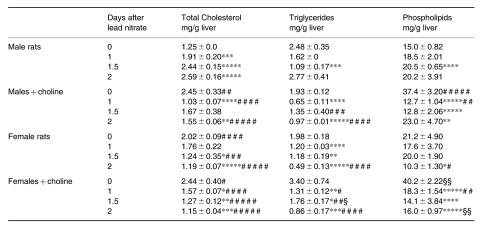
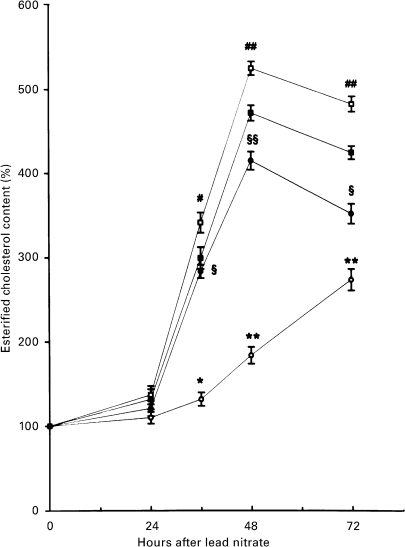
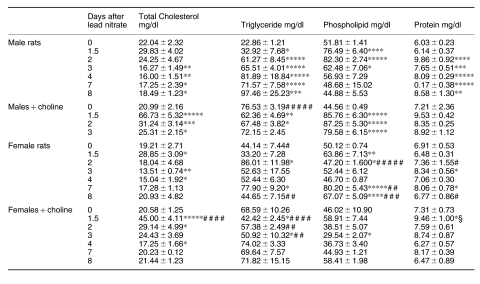
Female rats had a higher baseline hepatic content of total CH and PL than males (Table 1). Excess dietary choline increased the total CH and PL content in males and increased both TG and PL in females. Total CH content progressively increased during the adaptive growth of male rat livers, whereas it decreased in female livers and after choline feeding. PL content in males showed a growth-related pattern similar to that of total CH, although to a lesser extent. TG content decreased during mitogen-induced hyperplasia in all the experimental groups; it returned to normal levels at 48 h only in males (Table 1). Unlike the pattern of the total CH content, esterified CH in the liver increased during growth in all groups except for females that were not choline-treated, the accumulation being minimum and delayed (Figure 2).
Table 1.
Cholesterol and lipid content during lead nitrate-induced liver hyperplasia
data are means ± SEM; n = 4; * P < 0.05, ** P < 0.025, *** P < 0.01, **** P < 0.005 and ***** P < 0.0005 vs. 0 day; # P < 0.05, ## P < 0.025, ### P < 0.01, #### P < 0.005 and ##### P < 0.0005 vs. males; § P < 0.05 and §§ P < 0.01 vs. females.
Figure 2.
Effect of lead nitrate on the content of liver esterified CH in rats of both sexes. Vertical bars denote S.D.; n = 4. (○) females (•) choline females (□) males (▪) choline-males. # P < 0. 01 and ##P < 0. 001 male vs. h 0 after lead nitrate administration *P < 0.01 and **P < 0.001 female vs. male; §P < 0.01 and § §P < 0.001 choline-female vs. female.
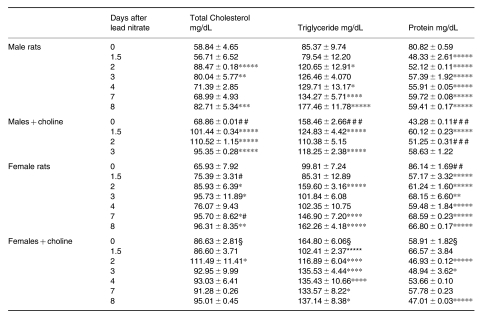
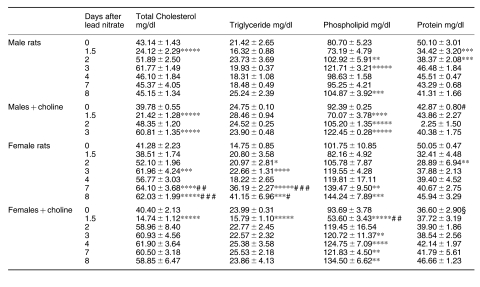
While continuous feeding of excess choline for 3 weeks decreased the plasma protein, it increased the levels of CH, and particularly of TG, in rats of either sex (Table 2); lead nitrate induced similar changes in the plasma levels of rats of either sex, CH and TG increasing while protein decreased. However, the plasma levels of protein were slightly elevated after lead in rats receiving choline. After excess choline feeding, the high basal plasma TG levels dropped to below time 0 values during liver hyperplasia.
Table 2.
Cholesterol, triglyceride and protein content in the plasma during lead nitrate-induced liver hyperplasia
data are means ± SEM; n = 4; §P < 0.0005 vs. females; # P < 0.05 and ## P < 0.025 vs. males; * P < 0.05, ** P < 0.025, *** P < 0.01, **** P < 0005 and ***** P < 0.0005 vs. 0 day.
The distribution of plasma CH, lipids and proteins between the very low density lipoproteins (VLDL) and the HDL fractions is shown in Tables 3 and 4, respectively. Although total plasma triglycerides (TG) were similar, females had higher TG in VLDL + LDL and lower TG in HDL. The hypertriglyceridemia induced by choline was due to increased TG levels of VLDL + LDL, while hypoproteinemia was only partially ascribed to reduced protein content of HDL. All components of VLDL + LDL were elevated during liver hyperplasia in rats of all experimental groups, except for a drop in TG in choline-fed animals; CH and TG returned to normal values from day 2 (Table 3). In contrast, the HDL fraction revealed a significant drop in the CH level at 36 h after lead administration in males and choline-fed rats of either sex but not in choline-free females (Table 4).
Table 3.
Cholesterol, lipid and protein composition of VIDL-IDL lipoproteins during lead nitrate-induced liver hyperplasia
data are means ± SEM; n = 4; * P < 0.05 ** P < 0.005 *** P < 0.001 **** P < 0.0005 and *****P < 0.0001 vs. 0 day; P < 0.05, ## P < 0.005 ### P < 0.001 #### P < 0.0005 ##### P < 0.0001 vs. males; § P < 0.025 vs. females.
Table 4.
Cholesterol, lipid and protein composition of HDL lipoproteins during lead nitrate-induced liver hyperplasia
data are means ± SEM; n = 4; * P < 0.05, ** P < 0.025, *** P < 0.01 and **** P < 0.005, ***** P < 0.0005 vs. 0 day; P < 0.05, P < 0.01 and P < 0.0005 vs. males; § P < 0.005 vs. females.
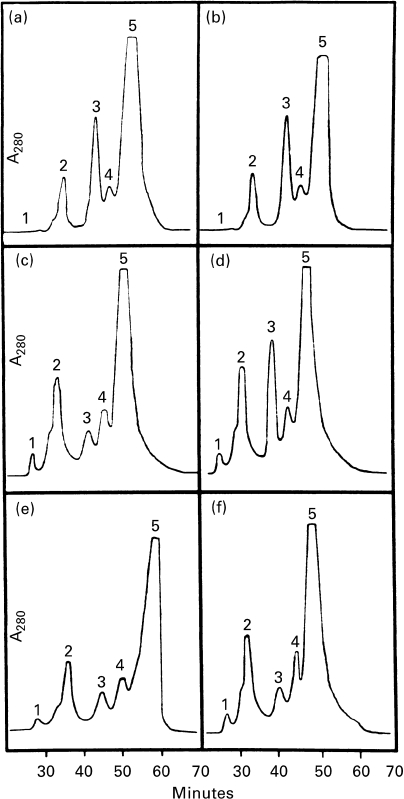
HPLC analysis of plasma lipoproteins revealed a drop of HDL2, the main lipoprotein subfraction in rat plasma, and an increase in the HDL3 subfraction at 36 h after lead administration in correspondence with the peak of 3H thymidine incorporation into DNA, in male but not in female rats (Figure 3). No significant changes in the HPLC profiles of VLDL-LDL were observed. Choline feeding displaced the female HPLC pattern of plasma lipoprotein towards that of males (Figure 3).
Figure 3.
Elution pattern (min) by HPLC of serum lipoprotein. Peaks: 1, VLDL; 2, LDL; 3, HDL2; 4, HDL3; 5, other serum proteins. a, b, male and female patterns at the beginning of lead nitrate administration, respectively; c, d, male and female patterns 36 h after lead nitrate administration; e, f, choline-male and choline-female patterns 36 h after lead nitrate administration.
Discussion
The results indicate that the different PL pattern in male and female rats resulted in a different proliferative response to lead nitrate accompanied by differences in CH metabolism. The earlier and more marked liver hyperplasia in males was associated with an earlier and more marked accumulation of intracellular CH esters and lower HDL CH levels. Increased dietary choline in females shifted both the proliferative response and cholesterol metabolism changes towards those of their male counterparts.
The content of CH, TG and PL in the liver as well as that of CH, TG and protein in the plasma was not correlated with the different growth responses to lead nitrate in male and female rats, nor in animals of either sex fed with excess choline, suggesting that these parameters are not necessarily implied in the regulation of the rate of cell proliferation.
It has been reported that an increase in the activity of CT, the key enzyme of the CDP-choline pathway, is essential for cell division (Jackowski 1994; Sesca et al. 1996; Tessitore et al. 1997), while an increase of PEMT, the enzyme catalysing the conversion of phosphatidylethanolamine (PE) into PC, has the opposite effect (Cui et al. 1994; Cui et al. 1995; Sesca et al. 1996; Tessitore et al. 1997; Tessitore et al. 1999a; Tessitore et al. 1999b). Similar results have also been obtained during liver hyperplasia induced by lead nitrate, where a transient PEMT inactivation associated with an enhanced CT activity was found to coincide with the peak in DNA synthesis in male and female rats (Tessitore et al. 1997), suggesting a possible involvement of PC metabolism during cell proliferation.
Modulation of CH metabolism, mainly consisting of an increase of CH synthesis, high expression/activity of 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) reductase and an increase in the number of LDL receptors per cell, has consistently been observed when cell proliferation is triggered by a mitogenic stimulus (Witte et al. 1982; Siperstein 1984; Brown & Goldstein 1986; Dessì et al. 1997). In addition, in a variety of human tumours and in various experimental models of cell proliferation including liver hyperplasia induced by lead nitrate, a decrease in plasma HDL CH associated with intracellular accumulation of CH esters has consistently been found (Dessi et al. 1984; Dessi et al. 1989; Dessi et al. 1991; Dessi et al. 1992a; Dessi et al. 1992b; Dessi et al. 1994; Dessi et al. 1995).
We recently found that cell growth rate correlates with the ability of cells to esterify cholesterol in different lymphoblastoid cell lines (Dessi et al. 1997; Batetta et al. 1999); the absolute levels of cholesterol synthesis and uptake did not appear to be directly correlated with the difference in growth rate. On the basis of these results we hypothesized that a larger store of cholesterol esters within the cell would allow for a more rapid mobilization and utilization of cholesterol for new membrane biogenesis and that cholesterol esterification may have a role in the regulation of cell growth and division.
In this study, we have demonstrated that, in liver hyperplasia induced by lead nitrate, derangement of PC metabolism may affect cholesterol turnover and may be associated with changes in cell growth rates. Since the cellular FC:PL molar ratio is an important aspect of normal cell function, overall our results suggest that, in order to ensure an appropriate FC:PL ratio the biological response to mitogenic stimulus might include concomitant changes in both PL and CH metabolism.
Acknowledgments
This work was supported by grants from MURST and Associazione Italiana per la Ricerca sul Cancro, Milan, Italy (40% and 60%).
References
- 1.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 2.Cui Z, Houweling M, Vance DE. Suppression of rat hepatoma cell growth by expression of phosphatidylethanolamine N methyltransferase 2. J. Biol. Chem. 1994;269:24531–24533. [PubMed] [Google Scholar]
- 3.Cui Z, Houweling M, Vance DE. Expression of phosphatidyl–ethanolamine N methyltransferase 2 in Mc Ardle RH7777 hepatoma cells inhibits the CDP-choline pathway for phosphatidylcholine biosynthesis via decreased gene expression of CTP: phosphocholinecytidylyltransferase. Biochem. J. 1995;312:939–945. doi: 10.1042/bj3120939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dessi S, Batetta B, Laconi E, Ennas C, Pani P. Hepatic cholesterol in lead nitrate induced liver hyperplasia. Chem. Biol. Interact. 1984;48:271–279. doi: 10.1016/0009-2797(84)90140-6. [DOI] [PubMed] [Google Scholar]
- 5.Dessi S, Batetta B, Carrucciu A, Pulisci D, Laconi S, Fadda AM, Anchisi C, Pani P. Variations of serum lipoproteins during cell proliferation induced by lead nitrate. Exp. Mol. Pathol. 1989;51:97–102. doi: 10.1016/0014-4800(89)90010-5. [DOI] [PubMed] [Google Scholar]
- 6.Dessi S, Batetta B, Pulisci D, Accogli P, Broccia G, Pani P. Total and HDL cholesterol in human hematologic neoplasm. Int. J. Hematol. 1991;54:483–486. [PubMed] [Google Scholar]
- 7.Dessi S, Batetta B, Anchisi C, Pani P, Costelli P, Tessitore L, Baccino FM. Cholesterol metabolism during the growth of a rat ascites hepatoma (Yoshida AH-130) Br. J. Cancer. 1992a;66:787–793. doi: 10.1038/bjc.1992.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dessi S, Batetta B, Spano O, Pulisci D, Mulas MF, Muntoni SA, Armeni M, Sanna C, Antonucci R, Pani P. Serum lipoprotein pattern as modified in G6PD-deficient children during haemolytic anaemia induced by fava bean ingestion. Int. J. Exp. Pathol. 1992b;73:157–160. [PMC free article] [PubMed] [Google Scholar]
- 9.Dessi S, Batetta B, Pulisci D, Spano O, Anchisi C, Tessitore L, Costelli P, Baccino FM, Aroasio E, Pani P. Cholesterol content in tumor tissues is inversely associated with HDL lipoprotein cholesterol in serum in patients with gastrointestinal cancer. Cancer. 1994;73:253–258. doi: 10.1002/1097-0142(19940115)73:2<253::aid-cncr2820730204>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 10.Dessi S, Batetta B, Spano O, Sanna F, Tonello M, Giacchino M, Tessitore L, Costelli P, Baccino FM, Madon E, Pani P. Clinical remission is associated with restoration of normal high-density lipoprotein cholesterol levels in children with malignancies. Clin. Science. 1995;89:505–510. doi: 10.1042/cs0890505. [DOI] [PubMed] [Google Scholar]
- 11.Dessi S, Batetta B, Pani A, Spano O, Sanna F, Putzolu M, Bonatesta R, Piras S, Pani P. Role of cholesterol synthesis and esterification in the growth of CEM and MOLT4 lymphoblastic cells. Biochem. J. 1997;321:603–608. doi: 10.1042/bj3210603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497. [PubMed] [Google Scholar]
- 13.Jackowski S. Coordination of membrane phospholipid synthesis with the cell cycle. J.Biol. Chem. 1994;267:1752–1760. [PubMed] [Google Scholar]
- 14.Nakamura S, Nishizuka Y. Lipid mediators and protein kinase C activation for the intracellular signaling network. J. Biochem. 1994;115:1029–1034. doi: 10.1093/oxfordjournals.jbchem.a124451. [DOI] [PubMed] [Google Scholar]
- 15.Okazaki M, Ohno Y, Hera I. High performance aqueous gel permeation chromatography of human serum lipoproteins. J. Chromatogr. 1980;221:257–264. doi: 10.1016/s0378-4347(00)84310-8. [DOI] [PubMed] [Google Scholar]
- 16.Sesca E, Perletti GP, Binasco V, Chiara M, Tessitore L. Phosphatidylethanolamine N methyltransferase 2 and CTP phosphocholine cytidyltransferase expression are related with protein kinase C isozymes in developmental liver growth. Biochem. Biophys. Res. Comm. 1996;229:158–162. doi: 10.1006/bbrc.1996.1773. [DOI] [PubMed] [Google Scholar]
- 17.Siperstein MD. Role of cholesterogenesis and isoprenoid synthesis in DNA replication and cell growth. J. Lipid. Res. 1984;25:1462–1468. [PubMed] [Google Scholar]
- 18.Tessitore L, Pani P, Dianzani MU. Mechanisms of the enhanced liver carcinogenesis by choline in female rats: delay in liver growth after partial hepatectomy and stimulation of 2 AAF mitoinhibition. Carcinogenesis. 1992a;13:1929–1932. doi: 10.1093/carcin/13.10.1929. [DOI] [PubMed] [Google Scholar]
- 19.Tessitore L, Pani P, Dianzani MU. Choline enhances acetyl-aminofluorene promotion of liver carcinogenesis in female but not in male rats. Carcinogenesis. 1992b;10:385–389. doi: 10.1093/carcin/13.3.385. [DOI] [PubMed] [Google Scholar]
- 20.Tessitore L, Perletti GP, Sesca E, Pani P, Dianzani MU, Piccinini F. Protein kinase C isozyme pattern in liver hyperplasia. Biochem. Biophys. Res. Comm. 1994;205:208–214. doi: 10.1006/bbrc.1994.2651. [DOI] [PubMed] [Google Scholar]
- 21.Tessitore L, Sesca E, Pani P, Dianzani MU. Sexual dimorphism in the regulation of cell turnover during liver hyperplasia. Chem. Biol. Interact. 1995;97:1–10. doi: 10.1016/0009-2797(94)03602-1. [DOI] [PubMed] [Google Scholar]
- 22.Tessitore L, Cui Z, Vance DE. Transient inactivation of phosphatidylethanolamine N methyltransferase 2 and activation of cytidine triphosphate: phosphocholine cytidyltransferase during non neoplastic liver growth. Biochem. J. 1997;322:151–154. doi: 10.1042/bj3220151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tessitore L, Sesca E, Bosco M, Vance DE. Expression of phosphatidylethanolamine N methyltransferase in Yoshida ascites hepatoma cells and the livers of host rats. Carcinogenesis. 1999a;20:561–567. doi: 10.1093/carcin/20.4.561. [DOI] [PubMed] [Google Scholar]
- 24.Tessitore L, Dianzani I, Cui Z, Vance DE. Diminished expression of phosphatidylethanolamine N methyltransferase 2 during hepatocarcinogenesis. Biochem. J. 1999b;337:23–27. [PMC free article] [PubMed] [Google Scholar]
- 25.Witte LD, Cornicelly R, Miller W, Goodman DS. Effects of platelet-derived and endothelial cell derived growth factors on the LDL receptor pathway in cultured human fibroblast. J. Biol. Chem. 1982;257:5392–5401. [PubMed] [Google Scholar]