Abstract
While the control or progression of leishmaniasis depends on host immune responses, the initial inflammatory process represents a key event. This process involves the participation of several cytokines and growth factors induced during inflammation as well as factors already present at the site of infection such as insulin-like growth factor (IGF)-I. We have previously demonstrated a potential role for IGF-I in experimental cutaneous leishmaniasis based on the significant increase in lesion size seen in mice injected with Leishmania promastigotes preactivated with IGF-I. In the present study we show that preactivation of Leishmania (Leishmania) amazonensis promastigotes with IGF-I induces an increase in the actual number of parasites at the lesion site from seven days postinfection, in addition to a more intense inflammatory infiltrate. There was a higher numerical density of polymorphonuclear neutrophils from 3 to 24 h, and of mononuclear cells from 48 h of infection onward. A higher density of polymorphonuclear neutrophils and mononuclear cells harboring parasites was also observed. The most important observation, however, was that more parasites per cell were present, revealing that IGF-I appears to favour parasite growth within the macrophages. These results strongly suggest an important role for IGF-I in the development of cutaneous leishmaniasis, where it influences both the inflammatory process and parasite growth.
Keywords: growth factor, inflammation, pathology, morphometry, phagocytosis
Leishmaniasis is an important public health concern in many countries and is caused by protozoan parasites of the genus Leishmania (Kinetoplastida: Trypanosomatidae). The host–parasite interaction begins when Leishmania parasites are injected into the skin of the mammalian host by the sand fly. The evolution of infection depends on innate and specific immune responses of the host (Reiner & Locksley 1995). The initial inflammatory events involve the participation of factors induced during infection such as transforming growth factor β (Barral-Neto et al. 1992), granulocyte-macrophage colony-stimulating factor (Barcinski et al. 1992) and TNF-α (Bogdan et al. 1990) as well as factors constitutively present such as IGF-I (Goto et al. 1998).
Since IGF-I is present in the blood, in many tissues and particularly in macrophages (Nagaoka et al. 1990; Arkins et al. 1993), it is one of the first factors encountered by the Leishmania promastigotes as soon as they are injected into the skin, and subsequently after internalization by macrophages. IGF-I is a polypeptide stimulating proliferation and differentiation of a wide variety of cell types (Jones & Clemmons 1995). IGF-I has been shown to affect cell metabolism and to be an important endocrine factor in inflammation, immune activation, and wound healing (Kratz & Gidlund 1993; Jones & Clemons 1995). Most cell types have the ability to produce IGF although the main site of production is the liver. It is detectable both in circulation and in the tissues.
The biological importance of IGF-I to Leishmania has been demonstrated by our observations showing that IGF-I induces a rapid growth response in Leishmania promastigotes and in cell-free amastigotes in vitro; IGF-I binds specifically to Leishmania and induces phosphorylation of intracellular molecules on stimulation (Gomes et al. 1997; Gomes et al. 1998; Goto et al. 1998).
In the initial in vivo study, we observed a significant increase in lesion size and in number of parasites in skin sections from mice injected with Leishmania promastigotes preactivated with IGF-I (Goto et al. 1998). However in vivo interactions are complex and IGF-I has a pleiotropic effect affecting metabolism and cell proliferation and differentiation (Kratz & Gidlund 1993; Jones & Clemmons 1995).
To distinguish between the effect of IGF-I on the parasite and on the host cells, we now provide a more detailed analysis of the effect of IGF-I on both inflammation and parasites. In the present study in mice injected with L. (L.) amazonensis preactivated with IGF-I, we observed an increase in lesion size related to the increase in the number of parasites, and an increase in the inflammatory cell infiltrate at the inoculation site. We also provide clear evidence that parasites pre-incubated with IGF-I affect the parasite–host interaction, favouring parasite growth within the macrophages.
Materials and methods
Mice. 6–10-week-old, inbred, BALB/c strain mice of both sexes were obtained from our breeding facility at the Medical School of the University of Sao Paulo and were maintained in our laboratory during the experiments.
Parasites. Leishmania (Leishmania) amazonensis, HSJD-1 strain, was isolated from a patient in Costa Rica and kindly characterized by Prof. J. J. Shaw (Instituto Evandro Chagas, Brazil) according to its reactivity to monoclonal antibodies specific for L. (L.) amazonensis, L. (V.) panamensis and for the subgenus Viannia. The strain was also characterized by Dr S.R. Uliana (Department of Parasitology, ICB, University of São Paulo, Brazil) according to its reactivity to subunit ribosomal DNA probes for L. amazonensis and the subgenus Viannia(Uliana et al. 1994). Parasites were maintained at 25 °C in NNN (Novy, Nicole, McNeal) medium over-layered by RPMI 1640 medium (Gibco Laboratories, Grand Island, NY, USA) and supplemented with 10% foetal calf serum (FCS) (W.L. Imunoquímica, Rio de Janeiro, Brazil). The parasites were inoculated periodically in vivo in the footpad of BALB/c mice. The parasite culture was expanded in RPMI 1640 medium supplemented with 10% FCS until the stationary phase of growth was attained. The parasites were washed in 0.01 m phosphate-buffered saline (PBS), pH 7.2, for use in the experiments.
Experimental protocol. After washing, the concentration of promastigotes was adjusted to 2 × 108 parasites/ml in PBS and incubated for 5 min with or without 50 ng/ml (previously established in Goto et al. 1998) human rIGF-I (kindly provided by M. Lake, Kabi-Pharmacia, Uppsala, Sweden or purchased from R & D Systems, Minneapolis, USA). 50 μl of parasite suspension were injected subcutaneously into the hind footpad of the mice. The contralateral footpad was inoculated with sterile PBS or rIGF-I as a control. At 3, 24 and 48 h, and 7, 30, 60 and 90 days postinfection (PI), five animals from each group were killed and skin specimens from the inoculation site were taken for qualitative and quantitative histopathological studies and quantification of the number of parasites in the lesion.
Histopathological studies and quantitative morphometric analysis. Samples were fixed in 0.01 m phosphate-buffered 4% paraformaldehyde and embedded in plastic resin (Technovit 7100, Kulzer, GmbH, Friedrichsdorf, Germany). Semi-thin sections were cut on a 11800 Pyramitome (LKB-BROMMA, Stockholm, Sweden) and stained with haematoxylin-eosin (H & E) for histological studies.
For quantitative morphometric analysis, semi-thin sections from three different levels in each sample were obtained and the cells and parasites were counted in 0.01 mm2 area using an Olympus planachromatic immersion objective lens ( × 100) and an eyepiece graticule. 900 cells were counted in each level where each cell type constituted at least 10% of the total to maintain the relative standard error below 0.1% (Weibel 1979). Samples from five experimental animals were analysed up to 30 days PI and the data analysed using the Mann–Whitney test.
Limiting dilution assay for parasite quantification. This assay was performed according to that originally described by Titus et al. (1985) and modified by Lima et al. (1997). Briefly, the skin from the inoculation site between the heel and toes was taken from the footpad, weighed and cut into several pieces in Schneider's insect medium (Gibco Laboratories, Grand Island, NY, USA) supplemented with 10% foetal calf serum, 2% normal human urine, 5 μg/ml phenol red, 5 mm HEPES pH 7.3, 50 μg/ml gentamicin, 100 UI/ml penicillin. The pieces were then homogenized vigorously for several minutes using a glass tissue glinder until the tissue was completely disrupted.
After serial dilutions in the same medium 100 μl/well from each dilution were dispensed into at least 16 wells of 96-well flat-bottom culture plates (Costar, Cambridge, USA). Each plate was covered with Parafilm®g42 (American National Can, Greenwich, CT, USA) which was pressed to tightly seal each well, covered with the lid and incubated at 25 °C for 10 days.
Absorbance at 450 nm was measured in a microtiter Multiscan (Bio-Rad, CA, USA). Parasite growth was confirmed concomitantly by examining the plate in an inverted microscope (Zeiss, Oberkochen, Germany). The data were analysed using the computer program ELIDA based on Poisson statistics.
Results
Effect of IGF-I on the lesion size and on the parasite growth in the experimental cutaneous leishmaniasis. IGF-I activation of L. (L.) amazonensis promastigotes before injection results in visibly increased lesion size in BALB/c mice. Figure 1 shows the lesions from mice after 70 days of infection.
Figure 1.
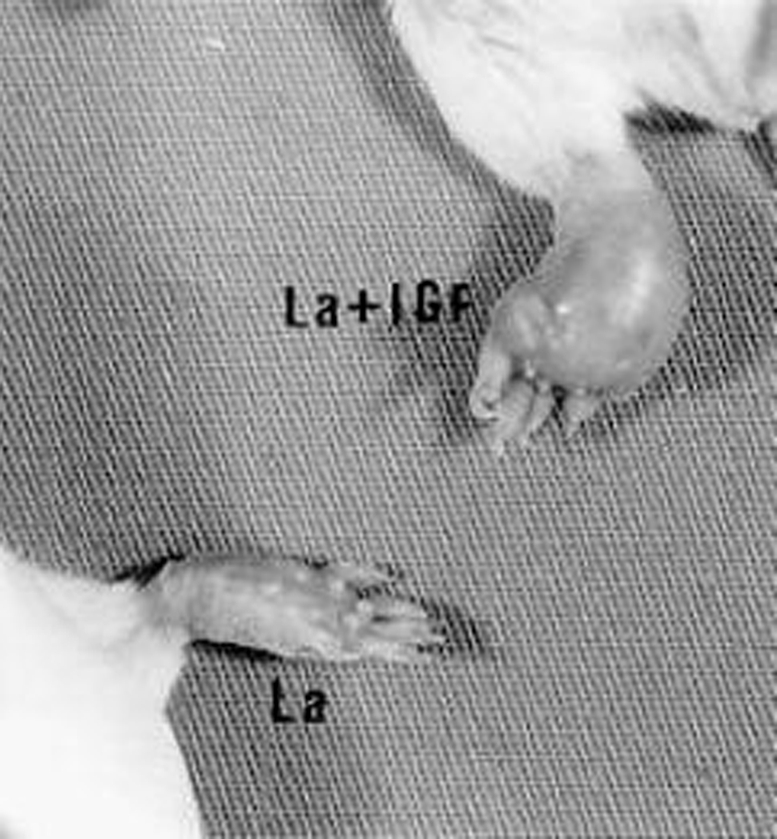
Cutaneous lesions in BALB/c mouse after 70 days infection with 107 Leishmania (L.) amazonensis promastigotes (LP) pre-incubated with 50 ng/ml IGF-I and control mouse infected with nonactivated parasites.
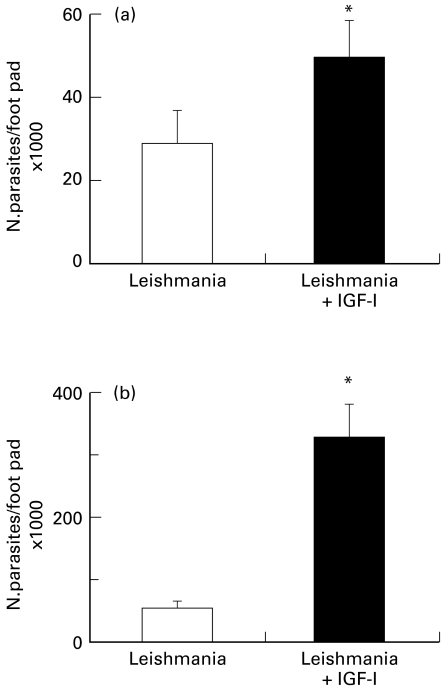
To better understand the effect of IGF-I on the host–parasite interaction in the lesion we studied parasite growth and the inflammatory process at different infection intervals. Skin lesions were taken 7 and 60 days post-infection (PI) and the parasites were quantified using the limiting dilution assay. The number of parasites in the lesion from animals injected with IGF-I-stimulated Leishmania was already significantly higher after 7 days of infection (Figure 2a). The difference between groups increased substantially by 60 days PI (Figure 2b).
Figure 2.
Number of parasites (mean ± SE) per footpad in BALB/c mice injected with 107Leishmania (L.) amazonensis promastigotes pre-incubated with 50 ng/ml of IGF-I. Control mice were infected with nonactivated parasites. Number of parasites was quantified using the limiting dilution assay as given in the Materials and Methods. (a) 7 days postinfection; (b) 60 days postinfection. *P ≤ 0.05 (Mann–Whitney test).
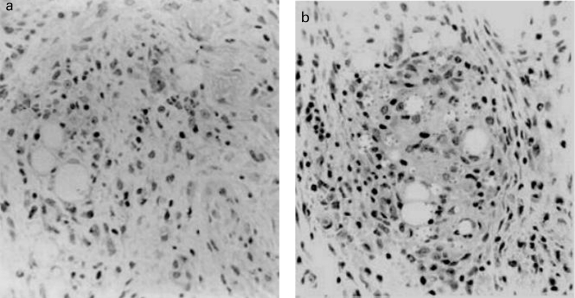
Effect of IGF-I on the inflammatory infiltrate and on the parasite–host cell interaction. Figure 3a,b shows the histopathology of the lesion in mice injected with IGF-I-activated or nonactivated Leishmania where the activated parasites caused a more intense inflammatory infiltrate. However, the inflammatory infiltrate was qualitatively similar showing an influx of polymorphonuclear neutrophils (PNMs) from 3 to 24 h and the subsequent appearance of mononuclear cells after 48 h of infection with or without pre-activation of the parasites by IGF-I. When IGF-I alone was injected into the footpad, a very mild mononuclear cell infiltrate was seen at 48 h postinoculation when compared with PBS (data not shown).
Figure 3.
Skin lesions showing inflammation constituted by a mixed cell infiltrate in the footpad of BALB/c mice 48 h after infection with 107Leishmania (L.) amazonensis promastigotes with or without IGF-I. (a) control footpad injected with promastigotes. (b) footpad injected with promastigotes pre-incubated with 50 ng/ml IGF-I. H & E × 40
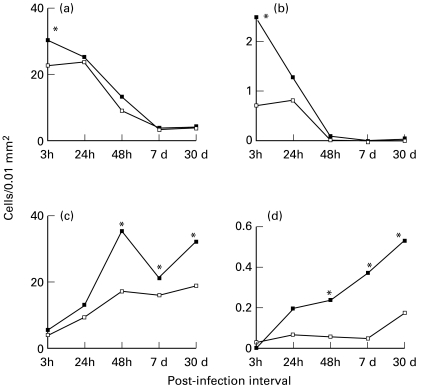
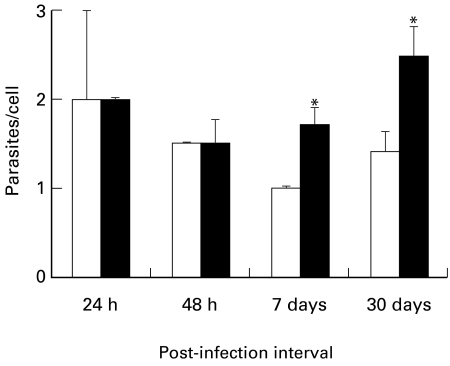
Since a difference in the intensity of the inflammatory cellular response was apparent, quantitative morphometric analysis of the cells and parasites was performed on skin sections. This analysis revealed an increase in the density of both PMNs and of PMN with ingested parasites (Figure 4a,b) during the early phase at 3 h PI. After 48 h, a similar difference was seen in mononuclear cell density and in cells harboring parasites (Figure 4c,d). Finally, we quantified the number of parasites per mononuclear cell. As seen in Figure 5, pre-activation of the parasite led to a much higher parasite/cell ratio at both 7 and 30 days PI.
Figure 4.
Numerical density (cells/0.01 mm2) of inflammatory cells and cells harbouring parasites in the skin of BALB/c mice injected with 107Leishmania (L.) amazonensis promastigotes pre-incubated with 50 ng/ml of IGF-I (▪) and control mice infected with nonactivated parasites (□). (a) Polymorphonuclear neutrophils (b) Polymorphonuclear neutrophils harbouring parasites. (c) Mononuclear cells. (d) Mononuclear cells harbouring parasites. Number of cells was quantified using the quantitative morphometric analysis on semi-thin sections from three different levels of the skin. Values are expressed as mean ± SE. *P ≤ 0.05 (Mann–Whitney test).
Figure 5.
Number of parasites per cell (mean ± SE) from skin lesions in BALB/c mice injected with 107Leishmania (L.) amazonensis promastigotes pre-incubated with 50 ng/ml of IGF-I (solid column). Control mice were infected with nonactivated parasites (open column). For further details see Materials and Methods. *P ≤ 0.05 (Mann–Whitney test).
Discussion
In a previous study, we demonstrated that IGF-I exacerbates the skin lesion of mice injected with Leishmania pre-activated with IGF-I (Goto et al. 1998). In the present study, we expand these findings, evaluating the effect of IGF-I on the parasite burden in the skin lesion, on the dynamics of the inflammatory process, and on the parasite–host cell interaction.
The size of the leishmanial lesion depends mainly on the multiplication of the parasites and on the inflammatory infiltrate present in the dermis where the parasites were injected. The present study shows that the increase in lesion size with IGF-I-activated parasites was due to the presence of a greater number of parasites, unequivocally demonstrated by quantification of the number of parasites in the lesion by the limiting dilution assay, although in addition to a more intense inflammatory cellular infiltrate. The inflammatory process was qualitatively similar in both groups but more intense in mice injected with IGF-I-activated parasites. Polymorphonuclear neutrophils predominated in the infiltrate at the beginning of infection, and mononuclear cells from 48 h PI and during the course of experiment up to 30 days of infection.
How the pre-activated parasite contributes to the increase in the early and later cellular infiltrate is not well understood. Leishmania can rapidly interact and induce host cell responses including phosphorylation (Love et al. 1998) and presumably protein synthesis and secretory events in the host cells (Racoosin & Beverley 1997). The IGF-I-activated parasite may better promote the release of migration-inducing chemotactic factors such as chemokines (Luster 1998), leukotriene B4 and C5a (Gallin 1989) promoting PMN migration. The increase in infiltration by the mononuclear cells that follows the PMN migration may thus be partly due to a mechanism involving the neutrophil-dependent regulation of mononuclear cell migration (Gallin 1989). Since the injection of IGF-I alone promoted a mild infiltrate in the footpad skin of noninfected control mice, the effect may partially reside in the direct action of IGF-I on migration.
Analysis of the parasite–host cell interaction at different postinfection intervals reveals several interesting points. Different cell types harboring parasites increased, accompanying the more intense cellular infiltrate seen at the same time periods. The increased phagocytosis of parasites by PMNs at the onset of infection contradicts the finding of a more intense parasitism at later intervals since such cells are known to effectively kill the parasites (Chang 1981). However this effect may have been counteracted by the subsequently more intense mononuclear cell infiltration and phagocytosis of parasites by these cells, allowing evasion by the parasites.
Our major finding was an increase in the parasite/cell ratio after IGF-I activation. This shows that the increased parasitism with IGF-I-activated parasites was due not only to the availability of more macrophages in the lesion but also to the increased growth of the parasites within the macrophages. The mechanisms and factors underlying this phenomenon may reside in the less explored interactions governing parasite–host interplay. A direct, growth-inducing effect on the parasites seen in vitro (Goto et al. 1998) is possible although the selection of parasite subpopulations with an advantage for intracellular growth, or protection of parasites in the macrophage enviroment against heat-induced death as shown with GM-CSF on Leishmania promastigotes (Barcinski et al. 1992) are other possibilities.
Considering the effect on the host cells, the fate of Leishmania within the macrophages is dependent on either macrophage-activating cytokines such as IFN-γ, TNF-α, macrophage migration inhibitory factor and granulocyte-macrophage colony–stimulating factor (Weiser et al. 1987; Heinzel et al. 1989; Bogdan et al. 1990; Juttner et al. 1998) or macrophage inactivating cytokines such as IL-10 (Vouldoukis et al. 1997), IL-13 (Juttner et al. 1998) and TGF-β (Barral et al. 1992). Thus, either a direct effect of IGF-I or an effect of IGF-I-activated parasites on the downregulation of the microbicidal mechanism of macrophages through effect of cytokine thus favouring parasite growth may operate.
To clearly define the in vivo effect of IGF-I we used IGF-I-preactivated parasites; however, data are available showing the presence of IGF-I in macrophages (Abbound et al. 1991; Arkins et al. 1993) and the increased production of IGF-I in activated macrophages (Arkins et al. 1993) and T cells (Baxter et al. 1991) on mitogen-stimulation and during wound healing (Antoniades et al. 1993). Thus in the lesion, IGF-I may act physiologically on the parasite both intracellularly and extracellularly during leishmaniasis.
In conclusion, we have shown that the enhancing effect of IGF-I on the lesion seen during Leishmania infection results from its effects on both the inflammatory process and on better parasite growth/survival within the host cell. These results strongly suggest an important role for IGF-I in cutaneous leishmaniasis.
Acknowledgments
We are grateful to Dr M. Lake for the gift of IGF-I, and Cleyton Alves, Daniela Mayumi Ura and Regiane Mathias for excellent technical assistance. This study was supported by the Fundacäo de Amparo a Pesquisa do Estado de Säo Paulo, Conselho Nacional de Pes-quisa and LIM/50 of the Hospital das Clínicas, Faculty of Medicine, University of Sao Paulo.
References
- 1.Abbound SL, Bethel CR, Aron DC. Secretion of insulin-like growth factor I and insulin-like growth factor- binding proteins by murine bone marrow stromal cells. J. Clin. Invest. 1991;88:470–475. doi: 10.1172/JCI115327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antoniades HN, Galanopoulos T, Neville-Golden J, Kiristsy CP, Lynch SE. Expression of growth factor and receptor mRNAs in skin epithelial cells following acute cutaneous injury. Am. J. Pathol. 1993;142:1099–1110. [PMC free article] [PubMed] [Google Scholar]
- 3.Arkins S, Rebeiz N, Biragyn A, Reese DL, Kelley KW. Murine macrophages express abundant Insulin-like Growth Factor class Ea and Eb transcripts. Endocrinology. 1993;133:2334–2343. doi: 10.1210/endo.133.5.8404686. [DOI] [PubMed] [Google Scholar]
- 4.Barcinski MA, Schehtman D, Quintão LG, Costa DA, Soares LRB, Moreira MEC, Charlab R. Granulocyte-macrophage colony-stimulating factor increases the infectivity of L amazonensis by protecting promastigote from heat-induced death. Infect. Immun. 1992;60:3523–3527. doi: 10.1128/iai.60.9.3523-3527.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barral-Neto M, Barral A, Brownell CE, Sheik YYA, Ellingsworth LR, Twardzik DR, Reed SG. Transforming growth factor-beta in leishmanial infection: a parasite escape mechanism. Science. 1992;257:545–548. doi: 10.1126/science.1636092. [DOI] [PubMed] [Google Scholar]
- 6.Baxter JB, Blalock JE, Weighent DA. Characterization of immunoreactive insulin-like growth factor from leukocytes and its regulation by growth hormone. Endocrinology. 1991;129:1727–1734. doi: 10.1210/endo-129-4-1727. [DOI] [PubMed] [Google Scholar]
- 7.Bogdan C, Moll H, Solbach W, Rollingoff M. Tumor necrosis factor -α in combination with interferon-gamma, but not interleukin-4 activates murine macrophages for elimination of Leishmania major amastigotes. Eur. J. Immunol. 1990;20:1131. doi: 10.1002/eji.1830200528. [DOI] [PubMed] [Google Scholar]
- 8.Chang KP. Leishmanicidal mechanisms of human polymorphonuclear phagocytes. Am. J. Trop. Med. Hyg. 1981;30:322–333. doi: 10.4269/ajtmh.1981.30.322. [DOI] [PubMed] [Google Scholar]
- 9.Gallin JI. Inflammation. In: Paul W E, editor. Fundamental Immunology. New York: Raven Press; 1989. pp. 721–733. [Google Scholar]
- 10.Gomes CMC, Goto H, Corbett CEP, Gidlund M. Insulin-like growth factor (IGF)-1 is a growth promoting factor for Leishmania promastigotes. Acta Trop. 1997;64:225–228. doi: 10.1016/s0001-706x(96)00633-x. [DOI] [PubMed] [Google Scholar]
- 11.Gomes CMC, Monteir HP, Gidlund M, Corbett CEP, Goto H. Insulin-like growth factor (IGF) -I induces phosphorylation in Leishmania (Leishmania) mexicana promastigotes and amastigotes. J. Euk. Microl. 1998;45:352–355. doi: 10.1111/j.1550-7408.1998.tb04548.x. [DOI] [PubMed] [Google Scholar]
- 12.Goto H, Gomes CMC, Corbett CEP, Monteiro HP, Gidlund M. Insulin-like growth factor I is a growth-promoting factor for Leishmania promastigotes and amastigotes. Proc. Natl. Acad. Sci. USA. 1998;95:13211–13216. doi: 10.1073/pnas.95.22.13211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, Locksley RM. Reciprocal expression of interferon γ or interleukin 4 during the resolution or progression of murine leishmaniasis. J. Exp. Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones JI, Clemmons DR. Insulin-like Growth Factors and their binding proteins: biological actions. Endocrine Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 15.Juttner S, Bernhagen J, Metz CN, Rollinghoff M, Bucala R, Gessner A. Migration inhibitory factor induces killing of Leishmania major by macrophages: dependence on reactive nitrogen intermediates and endogenous TNF-alpha. J. Immunol. 1998;161:2383–2390. [PubMed] [Google Scholar]
- 16.Kratz G, Gidlund M. The role of insulin-like growth factor-I and II in human wound healing. Modulating effect of IGF binding protein-1. Scand. J. Plast. Reconstr. Surg. 1993;28:107–112. doi: 10.3109/02844319409071187. [DOI] [PubMed] [Google Scholar]
- 17.Lima HC, Bleyenberg JA, Titus RG. A simple method for quantifying Leishmania in tissues of infected animals. Parasitol. Today. 1997;13:80–82. doi: 10.1016/s0169-4758(96)40010-2. [DOI] [PubMed] [Google Scholar]
- 18.Love DC, Mentink K, Mosser DM. Leishmania amazonensis: the phagocytosis of amastigotes by macrophages. Exp. Parasitol. 1998;88:161–171. doi: 10.1006/expr.1998.4232. [DOI] [PubMed] [Google Scholar]
- 19.Luster AD. Chemokines - Chemotactic cytokines that mediate inflammation. New. Engl. J. Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 20.Nagaoka I, Trapnell BC, Crystal RG. Regulation of Insulin-like Growth Factor-1 gene expression in the human macrophage cell line U937. J. Clin. Invest. 1990;85:448–455. doi: 10.1172/JCI114458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Racoosin EL, Beverley SM. Leishmania major: promastigotes induce expression of a subset of genes in murine macrophages. Exp. Parasitol. 1997;85:283–295. doi: 10.1006/expr.1996.4139. [DOI] [PubMed] [Google Scholar]
- 22.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 23.Titus RG, Marchand MT, Boon, Louis JA. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985;7:545–555. doi: 10.1111/j.1365-3024.1985.tb00098.x. [DOI] [PubMed] [Google Scholar]
- 24.Uliana SR, Nelson K, Beverly SM, Camargo EP, Floeter-Winter LM. Discrimination amongst Leishmania by polymerase chain reaction and hybridization with small subunit ribosomal DNA derived oligonucleotides. J. Euk. Microbiol. 1994;41:324–330. doi: 10.1111/j.1550-7408.1994.tb06085.x. [DOI] [PubMed] [Google Scholar]
- 25.Vouldoukis I, Becherel PA, Riveros-Moreno V, Arock M, Silva O, Debre P, Mazier D, Mossalayi MD. Interleukin-10 and interleukin-4 inhibit intracellular killing of Leishmania infantum and Leishmania major by human macrophages by decreasing nitric oxide generation. Eur. J. Immunol. 1997;27:860–865. doi: 10.1002/eji.1830270409. [DOI] [PubMed] [Google Scholar]
- 26.Weibel ER. Steriological Methods. London: Academic Press, Inc.; 1979. Practical methods for biological morphometry; pp. 1–415. [Google Scholar]
- 27.Weiser WY, Van Niel A, Clark SC, David JR, Remold HG. Recombinant human granulocyte/macrophage colony-stimulating factor activates intracellular killing of Leishmania donovani by human monocyte-derived macrophages. J. Exp. Med. 1987;166:1436–1446. doi: 10.1084/jem.166.5.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]