Abstract
GB Virus C and Hepatitis G Virus (GBV-C/HGV) are positive, single-stranded flaviviruses. GBV-C and HGV are independent isolates of the same virus. Transmission via the blood-borne route is the commonest mode, although vertical and sexual transmission is well documented. GBV-C/HGV is distributed globally; its prevalence in the general population is 10 fold higher in African countries than in non-African countries. High prevalences of GBV-C/HGV have been found in subjects with frequent parenteral exposure and in groups at high risk of exposure to blood and blood products. The clinical significance of human infection with GBV-C/HGV is currently unclear. The virus can establish both acute and chronic infection and appears to be sensitive to interferon. Only some 12–15% of chronic Non-A, B, C hepatitis cases are infected with GBV-C/HGV. A direct association with liver pathology is still lacking and it is not yet clear as to whether GBV-C/HGV is indeed a hepatotropic virus. Current evidence suggests that the spectrum of association of GBV-C/HGV infection with extrahepatic diseases ranges from haematalogical diseases, aplastic anaemia, human immunodeficiency virus (HIV)-positive idiopathic thrombocytopenia and thalassemia, through to common variable immune deficiency and cryoglobunemia.
Keywords: Flavivirus, PCR, Anti-E2, hepatitis, liver disease, clinical features
Introduction
Discovery of GBV-C and HGV
The first successful transmission of viral hepatitis from humans to nonhuman primates was achieved by Deinhardt et al. (Deinhardt et al. 1967), when serum from a 34-year-old surgeon (whose initials were GB) with acute hepatitis was inoculated into tamarins (Saguinus spp.). Animals inoculated with GB serum developed hepatitis, as did animals inoculated with sera of tamarins with GB serum-induced hepatitis. GB Virus C (GBV-C) was identified in the serum of a human in West Africa that contained recombinant nonstructural proteins of two other novel flaviviruses, designated GBV-A and GBV-B (Simons et al. 1995a). These viruses were cloned from the serum of a tamarin inoculated with the GB agent (Deinhardt et al. 1967) and are now known to be of animal origin. Using degenerate primers derived from the homologous sequences shared by GBV-A, GBV-B, and HCV from the NS3/helicase region of these viruses, amplification products were obtained from immunoreactive sera whose sequence was different from the other viruses. The new virus was named GBV-C (Simons et al. 1995b; Leary et al. 1996). Independently, in an effort to identify additional agents for post-transfusion non-A-non-B hepatitis (NANB), Linnen et al. (Linnen et al. 1996) performed molecular cloning with plasma from a patient with presumed NANB hepatitis, and a virus like RNA sequence was identified and designated Hepatitis G Virus (HGV).
Genomic organization of GBV-C/HGV
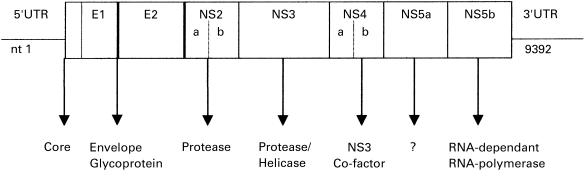
The genomic organization of GBV-C/HGV and HCV is similar and both viruses belong to the family of Flaviviridae. They are both linear, positive single-stranded RNA molecules composed of about 9500 nucleotides (nt)(Figure 1).
Figure 1.
HGV (PNF2161) genome. Proteolytic processing and functions of the structural and non-structural proteins (nt = nucleotide; UTR = untranslated region; E = envelope; NS = nonstructural).
The viral polyprotein is preceded by a 5′ untranslated region (UTR), followed by a long open reading frame (ORF) terminating with 3′ UTR (Figure 1)(Linnen et al. 1996). The polyprotein is cleaved into smaller fragments with different functions by host-encoded signal peptidases and viral proteases. These fragments include the envelope proteins (E1 and E2) at the amino or N-terminal end followed by nonstructural (NS) proteins (NS2, NS3, NS4, and NS5) at the carboxy or C-terminal end (Figure 1). The GBV-C/HGV genome is unusual in that the region between the 5′ UTR and the envelope proteins is absent or truncated (Simons et al. 1995a; Leary et al. 1996; Linnen et al. 1996). This region normally encodes the nucleocapsid/core protein that encases the viral genome. The 5′ UTR contains an internal ribosome entry site (IRES) that is capable of directing CAP-independent translation of the polyprotein (Simons et al. 1996). Sequence comparisons of the two prototype isolates, HGV and GBV-C, show that they have 86% and 95% homology at the nucleotide and amino-acid levels, respectively. They are therefore considered to be independent isolates of the same virus (Leary et al. 1996; Linnen et al. 1996).
Phylogenetic analysis of GBV-C/HGV
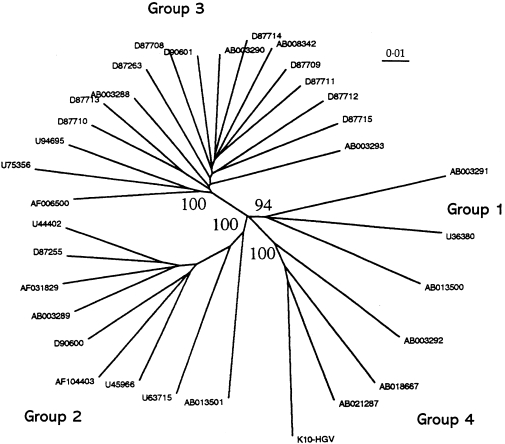
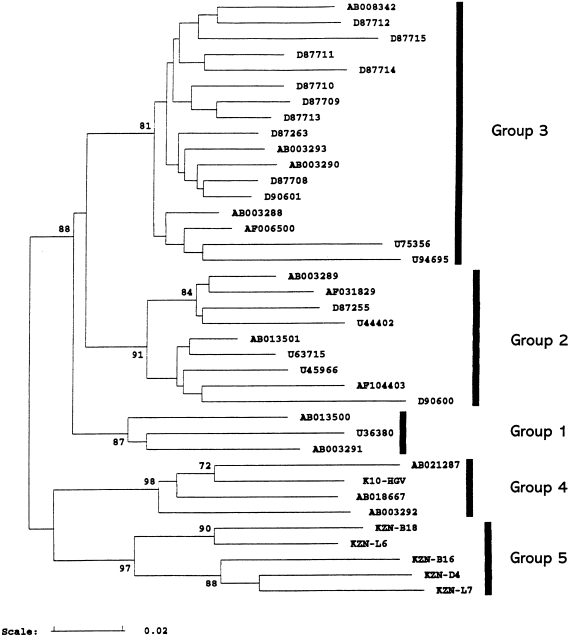
Comparisons of 33 epidemiologically distinct complete or near complete genomic sequences of GBV-C/HGV suggest the existence of four major phylogenetic groups that are equally divergent from the chimpanzee isolate, GBV-Ctrop (Birkenmeyer et al. 1998), and have distinct geographical distribution (Figure 2) (Smith et al. 2000). Group 1 includes isolates from Ghana, West Africa and a single Japanese isolate; Group 2 includes isolates from Europe, North and South America and Japan; Group 3 includes isolates from Japan and China and the fourth group consists of isolates from South-east Asia (Figure 2) (Smith et al. 2000). With the sole exception of E2 gene segments, phylogenetic analysis of individual genes and subgenomic regions have failed to consistently produce congruent phylogenetic trees of the four major groups that correlate with the geographical origin of the isolates (Smith et al. 2000). Recently, a new variant of GBV-C/HGV whose sequences of the 5′ NCR were different from all other known GBV-C/HGV sequences was identified in the province of KwaZulu Natal (KZN), South Africa (Sathar et al. 1999a). Phylogenetic analysis of the E2 gene segment from certain KZN isolates is consistent with previous analysis of the 5′ NCR(Sathar et al. 1999a; Smith et al. 2000), suggesting that these belong to a fifth group (Smith et al. 2000)(Figure 3).The spread of geographically distinct GBV-C/HGV groups has been associated with human migration (Smith et al. 2000).The greater diversity amongst Group 1 African isolates compared to the other major groups, and the confirmation of a fifth group in South Africa (Smith et al. 2000), is consistent with the possibility that GBV-C/HGV may have emerged in Africa (Tanaka et al. 1998) and evolved together with its human host during their prehistoric migration.
Figure 2.
Phylogenetic tree of GBV-C/HGV complete coding region sequences (with permission, Smith et al. 2000).
Figure 3.
South African isolates (KZN) form an additional E2 phylogenetic grouping (Group 5) (with permission, Smith et al. 2000).
Detection of GBV-C/HGV
GBV-C/HGV MA
The reverse-transcription polymerase chain reaction (RT-PCR) is the only diagnostic tool available to detect current GBV-C/HGV infection. Initially, primers from the NS3 region of the genome were used to detect viral RNA (Simons et al. 1995b; Linnen et al. 1996). Degenerate primers to the NS region have also been shown to be sensitive in detecting GBV-C/HGV RNA (Yoshiba et al. 1995). Primers from the NS5A (Linnen et al. 1996) or 5′ UTR (Schlueter et al. 1996) have been shown to be more reliable; the latter region appears to be more sensitive and more widely used in this respect (Kao et al. 1997a). Detection of PCR products can be accomplished by the relatively simple procedure of agarose gel electrophoresis and staining the gel with ethidium bromide, or alternatively, by a single tube assay based on RT-PCR amplification of the 5′ UTR, followed by oligomer hybridization. Detection employs a microparticle immunoassay in the automated LCx system (Marshall et al. 1998). While RT-PCR remains the ‘gold standard’ for detecting GBV-C/HGV, its sensitivity is not fully resolved.
GBV-C/HGV Antibodies
An antibody response to GBV-C/HGV directed against the envelope glycoprotein, E2, has been detected following its expression as a recombinant protein in Chinese hamster ovary cells (Pilot-Matias et al. 1996; Tacke et al. 1997a). The secreted E2 protein has been purified and used in a solid phase enzyme-linked immunosorbent assay (ELISA) for the detection of anti-E2 (Dille et al. 1997). Interestingly, almost all sera positive for anti-E2 are negative for viral RNA, and vice versa, implying that anti-E2 is associated with virus clearance and is perhaps, protective or neutralizing (Pilot-Matias et al. 1996; Tacke et al. 1997a). Testing for anti-E2 greatly extends the ability of RT-PCR to define the epidemiology of GBV-C/HGV (Sathar et al. 1999b). However, the specificity for anti-E2 has not been established.
Routes of GBV-C/HGV transmission
Since the discovery of GBV-C/HGV, attempts have been made to clarify its principle mode of transmission. In non-African countries the predominant route of transmission of GBV-C/HGV is parenteral. High prevalences of GBV-C/HGV have been found in subjects with frequent parenteral exposure and in groups at high risk of exposure to blood and blood products, including intravenous drug abusers (IVDA), patients on maintenance haemodialysis, multitransfused individuals and haemophiliacs. The high prevalence in blood donors worldwide suggests that the principle route of transmission is via contaminated blood and blood products. However, maintenance of the virus at high levels in blood donors and the general population requires an effective nonparenteral route of transmission. Blood donors, however, are not representative of the general population since they are highly selective.
Various studies have pointed to the important role of sexual exposure as a likely route of transmission of GBV-C/HGV, in both non-HIV infected subjects without the risk for parenteral transmission (IVDU and multitransfused individuals including haemophiliacs)(Kao et al. 1997b; Scallan et al. 1998; Sawayama et al. 1999) and HIV-infected individuals with the risk for parenteral and sexual transmission (homosexuals, heterosexuals and prostitutes)(Bourlet et al. 1999; Nubling et al. 1997; Ibanez et al. 1998; Nerurkar et al. 1998). Infection with GBV-C/HGV appears to be more frequent in patients with a sexual risk than those with parenteral exposure (Bourlet et al. 1999; Ibanez et al. 1998). In a study of 600 antenatal patients there was an overall prevalence (GBV-C/HGV RNA and/or Anti-E2 positive) of 11.8%. Since this group represents a young, sexually active population, the authors concluded that sexual or close contact might play a role in the transmission of GBV-C/HGV (Skidmore & Collingham 1999). Rubio et al. (Rubio et al. 1997) reported a GBV-C/HGV prevalence of 21.7% among heterosexual partners of 150 index cases. Stark et al. (Stark et al. 1996) found GBV-C/HGV prevalence of 10.9% among non-drug injecting homosexual and bisexual men. Scallan et al. (Scallan et al. 1998) found a high prevalence of markers for GBV-C/HGV in non-intravenous drug using prostitutes (40%) and male homosexuals (47%). A positive correlation was demonstrated between GBV-C/HGV infection in prostitutes and the number of years of prostitution (Kao et al. 1997b; Sawayama et al. 1999) and the high frequency of paid sex (Wu et al. 1997). The near absence of GBV-C/HGV infection among heterosexual men (4%) and the comparatively higher prevalence among heterosexual women (15%) suggests that, as in HIV infection, the receptive partner is at high risk for acquiring GBV-C/HGV (Nerurkar et al. 1998). Interspousal transmissions of GBV-C/HGV have been reported (Kao et al. 1997c; Sarrazin et al. 1997). Although the role of semen in the transmission of GBV-C/HGV is controversial (Semprini et al. 1997; Hollingsworth et al. 1998), recent reports have suggested that human saliva may contribute to the spread of GBV-C/HGV RNA (Seemayer et al. 1998; Chen et al. 1997). Tucker et al. (Tucker et al. 2000) did not detect GBV-C/HGV replicative intermediaries in the cadaver biopsies of salivary glands and the gonads of GBV-C/HGV positive patients, implying that the virus may be present in the saliva and semen of infected individuals, but not transmitted by these routes. Despite the evidence for increased frequencies of GBV-C/HGV infection in association with sexual exposure, the mechanism of transmission remains unclear.
There is a higher risk of mother to infant transmission in high risk groups (Feucht et al. 1996; Fischler et al. 1997; Viazov et al. 1997; Zanetti et al. 1997; Wejstal et al. 1999). However, it is not clear whether co-infection with HCV, HIV-1 (or both), or IVDU are the underlying cause for transmission of GBV-C/HGV from mother to infant. Nor is it clear as to whether transmission of GBV-C/HGV is influenced by breastfeeding or by the mode of delivery (Viazov et al. 1997; Zanetti et al. 1997; Wejstal et al. 1999). Although the rate of perinatal transmission of GBV-C/HGV exceeds that of HCV, in most studies GBV-C/HGV did not induce liver disease in the infants studied (Wejstal et al. 1999; Zanetti et al. 1997).
Among 220 cases of needle-stick injuries, GBV-C/HGV RNA was detected in 21 (9.5%) donors (Shibuya et al. 1998). At the time of injury none of the 21 recipients were positive for GBV-C/HGV RNA or anti-E2; only 1/14 (7.1%) recipients was positive for GBV-C/HGV RNA which persisted for approximately 3 years without any evidence of liver disease (Shibuya et al. 1998). It has been suggested that iatrogenic infection with GBV-C/HGV could possibly occur through insufficient sterilization of needles and syringes (Ohshima et al. 2000). Confirmation that GBV-C/HGV is indeed an occupational hazard in hospital employees (Gartner et al. 1999; Schaade et al. 2000) will require more comprehensive longitudinal studies.
Prevalence of GBV-C/HGV infection
The prevalences of GBV-C/HGV infection in selected groups of subjects from some published studies are listed in Tables 1–4. The frequency of positivity for RNA or anti-E2 varies among groups, depending on the subjects' origins and the methods used to detect GBV-C/HGV markers. Generally, infection with GBV-C/HGV is significantly associated with a history of IVDA, exposure to blood transfusions, dialysis and with HCV infection. There is a higher prevalence of GBV-C/HGV RNA in blood donors and the general population of African countries (10–19%) compared to non-African countries (1–6%) (Table 1). The high prevalence in commercial blood donors (5–26%) (Table 2) is probably due to the increased risk of parenteral acquisition in this group. The prevalence of GBV-C/HGV anti-E2 antibodies in healthy individuals ranges from 3–15.1% (Table 4).
Table 1.
Reported prevalences of GBV-C/HGV RNA in blood donors in some published studies
| Continent | Country | n | RNA + (%) | References |
|---|---|---|---|---|
| N America | USA | 769 | 13(1.7) | (Linnen et al. 1996) |
| S America | Brazil | 11 | 2(1.8) | (Lampe et al. 1997) |
| Africa | Egypt | 82 | 16(12.2) | (El-Zayadi et al. 1999) |
| South Africa | 248 | 32(12.9) | (Mphahlele et al. 1998) | |
| South Africa | 249 | 26(10.4) | (Tucker et al. 1997) | |
| South Africa | 167 | 21(12.6) | (Lightfoot et al. 1997) | |
| South Africa | 532 | 59(11.1) | (Castelling et al. 1998) | |
| South Africa | 232 | 44(18.9) | (Sathar et al. 1999b) | |
| Caribbean | Martinique | 221 | 9 (4.1) | (Cesaire et al. 1999) |
| Asia | Japan | 448 | 4(0.9) | (Masuko et al. 1996) |
| China | 205 | 2(1) | (Wang et al. 1997b) | |
| Thailand | 69 | 3(4.3) | (Raengsakulrach et al. 1997) | |
| Vietnam | 890 | 11(1.2) | (Kakumu et al. 1998) | |
| Nepal | 181 | 4(2) | (Shrestha et al. 1997) | |
| Mongolia | 121 | 8(6.6) | (Kondo et al. 1997) | |
| Australia | 120 | 5(4) | (Moaven et al. 1996) | |
| Europe | Austria | 92 | 3(3) | (Schlueter et al. 1996) |
| Germany | 1048 | 14(1.34) | (Roth et al. 1997) | |
| Germany | 106 | 59(4.7) | (Heringlake et al. 1996) | |
| UK | 125 | 4(3.2) | (Jarvis et al. 1996) | |
| Italy | 100 | 1(1) | (Fiordalisi et al. 1996) | |
| Spain | 200 | 6(3) | (Saiz et al. 1997) |
Table 4.
Reported prevalences of GBV-C/HGV RNA and Anti-E2 antibodies in some published studies
| Clinical Group | Country | n | RNA+ (%) | Anti-E2+ (%) | Exposure (%) | References |
|---|---|---|---|---|---|---|
| Blood Donors | Japan | 200 | 2(1) | 10(5) | 12(6) | (Tanaka et al. 1998) |
| Germany | 200 | 5(2.5) | 7(9) | 33(16.5) | (Tacke et al. 1997a) | |
| US | 199 | 3(1.5) | 9(4.5) | 11(5.5) | (Gutierrez et al. 1997) | |
| US | 100 | 1(1) | 3(3) | 4(4) | (Dille et al. 1997) | |
| Spain | 200 | 5(2.5) | 28(14) | 32(16) | (Tacke et al. 1997b) | |
| South Africa | 248 | 32(12.9) | 30(12.1) | 52(21.1) | (Mphahlele et al. 1999) | |
| South Africa | 232 | 44(18.9) | 35(15.1) | 74(31.9) | (Sathar et al. 1999b) | |
| Commercial Donors | US | 711 | 93(13.1) | 195(27.4) | 288(40.5) | (Gutierrez et al. 1997) |
| Plasmapheresis Donors | US | 50 | 13(26) | 17(34) | 30(60) | (Dille et al. 1997) |
| West Africa | 30 | 10(33.3) | 4(13.3) | 14(46.7) | (Dille et al. 1997) | |
| IVDU | Germany | 99 | 38(38) | 41(41) | 75(75) | (Tacke et al. 1997a) |
| US | 27 | 1(3.7) | 23(85.2) | 24(88.9) | (Dille et al. 1997) | |
| US | 102 | 15(14.7) | 76 (74.5) | 91(89.2) | (Gutierrez et al. 1997) | |
| Haemophiliacs | Spain | 62 | 22(34%) | 20(32) | 33(53) | (Tacke et al. 1997b) |
| France | 92 | 16(17.4) | 33(35) | 47(51) | (Gerolami et al. 1997) | |
| Haemodialysis | South Africa | 70 | 17(24.3) | 18(25.7) | 33(47.1) | (Sathar et al. 1999b) |
| Renal Transplant | Germany | 221 | 31(14) | 89(40) | 118(53) | (Stark et al. 1997) |
| Chronic liver disease | South Africa | 98 | 12(12.2) | 32(32.7) | 33(47.1) | (Sathar et al. 1999b) |
Table 2.
Reported prevalences of GBV-C/HGV RNA in high risk groups in some published studies
| Clinical group | Country | RNA+No. (%) | References |
|---|---|---|---|
| Haemodialysis | Egypt | 79 (30) | (El-Zayadi et al. 1999) |
| South Africa | 70(24.3) | (Sathar et al. 1999b) | |
| Brazil | 65(15.4) | (Lampe et al. 1997) | |
| China | 79(54) | (Wang et al. 1997) | |
| France | 61(57.5) | (Lamballerie et al. 1996) | |
| Japan | 519(3.1) | (Masuko et al. 1996) | |
| Indonesia | 58(55) | (Tsuda et al. 1996) | |
| Haemophiliacs | Scotland | 95(14) | (Jarvis et al. 1996) |
| Europe | 49(9) | (Linnen et al. 1996) | |
| France | 92(17.4) | (Gerolami et al. 1997) | |
| Japan | 63(24) | (Kinoshita et al. 1997) | |
| Nicaragua | 45(38) | (Gonzales-Prez et al. 1997) | |
| South Africa | 102(23.5) | (Castelling et al. 1998) | |
| IVDUs | Greece | 106(32.1) | (Anastassopoulou et al. 1998) |
| US | 27(4) | (Dille et al. 1997) | |
| US | 102 (14.7) | (Gutierrez et al. 1997) | |
| Sweden | 19(16) | (Shev et al. 1998) | |
| Europe | 60(33.3) | (Linnen et al. 1996) | |
| Germany | 99(38) | (Tacke et al. 1997a) | |
| Japan | 49(12) | (Aikawa 1996) | |
| Commercial blood donors | China | 205(8) | (Roth et al. 1997) |
| US | 50(26) | (Dille et al. 1997) | |
| US | 42(5) | (Pilot-Matias et al. 1996) | |
| US | 711(13.1) | (Gutierrez et al. 1997) | |
| Healthcare workers | Egypt | 30(6,6) | (El-Zayadi et al. 1999) |
| Drug addicts | Nepal | 72(44) | (Shrestha et al. 1997) |
| Prostitutes | UK | 50(18) | (Scallan et al. 1998) |
| China | 140(21) | (Wu et al. 1997) | |
| Homosexuals | UK | 52(17) | (Scallan et al. 1998) |
| Homosexual & bisexual men | Germany | 101(11) | (Schlueter et al. 1996) |
The simultaneous detection of Anti-E2 greatly extends the ability of RT-PCR to define the epidemiology of GBV-C/HGV (Table 4). For example, in non-African countries, 1–2.5% of blood donors is GBV-C/HGV RNA positive. Using Anti-E2 assays, the same population of blood donors showed 3–9% seroprevalence. The overall prevalence of GBV-C/HGV in non-African blood donors was 4–16%, compared to 20–30% in Africa (Table 4). In the high risk group of patients the overall prevalence of GBV-C/HGV infection ranged from 20 to 89% (Table 4). The combined overall prevalence of GBV-C/HGV infection is higher in African countries than in non-African countries (Table 4). The simultaneous detection of GBV-C/HGV RNA and Anti-E2 may represent the seroconversion state.
Thus, the total exposure to GBV-C/HGV should take into account both the number of PCR-positive samples (i.e. viraemic/RNA positive) and anti-E2 positive samples (i.e. previously infected but cleared) in a given population. GBV-C/HGV infection appears to be a common infection globally. The reason for the high prevalence of GBV-C/HGV in blood donors worldwide and the basis for the racial differences in GBV-C/HGV infection in blood donor populations are not known. Whether socio-economic factors are associated with prevalence of GBV-C/HGV is not known for certain, although a relationship was noted between GBV-C/HGV infection and the lack of water-borne sewage (Tucker et al. 1997). The differences in the prevalence of detecting GBV-C/HGV infection (Tables 1–4) may be due to the differences in the sensitivity of the various PCR protocols and primers (derived from various regions of the genome) used by various investigators, and preselection of patients in terms of status for other viral markers as well as different patient histories. Further investigations are required to determine whether genetically distinct isolates from different geographical regions of the world escape detection by current PCR methods and anti-E2 assays.
GBV-C/HGV Anti-E2: A protective/neutralizing antibody and a marker for recovery
Analysis of serial samples for both RNA and anti-E2 suggests that GBV-C/HGV infection follows one of two paths: acute infection followed by recovery (appearance of GBV-C/HGV E2 antibody), or acute infection progressing to chronicity (persistence of GBV-C/HGV RNA). Follow-up of 16 post-transfusion patients for up to 16 years revealed that individuals who develop an anti-E2 response become GBV-C/HGV-RNA negative, while those who do not develop anti-E2 are persistently infected (Tacke et al. 1997b). The presence of anti-E2 and the subsequent loss of viraemia have been confirmed by other investigators (Dille et al. 1997; Gutierrez et al. 1997; Hassoba et al. 1997). Anti-E2 appears to be long-lasting, circulating antibodies and once acquired generally tends to persist (Masuko et al. 1996; Lefrere et al. 1997)
In chronic HCV infection the co-existence of E2/NS1 antibody and viraemia suggests that anti-E2 is not a neutralizing/protective antibody, but serves as a marker of active HCV replication (Yuki et al. 1996). GBV-C/HGV Anti-E2 on the other hand, has been described as a marker of viral clearance (recovery/past) and is considered to be protective against GBV-C/HGV reinfection. In 54 recipients who underwent orthotopic liver transplantation (OLT), the presence of anti-E2 pre-transplant was associated with a relatively low rate (15%) of post-transplantation GBV-C/HGV infection compared to 46% in anti-E2 negative (pre-transplant) patients (Hassoba et al. 1998). Post-transplantation immune suppression apparently had only a minor effect on the prevalence of anti-E2 in patients who were anti-E2 positive prior to transplantation (Hassoba et al. 1998). A negative association between the presence of GBV-C/HGV RNA and the presence of anti-E2 was found in all patients tested pre- and post-transplantation, suggesting viral clearance (Hassoba et al. 1998). Anti-E2 appears to be a neutralizing antibody whose presence at the time of liver transplantation protects against acquisition of GBV-C/HGV infection post-OLT (Bizollon et al. 1998; Hassoba et al. 1998; Silini et al. 1998; Tillmann et al. 1998). No new GBV-C/HGV infections were noted among subjects with anti-E2, despite ongoing drug use (Thomas et al. 1998).
Site(s) of replication
The site of GBV-C/HGV replication has been an area of intense interest and remains uncertain. A true hepatotropic virus replicates in the liver. GBV-C/HGV is a positive-stranded flavivirus whose genomic organization is similar to HCV, as such replication should proceed via a negative-strand RNA intermediate, the detection of which should be possible in the liver. GBV-C/HGV RNA was detected by RT-PCR in washed hepatocytes of 9/58 (15%) children with chronic viral hepatitis (Lopez-Alcorocho et al. 1997). Madejon et al. (Madejon et al. 1997) and Saito et al. (Saito et al. 1997) detected GBV-C/HGV antigenomic RNA in 12/13 livers and peripheral blood mononuclear cells (PBMCs) of one of the same 13 patients examined. Because hepatocytes and PBMCs are bathed in blood, it is possible that the PCR signal noted may be due to cell-bound virus rather than active replication occurring in these cells (Laras et al. 1999). Using RT-PCR with tagged primers and southern blot analysis, antigenomic GBV-C/HGV RNA was detected in 4/6 liver specimens; using in situ hybridization in two such specimens GBV-C/HGV infection was restricted to hepatocytes (Seipp et al. 1999). Hepatotropism of GBV-C/HGV has been demonstrated by in vitro infection of PBMC and cells of human hepatoma cell lines (Ikeda et al. 1997; Fogeda et al. 1999; Seipp et al. 1999). Hepatocytes may not be the only site of viral replication; antigenomic GBV-C/HGV RNA was also detected in the mononuclear cell infiltrates in the portal areas of the liver (Kobayashi et al. 1999). These results suggest that GBV-C/HGV replicates in the liver.
Table 3.
Reported prevalences of GBV-C/HGV RNA in liver diseases in some published studies
| Clinical Group | Country | RNA+ No. (%) | References |
|---|---|---|---|
| Acute/Chronic HBV | Europe | 72(9.7) | (Linnen et al. 1996) |
| Egypt | 63(11,1) | (El-Zayadi et al. 1999) | |
| Japan | 83(4) | (Sugai et al. 1997) | |
| US | 100(32) | (Alter et al. 1997a) | |
| South Africa | 106(26.4) | (Mphahlele et al. 1998) | |
| Acute/Chronic HCV | Egypt | 100(14) | (El-Zayadi et al. 1999) |
| Germany | 100(9) | (Schleicher et al. 1996) | |
| Italy | 83(26.5) | (Francesconi 1997) | |
| Japan | 88(8) | (Sugai et al. 1997) | |
| Russia | 22(41) | (Yashina et al. 1997) | |
| Spain | 143(5.6) | (Saiz et al. 1997) | |
| Taiwan | 52(10) | (Hwang et al. 1997) | |
| US | 116(20) | (Alter et al. 1997a) | |
| South Africa | 82(30.5) | (Mphahlele et al. 1998) | |
| Acute/Chronic HAV | Japan | 21(0) | (Nakatsuji et al. 1996) |
| US | 100(25) | (Alter et al. 1997a) | |
| Non A-E hepatitis | China | 108(16.7) | (Wang & Jin 1997) |
| Japan | 43(0) | (Nakatsuji et al. 1996) | |
| Russia | 28(3.6) | (Yashina et al. 1997) | |
| US | 149(8.7) | (Dawson et al. 1996) | |
| Chronic Liver Disease | Indonesia | 149(5) | (Tsuda et al. 1996) |
| Nepal | 145(3) | (Shrestha et al. 1997) | |
| South Africa | 92(12) | (Sathar et al. 1999b) | |
| Japan | 226(7.5) | (Nakatsuji et al. 1996) | |
| US | 326(12.2) | (Linnen et al. 1996) | |
| Italy | 36(39) | (Fiordalisi et al. 1996) | |
| Hepatocellular Carcinoma | Japan | 111(10) | (Kanda et al. 1997) |
| Japan | 109(10) | (Nishiyama et al. 1999) | |
| Thailand | 101(6) | (Tangkijvanich et al. 1999) | |
| China | 114(14,9) | (Cao et al. 1998) | |
| Europe | 57(7) | (Brechot et al. 1998) | |
| South Africa | 135(14) | (Lightfoot et al. 1997) | |
| Fulminant hepatitis | Japan | 6(50) | (Yoshiba et al. 1995) |
| Japan | 10(0) | (Kanda et al. 1997) | |
| Germany | 22(50) | (Heringlake et al. 1996) | |
| UK | 23(21.7) | (Haydon et al. 1997) | |
| UK | 20(0) | (Sallie et al. 1996) | |
| United States | 36(38.8) | (Munoz et al. 1999) | |
| Taiwan | 32(9) | (Liu et al. 1999) |
Using RT-PCR with tagged primers and in vitro derived templates, Mellor et al. (Mellor et al. 1998) were unable to detect antigenomic GBV-C/HGV RNA in either liver biopsies or in the PBMCs of 20 GBV-C/HGV infected individuals. Radkowski et al. suggested that PBMCs may not be the replication site of GBV-C/HGV (Radkowski et al. 1998). In 5/17 patients undergoing liver transplantation, GBV-C/HGV RNA was detected in sera and not in the liver on repeated testing for viral RNA from different portions of the liver (Fan et al. 1999). In patients co-infected with GBV-C/HGV and HCV, the hepatotropism of HCV and not GBV-C/HGV was consistently proven (Kudo et al. 1997; Laskus et al. 1997; Pessoa et al. 1998). These findings suggest that GBV-C/HGV is not a hepatotropic virus and that neither the liver nor PBMCs may be the actual site of GBV-C/HGV replication.
In their study of six cadaver biopsies from one GBV-C/HGV positive patient co-infected with HIV, Mushahwar et al. (Mushahwar et al. 1998) detected glyceraldehyde-3-phosphate dehydrogenase (GAP-DH) mRNA and GBV-C/HGV RNA only in the liver, which was localized to individual hepatocytes. In multiple cadaver autopsies of 12 patients (four with AIDS, six HIV positive and two with end-stage liver disease) (Laskus et al. 1998; Radkowski et al. 1999), GBV-C/HGV RNA intermediaries were consistently demonstrated in the bone marrow and spleen. However, these results are difficult to interpret in immunocompromised patients. In a preliminary study of 23 cadaver biopsies from four GBV-C/HGV positive patients who were HIV negative, the spleen and bone marrow biopsies were uniformly positive for both negative- and positive-strand GBV-C/HGV RNA (Tucker et al. 2000). The authors (Tucker et al. 2000) concluded that GBV-C/HGV is a lymphotropic virus that replicates primarily in the spleen and bone marrow. These findings require confirmation using in situ hybridization and immunohistochemical staining.
Strand-specific detection of RNA is fraught with problems such as false priming of the incorrect strands or self-priming related to RNA secondary structure. All of the strand-specific studies used methods to reduce false-priming and self-priming events viz. chemical modification of the 3′ ends (Madejon et al. 1997; Saito et al. 1997); conducting cDNA synthesis at high temperature with the thermostable enzyme (Tth) (Laskus et al. 1997; Tucker et al. 2000); using in vitro derived templates (Laskus et al. 1997; Mellor et al. 1998;Tucker et al. 2000); using ‘tagged’ primers (Mellor et al. 1998; Seipp et al. 1999); in situ hybridization of liver biopsies (Kobayashi et al. 1999; Seipp et al. 1999) and in vitro infection of human hepatoma cells with GBV-C/HGV monoinfected serum (Seipp et al. 1999). Only Laskus et al. (Laskus et al. 1997) and Mellor et al. (Mellor et al. 1998) qualified their reactions using in vitro derived templates and provided end point titration data.
GBV-C/HGV infection and liver disease
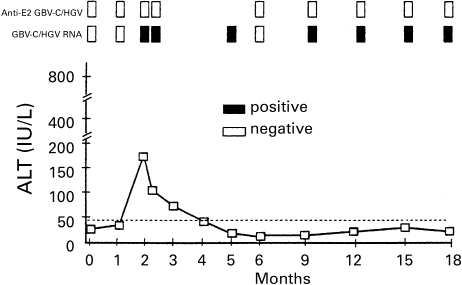
Most GBV-C/HGV infections appear to be asymptomatic, transient, and self-limiting, with slight or no elevation of alanine aminotransferase (ALT) (Alter et al. 1997a; Alter et al. 1997b). Co-infection with GBV-C/HGV does not alter the clinical course of community-acquired hepatitis A, B or C (Alter et al. 1997a; Alter et al. 1997b). Most of these subclinical cases resolve after loss of serum GBV-C/HGV RNA with a concomitant appearance of anti-E2 (Dille et al. 1997; Gutierrez et al. 1997). Figure 4 depicts a typical clinical picture of a patient with acute post-transfusion GBV-C/HGV infection. To evaluate the clinical course of GBV-C/HGV infection, patients who were infected with GBV-C/HGV only were studied by Wang et al. (Wang et al. 1996). Among 25 such patients who acquired GBV-C/HGV infection by transfusion, 20 patients who were followed up at 2–4 week intervals over six months maintained normal ALT activities. The other five patients showed only moderate elevations in ALT (< 124 IU/l) over the first six months, with no further elevations in the subsequent follow-up period of two years. In these five patients, there were no other clinical signs of liver disease. Jaundice was absent in the 25 patients, whereas it was present in two out of the seven patients with HCV co-infection (Wang et al. 1996). GBV-C/HGV is capable of inducing persistent infection in about 5–10% of infected individuals. Masuko et al. (Masuko et al. 1996) retrospectively followed eight haemodialysis patients with GBV-C/HGV infection for 7–16 years. In two patients, the virus was present at the start of haemodialysis. One had a history of transfusion, and GBV-C/HGV RNA persisted over a period of 16 years, the other cleared GBV-C/HGV RNA after 10 years. In five patients, GBV-C/HGV RNA was first detected 3–20 weeks after blood transfusion and persisted for up to 13 years. No elevations in serum ALT or signs of active liver disease were found in these patients. It would appear that in many patients infected with GBV-C/HGV, virus replication could occur without detectable damage to the liver. GBV-C/HGV transmission to chimpanzees and tamarins resulted in infection without elevation in ALT (Bukh et al. 1998). On the contrary, GBV-C/HGV infection in macaques (Cheng et al. 2000) produced mildly elevated ALT levels with mild hepatitis and positive antigenic expression in hepatocytes, suggesting that GBV-C/HGV may be pathogenic to primates.
Figure 4.
Clinical picture of a patient with acute post-transfusion GBV-C/HGV infection (with permission, Hwang et al. 1999).
A strong association between GBV-C/HGV and fulminant hepatitis has been suggested (Yoshiba et al. 1995; Heringlake et al. 1996) which may be associated with a specific strain of GBV-C/HGV (Heringlake et al. 1996). However, these studies did not clearly define whether GBV-C/HGV was transmitted by the transfusions they received prior to the onset of fulminant hepatitis. Additional studies by Yoshiba et al. (Yoshiba et al. 1996) showed that only a few of the fulminant hepatitis patients studied had received a blood transfusion prior to the onset of fulminant hepatitis. In a similar study, GBV-C/HGV RNA was detected in 3/15 (20%) patients with HBV infection and in 3/25 (12%) patients without markers of hepatitis A–E infection (Tameda et al. 1996). Of the six patients with GBV-C/HGV RNA, only three had a history of transfusion and all of these patients were co-infected with HBV. According to Tameda et al. (Tameda et al. 1996) these results indicate a role of GBV-C/HGV in inducing fulminant hepatitis either by itself or in concert with other hepatitis viruses. Using real time detection polymerase chain reaction (RTD-PCR), GBV-C/HGV RNA was measured serially in the sera of three Japanese patients with non A–E fulminant hepatitis, none of whom received any therapeutic transfusions before admission (Inoue et al. 1999). Serum ALT levels paralleled GBV-C/HG RNA in all three cases and sequence analysis revealed that the same GBV-C/HGV strain infected the patients during their entire clinical course, despite plasma exchange therapy. In one patient, hepatocyte destruction continued with persistent viraemia, although ALT levels decreased. The authors (Inoue et al. 1999) concluded that their assumption of an association of GBV-C/HGV with fulminant hepatitis in these three patients was further strengthened by the disappearance or persistence of GBV-C/HGV RNA in serum which appeared to be linked to the prognosis. However, several other studies have provided no evidence of an association of GBV-C/HGV with fulminant hepatitis (Hadziyannis 1997). The discrepancies in the association of GBV-C/HGV with fulminant hepatitis may be influenced by the sensitivity of the detection system and the differences in the GBV-C/HGV infection rates in the different populations studied. The role of GBV-C/HGV in the aetiology of fulminant hepatitis remains controversial.
Some investigators have reported histological features in liver biopsies of GBV-C/HGV-infected individuals. Among six chronic hepatitis patients with GBV-C/HGV RNA only, the histology of the liver samples revealed chronic active hepatitis in one patient and chronic persistent hepatitis in five others (Fiordalisi et al. 1996). All patients with chronic hepatitis had elevated ALT levels between 89 and 478 U/l. In contrast, among the 11 acute hepatitis cases positive for GBV-C/HGV RNA, the ALT levels varied between 615 and 2477 U/l. Colombatto et al. (Colombatto et al. 1997) studied GBV-C/HGV in 67 patients with liver disease without any markers for hepatitis A–E. They reported an association between nonspecific inflammatory bile duct lesions and elevated cholestatic enzymes (gamma glutamyl transpeptidase and alkaline phosphatase) in 50% of patients. Ross et al. (Ross et al. 1997) showed that GBV-C/HGV infection might affect the clinical course and outcome after orthotopic liver transplantation (OLT) by the development of severe cholestasis, which could result from bile duct damage and bile duct loss. In a preliminary study, an association between recurrent or de novo GBV-C/HGV infection and severe post-transplant cholestasis and ductopenia was also observed in the grafts of GBV-C/HGV-positive liver organ transplant patients (Dhillon et al. 1996). However, in many studies no correlation between GBV-C/HGV infection and elevation of cholestatic enzymes were noted. Further investigations are needed to substantiate these findings.
Manolakopoulos et al. (Manolakopoulos et al. 1998) found an association between GBV-C/HGV and HCV-viremia and portal and periportal inflammation. They reported that the duration of HCV/GBV-C/HGV co-infection may be an important factor in the progression of liver disease and that inflammation with necrosis in the portal and periportal tracts was significantly higher in patients with combined viremia compared to those with HCV infection alone. The authors suggested that GBV-C/HGV in patients with HCV infection might accelerate liver injury toward more severe fibrosis in patients with dual infection. Diamantis et al. (Diamantis et al. 1997) reported that mild fibrosis correlated with GBV-C/HGV whilst Francesconi et al. (Francesconi et al. 1997) observed subtleties in histological appearance in HCV co-infected patients. However, numerous studies have shown that in HCV co-infected individuals, GBV-C/HGV does not affect HCV replication, HCV RNA concentration, and liver disease (Tanaka et al. 1996; Bralet et al. 1997; Enomoto et al. 1998; Pawlotsky et al. 1998; Petrik et al. 1998; Slimane et al. 2000).
During OLT, pre-transplant GBV-C/HGV has been reported to be associated with post-transplant viraemia (Fried et al. 1997; Feucht et al. 1997; Haagsma et al. 1997). In the absence of HBV or HCV in liver transplant recipients, the prevalence of GBV-C/HGV infection has no influence on the graft (Haagsma et al. 1997). Berg et al. (Berg et al. 1996) found a significantly higher percentage of hepatocellular carcinoma in patients with pre-OLT GBV-C/HGV co-infection compared with patients with HCV infection alone (5/6 vs. 16/68; P < 0.01). Bizollon et al. (Bizollon et al. 1998), on the other hand, showed that the prevalence of hepatocellular carcinoma was not different in patients with pre-transplantation GBV-C/HGV co-infection or with HCV infection. In addition, GBV-C/HGV co-infection did not seem to have a significant impact on the course of HCV infection after transplantation.
Hepatocarcinogenecity of GBV-C/HGV
GBV-C/HGV RNA was detected in 11/111 (10%) of cases of hepatocellular carcinoma (HCC) (Kanda et al. 1997). The authors concluded that GBV-C/HGV was unlikely to be a major aetiological agent of non-B non-C HCC. In a large series of 503 patients with HCC in Europe, Brechot et al. (Brechot et al. 1998) demonstrated a major impact of HBV (19% positive) and HCV (40%) but not GBV-C/HGV (7%) in HCC. In a study of 167 Black South Africans with HCC and 167 matched controls, Lightfoot et al. (Lightfoot et al. 1997) showed that patients infected with GBV-C/HGV did not have an increased relative risk of developing HCC. In addition, co-infection with GBV-C/HGV did not further increase the risk of HCC in patients chronically infected with HBV and HCV. In a retrospective study of GBV-C/HGV in formalin-fixed, paraffin-embedded (FFPE) tissues of HCC patients from various geographical areas (Japan, Spain, Korea, United States, Japanese Americans in Hawaii), GBV-C/HGV was neither detected nor was there any evidence of any association of GBV-C/HGV with HCC (Abe et al. 1998). In this study HCV genotype II/1b and HBV were significantly associated with HCC.
In a population-based study of non-Asian patients with HCC and community controls in Los Angeles, California, Yuan et al. concluded that GBV-C/HGV infection may account for approximately 8% of HCC (Yuan et al. 1999). GBV-C/HGV RNA was detected in 12/144 (8.3%) non-Asian patients with HCC and 5/225 (2%) community controls. The presence of GBV-C/HGV RNA was associated with a statistically significant 5.4 fold risk, which was independent of the effects of HBV and HCV infections (Yuan et al. 1999). In a hospital-based case-controlled study the relative risk factor suggested a fair association between GBV-C/HGV infection and HCC (Tagger et al. 1997). However, GBV-C/HGV did not seem to be a major aetiological agent of HCC because the population-attributable risk was lower (4%) than those for HbsAg (52%), HCV RNA (36%) and excessive alcohol intake (52%) (Tagger et al. 1997). Among subjects with GBV-C/HGV exposure (RNA and anti-E2 positive) a greater proportion of cases (40%) than controls (14%) had a transfusion history (Tagger et al. 1997). Hepatocarcinogenicity of GBV-C/HGV is an important key question that remains controversial.
Extrahepatic manifestations of GBV-C/HG infection
Hepatitis-associated aplastic anaemia is a rare but well-documented phenomenon, unlikely to be caused by any of the known hepatitis viruses (Byrnes et al. 1996; Brown et al. 1997a). In some cases of hepatitis-associated aplastic anaemia, GBV-C/HGV was the only aetiological agent detected, even if the patients had not received any transfusions before diagnosis (Crespo et al. 1999; Kiem et al. 1997; Zaidi et al. 1996). Moriyama et al. (Moriyama et al. 1997) detected GBV-C/HGV RNA in 5/18 (27.7%) patients with aplastic anaemia who received blood transfusions before diagnosis but not in eight patients who did not receive transfusions. Similarly, Brown et al. (Brown et al. 1997b) detected GBV-C/HGV RNA in 26.3% and 23.1% of patients with aplastic anaemia and multi-transfused control patients, respectively. Kiem et al. (Kiem et al. 1997) detected GBV-C/HGV RNA in 26.1% of patients with hepatitis-associated aplastic anaemia and idiopathic aplastic anaemia who did not receive transfusions. The authors concluded that although transfusions are a major source of GBV-C/HGV infection, the high prevalence in those who did not receive transfusions suggests an association of GBV-C/HGV with aplastic anaemia, whether associated with hepatitis or not. Further studies in serial serum samples and meticulous evaluation of the disorders associated with the infection will be needed to prove or disprove a causal association of GBV-C/HGV and aplastic anaemia.
It is interesting to note that GBV-C/HGV replication has been consistently shown in bone marrow and spleen, and not in the lymph nodes and tonsils (Laskus et al. 1998; Radkowski et al. 1999; Tucker et al. 2000), suggesting a haematological cell tropism. Because the genomic organization, structural and biological characteristics of GBV-C/HGV are similar to that of HCV, GBV-C/HGV has been investigated as a possible aetiological agent in the development of haematological disorders. Ongoing GBV-C/HGV infection was detected in 29 of 60 (48%) multi-transfused patients with haematological malignancies (Skidmore et al. 1997). GBV-C/HGV prevalence in patients with B-cell non-Hodgkin's lymphoma was significantly higher than in healthy controls (Zignego et al. 1997; Ellenrieder et al. 1998). All patients were asymptomatic and without clinical or sonographic signs of chronic liver disease (Ellenrieder et al. 1998). GBV-C/HGV prevalence in lymphoma or cryoglobulinemia patients do not support the hypothesis that this virus also may play a major role in lymphomagenesis or in the production of mixed cryoglobulinemia (Cacoub et al. 1997; Nakamura et al. 1997; Ellenrieder et al. 1998). Pavlova et al. (Pavlova et al. 1999) investigated two groups of patients, one with clonal stem cell disease with long latency period (myelodysplasia, myeloproliferative disease) and one with malignant haematological diseases (Hodgkin's lymphoma, non-Hodgkin's lymphoma, acute leukaemia, multiple myeloma). The prevalence of GBV-C/HGV RNA in the group of oncological cases (72%) was significantly higher (P = 0.02) than in the patients with clonal stem cell diseases (28%). A correlation could not be confirmed between GBV-C/HGV and liver enzyme levels, blood transfusions, chemotherapy, or viral co-infection (Pavlova et al. 1999). GBV-C/HGV infection in these patients is most likely to have originated from exposure to blood products, and to persist because of deficient immune surveillance. However, the clinical significance of these findings with respect to liver dysfunction is not yet clear. The pathogenetic consequences of GBV-C/HGV infection in lymphoproliferative disorders must be conclusively proven in additional studies.
Viral infections are presumed to trigger auto-immune processes. Heringlake et al. (Heringlake et al. 1996) observed that the prevalence of GBV-C/HGV in autoimmune hepatitis (AIH) type I-III was higher (9.8%) than in blood donors (4.7%). In contrast, patients with viral hepatitis B, C, and D were more frequently infected with GBV-C/HGV (16%, 20%, 36%, respectively). In contrast, Tribl et al. (Tribl et al. 1999) found a significantly increased prevalence of GBV-C/HGV in patients with AIH (11%), HBV (16%), and HCV (21%) than in healthy controls (2%). However, it remains unclear whether infection with GBV-C/HGV has an impact on the course of disease in patients with AIH. Persistent GBV-C/HGV RNA detected in 7/36 (19.4%) thalassemic patients was not associated with significant biochemical evidence of liver damage (Zemel et al. 1998). Patients with common variable immunodeficiency (CVID) are prone to unexplained chronic hepatitis whilst patients with X-linked agammaglobulinemia (XLA) who have a similar primary antibody deficiency are not prone to hepatitis (Morris et al. 1998). In their study of 78 CVID and 28 XLA patients, Morris et al. (Morris et al. 1998) concluded that the high prevalence of GBV-C/HGV viremia is due to the long-term exposure to blood products and that GBV-C/HGV does not cause chronic hepatitis in immunocompromised XLA patients. In addition, the authors suggested that, in the majority of CVID patients, GBV-C/HGV is not the cause of chronic non-B or -C hepatitis. In Japanese leprous patients the prevalence of GBV-C/HGV was higher (5.2%) than in blood donors (1%) (Egawa et al. 1996).Tucker et al. (Tucker et al. 1998) suggested an association with glomerulonephritis, hinting that virus replication may occur in the kidney.
Interferon (IFN) treatment of GBV-C/HGV infection
There are conflicting reports concerning the sensitivity of GBV-C/HGV to interferon (IFN) therapy. In some studies it seems to be similar to HCV (Berg et al. 1996; Tanaka et al. 1996; Orito et al. 1997; Jarvis et al. 1999), but in others it appears to be independent (McHutchison et al. 1997; Nagayama et al. 1997; Umlauft et al. 1997). However, the response may be different (Saiz et al. 1997). During IFN-α therapy, serum GBV-C/HGV RNA levels decrease in most patients treated, and it may become undetectable (Tanaka et al. 1996; Martinot et al. 1997; Nagayama et al. 1997; Saiz et al. 1997; Brandhagen et al. 1999; Jarvis et al. 1999). In only a small percentage of patients the response is sustained, and in most cases the GBV-C/HGV RNA concentration returned to pre-treatment levels after therapy was stopped (Berg et al. 1996; Tanaka et al. 1996; Karayiannis et al. 1997; Martinot et al. 1997; Saiz et al. 1997; Pawlotsky et al. 1998). Genotype, viral load, IFN dose, and the amino acid substitutions in the NS5A region (designated as the interferon sensitivity determining region (ISDR)) are considered to be some of the predictors for the efficacy of IFN therapy on HCV (Shiratori et al. 1997). However, most researchers detect no influence of GBV-C/HGV infection in response to IFN-α in patients with chronic HCV (Tanaka et al. 1996; Martinot et al. 1997; Orito et al. 1997; Saiz et al. 1997; Kato et al. 1999). No correlation between the amino acid sequence in the GBV-C/HGV NS5A region and response to IFN therapy was found, indicating that the GBV-C/HGV NS5A region does not act as the ISDR (Kato et al. 1999). A sustained response is predictable in patients with a low pre-treatment GBV-C/HGV viral load (Nagayama et al. 1997; Orito et al. 1997; Saiz et al. 1997; Enomoto et al. 1998; Jarvis et al. 1999).
Conclusions
GBV-C and HGV are closely related Flaviviruses of human origin. The detection methods for GBV-C/HGV need to be standardized and subjected to repeated quality control studies. GBV-C/HGV has a relatively high prevalence in the general population and a higher prevalence in certain high-risk groups. Transmission by blood transfusions, parenterally, sexually and from infected mothers to their new-born infants has been documented, and can induce persistent viraemia in humans. Most studies on GBV-C/HGV tropism have been limited to PBMCs and liver biopsies. Clearly, additional tissues from different organs of non-immunocompromised patients need to be studied using highly specific techniques to resolve the site(s) of replication for GBV-C/HGV. The role of GBV-C/HGV in human pathology needs to be examined more thoroughly in the absence of co-infection with other viruses. More information about its immune responses against the viral antigens and the virus pathogenicity is needed to better understand the clinical significance of GBV-C/HGV. However, some findings suggest that GBV-C/HGV is involved with some cases of acute and chronic hepatitis; that GBV-C/HGV may be pathogenic to primates considered to be appropriate non-human hosts for viral hepatitis studies; and that GBV-C/HGV does indeed replicate in human liver. It would be premature to screen blood donors for GBV-C/HGV and exclude a large proportion of blood donors from the donation pool without solid evidence that GBV-C/HGV is indeed pathogenic to humans.
Current evidence suggests that other viral agents or other factors may be responsible for a large majority of post-transfusion or community-acquired non-A–E hepatitis. A new pathogen, namely, Transfusion Transmissible Virus (TTV) has become a new focus of viral hepatitis research. This DNA non-enveloped virus has numerous similarities to GBV-C/HGV. Several independent studies have cast doubts on the pathogenicity of TTV. More recently, a novel hepatitis virus code named SEN-V has been isolated. SEN-V is considered by some leading researchers in hepatitis, to be the ‘best candidate virus to account for previously unexplained hepatitis.’ However, no data on SEN-V (The New York Times, 20th July 1999) has been presented in peer-reviewed publications.
Acknowledgments
M. A. Sathar was the recipient of the International Journal of Experimental Pathology, the SAGES-Roussell and SAGES-Abbott Overseas Research Fellowships.
References
- Abe K, Edamoto Y, Park YN, et al. In situ detection of hepatitis B, C, and G virus nucleic acids in human hepatocellular carcinoma tissues from different geographic regions. Hepatol. 1998;28:568–572. doi: 10.1002/hep.510280239. [DOI] [PubMed] [Google Scholar]
- Aikawa T, Sugai Y, Okamoto H. Hepatitis C infection in drug abusers with chronic hepatitis C. N Engl J Med. 1996;334:193–194. doi: 10.1056/NEJM199601183340316. [DOI] [PubMed] [Google Scholar]
- Alter HJ, Nakatsuji Y, Melpolder J, et al. The incidence of transfusion-associated hepatitis G virus infection and its relation to liver disease. N Engl J Med. 1997a;336:747–754. doi: 10.1056/NEJM199703133361102. [DOI] [PubMed] [Google Scholar]
- Alter MJ, Gallagher M, Morris TY, et al. for the Sentinel Counties Viral Hepatitis Study Team. Acute non-A-E Hepatitis in the United States and the role of Hepatitis G Virus infection. N Eng J Med. 1997b;336:741–746. doi: 10.1056/NEJM199703133361101. [DOI] [PubMed] [Google Scholar]
- Anastassopoulou CG, Paraskevis D, Sypsa V, et al. Prevalence patterns and genotypes of GB Virus C/Hepatitis G Virus among imprisoned intravenous drug users. J Med Virol. 1998;56:246–252. 10.1002/(sici)1096-9071(199811)56:3<246::aid-jmv12>3.3.co;2-b. [PubMed] [Google Scholar]
- Berg T, Dirla U, Naumann U, et al. Responsiveness to interferon alpha treatment in patients with chronic hepatitis C coinfected with hepatitis G virus. J Hepatol. 1996;25:763–768. doi: 10.1016/s0168-8278(96)80250-9. [DOI] [PubMed] [Google Scholar]
- Birkenmeyer LG, Desai SM, Muerhoff AS, et al. Isolation of a GB virus-related genome from a chimpanzee. J Med Virol. 1998;56:44–51. doi: 10.1002/(sici)1096-9071(199809)56:1<44::aid-jmv8>3.0.co;2-n. 10.1002/(sici)1096-9071(199809)56:1<44::aid-jmv8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Bizollon T, Guichard S, Ahmed SAS, et al. Impact of hepatitis G virus on the course of hepatitis C virus infection before and after liver transplantation. J Hepatol. 1998;29:893–900. doi: 10.1016/s0168-8278(98)80116-5. [DOI] [PubMed] [Google Scholar]
- Bourlet T, Guglielminotti C, Evrard M, et al. Prevalence of GBV-C/Hepatitis G Virus RNA and E2 antibody among subjects infected with human immunodeficiency virus Type 1 after parenteral or sexual exposure. J Med Virol. 1999;58:373–377. 10.1002/(sici)1096-9071(199908)58:4<373::aid-jmv9>3.3.co;2-t. [PubMed] [Google Scholar]
- Bralet M-P, Roudot-Thoraval F, Pawlotsky J-M, et al. Histopathologic impact of GB virus C infection on chronic hepatitis C. Gastroenterol. 1997;112:188–192. doi: 10.1016/s0016-5085(97)70234-8. [DOI] [PubMed] [Google Scholar]
- Brandhagen DJ, Gross Jr JB, Poterucha JJ. The clinical significance of simultaneous infection with hepatitis G virus in patients with chronic hepatitis C. Am J Gastroenterol. 1999;94:1000–1005. doi: 10.1111/j.1572-0241.1999.01003.x. [DOI] [PubMed] [Google Scholar]
- Brechot C, Jaffedo F, Logorce D, et al. Impact of HBV, HCV and GBV-C/HGV on hepatocellular carcinomas in Europe: Results of a European concerted study. J Hepatol. 1998;29:173–183. doi: 10.1016/s0168-8278(98)80001-9. [DOI] [PubMed] [Google Scholar]
- Brown KE, Tisdale J, Barrett J, Dunbar CE, Young NS. Hepatitis-associated aplastic anaemia. N Eng J Med. 1997a;336:1059–1064. doi: 10.1056/NEJM199704103361504. [DOI] [PubMed] [Google Scholar]
- Brown KE, Wong S, Young NS. Prevalence of GBV-C/HGV, a novel ‘hepatitis’ virus in patients with aplastic anaemia. Br J Haematol. 1997b;97:492–496. doi: 10.1046/j.1365-2141.1997.822722.x. [DOI] [PubMed] [Google Scholar]
- Bukh J, Kim JP, Govindarajan S, et al. Experimental infection of chimpanzees with hepatitis G virus and genetic analysis of the virus. J Infect Dis. 1998;177:855–862. doi: 10.1086/515255. [DOI] [PubMed] [Google Scholar]
- Byrnes JJ, Banks AT, Piatack M, Kim JP. Hepatitis G associated with aplastic anaemia. Lancet. 1996;348:472.. doi: 10.1016/S0140-6736(05)64562-X. [DOI] [PubMed] [Google Scholar]
- Cacoub P, Frangeul L, Musset L. Hepatitis G and mixed cryoglobulinemia. Ann Intern Med. 1997;126:1022.. doi: 10.7326/0003-4819-126-12-199706150-00021. [DOI] [PubMed] [Google Scholar]
- Cao K, Mizokami M, Orito E, et al. GB virus C/hepatitis G virus infection among patients with hepatocellular carcinoma in the inshore area of the Yangtze River, China. J Gastroenterol Hepatol. 1998;13:1241–1248. [PubMed] [Google Scholar]
- Castelling A, Song E, Sim J, et al. GB Virus C prevalence in blood donors and high risk groups for parenterally transmitted agents from Gauteng, in South Africa. JMed Virol. 1998;5:103–108. doi: 10.1002/(sici)1096-9071(199806)55:2<103::aid-jmv4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Cesaire R, Martial J, Maier H, et al. Infection with GB virus C/hepatitis G virus among blood donors and hemophiliacs in Martinique, a Caribbean island. J Med Virol. 1999;59:160–163. 10.1002/(sici)1096-9071(199910)59:2<160::aid-jmv6>3.0.co;2-y. [PubMed] [Google Scholar]
- Chen M, Sonnerborg A, Johansson B, Sallberg M. Detection of hepatitis G virus (GB virus C) RNA in human saliva. J Clin Microbiol. 1997;35:973–975. doi: 10.1128/jcm.35.4.973-975.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Zhang W-Z, Li J, et al. Serological and histological findings in infection and transmission of GBV-C/HGV to Macaques. J Med Virol. 2000;60:28–33. doi: 10.1002/(sici)1096-9071(200001)60:1<28::aid-jmv5>3.0.co;2-s. 10.1002/(sici)1096-9071(200001)60:1<28::aid-jmv5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Colombatto P, Randone A, Civitico G, et al. A new hepatitis C virus-like flavivirus in patients with cryptogenic liver disease associated with elevated GGT and alkaline phosphatase serum levels. J Viral Hepat. 1997;4:55–60. doi: 10.1111/j.1365-2893.1997.tb00161.x. [DOI] [PubMed] [Google Scholar]
- Crespo J, de las Heras B, Rvero M, Lozano JL, Fabrega E, Pons-Romero F. Hepatitis G virus infection as a possible causative agent of community-acquired hepatitis and associated aplastic anaemia. Postgrad Med J. 1999;75:159–160. doi: 10.1136/pgmj.75.881.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson GJ, Schlauder GG, Pilot-Matias TJ, et al. Prevalence studies of GB Virus C infection using reverse transcriptase-polymerase chain reaction. JMed Virol. 1996;50:97–103. doi: 10.1002/(SICI)1096-9071(199609)50:1<97::AID-JMV16>3.0.CO;2-V. 10.1002/(sici)1096-9071(199609)50:1<97::aid-jmv16>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Deinhardt F, Holmes AW, Capps RB, Popper H. Studies on the transmission of human viral hepatitis to marmoset monkeys. Transmission of disease, serial passage and description of liver lesions. J Exp Med. 1967;125:673–687. doi: 10.1084/jem.125.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon AP, Savage K, Kim JP, et al. Histopathology of hepatitis G virus infection. IX Triennial International Symposium on Viral Hepatitis and Liver Disease, Rome (Abstract)
- Diamantis ID, Kouroumalis E, Koulentaki M, et al. Influence of HGV infection on liver disease. Eur J Clin Microbiol Infect Dis. 1997;16:916–919. doi: 10.1007/BF01700559. [DOI] [PubMed] [Google Scholar]
- Dille BJ, Surowy TK, Gutierrez RA, et al. An ELISA for detection of antibodies to the E2 protein of GB Virus C. JInfect Dis. 1997;175:458–461. doi: 10.1093/infdis/175.2.458. [DOI] [PubMed] [Google Scholar]
- Egawa K, Yukawa T, Arakawa S, et al. Infection with GB Virus C in leprous patients in Japan. J Med Virol. 1996;49:110–114. doi: 10.1002/(SICI)1096-9071(199606)49:2<110::AID-JMV7>3.0.CO;2-8. 10.1002/(sici)1096-9071(199606)49:2<110::aid-jmv7>3.3.co;2-s. [DOI] [PubMed] [Google Scholar]
- El-Zayadi AR, Selim O, Naito H, Hess G, Ahdy A. Prevalence of GBV-C/hepatitis G virus viraemia among blood donors, health care personnel, chronic non-B non-C hepatitis, chronic hepatitis C and hemodialysis patients in Egypt. JVirol Meth. 1999;80:53–58. doi: 10.1016/s0166-0934(99)00036-1. [DOI] [PubMed] [Google Scholar]
- Ellenrieder V, Weidenbach H, Frickhofen N, et al. HCV and HGV in B-cell non-Hodgkin's lymphoma. JHepatol. 1998;28:34–39. doi: 10.1016/s0168-8278(98)80199-2. [DOI] [PubMed] [Google Scholar]
- Enomoto M, Nishiguchi S, Fukuda K, et al. Characteristics of patients with Hepatitis C Virus with and without GB Virus C/Hepatitis G Virus co-infection and efficacy of interferon Alfa. Hepatol. 1998;27:1388–1393. doi: 10.1002/hep.510270528. [DOI] [PubMed] [Google Scholar]
- Fan X, Xu Y, Solomon H, Ramrakhiani S, Neuschwander-Tetri BA, Di Bisceglie AM. Is hepatitis G/GB virus-C virus hepatotropic? Detection of hepatitis G/GB virus-C viral RNA in liver and serum. JMed Virol. 1999;58:160–164. 10.1002/(sici)1096-9071(199906)58:2<160::aid-jmv10>3.3.co;2-0. [PubMed] [Google Scholar]
- Feucht H-H, Fischer L, Sterneck M, Knodler B, Broelsch CE, Laufs R. GB virus C infection and liver transplantation: increased risk of transfusion-transmitted infection. Blood. 1997;63:1695–1697. [PubMed] [Google Scholar]
- Feucht HH, Zollner B, Polywka S, Laufs R. Vertical transmission of hepatitis G. Lancet. 1996;347:615. [PubMed] [Google Scholar]
- Fiordalisi G, Zanella I, Mantero G, et al. High prevalence of GB virus C infection in a group of Italian patients with hepatitis of unknown etiology. J Infect Dis. 1996;174:181–183. doi: 10.1093/infdis/174.1.181. [DOI] [PubMed] [Google Scholar]
- Fischler B, Lara C, Chen M, Sonnerborg A, Nemeth A, Sallberg M. Genetic evidence for mother-to-infant transmission of hepatitis G virus. J Infect Dis. 1997;176:285. doi: 10.1086/517267. [DOI] [PubMed] [Google Scholar]
- Fogeda M, Navas S, Martin J, et al. In vitro infection of human peripheral blood mononuclear cells by GB virus C/Hepatitis G virus. JVirol. 1999;73:4052–4061. doi: 10.1128/jvi.73.5.4052-4061.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francesconi R, Giostra F, Ballardini G, et al. Clinical implications of GBV-C/HGV infection in patients with ‘HCV-related’ chronic hepatitis. J Hepatol. 1997;26:1165–1172. doi: 10.1016/s0168-8278(97)80448-5. [DOI] [PubMed] [Google Scholar]
- Fried MW, Khudyakov YE, Smallwood GA, et al. Hepatitis G virus co-infection in liver transplantation recipients with chronic hepatitis C and non-viral chronic liver disease. Hepatol. 1997;25:1271–1275. doi: 10.1002/hep.510250536. [DOI] [PubMed] [Google Scholar]
- Gartner BC, Kaul H, Neutzling AG, Sauter M, Mueller-Lantzsch N, Kohler H. High prevalence of hepatitis G virus (HGV) infections in dialysis staff. Nephrol Dial Transplant. 1999;14:406–408. doi: 10.1093/ndt/14.2.406. 10.1093/ndt/14.2.406. [DOI] [PubMed] [Google Scholar]
- Gerolami V, Haflon P, Chambost H, et al. Prevalence of hepatitis G virus RNA in a monocentric population of French haemophiliacs. Br J Haematol. 1997;99:209–214. doi: 10.1046/j.1365-2141.1997.3463160.x. [DOI] [PubMed] [Google Scholar]
- Gonzales-Prez MA, Norder H, Berstrom A, Lopez E, Visona KA, Magniu LO. High prevalence of GB Virus C strains genetically related to strains with Asian origin in Nicaraguan haemophiliacs. JMed Virol. 1997;52:155. [PubMed] [Google Scholar]
- Gutierrez RA, Dawson GJ, Knigge MF, et al. Seroprevalence of GB Virus C and persistence of RNA and antibody. J Med Virol. 1997;53:167–173. doi: 10.1002/(sici)1096-9071(199710)53:2<167::aid-jmv10>3.0.co;2-g. 10.1002/(sici)1096-9071(199710)53:2<167::aid-jmv10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Haagsma EB, Cuypers HTM, Gouw ASH, et al. High prevalence of hepatitis G virus after liver transplantation without apparent influence on long-term graft function. J Hepatol. 1997;26:921–925. doi: 10.1016/s0168-8278(97)80261-9. [DOI] [PubMed] [Google Scholar]
- Hadziyannis SJ. Fulminant hepatitis and the new G,GBV-C flavivirus. JViral Hepatol. 1997;5:15–19. doi: 10.1046/j.1365-2893.1998.00076.x. [DOI] [PubMed] [Google Scholar]
- Hassoba HM, Pessoa MG, Terrault NA, et al. Antienvelope antibodies are protective against GBV-C reinfection: Evidence from the liver transplant model. J Med Virol. 1998;56:253–258. 10.1002/(sici)1096-9071(199811)56:3<253::aid-jmv13>3.0.co;2-f. [PubMed] [Google Scholar]
- Hassoba HM, Terrault NA, El-Gohary A, et al. Antibody to GBV-C second envelope glycoprotein (Anti-GBV-C E2): Is it a marker of immunity? J Med Virol. 1997;53:354–360. 10.1002/(sici)1096-9071(199712)53:4<354::aid-jmv7>3.0.co;2-6. [PubMed] [Google Scholar]
- Haydon GH, Jarvis LM, Simpson KJ, Hayes PC, Simmonds P. The clinical significance of the detection of hepatitis GBV-C RNA in the serum of patients with fulminant, presumed viral, hepatitis. JViral Hepatol. 1997;4:45–49. doi: 10.1046/j.1365-2893.1997.00122.x. [DOI] [PubMed] [Google Scholar]
- Heringlake S, Osterkamp S, Trautwein C, et al. Association between fulminant hepatic failure and a strain of GB virus C. Lancet. 1996;348:1626–1629. doi: 10.1016/S0140-6736(96)04413-3. 10.1016/s0140-6736(96)04413-3. [DOI] [PubMed] [Google Scholar]
- Hollingsworth RC, Jameson L, Minton JE, et al. GBV-C/HGV coinfection in HIV-1 positive men: Frequent detection of viral RNA in blood plasma but absence from seminal fluid plasma. JMed Virol. 1998;56:321–326. doi: 10.1002/(sici)1096-9071(199812)56:4<321::aid-jmv6>3.0.co;2-v. 10.1002/(sici)1096-9071(199812)56:4<321::aid-jmv6>3.3.co;2-m. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Lu RH, Chan CY, Chang FY, Lee SD. Detection of antibodies to E2-protein of GB virus-C/hepatitis G virus in patients with acute posttransfusion hepatitis. J Med Virol. 1999;57:85–89. 10.1002/(sici)1096-9071(199901)57:1<85::aid-jmv13>3.3.co;2-u. [PubMed] [Google Scholar]
- Hwang SJ, Lu RH, Chan CY, Wang YJ, Wu JC, Lee SD. The role of hepatitis G virus infection in patients with acute posttransfusion hepatitis in Taiwan. Gastroenterol. 1997;112:1260–1264. doi: 10.1016/s0016-5085(97)70138-0. [DOI] [PubMed] [Google Scholar]
- Ibanez A, Gimenez-Barcons M, Tajahuerce A, et al. Prevalence and Genotyes of GB Virus C/Hepatitis G Virus (GBV-C/HGV) and Hepatitis C Among Patients infected with Human Immunodeficiency Virus: Evidence of GBV-C/HGV sexual transmission. JMed Virol. 1998;55:293–299. doi: 10.1002/(sici)1096-9071(199808)55:4<293::aid-jmv7>3.0.co;2-w. 10.1002/(sici)1096-9071(199808)55:4<293::aid-jmv7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Sugiyama K, Mizutani T, et al. Hepatitis G virus replication in human cultured cells displaying susceptibility to hepatitis C virus infection. Biochem Biophys Res Commun. 1997;235:505–508. doi: 10.1006/bbrc.1997.6818. 10.1006/bbrc.1997.6818. [DOI] [PubMed] [Google Scholar]
- Inoue K, Yoshiba M, Sekiyama K, Kohara M. Possible association between serum GB virus C RNA level and disease activity in fulminant hepatitis type G. J Hepatol. 1999;30:801–806. doi: 10.1016/s0168-8278(99)80132-9. [DOI] [PubMed] [Google Scholar]
- Jarvis LM, Davidson F, Hanley JP, Yap PL, Ludlam CA, Simmonds P. Infection with Hepatitis G Virus among recipients of plasma products. Lancet. 1996;348:352–1355. doi: 10.1016/s0140-6736(96)04041-x. [DOI] [PubMed] [Google Scholar]
- Jarvis LM, Bell H, Simmonds P, et al. The effect of treatment with alpha-interferon on hepatitis G/GBV-C viraemia. The CONSTRUCT Group. Scand JGastroenterol. 1999;33:195–200. doi: 10.1080/00365529850166941. [DOI] [PubMed] [Google Scholar]
- Kakumu S, Sato K, Morishita T, et al. Prevalence of Hepatitis B, Hepatitis C, and Hepatitis GB Virus C/Hepatitis G Virus Infections in liver disease patients and inhabitants in Ho Chi Minh, Vietnam. JMed Virol. 1998;54:243–248. 10.1002/(sici)1096-9071(199804)54:4<243::aid-jmv2>3.3.co;2-#. [PubMed] [Google Scholar]
- Kanda T, Yokosuka O, Imzeki F, et al. GB virus C RNA in Japanese patients with hepatocellular carcinoma and cirrhosis. JHepatol. 1997;27:464–469. doi: 10.1016/s0168-8278(97)80349-2. [DOI] [PubMed] [Google Scholar]
- Kao JH, Chen PJ, Chen W, Hsiang CS, Lai MY, Chen DS. Amplification of GB virus-C, hepatitis G virus RNA with primers from different regions of the viral genome. JMed Virol. 1997a;51:284–289. 10.1002/(sici)1096-9071(199704)51:4<284::aid-jmv5>3.0.co;2-1. [PubMed] [Google Scholar]
- Kao JH, Chen W, Chen PJ, Lai MY, Lin RY, Chen DS. GB Virus-C/hepatitis G virus infection in prostitutes: Possible role of sexual transmission. JMed Virol. 1997b;52:381–384. 10.1002/(sici)1096-9071(199708)52:4<381::aid-jmv6>3.0.co;2-y. [PubMed] [Google Scholar]
- Kao JH, Liu CJ, Chen PJ, et al. Interspousal transmission of GB virus C/hepatitis G virus: comparison with hepatitis C. J Med Virol. 1997c;53(3):48–353. [PubMed] [Google Scholar]
- Karayiannis P, Hadziyannis SJ, Kim J, et al. Hepatitis G virus infection: Clinical characteristics and response to interferon. JViral Hepatol. 1997;4:37–44. doi: 10.1046/j.1365-2893.1997.00128.x. [DOI] [PubMed] [Google Scholar]
- Kato T, Mizokami M, Orito S, et al. Amino acid substitutions in NS5A region of GB Virus C and response to interferon therapy. JMed Virol. 1999;57:376–382. doi: 10.1002/(sici)1096-9071(199904)57:4<376::aid-jmv9>3.0.co;2-#. 10.1002/(sici)1096-9071(199904)57:4<376::aid-jmv9>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Kiem HP, Myerson D, Storb R, McDonald GB, Spurgeon CL, Leisenring W. Prevalence of hepatitis G virus in patients with aplastic anaemia. Blood. 1997;90:1335–1336. [PubMed] [Google Scholar]
- Kinoshita T, Miyake K, Nakao H, et al. Molecular investigation of GB Virus C infection in haemophiliacs in Japan. J Infect Dis. 1997;175:454–457. doi: 10.1093/infdis/175.2.454. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Tanaka E, Nakayama J, et al. of GB Virus C/Hepatitis G Virus genome in periperalblood mononuclear cells and liver tissue. JMed Virol. 1999;57:114–121. doi: 10.1002/(sici)1096-9071(199902)57:2<114::aid-jmv5>3.0.co;2-0. 10.1002/(sici)1096-9071(199902)57:2<114::aid-jmv5>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Mizokami M, Nakao TEA. Prevelance and molecular epidemiology of GB virus C/hepatitis G virus infection in Mongolia. JMed Virol. 1997;52:143–148. 10.1002/(sici)1096-9071(199706)52:2<143::aid-jmv4>3.0.co;2-3. [PubMed] [Google Scholar]
- Kudo T, Morishima T, Shibata M. Hepatitis G Infection. N Eng J Med. 1997;337:276–277. doi: 10.1056/NEJM199707243370413. [DOI] [PubMed] [Google Scholar]
- Lamballerie X, Charrell RN, Dussol B. Hepatitis GB virus C in patients on haemodialysis. New Engl J Med. 1996;334:1549. doi: 10.1056/NEJM199606063342319. [DOI] [PubMed] [Google Scholar]
- Lampe E, Saback FL, Yoshida CF, Neil C. Infection with GB virus C/hepatitis G virus in Brazilian hemodialysis and hepatitis patients and asymptomatic individuals. J Med Virol. 1997;52:61–67. doi: 10.1002/(sici)1096-9071(199705)52:1<61::aid-jmv10>3.0.co;2-3. 10.1002/(sici)1096-9071(199705)52:1<61::aid-jmv10>3.3.co;2-p. [DOI] [PubMed] [Google Scholar]
- Laras A, Zacharakis G, Hadziyannis SJ. Absence of the negative strand of GBV-C/HGV RNA from the liver. J Hepatol. 1999;30:383–388. doi: 10.1016/s0168-8278(99)80094-4. [DOI] [PubMed] [Google Scholar]
- Laskus T, Radkowski M, Wang LF, Vargas H, Rakela J. Lack of hepatitis G virus replication in the livers of patients coinfected with hepatitis C and G viruses. J Virol. 1997;71:7804–7806. doi: 10.1128/jvi.71.10.7804-7806.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskus T, Radkowski M, Wang LF, Vargas H, Rakela J. Detection of hepatitis G virus replication sites by using highly strand-specific Tth-based reverse transcriptase PCR. J Virol. 1998;74:3072–3075. doi: 10.1128/jvi.72.4.3072-3075.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary TP, Muerhoff AS, Simons JN, et al. Sequence and genomic organisation of GBV-C: a novel member of the Flaviviridae associated with human non A-E hepatitis. J Med Virol. 1996;48:60–67. doi: 10.1002/(SICI)1096-9071(199601)48:1<60::AID-JMV10>3.0.CO;2-A. 10.1002/(sici)1096-9071(199601)48:1<60::aid-jmv10>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Lefrere JJ, Loiseau P, Maury J, et al. Natural history of GBV-C/hepatitis G virus infection through the follow-up of GBV-C/hepatitis G virus-infected blood donors and recipients studied by RNA polymerase chain reaction and anti-E2 serology. Blood. 1997;90:3776–3780. [PubMed] [Google Scholar]
- Lightfoot K, Skelton M, Kew MC, et al. Does hepatitis GB virus C infection cause hepatocellular carcinoma in black Africans? Hepatol. 1997;26:740–742. doi: 10.1002/hep.510260328. [DOI] [PubMed] [Google Scholar]
- Linnen J, Wages J, Zhang-Keck ZY, et al. Molecular cloning and disease association of hepatitis G virus: a transfusion transmissible agent. Science. 1996;271:505–508. doi: 10.1126/science.271.5248.505. [DOI] [PubMed] [Google Scholar]
- Liu CJ, Kao JH, Lai MY, et al. Minimal role of GB virus-C/hepatitis G virus in fulminant and subfulminant hepatitis in Taiwan. J Gastroenterol Hepatol. 1999;14:352–357. doi: 10.1046/j.1440-1746.1999.01855.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Alcorocho JM, Millan A, Garcia-Trevijano ER, et al. Detection of hepatitis GB virus type C RNA in serum and liver from children with chronic viral hepatitis B and C. Hepatol. 1997;25:1258–1260. doi: 10.1002/hep.510250533. [DOI] [PubMed] [Google Scholar]
- Madejon A, Fogeda M, Bartolome J, et al. GB virus C RNA in serum, liver and peripheral blood mononuclear cells from patients with chronic hepatitis B, C, and D. Gastroenterol. 1997;113:573–578. doi: 10.1053/gast.1997.v113.pm9247478. [DOI] [PubMed] [Google Scholar]
- Manolakopoulos S, Morris A, Davies S, Brown D, Hajat S, Dusheiko GM. Influence of GB virus C viraemia on the clinical, virological and histological features of early hepatitis C-related hepatic disease. J Hepatol. 1998;28:173–178. doi: 10.1016/0168-8278(88)80001-1. [DOI] [PubMed] [Google Scholar]
- Marshall RL, Cockerill J, Friedman P, et al. Detection of GB virus C by the RT-PCR LCx system. J Virol Meth. 1998;73:99–107. doi: 10.1016/s0166-0934(98)00050-0. [DOI] [PubMed] [Google Scholar]
- Martinot M, Marcellin P, Boyer N, et al. Influence of hepatitis G virus infection on the severity of liver disease and response to interferon-alpha in patients with chronic hepatitis C. Ann Intern Med. 1997;126:874–881. doi: 10.7326/0003-4819-126-11-199706010-00004. [DOI] [PubMed] [Google Scholar]
- Masuko K, Mitsui T, Iwano K, et al. Infection with Hepatitis GB Virus C in patients on maintenance haemodialysis. NEngl JMed. 1996;23:1485–1490. doi: 10.1056/NEJM199606063342301. [DOI] [PubMed] [Google Scholar]
- McHutchison J, Nainan OV, Alter MJ, et al. Hepatitis C and G co-infection: Response to interferon therapy and quantitative changes in serum HGV-RNA. Hepatol. 1997;26:132–1327. doi: 10.1002/hep.510260534. [DOI] [PubMed] [Google Scholar]
- Mellor J, Haydon G, Blair C, Livingstone W, Simmonds P. Low levels or absent in vivo replication of hepatitis C virus and hepatitis G virus/GB virus C in peripheral blood mononuclear cells. J Gen Virol. 1998;79:705–714. doi: 10.1099/0022-1317-79-4-705. [DOI] [PubMed] [Google Scholar]
- Moaven LD, Hyland CA, Young IF, McCaw R, Mison L, Locarnini SA. Prevalence of hepatitis G in Queensland blood donors. Med JAustralia. 1996;165:369–371. doi: 10.5694/j.1326-5377.1996.tb125019.x. [DOI] [PubMed] [Google Scholar]
- Moriyama K, Okamura T, Nakano S. Hepatitis GB virus C genome in the serum of aplastic anaemia patients receiving frequent blood transfusions. Br J Haematol. 1997;96:864–867. doi: 10.1046/j.1365-2141.1997.d01-2105.x. [DOI] [PubMed] [Google Scholar]
- Morris A, Webster AD, Brown D, Harrison TJ, Dusheiko G. GB virus C infection in patients with primary antibody deficiency. J Infect Dis. 1998;177:1719–1722. doi: 10.1086/517430. [DOI] [PubMed] [Google Scholar]
- Mphahlele MJ, Aspinall S, Spooner R, Carman WF. Age related prevalence of hepatitis G virus in South Africans. J Clin Pathol. 1999;52:752–757. doi: 10.1136/jcp.52.10.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mphahlele MJ, Lau GKK, Carman WF. HGV: the identification, biology and prevalence of an orphan virus. Liver. 1998;18:143–155. doi: 10.1111/j.1600-0676.1998.tb00142.x. [DOI] [PubMed] [Google Scholar]
- Munoz SJ, Alter HJ, Nakatsuji Y, et al. The significance of hepatitis G virus in serum of patients with sporadic fulminant and subfulminant hepatitis of unknown etiology. Blood. 1999;94:1460–1464. [PubMed] [Google Scholar]
- Mushahwar IK, Erker JC, Muerhoff AC, Desai SM. Tissue tropism of GBV-C and HCV in immunocompromised patients and protective immunity of antibodies to GB virus C second envelope (GBV-C E2) glycoprotein. Hepatol Clin. 1998;6:23–27. [Google Scholar]
- Nagayama R, Miyake K, Okamoto H. Effect of interferon on GB virus C and hepatitis C virus in hepatitis patients with the co-infection. J Med Virol. 1997;52:156–160. 10.1002/(sici)1096-9071(199706)52:2<156::aid-jmv6>3.0.co;2-2. [PubMed] [Google Scholar]
- Nakamura S, Takagi T, Matsuda T. Hepatitis G virus RNA in patients with B-cell non-Hodgkin's lymphoma. Br J Haematol. 1997;98:1051–1052. [PubMed] [Google Scholar]
- Nakatsuji Y, Shih W-K, Tanaka E, et al. Prevalence and disease association of hepatitis G virus infection in Japan. J Viral Hepat. 1996;36:307–316. doi: 10.1111/j.1365-2893.1996.tb00103.x. [DOI] [PubMed] [Google Scholar]
- Nerurkar VR, Chua PK, Hoffmann PR, Dashwood WM, Shikuma CM, Yanagihara R. High prevalence of GB Virus C/Hepatitis G Virus infection among homosexual men infected with Human Immunodeficiency Virus Type 1: Evidence for sexual transmission. J Med Virol. 1998;56:123–127. 10.1002/(sici)1096-9071(199810)56:2<123::aid-jmv4>3.0.co;2-a. [PubMed] [Google Scholar]
- Nishiyama Y, Hino K, Sawada N, Tsuda F. Little contribution of GB virus C to the development of primary hepatocellular carcinoma in Japan. J Gastroenterol. 1999;34:494–497. doi: 10.1007/s005350050302. 10.1007/s005350050302. [DOI] [PubMed] [Google Scholar]
- Nubling CM, Bialleck H, Fursch AJ, et al. Frequencies of GB Virus C/Hepatitis G Virus genomes and specific antibodies in German risk and non-risk populations. J Med Virol. 1997;53:218–224. 10.1002/(sici)1096-9071(199711)53:3<218::aid-jmv6>3.0.co;2-b. [PubMed] [Google Scholar]
- Ohshima S, Komatsu M, Nakane K, et al. Iatrogenic GB virus C/hepatitis G virus infection in an area endemic for hepatitis C virus. J Hosp Infect. 2000;44:179–185. doi: 10.1053/jhin.1999.0694. 10.1053/jhin.1999.0694. [DOI] [PubMed] [Google Scholar]
- Orito E, Mizokami M, Yasuda K, et al. Interferon-alpha therapy in patients dually infected with hepatitis C virus GB Virus C/hepatitis C virus-virological response to HGV and pretreatment HGV viraemia level. J Hepatol. 1997;27:603–612. doi: 10.1016/s0168-8278(97)80076-1. [DOI] [PubMed] [Google Scholar]
- Pavlova BG, Heinz R, Selim U, Tuchler H, Pittermann E, Eder G. Association of GB virus C (GBV-C) /hepatitis G virus (HGV) with haematological diseases of different malignant potential. J Med Virol. 1999;57:361–366. doi: 10.1002/(sici)1096-9071(199904)57:4<361::aid-jmv6>3.0.co;2-o. 10.1002/(sici)1096-9071(199904)57:4<361::aid-jmv6>3.3.co;2-f. [DOI] [PubMed] [Google Scholar]
- Pawlotsky J-M, Roudot-Thoraval F, Muerhoff AS, et al. GB Virus C (GBV-C) infection in patients with chronic hepatitis C. Influence on liver disease and on hepatitis virus behaviour: Effect of interferon alpha therapy. J Med Virol. 1998;54:26–37. doi: 10.1002/(sici)1096-9071(199801)54:1<26::aid-jmv5>3.0.co;2-r. 10.1002/(sici)1096-9071(199801)54:1<26::aid-jmv5>3.3.co;2-2. [DOI] [PubMed] [Google Scholar]
- Pessoa MG, Terrrault NA, Demter J, et al. Quantification of Hepatitis G and C viruses in the liver: Evidence that hepatitis G virus is not hepatotropic. Hepatol. 1998;27:877–880. doi: 10.1002/hep.510270335. [DOI] [PubMed] [Google Scholar]
- Petrik J, Guella L, Wight DGD, et al. Hepatic histology in hepatitis C virus carriers co-infected with hepatitis G virus. Gut. 1998;42:103–106. doi: 10.1136/gut.42.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilot-Matias TJ, Carrick RJ, Coleman PF, et al. Expression of the GB virus C E2 glycoprotein using the Semliki Forest virus vector system and its utility as a serologic marker. Virology. 1996;225:282–292. doi: 10.1006/viro.1996.0602. 10.1006/viro.1996.0602. [DOI] [PubMed] [Google Scholar]
- Radkowski M, Wang LF, Cianciara J, Rakela J, Laskus T. Analysis of hepatitis G virus/GB virus C quasispecies and replication sites in human subjects. Biochem Biophys Res Commun. 1999;258:296–299. doi: 10.1006/bbrc.1999.0632. 10.1006/bbrc.1999.0632. [DOI] [PubMed] [Google Scholar]
- Radkowski M, Wang LF, Vargas H, Rakela J, Laskus T. Lack of evidence for GB virus C/hepatitis G virus replication in peripheral blood mononuclear cells. J Hepatol. 1998;28:179–183. doi: 10.1016/0168-8278(88)80002-3. [DOI] [PubMed] [Google Scholar]
- Raengsakulrach B, Ong-Aj-Yooth L, Thaiprasert T, et al. High prevalence of hepatitis G viraemia among kidney transplant patients in Thailand. J Med Virol. 1997;53:162–166. doi: 10.1002/(sici)1096-9071(199710)53:2<162::aid-jmv9>3.0.co;2-7. 10.1002/(sici)1096-9071(199710)53:2<162::aid-jmv9>3.3.co;2-u. [DOI] [PubMed] [Google Scholar]
- Ross RS, Viazov S, Kruppenbacher JP, et al. GB Virus C infection in patients who under went liver transplantation. Liver. 1997;17:238–243. doi: 10.1111/j.1600-0676.1997.tb01024.x. [DOI] [PubMed] [Google Scholar]
- Roth WK, Waschk D, Marx S, et al. Prevalence of hepatitis G virus, its strain variant, the GB agent, in blood donations and their transmission to recipients. Transfusion. 1997;37:651–656. doi: 10.1046/j.1537-2995.1997.37697335162.x. [DOI] [PubMed] [Google Scholar]
- Rubio A, Rwy C, Sanchez-Quijano A, et al. Is hepatitis G virus transmitted sexually. J. Am. Med. Assoc. 1977;277:532–533. doi: 10.1001/jama.1997.03540310030026. [DOI] [PubMed] [Google Scholar]
- Saito T, Tanaka K, Kondo M, et al. Plus-and Minus stranded Hepatitis G Virus RNA in liver tissue and in peripheral blood mononuclear cells. Biochem Biophys Res Commun. 1997;237:288–291. doi: 10.1006/bbrc.1997.7103. 10.1006/bbrc.1997.7103. [DOI] [PubMed] [Google Scholar]
- Saiz JC, Ampurdanes S, Olmedo E, et al. Hepatitis G virus infection in chronic hepatitis C: frequency, features and response to interferon therapy. JHepatol. 1997;26:787–793. doi: 10.1016/s0168-8278(97)80243-7. [DOI] [PubMed] [Google Scholar]
- Sallie R, Shaw J, Mutimer D. GBV-C virus and fulminant hepatic failure. Lancet. 1996;347:68. doi: 10.1016/s0140-6736(96)90704-7. [DOI] [PubMed] [Google Scholar]
- Sarrazin C, Roth WK, Zeuzems S. Heterosexual transmission of GB virus-C/hepatitis G virus infection. Eur J Gastroenterol Hepatol. 1997;9:1117–1120. doi: 10.1097/00042737-199711000-00017. [DOI] [PubMed] [Google Scholar]
- Sathar MA, Soni PN, Pegoraro R, et al. A new variant of GB virus C/hepatitis G virus (GBV-C/HGV) from South Africa. Virus Res. 1999a;64:151–160. doi: 10.1016/s0168-1702(99)00090-8. 10.1016/s0168-1702(99)00090-8. [DOI] [PubMed] [Google Scholar]
- Sathar MA, Soni PN, Naicker S, Conradie J, Lockhat F, Gouws E. GB Virus C/Hepatitis G Virus infection in KwaKulu Natal, South Africa. JMed Virol. 1999b;59:38–44. 10.1002/(sici)1096-9071(199909)59:1<38::aid-jmv7>3.0.co;2-3. [PubMed] [Google Scholar]
- Sawayama Y, Hayaashi J, Etoh Y, Urabe H, Minami K, Kashiwagi S. Hetrosexual transmission of GB Virus C/Hepatitis G Virus infection to non-intravenous drug-using female prostitutes in Fkuoka, Japan. Dig Dis Sci. 1999;44:1937–1943. doi: 10.1023/a:1026664428194. [DOI] [PubMed] [Google Scholar]
- Scallan MF, Clutterbuck D, Jarvis LM, Scott GR, Simmonds P. Sexual Transmission of GB Virus C/Hepatitis G Virus. J Med Virol. 1998;55:203–208. doi: 10.1002/(sici)1096-9071(199807)55:3<203::aid-jmv4>3.0.co;2-5. 10.1002/(sici)1096-9071(199807)55:3<203::aid-jmv4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Schaade L, Platzer CA, Kleines M, Ritter K. GB virus/hepatitis G virus infection in medical staff. J Hosp Infect. 2000;44:153–154. doi: 10.1053/jhin.1999.0669. 10.1053/jhin.1999.0669. [DOI] [PubMed] [Google Scholar]
- Schleicher S, Chaves RL, Dehmer T, Gregor M, Hess G, Flehming B. Identification of GBV-C hepatitis G RNA in chronic hepatitis C patients. J Med Virol. 1996;50:71–74. doi: 10.1002/(SICI)1096-9071(199609)50:1<71::AID-JMV12>3.0.CO;2-0. 10.1002/(sici)1096-9071(199609)50:1<71::aid-jmv12>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Schlueter V, Schmolke S, Stark K, Hess G, Ofenloch-Haehnle B, Engel AM. Reverse transscription-PCR detection of hepatitis G virus. J Clin Microbiol. 1996;34:2660–2664. doi: 10.1128/jcm.34.11.2660-2664.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemayer CA, Viazov S, Philipp T, Roggendoef M. Detection of GBV-C/HGV RNA in saliva and serum, but not in urine of infected patients. Infection. 1998;26:39–41. doi: 10.1007/BF02768751. [DOI] [PubMed] [Google Scholar]
- Seipp S, Scheidel M, Hofmann WJ, et al. Hepatotropism of GB virus C (GBV-C): GBV-C replication in human hepatocytes of human hepatoma cell lines. J Hepatol. 1999;30:570–579. doi: 10.1016/s0168-8278(99)80186-x. [DOI] [PubMed] [Google Scholar]
- Semprini AE, Perisco T, Theirs V, et al. Absence of hepatitis C virus and detection of hepatitis G virus/GB virus C RNA sequences in the semen of infected men. JInfect Dis. 1997;177:848–854. doi: 10.1086/515257. [DOI] [PubMed] [Google Scholar]
- Shev S, Bjorkman P, Norkrans G, et al. GBV-C/HGV infection in Hepatitis C Virus-infected deferred Swedish blood donors. J Med Virol. 1998;54:75–79. doi: 10.1002/(sici)1096-9071(199802)54:2<75::aid-jmv1>3.0.co;2-l. 10.1002/(sici)1096-9071(199802)54:2<75::aid-jmv1>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Shibuya A, Takeuchi A, Sakurai K, Saigenji K. Hepatitis G virus infection from needle-stick injuries in hospital employees. J Hospt Infect. 1998;40:287–290. doi: 10.1016/s0195-6701(98)90305-x. [DOI] [PubMed] [Google Scholar]
- Shiratori Y, Kato N, Yokosuka O et al., for the Tokyo-Chiba Hepatitis Research Group. Predictors for the efficacy of interferon therapy in chronic hepatitis C virus infection. Gastroenterol. 1997;13:558–566. doi: 10.1053/gast.1997.v113.pm9247476. [DOI] [PubMed] [Google Scholar]
- Shrestha SM, Shrestha S, Tsuda F, et al. Infection with GB virus C and hepatitis C virus in drug addicts, patients on maintenance hemodialysis, or with chronic liver disease in Nepal. J Med Virol. 1997;53:157–161. doi: 10.1002/(sici)1096-9071(199710)53:2<157::aid-jmv8>3.0.co;2-8. 10.1002/(sici)1096-9071(199710)53:2<157::aid-jmv8>3.3.co;2-j. [DOI] [PubMed] [Google Scholar]
- Silini E, Belli L, Alberti AB, et al. HGV/GBV-C infection in liver transplant recipients: antibodies to the viral E2 envelope glycoprotein protect from de novo infection. J Hepatol. 1998;29:533–540. doi: 10.1016/s0168-8278(98)80147-5. [DOI] [PubMed] [Google Scholar]
- Simons JN, Pilot-Matias TJ, Leary TP, et al. Identification of two flavivirus-like genomes in the GB hepatitis agent. Proc Natl Acad Sci USA. 1995a;921:3401–3405. doi: 10.1073/pnas.92.8.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JN, Dawson GJ, Pilot-Matias TJ, et al. Isolation of novel virus-like sequences associated with human hepatitis. Nature Med. 1995b;1:564–569. doi: 10.1038/nm0695-564. [DOI] [PubMed] [Google Scholar]
- Simons JN, Desai SM, Schultz DE, Lemon SM, Mushahwar K. Translation initiation in GB viruses A and C: Evidence for internal ribosorne entry and implications for genome organisation. J Virol. 1996;70:6126–6135. doi: 10.1128/jvi.70.9.6126-6135.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidmore SJ, Collingham E. Prevalence of GB Virus – C/Hepatitis G Virus infection in an antenatal population. J Med Virol. 1999;57:235–237. 10.1002/(sici)1096-9071(199903)57:3<235::aid-jmv4>3.0.co;2-s. [PubMed] [Google Scholar]
- Skidmore SJ, Collingham KE, Harrison P, Neilson JR, Pillay D, Milligan DW. High prevalence of hepatitis G virus in bone marrow transplant recipients and patients treated for acute leukemia. Blood. 1997;89:3853–3856. [PubMed] [Google Scholar]
- Slimane SB, Albrecht JK, Fang JWS, et al & the Hepatitis Interventional Therapy Group. Clinical, virological and histological implications of GBV virus-C/hepatitis G virus infection in patients with chronic hepatitis C virus infection: a multicentre study based on 671 patients. J Viral Hepatol. 2000;7:51–55. doi: 10.1046/j.1365-2893.2000.00188.x. [DOI] [PubMed] [Google Scholar]
- Smith DB, Basaras M, Simon F, et al. Phylogenetic analysis of GBV-C/Hepatitis G virus. J Gen Virol. 2000;81:769–780. doi: 10.1099/0022-1317-81-3-769. [DOI] [PubMed] [Google Scholar]
- Stark K, Meyer CG, Tacke M, et al. Hepatitis G virus RNA and hepatitis G virus antibodies in renal transplant recipients: prevalence and risk factors. Transplant. 1997;64:608–612. doi: 10.1097/00007890-199708270-00011. [DOI] [PubMed] [Google Scholar]
- Stark K, Bienzle U, Hess G, Engel AM, Hegenscheid B, Schluter V. Detection of the hepatitis G virus genome among injecting drug users, homosexual and bisexual men, and blood donors. J Infect Dis. 1996;174:1320–1323. doi: 10.1093/infdis/174.6.1320. [DOI] [PubMed] [Google Scholar]
- Sugai Y, Nakayama H, Fukuda M, et al. Infection with GB virus C in patients with chronic liver disease. J Med Virol. 1997;51:175–181. 10.1002/(sici)1096-9071(199703)51:3<175::aid-jmv6>3.0.co;2-3. [PubMed] [Google Scholar]
- Tacke M, Kiyosawa K, Stark K, Schlueter V, Ofenloch-Haehnle B, Hess G. Detection of antibodies to a putative hepatitis G virus envelope protein. Lancet. 1997a;349:318–320. doi: 10.1016/S0140-6736(96)06461-6. 10.1016/s0140-6736(96)06461-6. [DOI] [PubMed] [Google Scholar]
- Tacke M, Schmolke S, Schlueter V, et al. Humoral Immune response to the E2 protein of the Hepatitis G Virus is associated with long-term recovery from infection and reveals a high frequency of Hepatitis G Virus exposure among healthy blood donors. Hepatol. 1997b;26:1626–1633. doi: 10.1002/hep.510260635. [DOI] [PubMed] [Google Scholar]
- Tagger A, Donato F, Ribero ML, et al. the Brescia HCC Group. A case-control study on GB virus C/hepatitis G virus infection and hepatocellular carcinoma. Hepatol. 26:1653–1657. doi: 10.1002/hep.510260639. [DOI] [PubMed] [Google Scholar]
- Tameda Y, Kosaka Y, Tagawa S, et al. Infection with GB virus C (GBV-C) in patients with fulminant hepatitis. J Hepatol. 1996;25:842–847. doi: 10.1016/s0168-8278(96)80287-x. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Alter HJ, Nakatsuji Y. Effect of hepatitis G virus infection on chronic hepatitis C. Ann Intern Med. 1996;125:740–743. doi: 10.7326/0003-4819-125-9-199611010-00007. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Mizokami M, Orito E, et al. African origin of GB virus C/hepatitis G virus. FEBS Lett. 1998;423:143–148. doi: 10.1016/s0014-5793(98)00083-0. 10.1016/s0014-5793(98)00083-0. [DOI] [PubMed] [Google Scholar]
- Tangkijvanich P, Hirsch P, Theamboonlers A, Nuchprayoon I, Povorawan Y. Association of hepatitis viruses with hepatocellular carcinoma in Thailand. J Gastroenterol. 1999;34:227–233. doi: 10.1007/s005350050248. 10.1007/s005350050248. [DOI] [PubMed] [Google Scholar]
- Thomas DL, Vlahov D, Alter HJ, et al. Association of antibody to GB Virus C (Hepatitis G Virus) with viral clearance and protection from reinfection. JInfect Dis. 1998;177:539–542. doi: 10.1086/514245. [DOI] [PubMed] [Google Scholar]
- Tillmann HL, Heringlake S, Trautwein C, et al. Antibodies against the GB Virus C envelope 2 protein before liver transplantation protect against GBVirus C de novo infection. Hepatol. 1998;28:379–384. doi: 10.1002/hep.510280213. [DOI] [PubMed] [Google Scholar]
- Tribl B, Schoniger-Hekele M, Peterman D, Bakos S, Penner E, Muller C. Prevalence of GBV-C/HGV RNA, virus genotypes, and Anti-E2 antibodies in Autoimmune Hepatitis. Am J Gastroenterol. 1999;94:3336–3340. doi: 10.1111/j.1572-0241.1999.01452.x. [DOI] [PubMed] [Google Scholar]
- Tsuda F, Hadiwandowo S, Sawada N, et al. Infection with GB virus C (GBV-C) in patients with chronic liver disease or on maintenance haemodialysis in Indonesia. J Med Virol. 1996;49:248–252. doi: 10.1002/(SICI)1096-9071(199607)49:3<248::AID-JMV15>3.0.CO;2-8. 10.1002/(sici)1096-9071(199607)49:3<248::aid-jmv15>3.3.co;2-q. [DOI] [PubMed] [Google Scholar]
- Tucker TJ, Louw SJ, Robson SC, Isaacs S, Kirsch R. High prevalence of GBV-C Hepatitis G Virus infection in a rural South African Population. JMed Virol. 1997;53:225–228. 10.1002/(sici)1096-9071(199711)53:3<225::aid-jmv7>3.0.co;2-a. [PubMed] [Google Scholar]
- Tucker TJ, Smuts HE, Eedes C, et al. Evidence that the GBV-C/hepatitis G virus is primarily a lymphotropic virus. J Med Virol. 2000;61:52–58. 10.1002/(sici)1096-9071(200005)61:1<52::aid-jmv8>3.0.co;2-l. [PubMed] [Google Scholar]
- Tucker TJ, Smuts HEM, Kirsch KE, Eickhaus P, Robson SC, Swanepoel C. The hepatitis G virus/GBV-C is associated with glomerula nephritis. S Afr Med J. 1998;88:285–286. [PubMed] [Google Scholar]
- Umlauft F, Wong DT, Underhill PA, et al. Hepatitis G virus infection in haemodialysis patients and the effects of interferon treatment. Am J Gastroenterol. 1997;92:1986–1991. [PubMed] [Google Scholar]
- Viazov S, Riffelmann M, Sarr S, Ballauff A, Meisel H, Roggendorf M. Transmission of GBV-C HGV from drug-addicted mothers to their babies. J Hepatol. 1997;27:85–90. doi: 10.1016/s0168-8278(97)80284-x. [DOI] [PubMed] [Google Scholar]
- Wang HL, Jin DY. Prevalence and genotypes of hepatitis G virus in Chinese professional blood donors and hepatitis patients. J Infect Dis. 1997;175:1229–1233. doi: 10.1086/593676. [DOI] [PubMed] [Google Scholar]
- Wang J-T, Tsai F-C, Lee C-Z, et al. A prospective study of transfusion-transmitted GB virus C infection: Similar frequency but different clinical presentation compared with hepatitis C virus. Blood. 1996;88:1881–1886. [PubMed] [Google Scholar]
- Wang Y, Chen HS, Fan MH, et al. Infection with GB Virus C and Hepatitis C Virus in haemodialysis patients and blood donors in Beijing. JMed Virol. 1997;52:26–30. doi: 10.1002/(sici)1096-9071(199705)52:1<26::aid-jmv5>3.0.co;2-t. 10.1002/(sici)1096-9071(199705)52:1<26::aid-jmv5>3.3.co;2-w. [DOI] [PubMed] [Google Scholar]
- Wejstal R, Manson AS, Widell A, Norkrans G. Perinatal transmission of hepatitis G virus (GB Virus type C) and hepatitis C virus infection-A comparison. Clin Infect Dis. 1999;28:816–821. doi: 10.1086/515187. [DOI] [PubMed] [Google Scholar]
- Wu JC, Sheng WY, Huang YH, Hwang SJ, Lee SD. Prevalence and risk factor analysis of GBV-C/HGV infection in prostitutes. J Med Virol. 1997;52:83–85. 10.1002/(sici)1096-9071(199705)52:1<83::aid-jmv13>3.0.co;2-1. [PubMed] [Google Scholar]
- Yashina TL, Favorov MO, Khudyakov YE, et al. Detection of hepatitis G virus (HGV) RNA: clinical characteristics of acute HGV infection. JInfect Dis. 1997;175:1302–1307. doi: 10.1086/516460. [DOI] [PubMed] [Google Scholar]
- Yoshiba M, Inoue K, Sekiyama K. Hepatitis GB virus. N Eng J Med. 1996;335:1392–1393. [PubMed] [Google Scholar]
- Yoshiba M, Okamoto M, Mishiro S. Detection of the GBV-C hepatitis virus genome in serum from patients with fulminant hepatitis of unknown etiology. Lancet. 1995;346:1131–1132. doi: 10.1016/s0140-6736(95)91802-7. [DOI] [PubMed] [Google Scholar]
- Yuan JM, Govindarajan S, Ross RK, Yu MC. Chronic infection with hepatitis G virus in relation to hepatocellular carcinoma among non-Asians in Los Angeles County, California. Cancer. 1999;86:936–943. doi: 10.1002/(sici)1097-0142(19990915)86:6<936::aid-cncr7>3.0.co;2-t. 10.1002/(sici)1097-0142(19990915)86:6<936::aid-cncr7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Yuki N, Hayashi N, Kasahara A, et al. Quantitative analysis of antibody to hepatitis C virus envelope 2 glycoprotein in patients with chronic hepatitis C virus infection. Hepatol. 1996;23:947–952. doi: 10.1053/jhep.1996.v23.pm0008621173. [DOI] [PubMed] [Google Scholar]
- Zaidi Y, Chapman CS, Myint S. Aplastic anaemia after HGV infection. Lancet. 1996;348:471–472. doi: 10.1016/s0140-6736(05)64561-8. 10.1016/s0140-6736(96)24033-4. [DOI] [PubMed] [Google Scholar]
- Zanetti AR, Tanzi E, Romano L, et al. The Lombardy Study Group on vertical/perinatal hepatitis viruses transmission of GBV-C virus. JMed Virol. 1997;54:107–111. doi: 10.1002/(sici)1096-9071(199802)54:2<107::aid-jmv7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Zemel R, Dickman R, Tamary H, Bukh J, Zaizov R, Tur-Kaspa R. Viremia, genetic heterogeneity, and immunity to hepatitis G/GB-C virus in multiply transfused patients with thalassemia. Transfusion. 1998;38:301–306. doi: 10.1046/j.1537-2995.1998.38398222876.x. [DOI] [PubMed] [Google Scholar]
- Zignego AL, Giannini C, Gentilini P, Bellesi G, Hadziyannis S, Ferri C. Could HGV infection be implicated in lymphomagenesis. Br J Haematol. 1997;98:778–779. doi: 10.1046/j.1365-2141.1997.d01-3531.x. [DOI] [PubMed] [Google Scholar]