Abstract
Over-activation of calpain, a ubiquitous calcium-sensitive protease, has been linked to a variety of degenerative conditions in the brain and several other tissues. Dozens of substrates for calpain have been identified and several of these have been used to measure activation of the protease in the context of experimentally induced and naturally occurring pathologies. Calpain-mediated cleavage of the cytoskeletal protein spectrin, in particular, results in a set of large breakdown products (BDPs) that are unique in that they are unusually stable. Over the last 15 years, measurements of BDPs in experimental models of stroke-type excitotoxicity, hypoxia/ischemia, vasospasm, epilepsy, toxin exposure, brain injury, kidney malfunction, and genetic defects, have established that calpain activation is an early and causal event in the degeneration that ensues from acute, definable insults. The BDPs also have been found to increase with normal ageing and in patients with Alzheimer's disease, and the calpain activity may be involved in related apoptotic processes in conjunction with the caspase family of proteases. Thus, it has become increasingly clear that regardless of the mode of disturbance in calcium homeostasis or the cell type involved, calpain is critical to the development of pathology and therefore a distinct and powerful therapeutic target. The recent development of antibodies that recognize the site at which spectrin is cleaved has greatly facilitated the temporal and spatial resolution of calpain activation in situ. Accordingly, sensitive spectrin breakdown assays now are utilized to identify potential toxic side-effects of compounds and to develop calpain inhibitors for a wide range of indications including stroke, cerebral vasospasm, and kidney failure.
Keywords: age, Alzheimer's disease, excitotoxicity, ischemia, review, spectrin breakdown
A rise in the concentration of intracellular calcium ([Ca2+]i) has been identified as a primary trigger of pathology in most paradigms used to study cellular responses to acute, definable insults (e.g. ischemia, excitotoxicity; see Mattson 1992, 1994). Disturbances in calcium homeostasis also have been observed in tissues from aged humans both with and without associated age-related pathologies such as Alzheimer's disease. Thus, it is likely that some of the actions of calcium in discrete pathological events are also relevant to less defined, and more protracted, degenerative conditions. Calcium-dependent processes which represent points of convergence among a heterogeneous set of degenerative mechanisms are therefore logical targets for intervention strategies, and have the potential to generate valuable markers of pathogenesis.
An observation common to the types of pathologies noted above is the activation of the cysteine protease calpain, a calcium-dependent, nonlysosomal enzyme known to exist widely in animal tissues (see Melloni & Pontremoli 1989 and Croall & DeMartino 1991 for reviews of calpain biochemistry). The activated protease acts on a host of endogenous proteins (Takahashi 1990) including the cytoskeletal element spectrin. Calpain-mediated proteolysis of spectrin leads to the immediate production of breakdown products (BDPs) of ∼150 kDa in size. The BDPs are relatively stable and can be readily measured after immunolabelling with antibodies raised to intact spectrin (Siman et al. 1984; Seubert et al. 1988a, b; Siman & Noszek 1988) or to peptide sequences on either side of the calpain cleavage site (Saido et al. 1993; Roberts-Lewis et al. 1994; Bahr et al. 1995). Rapid and sustained increases in the levels of spectrin BDPs have been observed across a broad spectrum of experimentally induced and insidious pathologies to be reviewed here. Taken together, these studies implicate calpain activation as a primary contributor to the onset and progression of degeneration in vulnerable populations of neurones and other cell types. Accordingly, assays for calpain activity provide a sensitive technique for detecting the earliest stages of pathogenesis.
Calpain-mediated proteolysis of spectrin
Baudry et al. (1981) first demonstrated that low concentrations of calcium stimulate leupeptin-sensitive proteolysis of a large protein doublet in brain synaptic membranes. The doublet was later identified as spectrin (α and β subunits), which is a major actin-binding cytoskeletal component and a preferred substrate for calpain (Siman et al. 1984; Boivin et al. 1990), particularly in the presence of calmodulin (Seubert et al. 1987). The α-subunit is cleaved by calpain between Tyr115 and Gly116 of the 11th spectrin repeat unit (Harris et al. 1989; Moon & McMahon 1990) resulting in two BDPs of nearly equal electrophoretic mobility. Polyclonal antibodies developed and affinity-purified against native spectrin recognize these fragments on western blots, and quantification of the immunolabelled cleavage products has been used extensively to measure calpain activation in situ.
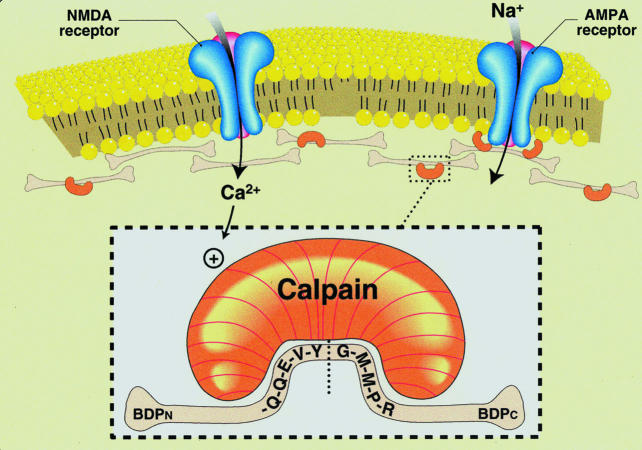
Spectrin breakdown-specific antibodies also have been developed to epitopes exposed by the calpain cleavage event (Saido et al. 1993; Roberts-Lewis et al. 1994; Bahr et al. 1995). As described in Figure 1, antibodies to the five amino acid sequence (QQEVY) at the carboxyl end of the amino-terminal fragment (anti-BDPN) recognize an antigen of ∼150 kDa in brain membrane samples that were treated with calcium to activate endogenous calpain. Similarly, anti-BDPC antibodies to the calpain-rendered epitope of the carboxy-terminal fragment of spectrin (residues GMMPR) label a 155-kDa antigen in calcium-treated, but not control, membranes. Thus, antibodies against new spectrin termini exposed by calpain proteolysis selectively label distinct proteolytic fragments. These fragments correspond to those described throughout much of the literature as BDP1 and BDP2 (Seubert et al. 1988a,b; Siman & Noszek 1988). Occasionally, antibodies to native spectrin also recognize a third calcium-induced cleavage product, forming a triplet pattern with the larger two BDPs (Roberts-Lewis et al. 1994; Bahr et al. 1995). The smaller proteolytic product may originate from the β-subunit of spectrin, which is also labelled by antispectrin antibodies.
Figure 1.
Excitotoxic activation of calpain. Over-stimulation of excitatory amino acid receptors can lead to a rise in intracellular calcium sufficient to activate calpain. This activation event can be monitored with antibodies selective for new terminal domains produced by the cleavage of key proteins. Shown is calpain targeting its recognition sequence in spectrin, a preferred substrate that makes up a large part of the cytoskeletal network, resulting in the production of aminoterminal (BDPN) and carboxyterminal (BDPC) breakdown products.
Threshold and subcellular locus of the calpain–spectrin interaction in glutamatergic neurones
The relevance of a particular enzymatic reaction to pathology depends largely on such factors as activation threshold, subcellular distribution, substrate selectivity, and the extent to which the proteolytic consequences are reversible. These considerations mirror a set of constraints which initially were used to implicate subtle levels of calpain-mediated spectrin proteolysis in the production of synaptic plasticity (Lynch & Baudry 1984; also see Vanderklish et al. 1995,1996), and which ultimately led to a consideration of calpain over-activation as a critical factor in neuropathology. There are two isoforms of calpain: µ-calpain which is activated by micromolar calcium levels and m-calpain activated by millimolar calcium. The µ-calpain species, or calpain 1, is found in dendrites (Fukuda et al. 1990; Perlmutter et al. 1990) and it undergoes autolytic activation (Suzuki et al. 1981; Inomata et al. 1988; Saido et al. 1992) at calcium concentrations (low µM) within the range of those achieved in dendritic spines during intense stimulation of glutamatergic synapses (Muller & Connor 1991; Guthrie et al. 1991; Faddis et al. 1997). Autolysis occurs at the plasma membrane (Pontremoli et al. 1985) so as to influence, for example, pp60Src, protein kinase C (PKC), phosphatase 1B, and focal adhesion kinase (Saido et al. 1994; Cooray et al. 1996; Rock et al. 1997) and several types of adhesion molecules (see Bahr et al. 1999) during calpain's many roles in cell-signalling cascades. The autolytic activation also allows the protease to contribute in signal transduction events related to cellular structure and synaptic organization (Fox et al. 1993; Earnest et al. 1995; Inomata et al. 1996; Capaldi et al. 1997; Kawasaki et al. 1997; Bahr, 2000).
The activation threshold of calpain 1 is well above the resting [Ca2+]i (∼80 nm), making it unlikely that the protease would be activated by fluctuations in calcium concentrations associated with routine synaptic activity mediated predominantly by AMPA receptors. Indeed, under control conditions BDPs are at low or undetectable levels in immunoblot assays of adult brain homogenate (1–2% of the total antispectrin immunoreactivity), a finding corroborated by immunohistochemical methods. However, western blot analyses have shown that BDPs are produced in the hippocampus during direct (Seubert et al. 1988b; del Cerro et al. 1994) or indirect (Bahr et al. 1995) stimulation of NMDA receptors. That calpain may become activated during physiological NMDA receptor stimulation is suggested by the potent blocking action of calpain inhibitors on long-term potentiation, a NMDA receptor-dependent form of synaptic plasticity (del Cerro et al. 1990; Denny et al. 1990; Oliver et al. 1990). The implied relationship between calpain activation and synaptic transmission conditions that evoke large increases in [Ca2+]i (i.e. high frequency activity) was confirmed in situ using BDP-specific antibodies (Vanderklish et al. 1995). Spectrin BDPs were found to accumulate in postsynaptic elements of CA1 pyramidal cells to an extent that correlated with the number of short, high frequency bursts of afferent stimulation delivered to Schaffer-collateral projections; low-frequency stimulation did not activate calpain. High resolution studies employing a calpain substrate that exhibits fluorescence resonance energy transfer indicate that activation of calpain occurs in dendritic spine heads following stimulation of NMDA receptors (Vanderklish et al. 2000). The above results emphasize that calpain activation in adult glutamatergic neurones likely has a physiological role but is a relatively infrequent event, potentially reserved for specialized synaptic modulation events such as long-term potentiation. The distinction is made here between calpain activation in neurones of the adult nervous system and in developing neurones. Studies conducted in vitro and in vivo suggest that calpain is involved in the regulation of process outgrowth and differentiation (Oshima et al. 1989; Pinter et al. 1994). They further indicate that calpain activity is controlled much more dynamically during development, and potentially ‘tolerated’ at a higher level. They also indicated that spectrin BDPs are induced by modest levels of NMDA receptor activation and associated calcium influx.
Pathogenic calpain activation
Functionally relevant calpain activation can be expected to be a highly localized and selective event in vivo for several reasons: (i) calcium elevation in neurones is tightly regulated by energy-dependent buffering and extrusion systems, and can be compartmentalized by dendritic structure; (ii) routes of calcium entry (e.g. voltage-gated and receptor-operated channels) exhibit distinct distributions, often restricted to particular lamina or neural populations; and (iii) inhibitory (calpastatin) and stimulatory factors (Takeyama et al. 1986; Melloni et al. 1998) modulate the activity and probably the substrate selectivity of calpain. However, given the broad list of calpain substrates and the irreversible nature of proteolysis, a challenge to any one of the above systems could permit more generalized calpain activation, resulting in inherently pathological rather than functional proteolysis. Release of intracellularly stored calcium and the autocatalytic priming of calpain are also likely to exacerbate the nature of the proteolytic response (Shea 1997a).
Considered next is evidence that over-activation of calpain is, in multiple circumstances, an early and causative event in the development of pathology. As will become evident, assays based on detection of spectrin BDPs can be of great value in describing the link between calpain activation and pathology.
Excitotoxicity
Intense stimulation of NMDA receptors leads to BDP formation in the hippocampus (Seubert et al. 1988b; Siman et al. 1989) (see Figure 1). This effect, as shown in studies utilizing organotypic hippocampal slice cultures (Stoppini et al. 1991; Bahr 1995), is correlated with the amount of receptor stimulation (del Cerro et al. 1994; Vanderklish et al. 1995) and can be detected after only 2 min of NMDA exposure (Bahr et al. 1995). This is far in advance of histological signs of degeneration observed in the hippocampus following administration of NMDA for hours to days in vivo (Siman & Noszek 1988; Siman et al. 1989) and precedes the onset of propidium iodide staining evident after ∼30 min of NMDA exposure in vitro (see Vornov et al. 1991). Both the proteolytic (Seubert et al. 1988b; del Cerro et al. 1994; Bahr et al. 1995) and degenerative (Siman et al. 1989) responses to excessive NMDA receptor stimulation are blocked by NMDA receptor channel antagonists. The rapid nature of calpain activation and the resultant BDP induction raises the question of what degree of NMDA receptor-induced proteolysis can be tolerated by neurones. Electrophysiological results suggest that the temporal limit is less than 15 min at saturating concentrations of NMDA (Blake et al. 1988). Correspondingly, a 15-min NMDA infusion caused persistent BDP formation in cultured hippocampal slices, while a 5-min infusion produced only transient breakdown of spectrin (Bahr et al. 1995). It also was demonstrated in cultured slices that a ∼50% reduction in calpain 1 levels using antisense techniques dramatically improved the recovery of evoked synaptic potentials following a 15-min infusion of NMDA (Bednarski et al. 1995). The translational suppression of calpain was associated with an expected reduction in NMDA-induced BDPs detected by immunoblot or immunocytochemistry. These studies indicate that calcium-dependent proteolysis induced at a large number of sites becomes pathogenic within a short time. With regard to the intensity of NMDA receptor stimulation, levels of BDP formation and neurodegeneration correlate with the amount of NMDA administered (see Siman et al. 1989). Moreover, there is an overlap among areas of unusual NMDA vulnerability, regions of high NMDA receptor density, and loci of spectrin BDP induction. The hippocampal field CA1 exhibits the greatest vulnerability to NMDA (Vornov et al. 1995), contains the highest concentration of NMDA receptors in the hippocampus, and manifests the most robust BDP staining following a short exposure to the excitotoxin (Bednarski et al. 1995). These data argue that the relative susceptibility of neurones to NMDA is defined by the extent to which calpain is activated by the associated calcium channel response.
An equally reliable, spatially restricted proteolytic response accompanies kainic acid (KA) neurotoxicity. Large accumulations of BDP have been found in the hippocampus and piriform cortex within 3–16 h of KA injection, persisting for as long as 9 days (Siman & Noszec 1988; Siman et al. 1989; Najm et al. 1992; Bi et al. 1996). In the hippocampus, the calpain-mediated proteolysis was maximal at 24 h, with the highest levels of BDP occurring in the CA3 region, and lesser amounts in CA2/CA1 regions, the dentate, and CA1/subicular regions. This pattern matches the profile of cell loss observed in the hippocampus a few days after the insult (Nadler et al. 1978,1980; Siman & Noszak 1988), and accords well with the distribution of high-affinity KA receptors. It is noteworthy that the magnitude of spectrin proteolysis and the extent of cell damage co-vary with the dosage of KA, much as observed when NMDA is used as the excitotoxin (Siman et al. 1989). In conjunction with the temporal and regional correlations between BDP formation and cell death, the findings argue that calpain plays an early and causal role in the development of excitotoxicity.
Of special mention with regard to the KA model of neurotoxicity is its use in understanding how separate, liminally toxic manipulations might act synergistically at the point of calpain activation to produce neuropathology. Elliot et al. (1993), in expanding a series of results implicating glucocorticoids in the augmentation of neuronal damage, found that daily injections of corticosterone (CORT) exacerbated KA-induced spectrin proteolysis: spectrin BDP formation was enhanced by 124% in CORT-injected rats relative to vehicle controls. In a complementary study, Lowy et al. (1994) demonstrated that adrenalectomy effectively reduced the proteolytic response to KA in the hippocampus and cortex by lowering baseline as well as KA-induced CORT levels. Glucocorticoids are known to inhibit glucose uptake in neurones, and it is thought that secondary metabolic effects, such as compromised calcium and glutamate regulation, are responsible for the amplifying effect of the steroids on other neuropathologies. As expected for this mechanism, mannose supplementation blocked the exacerbating influence of CORT on KA-induced spectrin breakdown (Elliot et al. 1993). A more direct influence on KA-induced calpain activation has been shown for polyamines. Spermine, spermidine, and putrescine were found to increase in concentration following KA lesions and other pathological circumstances associated with calpain activation (e.g. ischemia). With the above order of potency, the polyamines stimulated calpain-mediated hydrolysis of endogenous and exogenous protein substrates – most likely through modulation of an endogenous calpain activator (Najm et al. 1991a,b). This modulation of calpain by polyamines apparently occurs in vivo. As reported by Najm et al. (1992), injection of an inhibitor of ornithine decarboxylase, the rate-limiting enzyme in the polyamine metabolic pathway, reduces KA-induced spectrin proteolysis by approximately 75% in the hippocampus. In this experiment, putrescine and BDP levels were coordinately reduced by DFMO in the first 16 h after KA injection, but not at later time points. At four and seven days post-KA, concentrations of the two products were similarly elevated in DFMO-treated and control animals. It is noteworthy that DFMO was equally ineffective at preventing the delayed neurotoxicity elicited by KA. Binding of the gliosis marker 3H-RO5–4864, measured 7 days after KA lesions, also was unchanged by DFMO pretreatment; this parameter was highly correlated with the amount of spectrin breakdown whether or not animals received DFMO. Lastly, although DFMO did not completely abolish KA-induced proteolysis after 16 h and its effect at earlier time points remains undetermined, the results obtained with the compound illustrate an important conclusion: there is a clear dissociation when comparing the relationships between neurodegeneration and early vs. late phases of calpain-mediated proteolysis. This dissociation has been made with some experimental precision in paradigms of ischemic brain injury.
Ischemia and hypoxia
Calpain is one of the main suspected perpetrators in hypoxic/ischemic injury to the brain (see Lee 1995; Lipton 1999) and to other organs such as the liver (Bronk & Gores 1993), heart (Tolnadi & Korecky 1986), and kidney (Edelstein et al. 1995,1996; 1997a,b). The latter is a particularly important subject of study since renal failure is a very common problem affecting about 5% of all hospital admittants and carries a high mortality rate of > 50% (a figure that has not changed significantly in the last 40 years). Additionally, a great deal of research has focused on the brain since the ischemic condition known as stroke affects approximately 500 000 people each year in the United States alone, making it the third leading cause of death and the number one cause of disability. Patterns of regional vulnerability to ischemia in the brain and temporal characteristics of the degenerative response have been described in detail (see Hatakeyama et al. 1988). In a typical gerbil model of global forebrain ischemia, transient carotid artery occlusion elicited degeneration in a set of highly vulnerable neural populations. With increasing durations of arterial occlusion, cortical and subcortical structures of lesser susceptibility were recruited among the more vulnerable regions in a reliable sequence. Damage to these structures occurred at a threshold duration of ischemia, beneath which cells recovered, and beyond which neurones died after a delay of a few days. Ischemic lesions also were induced most readily in the CA1 subfield of the hippocampus, a region that, as noted, contains high levels of NMDA receptors. Channel and agonist blockers that disrupt NMDA receptor function have been shown to ameliorate ischemic injury (Kochhar et al. 1991; Kaku et al. 1993; Madden et al. 1993; Maier et al. 1995; Takaoka et al. 1997; Mueller et al. 1999). Also, given that ischemia leads to massive glutamate efflux, the particularly high density of NMDA receptors in CA1 appears to explain the unusual sensitivity of pyramidal cells in this region. Such results implicating the NMDA receptor as a variable in ischemic brain damage serve to strengthen the longstanding hypothesis that calcium influx is critically involved in pathogenesis.
In a study that linked the NMDA receptor-dependency of ischemia with the known downstream effects of the calcium ion on calpain activation, Seubert et al. (1989a) demonstrated that episodes of global ischemia lasting 10 min elicit a rapid proteolysis of spectrin. Using antibodies to native spectrin, BDPs were detected in immunoblot samples collected 15 min after reperfusion. Calpain activity was most pronounced in CA1, while areas of lesser vulnerability (e.g. thalamus and cerebellum) had correspondingly smaller accumulations of the spectrin fragments. At subsequent time points, BDPs were shown to persist only in CA1, and after 4 days this region experienced a second, more intense wave of proteolysis. In vitro models of hypoxia also have helped to define the early stages of calcium-dependent proteolysis and to identify which component of ischemia, hypoxia/hypoglycemia or reperfusion, is more influential in eliciting this pathogenic mechanism. Under conditions in which the baseline calpain activation that accompanies acute slice preparation was kept to a minimum, BDP concentrations were roughly doubled in hippocampal slices after 5 min of hypoxia (Arai et al. 1991; Lee et al. 1991). Thus, the initiating stage of calpain-mediated spectrin proteolysis after global ischemia appears to be unrelated to reperfusion, but rather seems a primary response to hypoxia. Interestingly, the proteolytic response is bi-phasic in nature: an early component initiated during or shortly after ischemia, followed by a later and larger wave correlating with delayed cell death. This general profile holds for multiple models of focal and global ischemia. Using similar techniques, Lee and coworkers (Hong et al. 1994b) have shown that focal neocortical ischemia (achieved by occlusion of both carotids and one middle cerebral artery) is associated with a rapid and sustained induction of spectrin BDPs in the infarct zone (also see Lee et al. 1991; Bartus et al. 1995; Yao et al. 1995; Blomgren et al. 1997,1999). Recently, Bartus et al. (1998) measured BDP formation in CA1 hippocampus following four-vessel occlusion in the rat and correlated this measure with histological indices of cell damage and loss of synaptic transmission. Observations made between 6 h and 4 days post-ischemia indicated that calpain activation correlates with the progressive compromise of pyramidal cell structure and function.
The above findings have been corroborated and extended by two groups using BDP-specific antibodies (Saido et al. 1993; Roberts-Lewis et al. 1994). In immunohistochemical assays, these antibodies labelled only those sites where BDPs accumulated, and thus provided greater spatial resolution of calpain activation, both at the regional and cellular level. Exploiting this specificity, the two groups showed that calpain becomes activated in the dendrites of pyramidal cells in the CA2/CA3 border zone after ischemic episodes as short as 1 min. Longer durations, 5 and 10 min, elicited the immunostaining in CA1 followed by spectrin proteolysis in cortical, thalamic, and other sites. Thus, sampling the post-ischemic brain throughout an extended period revealed that CA1 undergoes an early phase (within 15 min), then a later and more intense phase (between one and two days), of proteolytic activation after an intermediate ischemic duration. The longer episodes of carotid artery occlusion elicited the calpain response within additional cortical and thalamic areas (Roberts-Lewis et al. 1994). Analyses with the BDP-specific antibodies provide an important set of observations: (i) the studies document with great acuity the strict spatiotemporal correlations between calpain-mediated proteolysis and cellular pathology in vulnerable brain areas; (ii) they indicate that the proteolysis must persist beyond the initial stimulus-linked phase to irreversibly compromise cells; and (iii) they add to previous data in suggesting that protection of neurones from ischemia is achieved by inhibiting calpain after the primary insult. With regard to the latter, BDP assays have been used to determine the protectant nature of protease inhibitors in target neural populations (see Lee et al. 1991) as well as in other highly vulnerable cell types (Edelstein et al. 1996).
Pharmacological approaches have been used to test the receptor dependency of the calpain response and the prediction made with the BDP assay that the protease is a primary contributor to pathogenesis in the ischemic brain. Inhibition of NMDA receptors with MK-801 was shown to be effective in reducing both phases of proteolysis in the aforementioned global ischemia paradigm when administered in doses that afford significant neuroprotection (Seubert et al. 1990; Roberts-Lewis & Siman 1993; Roberts-Lewis et al. 1994). The coordinated reductions in cell death and calpain activation by MK-801 emphasize the importance of NMDA receptor-linked calcium entry in ischemia. Correspondingly, NMDA receptor antagonists also reduce the cellular toxicity and spectrin breakdown associated with NMDA administration in vivo (Siman et al. 1989), and prevent BDP formation elicited with the excitotoxin in vitro (Seubert et al. 1988b; del Cerro et al. 1994; Bahr et al. 1995). Thus, neuroprotection by NMDA receptor blockade is associated with reductions in calpain activity in two paradigms: ischemia and excitotoxicity.
Direct inhibition of calpain in vivo has been shown in several instances to reduce calpain-mediated proteolysis and protect vulnerable cells from hypoxia/ischemia (Lee et al. 1991; Rami & Krieglstein 1993; Bartus et al. 1994a,b; Hong et al. 1994a; Edelstein et al. 1996; Li et al. 1996; Yuen & Wang 1996; Yokota et al. 1999). Such a route for neuroprotection extends to the point of preserving synaptic function as calpain inhibitors improve the recovery of synaptic responses after hypoxia in acute hippocampal (Arai et al. 1990; Arlinghaus et al. 1991; Chen et al. 1997; also see Bednarski et al. 1995) and neocortical slices (Hiramatsu et al. 1993). Moreover, hippocampal slices prepared from animals treated with the calpain inhibitor leupeptin prior to ischemia retained the ability to express long-term potentiation (Lee et al. 1991). This result is of great significance therapeutically as long-term potentiation is thought to underlie the encoding of certain types of memory, and the phenomenon itself relies heavily on the structural and functional integrity of excitatory synapses. As predicted from early data obtained with BDP assays, blockade of the latter, persistent phase of ischemia-induced proteolysis affords significant neuroprotection (Bartus et al. 1994a,b). Such reports characterizing the phasic generation of spectrin BDPs after ischemia establish that the second, persistent wave of proteolysis starting within hours of the initial calpain response is correlated with and indicative of eventual cell death. This implies a ‘window of opportunity’ for therapeutically efficacious inhibition of calpain that may extend many hours after the ischemic insult. In fact, by measuring cerebral infarct volume, Markgraf et al. (1998) defined the outer limit of a calpain inhibitor's therapeutic benefit to be as great as six hours postischemia. It stands to reason, then, that a treatment for stroke that targets a point of convergence among many pathological mechanisms has the potential to prevent neuronal damage even when administered several hours after the insult.
Trauma and haemorrhage
Calpain-mediated proteolytic responses have been implicated in the neurodegeneration associated with brain trauma and related vascular problems that can lead to cerebral vasospasm and ischemic conditions. Spectrin BDPs were evident regionally after the trauma of experimental brain injury (Kampfl et al. 1996; Saatman et al. 1996a; Posmantur et al. 1997) and in the cerebrospinal fluid of humans following subarachnoid and intraventricular haemorrhages (see Lee et al. 1993). It is not known whether these types of haemorrhage are generated solely by neuronal or vascular tissue. However, increased calpain activation and spectrin fragmentation have been detected in smooth muscle cells of the rabbit basilar artery after vasospasm (Lee et al. 1993; Yamaura et al. 1993). It is thought that the proteolysis of spectrin, calponin, and regulatory kinases in these cells contributes to the morphological alterations of arterial cells after vasospasm, and perpetuates spastic contraction initiated by haemorrhage (Minami et al. 1992; Yamaura et al. 1993; Lee 1995). In particular, proteolytic conversion of PKC into a tonically catalytic form by calpain (Sato et al. 1997) occurs in the basilar artery after subarachnoid haemorrhage, leading to increased smooth muscle contractility. Evidence suggests that calpain 1 remains activated and levels of its endogenous inhibitor calpastatin are diminished in arterial smooth muscle cells for days after subarachnoid haemorrhage, thereby perpetuating stable contraction (Yamaura et al. 1993; Sato et al. 1997). With regard to this idea, it has been demonstrated that selective calpain inhibitors attenuate the development and progression of cerebral vasospasm (Minami et al. 1992; Lee et al. 1997). A dual action of calpain inhibitors on pathological processes in neurones and the vasculature that supports them could account for the efficacy of the inhibitors in models of brain trauma. A brain trauma model developed by McIntosh and colleagues allowed researchers to demonstrate that calpain inhibitors attenuate trauma-induced spectrin breakdown (Posmantur et al. 1997) as well as the associated motor and cognitive deficits (Saatman et al. 1996b). The above results indicate that measurements of BDP levels in cerebrospinal fluid may be of utility in detecting both the neuronal and vascular aspects of cerebrovascular accidents. They also suggest that calpain inhibition can be useful in decreasing the risk of haemorrhage-induced brain damage associated with certain surgical procedures.
Toxins
While the types of neural insults considered above – excitotoxicity and ischemia – have the common feature of glutamate receptor hyperstimulation at an early stage, it must be emphasized that routes to pathology which do not directly engage glutamatergic systems also lead to calpain activation. Colchicine, for example, causes neuronal damage by blocking critical transport processes. Intraventricular injections of the drug caused extensive death developing over several days among granule cells of the dentate gyrus, with much smaller effects in the other hippocampal subdivisions (Sutula et al. 1986). Spectrin BDPs appeared within 24 h of intrahippocampal colchicine injection, peaked at 4 days, and remained elevated for weeks (Seubert et al. 1989b). As was the case for cell death, the increase in the BDP response was largest in the dentate gyrus, particularly in the molecular layer. Note that these results, together with data on excitotoxicity and ischemia, establish a correlation between selective vulnerability and early appearance of BDPs that includes all of the major hippocampal subfields (see Table 1).
Table 1.
Correlation between cellular vulnerability and early appearance of spectrin BDPs in the hippocampus. Regional selectivity with regards to cellular damage and calpain-mediated spectrin proteolysis is shown for three pathogenic insults
| Treatment | Hippocampal region of greatest vulnerability | Region of earliest and largest BDP accumulation |
|---|---|---|
| Kainic acid | Field CA3 | Field CA3 |
| Ischemia | Field CA1 | Field CA1 |
| Colchicine | Dentate gyrus | Dentate gyrus |
The pathogenic action of some environmental toxins also appears to be mediated by calpain. This includes the action of trimethyltin (see Bahr et al. 1995; Munirathinam, Bahr, 2000), a neurotoxic pesticide known to cause seizures, amnesia, and selective damage to the hippocampus, as well as that of the heavy metal mercury (Elliget et al. 1994) and maitotoxin (Zhao et al. 1999). For example, peripheral injections of trimethyltin into rats produced calpain-mediated spectrin proteolysis in the hippocampus within 24 h (Seubert et al. 1988a); the BDP formation steadily increased thereafter for two weeks. The BDP response was more evident in the dentate gyrus relative to field CA1, thus correlating with the regional vulnerability to the toxin (Noraberg et al. 1998). Exposure of hippocampal slice cultures to trimethyltin, as well as to snake venoms, induced the calpain response and resultant spectrin fragments within 3 h, the shortest time tested (Bahr et al. 1995).
Partial deafferentation
Brain damaging insults not only cause local degeneration, they can also produce atrophy in neuronal populations that send electrical signals to or receive signals from the injured region. The magnitude and time course of experimentally induced forms of this effect have been measured carefully in a few cases, including the dentate gyrus. Dendritic shrinkage and spine loss were found in granule cells within 48 h of lesioning cortical inputs to the dentate gyrus, and were more substantial in degree by 96 h post-lesion (Ivy et al. 1988; Seubert et al. 1988a). Spectrin BDPs also appeared in the deafferented zones within 12 h and increased dramatically over the subsequent 24-h period. The calpain response occurred while the severed axons were still functional and prior to any signs of degeneration. These results confirm the sensitivity of the BDP markers as early indicators of pathogenesis, and they demonstrate that BDPs can be used to identify sites where atrophy (as opposed to cell death) has been initiated by a particular insult. Furthermore, in that surgical procedures involving removal or lesioning of brain tissue produce deafferentation, inhibition of calpain may be useful in limiting any associated loss of peripheral function. This application has been tested in an animal model involving ablation of a discrete portion of the visual cortex. Indeed, selective calpain inhibitors attenuated the spread of calpain activation outside the surgical zone, as well as reduced the atrophy in those areas which connected to the removed tissue (Bartus et al. 1999).
Genetic defects
The brindled mouse mutation is a widely studied example of how genetic defects can lead to the emergence of neuropathology. The mutation involves a problem in the regulation of copper levels that has profound effects on the activity of copper-dependent oxidative enzymes of mitochondria. Death usually occurs in mice bearing the mutation by postnatal day 16 unless the animals are given supplemental doses of CuCl2. As mitochondria are an important high-capacity buffering system in neurones, such deficits are expected to lead to altered calcium regulation. Experiments have shown that spectrin BDPs begin to appear between postnatal days 12 and 15 in those brain regions destined to undergo degeneration over the course of further maturation (Seubert et al. 1990). Brain regions that are less affected by the mutation, according to alternative criteria, exhibit less calpain activation at the time points assayed. Also in a corresponding manner, peripheral injection of CuCl2 forestalls both the proteolytic response and the neuropathology that ensues without the copper supplement.
Ageing
The gradual degenerative processes characteristic of brain ageing (see Masliah et al. 1993) were found to be associated with calpain-mediated proteolysis in a study using antispectrin antibodies (Bahr et al. 1991). Moreover, the study showed that spectrin BDP levels increase with age in the mouse brain in a region-specific manner. While the hindbrain had low concentrations of BDPs across mice of 3–30 months of age, a linear relationship with age was exhibited by BDPs from the telencephalon (r = 0.91). This is in correspondence with the fact that telencephalic neurones also selectively express age-related deficits in synaptic markers (Bahr et al. 1992,1993; Masliah et al. 1993). Another type of assay showed calpain activity to exhibit only small, statistically insignificant increases in aged human brains (Banay-Schwartz et al. 1994). However, this could be due to postmortem effects causing the proteolytic events to fall below detectable levels in many areas. Recent immunocytochemical studies with BDP-specific antibodies (Bahr et al. unpublished data) confirmed the region-specific increase in calpain activation in the aged mouse brain, and began to map with precision those cell types of high vulnerability (i.e. cortical and hippocampal neurones). The origins of the regional vulnerability are not known, but it has been proposed that deregulation of the calpain system may occur at a faster rate with age in those regions of the telencephalon that exhibit lasting forms of calcium-triggered synaptic plasticities (see Lynch & Seubert 1989). Overall, the above results suggest that pathogenic processes set in motion by acute neurological insults – such as those discussed earlier – may be recruited in a gradual and selective manner with normal ageing. Such pathogenic insults are probably promoted by age-related disturbances in calcium homeostasis (an issue discussed by Mattson 1992). Indeed, differences in calcium-regulated events including calpain activation have been noted in fibroblasts (Peterson et al. 1991) and erythrocytes (Glaser et al. 1994) harvested from young vs. aged human subjects. One hypothesis on the contribution of the calpain system to brain ageing posits that other age-related cellular disturbances (for instance, gradual loss of lysosomal function; see Bahr et al. 1994a) interact with ongoing but normally subthreshold neuronal ‘stresses’ (e.g. excitotoxicity), the consequences of which may produce detrimental positive feedback. It is noteworthy that spectrin BDPs increase gradually starting just after adulthood (Bahr et al. 1991), perhaps indicating that aberrant activation of calpain is among a set of primary age-related dysfunctions that precede the appearance of overt pathology and symptomology.
Alzheimer's Disease
Studies with postmortem tissue suggest that spectrin cleavage events are more pronounced in the hippocampus and cortex of subjects with Alzheimer's disease than in those regions of age-matched control subjects (Masliah et al. 1990). Assays for calpain and calpastatin have shown that there are indeed regionally specific increases in the net calpain activity with Alzheimer's disease (Nilsson et al. 1990; Saito et al. 1993; Saido 1996; also see Haug et al. 1996) and in the expression levels of calpain with another age-related disorder, Parkinson's disease (Mouatt-Prigent et al. 1996). Histological indices also have shown that spectrin BDPs increase in areas most affected by Alzheimer-type pathogenesis in terms of synapse loss. In this respect, induction of calpain-mediated proteolysis in areas targeted by the disease may involve mechanisms of the type associated with deafferentation (discussed earlier). In one particular study, degradation of spectrin was greatly enhanced in the vulnerable frontal cortex region of a rat model of cholinergic degeneration (Fernandez-Shaw et al. 1997). Abnormally high levels of BDPs also have been detected in the cerebrospinal fluid of patients with Alzheimer's or Pick's disease (Vanderklish et al. unpublished observation). It is noteworthy that the fundamental cellular disturbance underlying the increased calpain activation in Alzheimers may extend to other somatic contexts. For example, after stimulation with serum or mitochondrial uncoupling agents, skin fibroblasts collected from Alzheimer patients were found to have a significantly higher BDP content than fibroblasts from age-matched or young donors (Peterson et al. 1991). This difference was obtained despite indications of equal calpain and calpastatin concentrations among the three donor groups. There was also evidence for an age-related increase in fibroblast BDP content in healthy subjects. The increased BDP levels in Alzheimer's disease and normal ageing are most likely due to the alterations in calcium regulation identified in skin cells (Peterson & Goldman 1986). In another study, increases in membrane-bound calpain and related proteolytic activity were evident in peripheral blood lymphocytes from patients with early onset Alzheimer's disease (Karlsson et al. 1995).
Stemming from work by Nixon and colleagues, persistent calpain activation in Alzheimer's disease is thought to contribute to hallmark features of the disease prior to the occurrence of gross synaptic loss and cell death (see Saito et al. 1993). These hallmark features of Alzheimer-type pathogenesis refer to (1) the formation of neurofibrillary tangles containing hyperphosphorylated isoforms of the microtubule-associated protein tau, and (2) the abnormal processing of amyloid precursor protein which can lead to the production of the β-amyloid peptide and related deposition in the form of plaques. With regard to the first event, calpain has been shown to be involved in the proteolytic processing/turnover of tau (Litersky & Johnson 1992; Mercken et al. 1995; Yang & Ksiezak-Reding 1995; Shea 1997b; Yang et al. 1997; Xie & Johnson 1998) that experiences distinct alterations with age (Bahr et al. 1994b; Bahr & Vicente 1998). There is some dispute in regards to whether reduced tau turnover by calpain is a possible mechanism to facilitate neurofibrillary chemistries in Alzheimer's disease. However, calpain was implicated in early stage neurofibrillary degeneration due to an active form of the protease colocalizing with tangles, neuritic plaques, and neuropil threads in Alzheimer brains (Grynspan et al. 1997). Further evidence suggests that calpain contributes to the conversion of normal tau to pathogenic, hyperphosphorylated forms of the protein (Shea et al. 1996). Interestingly, the action of calpain in this study was determined to be indirect through the proteolytic conversion of PKC into a tonically catalytic kinase, the same event that is thought to underlie vasospasm during subarachnoid haemorrhage (Sato et al. 1997). A more recent study indicated that calpain causes the conversion of p35 to p25, a 25-kDa species that accumulates in Alzheimer brains and promotes tau hyperphosphorylation, cytoskeletal disruption, and cell death through the prolonged activation and mislocalization of the cyclin-dependent kinase 5 (cdk5) (see Lee et al. 2000). Interestingly, this study also showed that application of the β-amyloid peptide to primary cortical neurones causes corresponding production of p25 and spectrin breakdown products, thus further suggesting a significant role for calpain in the pathogenesis of Alzheimer's disease.
Regarding amyloidogenesis, Siman et al. (1990) proposed that calpain is active in the normal, and perhaps pathological, processing of the precursor protein for β-amyloid (also see Li et al. 1995). This led to the idea that modulation of calpain may merit consideration as a potential therapeutic strategy against amyloid production. Recent studies, however, found conflicting results using potent calpain inhibitors. Several groups showed that blocking calpain activity causes different effects with regard to the production of β-amyloid: reduction in β-amyloid species (Klafki et al. 1996), no change in β-amyloid secretion levels (Figueiredo-Pereira et al. 1999), as well as increased secretion of the amyloidogenic peptide from cultured cells (Yamazaki et al. 1997; Yamazaki & Ihara 1998). Thus, it is not clear whether inhibitors or activators of the calpain system can influence amyloidogenic chemistries.
β-amyloid not only is the major constituent of senile plaques, the peptide also can cause neuronal apoptosis possibly through calpain (Martin et al. 1995; Chi et al. 1999; Wolf et al. 1999; Lee et al. 2000; Ray et al. 2000; also see Kluck et al. 1997) or through members of another family of cysteine proteases, the caspases (see Chan & Mattson 1999). There is much interest in the relationship between the two types of proteases and their common targets during proteolytic and related pathogenic responses (Porn-Ares et al. 1998; Chan & Mattson 1999; Ruiz-Vela et al. 1999; Newcomb et al. 2000; Wang, 2000). For example, they both cleave AMPA-type glutamate receptors in a way that can potentially regulate excitatory transmission (see Bahr et al. 1996; Bahr, 2000) or determine pathogenic consequences (Glazner et al. 2000). Interestingly, calpain is thought to contribute to the degradation phase of apoptosis using a caspase-dependent activation mechanism (Wood & Newcomb 1999), possibly through caspase-mediated cleavage of calpastatin and resulting decontrol of calpain activity suppression (Porn-Ares et al. 1998; Kato et al. 2000). It should be mentioned, however, that there is evidence of caspase-independent involvement of calpain in apoptotic events in platelets (Wolf et al. 1999). Calpain inhibition was found to reduce cell death in cortical neurones treated with β-amyloid (Lee et al. 2000; also see Jordan et al. 1997), although another study showed such inhibition to have no effect on β-amyloid-induced apoptosis (Selznick et al. 2000). Calpain also was shown to directly cleave certain caspases and thereby block the activation of the pro-apoptotic species caspase-3 (Chua et al. 2000). Such negative regulation of caspase processing by calpain is in contrast to calpain's putative role in apoptosis. Such a discrepancy may stem from isoform-specific actions among calpain species. In fact, the m-calpain isoform was found to selectively cleave and, in effect, activate caspase-3 in a neonatal model of cerebral hypoxia-ischemia where sequential activation of the two proteases occurs (Blomgren et al. 2000). This study promotes the idea that the pro-apoptotic caspase-3 is synergistically activated by the early induction of m-calpain, a calpain isoform implicated in Alzheimer's disease (Grynspan et al. 1997; Tsuji et al. 1998) and Parkinson's (Mouatt-Prigent et al. 1996).
Other pathological conditions
Finally, it is worthwhile to list a variety of other pathogenic circumstances in which calpain has been implicated. The research described above indicates that the protease plays a major role in a wide spectrum of processes including those underlying synaptic plasticity, neurotoxicity, trauma, and age-related disorders. This spectrum is broadened by additional studies in nervous tissue which suggest that calpain is involved in the inflammatory reactions associated with allergic encephalomyelitis (Shields et al. 1998), the demyelination events of multiple sclerosis (see Shields et al. 1999), the neuronal damage after spinal cord injury (Li et al. 1995,1998; James et al. 1998; Ray et al. 1999), as well as in behavioural disorders such as depression (Banay-Schwartz et al. 1998) and obsessive-compulsive disorder (Mundo et al. 1997). Outside the nervous system, calpain also has been implicated in a great diversity of disorders including: (1) different forms of cardiac pathologies (Atsma et al. 1995; Di Lisa et al. 1995; Arthur & Belcastro 1997); (2) mechanisms of renal failure (Elliget et al. 1994; Kelton et al. 1996; Edelstein et al. 1997a,b); (3) certain types of respiratory disorders including injury to inspiratory muscles of the diaphragm (see Miller et al. 1995; Jiang et al. 1998); (4) tissue damage associated with organ transplantation and graft dysfunction (Aguilar et al. 1997; Kohli et al. 1997; Tijsen et al. 1997); (5) collagen destruction and resulting bone erosion in cholesteatoma (Amar et al. 1996); (6) cataract formation (Andersson et al. 1994; Sanderson et al. 1996; Wang et al. 1996); (7) the eye inflammatory disorder optic neuritis (see Shields & Banik 1998); (8) a putative cell death mechanism mediated by HIV (Corasaniti et al. 1996); (9) breast cancer (Shiba et al. 1996; Pink et al. 2000); and (10) various disorders involving skeletal muscle (see Kumamoto et al. 1995,1997). With regards to the latter, it should be added that many mutations in calpain genes have been found to co-segregate with certain forms of muscular dystrophy (Richard et al. 1995; Beckmann et al. 1996; also see Spencer et al. 1995). It has been further suggested that calpain is responsible for the early stage muscle degradation in these myopathies (Kumamoto et al. 1995). To summarize, the problems associated with unregulated, calpain-mediated proteolysis are not restricted to the central nervous system but are in fact widespread.
Concluding remarks
Calpain activation is an early event in the cellular response to a variety of stressful and potentially pathogenic conditions. Such a proteolytic response, if sustained, can lead to cell death in vulnerable populations of cells. Studies utilizing the spectrin BDPs as markers of calcium-activated proteolysis have elucidated the commonality of this pathogenic mechanism and described paradigm-specific aspects of its presentation that correlate with cellular vulnerability. Moreover, data based on BDP measurements have served as the basis of testable predictions and pharmaceutical development concerning the efficacy of calpain inhibition as a therapeutic strategy. It is clear that assays for calpain activity and BDP detection can be exploited as a tool for the study of degenerative mechanisms and related therapeutics.
Acknowledgments
Editorial work was provided by Ms. Alyson M. Blow and the authors are grateful for her excellent services. This work was supported in part by U.S. Army Medical Research Grant DAMD17–99-C9090.
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid
- AP5
DL-2-amino-5-phosphonopentanoic acid
- BDP
spectrin breakdown product
- BDPN
147-kDa amino-terminal BDP mediated by calpain I
- BDPC
152-kDa carboxy-terminal BDP mediated by calpain I
- CORT
corticosterone
- KA
kainic acid
- NMDA
N-methyl-d-aspartate
- PKC
protein kinase C.
- Single letter notation for amino acids are A
Ala
- C
Cys
- D
Asp
- E
Glu
- F
Phe
- G
Gly
- H
His
- I
Ile
- K
Lys
- L
Leu
- M
Met
- N
Asn
- P
Pro
- Q
Gln
- R
Arg
- S
Ser
- T
Thr
- V
Val
- W
Trp
- and Y
Tyr
References
- Aguilar HI, Steers JL, Wiesner RH, Krom RA, Gores GJ. Enhanced liver calpain protease activity is a risk factor for dysfunction of human liver allografts. Transplantation. 1997;63:612–614. doi: 10.1097/00007890-199702270-00023. [DOI] [PubMed] [Google Scholar]
- Amar MS, Wishahi HF, Zakhary MM. Clinical and biochemical studies of bone destruction in cholesteatoma. J Laryngol Otol. 1996;110:534–539. doi: 10.1017/s002221510013419x. [DOI] [PubMed] [Google Scholar]
- Andersson M, Sjostrand J, Andersson AK, Andersen B, Karlsson JO. Calpains in lens epithelium from patients with cataract. Exp Eye Res. 1994;59:359–364. doi: 10.1006/exer.1994.1118. 10.1006/exer.1994.1118. [DOI] [PubMed] [Google Scholar]
- Arai A, Kessler M, Lee K, Lynch G. Calpain inhibitors improve the recovery of synaptic transmission from hypoxia in hippocampal slices. Brain Res. 1990;523:313–315. doi: 10.1016/0006-8993(90)91742-y. [DOI] [PubMed] [Google Scholar]
- Arai A, Vanderklish P, Kessler P, Lee K, Lynch G. A brief period of hypoxia causes proteolysis of cytoskeletal proteins in hippocampal slices. Brain Res. 1991;555:276–280. doi: 10.1016/0006-8993(91)90352-v. [DOI] [PubMed] [Google Scholar]
- Arlinghaus L, Mehdi S, Lee KS. Improved posthypoxic recovery with a membrane-permeable calpain inhibitor. Eur J Pharmacol. 1991;209:123–125. doi: 10.1016/0014-2999(91)90022-i. [DOI] [PubMed] [Google Scholar]
- Arthur GD, Belcastro AN. A calcium stimulated cysteine protease involved in isoproterenol induced cardiac hypertrophy. Mol Cell Biochem. 1997;176:241–248. [PubMed] [Google Scholar]
- Atsma DE, Bastiaanse EML, Jerzewski A, Van der Valk LJM, Van der Laarse A. Role of calcium-activated neutral protease (calpain) in cell death in cultured neonatal rat cardiomyocytes during metabolic inhibition. Circ Res. 1995;76:1071–1078. doi: 10.1161/01.res.76.6.1071. [DOI] [PubMed] [Google Scholar]
- Bahr BA. Long-term hippocampal slices: a model system for investigating synaptic mechanisms and pathologic processes. J Neurosci Res. 1995;42:294–305. doi: 10.1002/jnr.490420303. [DOI] [PubMed] [Google Scholar]
- Bahr BA. Integrin-type signaling has a distinct influence on NMDA-induced cytoskeletal disassembly. J Neuroscience Res. 2000;59:827–832. doi: 10.1002/(SICI)1097-4547(20000315)59:6<827::AID-JNR15>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Bahr BA, Vicente JS. Age-related phosphorylation and fragmentation events influence the distribution profiles of distinct tau isoforms in mouse brain. J Neuropathol Exp Neurol. 1998;57:111–121. doi: 10.1097/00005072-199802000-00002. [DOI] [PubMed] [Google Scholar]
- Bahr BA, Vanderklish PW, Ha LT, Tin M, Kessler M, Lynch G. Spectrin breakdown products increase with age in telencephalon of mouse brain. Neurosci Lett. 1991;131:237–240. doi: 10.1016/0304-3940(91)90622-z. [DOI] [PubMed] [Google Scholar]
- Bahr BA, Godshall AC, Hall RA, Lynch G. Mouse telencephalon exhibits an age-related decrease in glutamate (AMPA) receptors but no change in nerve terminal markers. Brain Res. 1992;589:320–326. doi: 10.1016/0006-8993(92)91293-n. [DOI] [PubMed] [Google Scholar]
- Bahr BA, Godshall AC, Murray BA, Lynch G. Age-related changes in neural cell adhesion molecule (NCAM) isoforms in the mouse telencephalon. Brain Res. 1993;628:286–292. doi: 10.1016/0006-8993(93)90966-q. [DOI] [PubMed] [Google Scholar]
- Bahr BA, Abai B, Gall C, Vanderklish PW, Hoffman KB, Lynch G. Induction of β-amyloid containing polypeptides in hippocampus: evidence for a concomitant loss of synaptic proteins and interactions with an excitotoxin. Exp Neurol. 1994a;129:81–94. doi: 10.1006/exnr.1994.1149. 10.1006/exnr.1994.1149. [DOI] [PubMed] [Google Scholar]
- Bahr BA, Lam N, Lynch G. Changes in the concentrations of tau and other structural proteins in the brains of aged mice. Neurosci Lett. 1994b;175:49–52. doi: 10.1016/0304-3940(94)91075-8. [DOI] [PubMed] [Google Scholar]
- Bahr BA, Tiriveedhi S, Park GY, Lynch G. Induction of calpain-mediated spectrin fragments by pathogenic treatments in long-term hippocampal slices. J Pharmacol Exp Ther. 1995;273:902–908. [PubMed] [Google Scholar]
- Bahr BA, Hoffman KB, Kessler M, et al. Distinct distributions of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptor subunits and a related 53,000 MR antigen (GR53) in brain tissue. Neuroscience. 1996;74:707–721. doi: 10.1016/0306-4522(96)00133-9. 10.1016/0306-4522(96)00133-9. [DOI] [PubMed] [Google Scholar]
- Bahr BA, Yilma S, Suppiramaniam V. Structural chemistries underlying synaptic signaling and regulation. J Mol Biol Biotech. 1999;1:35–48. [Google Scholar]
- Banay-Schwartz M, Deguzman T, Palkovits M, Lajtha A. Calpain activity in adult and aged human brain regions. Neurochem Res. 1994;19:563–567. doi: 10.1007/BF00971331. [DOI] [PubMed] [Google Scholar]
- Banay-Schwartz M, Deguzman T, Faludi G, Lajtha A, Palkovits M. Alteration of protease levels in different brain areas of suicide victims. Neurochem Res. 1998;23:953–959. doi: 10.1023/a:1021028304481. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Baker KL, Heiseries AD, et al. Postischemic administration of AK275, a calpain inhibitor, provides substantial protection against focal ischemic brain damage. J Cereb Blood Flow Metab. 1994a;14:537–544. doi: 10.1038/jcbfm.1994.67. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Hayward NJ, Elliot PJ, et al. Calpain inhibitor AK295 protects neurons from focal brain ischemia. Stroke. 1994b;25:2265–2270. doi: 10.1161/01.str.25.11.2265. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, Cavanaugh K, Eveleth D, Carriero DL, Lynch G. Time-related neuronal changes following middle cerebral artery occlusion: Implications for therapeutic intervention and the role of calpain. J Cereb Blood Flow Metab. 1995;15:969–979. doi: 10.1038/jcbfm.1995.123. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, Mennerick S, Eveleth D, Lynch G. Temporal ordering of pathogenic events following transient global ischemia. Brain Res. 1998;790:1–13. doi: 10.1016/s0006-8993(97)01414-5. 10.1016/s0006-8993(97)01414-5. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Chen EY, Lynch G, Kordower JH. Cortical ablation induces a spreading calcium-dependent, secondary pathogenesis which can be reduced by inhibiting calpain. Exp Neurol. 1999;155:315–326. doi: 10.1006/exnr.1998.7001. 10.1006/exnr.1998.7001. [DOI] [PubMed] [Google Scholar]
- Baudry M, Bundman M, Smith E, Lynch G. Micromolar calcium stimulates proteolysis and glutamate binding in rat brain synaptic membranes. Science. 1981;212:937–938. doi: 10.1126/science.7015504. [DOI] [PubMed] [Google Scholar]
- Beckmann JS, Richard I, Broux O, et al. Identification of muscle-specific calpain and beta-sarcoglycan genes in progressive autosomal recessive muscular dystrophies. Neuromuscul Disord. 1996;6:455–462. doi: 10.1016/s0960-8966(96)00386-0. 10.1016/s0960-8966(96)00386-0. [DOI] [PubMed] [Google Scholar]
- Bednarski E, Vanderklish P, Gall C, Saido TC, Bahr BA, Lynch G. Translational suppression of calpain 1 reduces NMDA-induced spectrin proteolysis and pathophysiology cultured hippocampal slices. Brain Res. 1995;694:147–157. doi: 10.1016/0006-8993(95)00851-g. 10.1016/0006-8993(95)00851-g. [DOI] [PubMed] [Google Scholar]
- Bi X, Chang V, Siman R, Tocco G, Baudry M. Regional distribution and time-course of calpain activation following kainate-induced seizure activity in adult rat brain. Brain Res. 1996;726:98–108. doi: 10.1016/0006-8993(95)01360-1. 10.1016/0006-8993(95)01360-1. [DOI] [PubMed] [Google Scholar]
- Blake JF, Brown MW, Collingridge GL. A quantitative study of the actions of excitatory amino acids and antagonists in rat hippocampal slices. Br J Pharmacol. 1988;95:291–299. doi: 10.1111/j.1476-5381.1988.tb16576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomgren K, Mcrae A, Elmered A, et al. The calpain proteolytic system in neonatal hypoxic-ischemia. Ann NY Acad Sci. 1997;825:104–119. doi: 10.1111/j.1749-6632.1997.tb48420.x. [DOI] [PubMed] [Google Scholar]
- Blomgren K, Hallin U, Andersson AL, et al. Calpastatin is up-regulated in response to hypoxia and is a suicide substrate to calpain after neonatal cerebral hypoxia-ischemia. J Biol Chem. 1999;274:14046–14052. doi: 10.1074/jbc.274.20.14046. [DOI] [PubMed] [Google Scholar]
- Blomgren K, Zhu C, Wang X, et al. Synergism between caspase-3 and m-calpain: a possible mechanism of ‘pathological apoptosis’? Submitted.
- Boivin P, Galand C, Dhermy D. In vitro digestion of spectrin, protein 4.1 and ankyrin by erythrocyte calcium dependent neutral protease (calpain I) Int J Biochem. 1990;22:1479–1489. doi: 10.1016/0020-711x(90)90240-4. [DOI] [PubMed] [Google Scholar]
- Bronk SF, Gores GJ. pH-dependent nonlysosomal proteolysis contributes to lethal anoxic injury in rat hapatocytes. Am J Physiol. 1993;264:G744–G751. doi: 10.1152/ajpgi.1993.264.4.G744. [DOI] [PubMed] [Google Scholar]
- Capaldi D, Rosario R, Esteban ET, Bahr BA. A 27-kDa matrix receptor from rat brain synaptosomes: selective recognition of the Arg-Gly-Asp-Ser domain and unique resistance to calcium-dependent proteolysis. Neuroscience Res. 1997;28:275–279. doi: 10.1016/s0168-0102(97)00049-7. [DOI] [PubMed] [Google Scholar]
- Chan SL, Mattson MP. Caspase and calpain substrates: Roles in synaptic plasticity and cell death. J Neurosci Res. 1999;58:167–190. [PubMed] [Google Scholar]
- Chen ZF, Schottler F, Lee KS. Neuronal recovery after moderate hypoxia is improved by the calpain inhibitor MDL28170. Brain Res. 1997;769:188–192. doi: 10.1016/s0006-8993(97)00848-2. 10.1016/s0006-8993(97)00848-2. [DOI] [PubMed] [Google Scholar]
- Chi XJ, Hiwasa T, Maki M, et al. Suppression of okadaic acid-induced apoptosis by overexpression of calpastatin in human UVr-1 cells. FEBS Lett. 1999;459:391–394. doi: 10.1016/s0014-5793(99)01281-8. 10.1016/s0014-5793(99)01281-8. [DOI] [PubMed] [Google Scholar]
- Chua BT, Guo K, Li P. Direct cleavage by the calcium-activated protease calpain can lead to inactivation of caspases. J Biol Chem. 2000;275:5131–5135. doi: 10.1074/jbc.275.7.5131. [DOI] [PubMed] [Google Scholar]
- Cooray P, Yuan Y, Schoenwaelder SM, Mitchell CA, Salem HH, Jackson SP. Focal adhesion kinase (pp. 125FAK) cleavage and regulation by calpain. Biochemistry. 1996;318:41–47. doi: 10.1042/bj3180041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corasaniti MT, Navarra M, Catani MV, Melino G, Nistico G, Finazzi-Agro A. NMDA and HIV-1 coat protein, GP120, produce necrotic but not apoptotic cell death in human CHP100 neuroblastoma cultures via a mechanism involving calpain. Biochem Biophys Res Commun. 1996;229:299–304. doi: 10.1006/bbrc.1996.1796. 10.1006/bbrc.1996.1796. [DOI] [PubMed] [Google Scholar]
- Croall DE, Demartino GN. Calcium-activated neutral protease (calpain) system: structure, function, and regulation. Physiol Rev. 1991;71:813–847. doi: 10.1152/physrev.1991.71.3.813. [DOI] [PubMed] [Google Scholar]
- Del Cerro S, Larson J, Oliver MW, Lynch G. Development of hippocampal long-term potentiation is reduced by recently introduced calpain inhibitors. Brain Res. 1990;523:313–315. doi: 10.1016/0006-8993(90)90660-4. [DOI] [PubMed] [Google Scholar]
- Del Cerro S, Arai A, Kessler M, et al. Stimulation of NMDA receptors activates calpain in cultured hippocampal slices. Neurosci Lett. 1994;167:149–152. doi: 10.1016/0304-3940(94)91049-9. [DOI] [PubMed] [Google Scholar]
- Denny JB, Polan-Curtain J, Ghuman A, Wayner MJ, Armstrong DL. Calpain inhibitors block long-term potentiation. Brain Res. 1990;534:317–320. doi: 10.1016/0006-8993(90)90148-5. [DOI] [PubMed] [Google Scholar]
- Di Lisa F, De Tullio R, Salamino F, et al. Specific degradation of troponin T and I by Mu-calpain and its modulation by substrate phosphorylation. Biochem J. 1995;308:57–61. doi: 10.1042/bj3080057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnest JP, Santos GF, Zuerbig S, Fox JE. Dystrophin-related protein in the platelet membrane skeleton. Integrin-induced change in detergent-insolubility and cleavage by calpain in aggregating platelets. J Biol Chem. 1995;270:27259–27265. doi: 10.1074/jbc.270.45.27259. [DOI] [PubMed] [Google Scholar]
- Edelstein CL, Wieder ED, Yaqoob MM, et al. The role of cysteine proteases in hypoxia-induced rat renal proximal tubular injury. Proc Natl Acad Sci USA. 1995;92:7662–7666. doi: 10.1073/pnas.92.17.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein CL, Yaqoob MM, Alkhunaizi A, et al. Modulation of hypoxia-induced calpain activity in rat renal proximal tubules. Kidney Int. 1996;50:1150–1157. doi: 10.1038/ki.1996.422. [DOI] [PubMed] [Google Scholar]
- Edelstein CL, Ling H, Gengaro PE, Nemenoff RA, Bahr BA, Schrier RW. Effect of glycine on prelethal and postlethal increases in calpain activity in rat renal proximal tubules. Kidney Int. 1997a;52:1271–1278. doi: 10.1038/ki.1997.452. [DOI] [PubMed] [Google Scholar]
- Edelstein CL, Ling H, Schrier RW. The nature of renal cell injury. Kidney Int. 1997b;51:1341–1351. doi: 10.1038/ki.1997.183. [DOI] [PubMed] [Google Scholar]
- Elliget KA, Phelps PC, Trump BF. Cytosolic Ca2+ elevation and calpain inhibitors in HgCl2 injury to rat kidney proximal tubule epithelial cells. Pathobiology. 1994;62:298–310. doi: 10.1159/000163923. [DOI] [PubMed] [Google Scholar]
- Elliott EM, Mattson MP, Vanderklish P, Lynch G, Change I, Sapolsky RM. Corticosterone exacerbates kainate-induced alterations in hippocampal tau immunoreactivity and spectrin proteolysis in vivo. J Neurochem. 1993;61:57–67. doi: 10.1111/j.1471-4159.1993.tb03537.x. [DOI] [PubMed] [Google Scholar]
- Faddis BT, Hasbani MJ, Goldberg MP. Calpain activation contributes to dendritic remodeling after brief excitotoxic injury in vitro. J Neurosci. 1997;17:951–959. doi: 10.1523/JNEUROSCI.17-03-00951.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Shaw C, Marina A, Cazorla P, Valdivieso F, Vazquez J. Anti-brain spectrin immunoreactivity in Alzheimer's disease: degradation of spectrin in an animal model of cholinergic degeneration. J Neuroimmunol. 1997;77:91–98. doi: 10.1016/s0165-5728(97)00066-0. 10.1016/s0165-5728(97)00066-0. [DOI] [PubMed] [Google Scholar]
- Figueiredo-Pereira ME, Efthimiopoulos S, Tezapsidis N, et al. Distinct secretases, a cysteine protease and a serine protease, generate the C termini of amyloid beta-proteins. J Neurochem. 1999;72:1417–1422. doi: 10.1046/j.1471-4159.1999.721417.x. [DOI] [PubMed] [Google Scholar]
- Fox JE.B, Taylor RG, Taffarel M, Boyles JK, Goll DE. Evidence that activation of platelet calpain is induced as a consequence of binding of adhesive ligand to the integrin glycoprotein IIbIIIa. J Cell Biol. 1993;120:1501–1507. doi: 10.1083/jcb.120.6.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Adachi E, Kawashima S, Yoshiya I, Hashimoto PH. Immunohistochemical distribution of calcium-activated neutral proteinases and endogenous CANP inhibitor in the rabbit hippocampus. J Comp Neurol. 1990;302:100–109. doi: 10.1002/cne.903020108. [DOI] [PubMed] [Google Scholar]
- Glaser T, Schwarz-Benmeir N, Barnoy S, Barak S, Eshhar Z, Kosower NS. Calpain (Ca (2+) -dependent thiol protease) in erythrocytes of young and old individuals. Proc Natl Acad Sci USA. 1994;91:7879–7883. doi: 10.1073/pnas.91.17.7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazner GW, Chan SL, Lu C, Mattson MP. Caspase-mediated degradation of AMPA receptor subunits: a mechanism for preventing excitotoxic necrosis and ensuring apoptosis. J Neurosci. 2000;20:3641–3649. doi: 10.1523/JNEUROSCI.20-10-03641.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynspan F, Griffin WR, Cataldo A, Katayama S, Nixon RA. Active site-directed antibodies identify calpain II as an early-appearing and pervasive component of neurofibrillary pathology in Alzheimer's disease. Brain Res. 1997;763:145–158. doi: 10.1016/s0006-8993(97)00384-3. 10.1016/s0006-8993(97)00384-3. [DOI] [PubMed] [Google Scholar]
- Guthrie PB, Segal M, Kater SB. Independent regulation of calcium revealed by imaging dendritic spines. Nature. 1991;354:76–80. doi: 10.1038/354076a0. [DOI] [PubMed] [Google Scholar]
- Harris AS, Croall DE, Morrow JS. The calmodulin-binding site in α-fodrin is near the calcium-dependant protease-I cleavage site. J Biol Chem. 1989;263:15754–15761. [PubMed] [Google Scholar]
- Hatakeyama T, Matsumoto M, Brengman JM, Yanagihara T. Immunohistochemical investigation of ischemic and postischemic damage after bilateral carotid occlusion in gerbils. Stroke. 1988;19:1526–1534. doi: 10.1161/01.str.19.12.1526. [DOI] [PubMed] [Google Scholar]
- Haug LS, Ostvold AC, Cowburn RF, et al. Decreased inositol (1,4,5) -trisphosphate receptor levels in Alzheimer's disease cerebral cortex: selectivity of changes and possible correlation to pathological severity. Neurodegeneration. 1996;5:169–176. doi: 10.1006/neur.1996.0024. 10.1006/neur.1996.0024. [DOI] [PubMed] [Google Scholar]
- Hiramatsu K, Kassell NF, Lee KS. Improved posthypoxic recovery of synaptic transmission in gerbil neocortical slices treated with a calpain inhibitor. Stroke. 1993;24:1725–1728. doi: 10.1161/01.str.24.11.1725. [DOI] [PubMed] [Google Scholar]
- Hong SC, Goto Y, Lanzino G, Soleau S, Kassell NF, Lee KS. Neuroprotection with a calpain inhibitor in a model of focal cerebral ischemia. Stroke. 1994a;25:663–669. doi: 10.1161/01.str.25.3.663. [DOI] [PubMed] [Google Scholar]
- Hong SC, Lanzino G, Goto Y, et al. Calcium-activated proteolysis in rat neocortex induced by transient focal ischemia. Brain Res. 1994b;661:43–50. doi: 10.1016/0006-8993(94)91178-9. [DOI] [PubMed] [Google Scholar]
- Inomata M, Kasai Y, Nakamura M, Kawashima S. Activation mechanism of calcium-activated neutral protease. Evidence for the existence of intramolecular and intermolecular autolyses. J Biol Chem. 1988;263:19783–19787. [PubMed] [Google Scholar]
- Inomata M, Hayashi M, Ohno-Iwashita Y, Tsubuki S, Saido TC, Kawashima S. Involvement of calpain in integrin-mediated signal transduction. Arch Biochem Biophys. 1996;328:129–134. doi: 10.1006/abbi.1996.0152. 10.1006/abbi.1996.0152. [DOI] [PubMed] [Google Scholar]
- Ivy GO, Seubert P, Baudry M, Lynch G. Presence of brain spectrin in dendrites of mammalian brain: technical factors involved in immunocytochemistry detection. Synapse. 1988;2:329–333. doi: 10.1002/syn.890020324. [DOI] [PubMed] [Google Scholar]
- James T, Matzelle D, Bartus R, Hogan EL, Banik NL. New inhibitors of calpain prevent degradation of cytoskeletal and myelin proteins in spinal cord in vitro. J Neurosci Res. 1998;51:218–222. doi: 10.1002/(SICI)1097-4547(19980115)51:2<218::AID-JNR10>3.0.CO;2-4. 10.1002/(sici)1097-4547(19980115)51:2<218::aid-jnr10b3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Jiang TX, Reid WD, Belcastro A, Road JD. Load dependence of secondary diaphragm inflammation and injury after acute inspiratory loading. Am J Respir Crit Care. 1998;157:230–236. doi: 10.1164/ajrccm.157.1.9702051. [DOI] [PubMed] [Google Scholar]
- Jordan J, Galindo MF, Miller RJ. Role of calpain- and interleukin-1 beta converting enzyme-like proteases in the beta-amyloid-induced death of rat hippocampal neurons in culture. J Neurochem. 1997;68:1612–1621. doi: 10.1046/j.1471-4159.1997.68041612.x. [DOI] [PubMed] [Google Scholar]
- Kaku DA, Giffard RG, Choi DW. Neuroprotective effects of glutamate antagonists and extracellular acidity. Science. 1993;260:1516–1518. doi: 10.1126/science.8389056. [DOI] [PubMed] [Google Scholar]
- Kampfl A, Posmantur R, Nixon R, et al. mu-calpain activation and calpain-mediated cytoskeletal proteolysis following traumatic brain injury. J Neurochem. 1996;67:1575–1583. doi: 10.1046/j.1471-4159.1996.67041575.x. [DOI] [PubMed] [Google Scholar]
- Karlsson JO, Blennow K, Janson I, et al. Increased proteolytic activity in lymphocytes from patients with early onset Alzheimer's disease. Neurobiol Aging. 1995;16:901–906. doi: 10.1016/0197-4580(95)02004-7. 10.1016/0197-4580(95)02004-7. [DOI] [PubMed] [Google Scholar]
- Kato M, Nonaka T, Maki M, Kikuchi H, Imajoh-Ohmi S. Caspases cleave the amino-terminal calpain inhibitory unit of calpastatin during apoptosis in human Jurkat T cells. J Biochem. 2000;127:297–305. doi: 10.1093/oxfordjournals.jbchem.a022607. [DOI] [PubMed] [Google Scholar]
- Kawasaki BT, Hoffman KB, Yamamoto RS, Bahr BA. Variants of the receptor/channel clustering molecule gephyrin in brain: distinct distribution patterns, developmental profiles, and proteolytic cleavage by calpain. J Neurosci Res. 1997;49:381–388. doi: 10.1002/(sici)1097-4547(19970801)49:3<381::aid-jnr13>3.0.co;2-2. 10.1002/(sici)1097-4547(19970801)49:3<381::aid-jnr13b3.3.co;2-1. [DOI] [PubMed] [Google Scholar]
- Kelton JG, Moore JC, Warkentin TE, Hayward CP. Isolation and characterization of cysteine proteinase in thrombotic thrombocytopenic purpura. Br J Haematol. 1996;93:421–426. doi: 10.1046/j.1365-2141.1996.4891031.x. [DOI] [PubMed] [Google Scholar]
- Klafki H, Abramowski D, Swoboda R, Paganetti PA, Staufenbiel M. The carboxyl termini of beta-amyloid peptides 1–40 and 1–42 are generated by distinct gamma-secretase activities. J Biol Chem. 1996;271:28655–28659. doi: 10.1074/jbc.271.45.28655. [DOI] [PubMed] [Google Scholar]
- Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- Kochhar A, Zivin JA, Mazzarella V. Pharmacologic studies of the neuroprotective actions of a glutamate antagonistin ischemia. J Neurotrauma. 1991;8:175–186. doi: 10.1089/neu.1991.8.175. [DOI] [PubMed] [Google Scholar]
- Kohli V, Gao W, Camargo Jr CA, Clavien PA. Calpain is a mediator of preservation-reperfusion injury in rat liver transplantation. Proc Natl Acad Sci USA. 1997;94:9354–9359. doi: 10.1073/pnas.94.17.9354. 10.1073/pnas.94.17.9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto T, Ueyama H, Watanabe S, et al. Immunohistochemical study of calpain and its endogenous inhibitor in the skeletal muscle of muscular dystrophy. Acta Neuropathol (Berl) 1995;89:399–403. doi: 10.1007/BF00307642. 10.1007/s004010050264. [DOI] [PubMed] [Google Scholar]
- Kumamoto T, Ueyama H, Sugihara R, Kominami E, Goll DE, Tsuda T. Calpain and cathepsins in the skeletal muscle of inflammatory myopathies. Eur Neurol. 1997;37:176–181. doi: 10.1159/000117430. [DOI] [PubMed] [Google Scholar]
- Lee KS. The role of calcium-activated proteolysis in ischemic brain injury. In: Peterson PL, Phillis JW, editors. Novel Therapeutics for CNS Injuries: Rationale & Results. Boca Raton: CRC Press, Inc.; 1995. pp. 347–361. [Google Scholar]
- Lee KS, Frank S, Vanderklish P, Arai A, Lynch G. Inhibition of proteolysis protects hippocampal neurons from ischemia. Proc Natl Acad Sci USA. 1991;88:7233–7237. doi: 10.1073/pnas.88.16.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Foley PL, Vanderklish P, Lynch G, Goto Y, Kassell NF. The role of calcium-activated proteolysis in vasospasm after subarachnoid hemorrhage. In: Findlay JM, editor. Plasticity and Pathology in the Damaged Brain: Cerebral Vasospasm. Elsevier Science Publishers BV; 1993. pp. 85–88. [Google Scholar]
- Lee KS, Yanamoto H, Fergus A, et al. Calcium-activated proteolysis as a therapeutic target in cerebrovascular disease. Ann NY Acad Sci. 1997;825:95–103. doi: 10.1111/j.1749-6632.1997.tb48419.x. [DOI] [PubMed] [Google Scholar]
- Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai L-H. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–364. doi: 10.1038/35012636. 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- Li QX, Evin G, Small DH, Multhaup G, Beyreuther K, Masters CL. Proteolytic processing of Alzheimer's disease beta A4 amyloid precursor protein in human platelets. J Biol Chem. 1995;270:14140–14147. doi: 10.1074/jbc.270.23.14140. [DOI] [PubMed] [Google Scholar]
- Li Z, Ortega-Vilain AC, Patil GS, et al. Novel peptidyl α-keto amide inhibitors of calpains and other cysteine proteases. J Med Chem. 1996;39:4089–4098. doi: 10.1021/jm950541c. [DOI] [PubMed] [Google Scholar]
- Li J, Nixon R, Messeries A, Berman S, Bursztajn S. Altered gene expression for calpain/calpastatin system in motor neuron degeneration (Mnd) mutant mouse brain and spinal cord. Brain Res Mol Brain Res. 1998;53:174–186. doi: 10.1016/s0169-328x(97)00295-7. 10.1016/s0169-328x(97)00295-7. [DOI] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Litersky JM, Johnson GV. Phosphorylation by cAMP-dependent protein kinase inhibits the degradation of tau by calpain. J Biol Chem. 1992;267:1563–1568. [PubMed] [Google Scholar]
- Lowy MT, Wittenberg L, Novotney S. Adrenalectomy attenuates kainic acid-induced spectrin proteolysis and heat shock protein 70 induction in hippocampus and cortex. J Neurochem. 1994;63:886–894. doi: 10.1046/j.1471-4159.1994.63030886.x. [DOI] [PubMed] [Google Scholar]
- Lynch G, Baudry M. The biochemistry of memory: a new and specific hypothesis. Science. 1984;224:1057–1063. doi: 10.1126/science.6144182. [DOI] [PubMed] [Google Scholar]
- Lynch G, Seubert P. Links between long-term potentiation and neuropathology. An hypothesis involving calcium-activated proteases. Ann NY Acad Sci. 1989;568:171–180. doi: 10.1111/j.1749-6632.1989.tb12505.x. [DOI] [PubMed] [Google Scholar]
- Madden KP, Clark WM, Zivin JA. Delayed therapy of experimental ischemia with competitive N-methyl-d-aspartate antagonists in rabbits. Stroke. 1993;24:1068–1071. doi: 10.1161/01.str.24.7.1068. [DOI] [PubMed] [Google Scholar]
- Maier CM, Sun GH, Kunis DM, Giffard RG, Steinberg GK. Neuroprotection by the N-methyl-d-aspartate receptor antagonist CGP 40116 in vivo and in vitro studies. J Neurochem. 1995;65:652–659. doi: 10.1046/j.1471-4159.1995.65020652.x. [DOI] [PubMed] [Google Scholar]
- Markgraf CG, Velayo NL, Johnson MP, et al. Six-hour window of opportunity for calpain inhibition in focal cerebral ischemia in rats. Stroke. 1998;29:152–158. doi: 10.1161/01.str.29.1.152. [DOI] [PubMed] [Google Scholar]
- Martin SJ, O'Brien GA, Nishioka WK, et al. Proteolysis of fodrin (non-erythroid spectrin) during apoptosis. J Biol Chem. 1995;270:6425–6428. doi: 10.1074/jbc.270.12.6425. [DOI] [PubMed] [Google Scholar]
- Masliah E, Iimoto DS, Saitoh T, Hanson LA, Terry RD. Increased immunoreactivity of brain spectrin in Alzheimer disease: a marker for synapse loss. Brain Res. 1990;531:55–65. doi: 10.1016/0006-8993(90)90755-z. [DOI] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Hansen L, Deteresa R, Terry RD. Quantitative synaptic alterations in the human neocortex during normal aging. Neurology. 1993;43:192–197. doi: 10.1212/wnl.43.1_part_1.192. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Calcium as sculptor and destroyer of neural circuitry. Exp Gerontol. 1992;27:29–49. doi: 10.1016/0531-5565(92)90027-w. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Calcium and neuronal injury in Alzheimer's disease. Contributions of beta-amyloid precursor protein mismetabolism, free radicals, and metabolic compromise. Ann NY Acad Sci. 1994;747:50–76. [PubMed] [Google Scholar]
- Melloni E, Pontremoli S. The calpains. Trends Neurosci. 1989;12:438–444. doi: 10.1016/0166-2236(89)90093-3. [DOI] [PubMed] [Google Scholar]
- Melloni E, Michetti M, Salamino F, Sparatore B, Pontremoli S. Mechanism of action of a new component of the Ca (2+) -dependent proteolytic system in rat brain: the calpain activator. Biochem Biophys Res Commun. 1998;249:583–588. doi: 10.1006/bbrc.1998.9200. 10.1006/bbrc.1998.9200. [DOI] [PubMed] [Google Scholar]
- Mercken M, Grynspan F, Nixon RA. Differential sensitivity to proteolysis by brain calpain of adult human tau, fetal human tau, and PHF-tau. FEBS Lett. 1995;368:10–14. doi: 10.1016/0014-5793(95)00590-6. 10.1016/0014-5793(95)00590-6. [DOI] [PubMed] [Google Scholar]
- Miller AC.K, Dwyer-Nield LD, Malkinson AM. Very early changes in pulmonary protein kinase C-alpha and calpain II contents following injection of butylated hydroxytoluene (BHT) into mice. Toxicology. 1995;97:141–149. doi: 10.1016/0300-483x(94)02943-o. 10.1016/0300-483x(94)02943-o. [DOI] [PubMed] [Google Scholar]
- Minami N, Tani E, Maeda Y, Yamaura I, Fukami M. Effects of inhibitors of protein kinase C and calpain in experimental delayed cerebral vasospasm. J Neurosurg. 1992;76:111–118. doi: 10.3171/jns.1992.76.1.0111. [DOI] [PubMed] [Google Scholar]
- Moon RT, Mcmahon AP. Generation of diversity in nonerythroid spectrin. Multiple polypeptides are predicted by sequence analysis of cDNAs encompassing the coding region of human nonerythroid alpha-spectrin. J Biol Chem. 1990;265:4427–4433. [PubMed] [Google Scholar]
- Mouatt-Prigent A, Karlsson JO, Agid Y, Hirsch EC. Increased M-calpain expression in the mesencephalon of patients with Parkinson's disease but not in other neurodegenerative disorders involving the mesencephalon: a role in nerve cell death? Neuroscience. 1996;73:979–987. doi: 10.1016/0306-4522(96)00100-5. 10.1016/0306-4522(96)00100-5. [DOI] [PubMed] [Google Scholar]
- Mueller AL, Artman LD, Balandrin MF, et al. NPS 1506, a novel NMDA receptor antagonist and neuroprotectant. Review of preclinical and clinical studies. Ann NY Acad Sci. 1999;890:450–457. doi: 10.1111/j.1749-6632.1999.tb08023.x. [DOI] [PubMed] [Google Scholar]
- Muller W, Connor JA. Dendritic spines as individual neuronal compartments for synaptic Ca2+ responses. Nature. 1991;354:73–76. doi: 10.1038/354073a0. [DOI] [PubMed] [Google Scholar]
- Mundo E, Soldati L, Bellodi L, Bianchi G. The calpain-calpastatin system in obsessive-compulsive disorder. Biol Psychiatry. 1997;42:228–229. doi: 10.1016/S0006-3223(97)00251-5. 10.1016/s0006-3223(97)00251-5. [DOI] [PubMed] [Google Scholar]
- Munirathinam S, Bahr BA. Positive modulation of hippocampal AMPA receptors promotes recovery several hours after trimethyltin-induced excitotoxicity (Abstract) Soc Neurosci. 2000;26:c3459. [Google Scholar]
- Nadler JV, Perry BW, Cotman CW. Intraventricular kainic acid preferentially destroys hippocampal pyramidal cells. Nature. 1978;271:676–677. doi: 10.1038/271676a0. [DOI] [PubMed] [Google Scholar]
- Nadler JV, Perry BW, Gentry C, Cotman CW. Degeneration of hippocampal CA3 pyramidal cells induced by intraventricular kainic acid. J Comp Neurol. 1980;192:333–359. doi: 10.1002/cne.901920209. [DOI] [PubMed] [Google Scholar]
- Najm I, Vanderklish P, Etebari A, Lynch G, Baudry M. Complex interactions between polyamines and calpain-mediated proteolysis in rat brain. J Neurochem. 1991a;57:1151–1158. doi: 10.1111/j.1471-4159.1991.tb08273.x. [DOI] [PubMed] [Google Scholar]
- Najm I, Vanderklish P, Lynch G, Baudry M. Effect of treatment with difluoromethylornithine on polyamine and spectrin breakdown levels in neonatal rat brain. Brain Res Dev Brain Res. 1991b;63:287–289. doi: 10.1016/0165-3806(91)90088-z. [DOI] [PubMed] [Google Scholar]
- Najm I, El-Skaf G, Massicotte G, Vanderklish P, Lynch G, Baudry M. Changes in polyamine levels and spectrin degradation following kainate-induced seizure activity: effect of difluoromethylornithine. Exp Neurol. 1992;116:345–354. doi: 10.1016/0014-4886(92)90013-g. [DOI] [PubMed] [Google Scholar]
- Newcomb JK, Pike BR, Zhao XR, Hayes RL. Concurrent assessment of calpain and caspase-3 activity by means of Western blots of protease-specific spectrin breakdown products. Calpain Meth Protocols. 2000;144:219–223. doi: 10.1385/1-59259-050-0:219. [DOI] [PubMed] [Google Scholar]
- Nilsson E, Alafuzoff I, Blennow K, et al. Calpain and calpastatin in normal and Alzheimer-degenerated human brain tissue. Neurobiol Aging. 1990;11:425–431. doi: 10.1016/0197-4580(90)90009-o. [DOI] [PubMed] [Google Scholar]
- Noraberg J, Bert JP, Fonnum F, Zimmer J. Trimethyltin (TMT) neurotoxicity in organotypic rat hippocampal slice cultures. Brain Res. 1998;783:305–315. doi: 10.1016/s0006-8993(97)01358-9. 10.1016/s0006-8993(97)01358-9. [DOI] [PubMed] [Google Scholar]
- Oliver M, Baudry M, Lynch G. The protease inhibitor leupeptin interferes with the development of LTP in hippocampal slices. Brain Res. 1990;505:233–238. doi: 10.1016/0006-8993(89)91448-0. [DOI] [PubMed] [Google Scholar]
- Oshima M, Koizumi S, Fujita K, Guroff G. Nerve growth factor‐induced decrease in the calpain activity of PC12 cells. J. Biol. Chem. 1989;264:20811–20816. [PubMed] [Google Scholar]
- Perlmutter LS, Gall C, Baudry M, Lynch G. Distribution of calcium-activated protease calpain in rat brain. J Comp Neurol. 1990;296:269–276. doi: 10.1002/cne.902960207. [DOI] [PubMed] [Google Scholar]
- Peterson C, Goldman JE. Alterations in calcium content and biochemical processes in cultured skin fibroblasts from aged and Alzheimer donors. Proc Natl Acad Sci USA. 1986;83:2758–2762. doi: 10.1073/pnas.83.8.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson C, Vanderklish P, Seubert P, Cotman C, Lynch G. Increased spectrin proteolysis in fibroblasts from aged and Alzheimer donors. Neurosci Lett. 1991;121:239–243. doi: 10.1016/0304-3940(91)90694-o. [DOI] [PubMed] [Google Scholar]
- Pink JJ, Wuerzberger-Davis S, Tagliarino C, et al. Activation of a cysteine protease in MCF-7 and T47D breast cancer cells during beta-lapachone-mediated apoptosis Exp. Cell Res. 2000;255:144–155. doi: 10.1006/excr.1999.4790. [DOI] [PubMed] [Google Scholar]
- Pinter M, Aszodi A, Friedrich P, Ginzburg I. Calpeptin, a calpain inhibitor, promotes neurite elongation in differentiating PC12 cells. Neurosci. Lett. 1994;170:91–93. doi: 10.1016/0304-3940(94)90246-1. [DOI] [PubMed] [Google Scholar]
- Pontremoli S, Salamino F, Sparatore B, Michetti M, Sacco O, Melloni E. Following association to the membrane, human erythrocyte procalpain is converted and released as fully active calpain. Biochim Biophys Acta. 1985;831:335–339. doi: 10.1016/0167-4838(85)90116-5. [DOI] [PubMed] [Google Scholar]
- Porn-Ares MI, Samali A, Orrenius S. Cleavage of the calpain inhibitor, calpastatin, during apoptosis. Cell Death Differ. 1998;5:1028–1033. doi: 10.1038/sj.cdd.4400424. [DOI] [PubMed] [Google Scholar]
- Posmantur R, Kampfl A, Siman R, et al. A calpain inhibitor attenuates cortical cytoskeletal protein loss after experimental traumatic brain injury in the rat. Neuroscience. 1997;77:875–888. doi: 10.1016/s0306-4522(96)00483-6. 10.1016/s0306-4522(96)00483-6. [DOI] [PubMed] [Google Scholar]
- Rami A, Krieglstein J. Protective effects of calpain inhibitors against neuronal damage caused by cytotoxic hypoxia in vitro and ischemia in vivo. Brain Res. 1993;609:67–70. doi: 10.1016/0006-8993(93)90856-i. [DOI] [PubMed] [Google Scholar]
- Ray SK, Shields DC, Saido TC, et al. Calpain activity and translational expression increased in spinal cord injury. Brain Res. 1999;816:375–380. doi: 10.1016/s0006-8993(98)01128-7. 10.1016/s0006-8993(98)01128-7. [DOI] [PubMed] [Google Scholar]
- Ray SK, Fidan M, Nowak MW, Wilford GG, Hogan EL, Banik NL. Oxidative stress and Ca2+ influx upregulate calpain and induce apoptosis in PC12 cells. Brain Res. 2000;852:326–334. doi: 10.1016/s0006-8993(99)02148-4. 10.1016/s0006-8993(99)02148-4. [DOI] [PubMed] [Google Scholar]
- Richard I, Broux O, Allamand V, et al. Mutations in the proteolytic enzyme calpain 3 cause limb-girdle muscular dystrophy type 2A. Cell. 1995;81:27–40. doi: 10.1016/0092-8674(95)90368-2. [DOI] [PubMed] [Google Scholar]
- Roberts-Lewis JM, Siman R. Spectrin proteolysis in the hippocampus: a biochemical marker for neuronal injury and neuroprotection. Ann NY Acad Sci. 1993;679:78–86. doi: 10.1111/j.1749-6632.1993.tb18290.x. [DOI] [PubMed] [Google Scholar]
- Roberts-Lewis JM, Savage MJ, Marcy VR, Pinsker LR, Siman R. Immunolocalization of calpain I-mediated spectrin degradation to vulnerable neurons in the ischemic gerbil brain. J Neurosci. 1994;14:3934–3944. doi: 10.1523/JNEUROSCI.14-06-03934.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock MT, Brooks WH, Roszman TL. Calcium-dependent signaling pathways in T cells. Potential role of calpain, protein tyrosine phosphatase 1b, and p130Cas in integrin-mediated signaling events. J Biol Chem. 1997;272:33377–33383. doi: 10.1074/jbc.272.52.33377. [DOI] [PubMed] [Google Scholar]
- Ruiz-Vela A, de Buitrago GG, Martinez AC. Implication of calpain in caspase activation during B cell clonal deletion. EMBO J. 1999;18:4988–4998. doi: 10.1093/emboj/18.18.4988. 10.1093/emboj/18.18.4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saatman KE, Bozyczko-Coyne D, Marcy V, Siman R, Mcintosh TK. Prolonged calpain-mediated spectrin breakdown occurs regionally following experimental brain injury in the rat. J Neuropathol Exp Neurol. 1996a;55:850–860. doi: 10.1097/00005072-199607000-00010. [DOI] [PubMed] [Google Scholar]
- Saatman KE, Murai H, Bartus RT, et al. Calpain inhibitor AK295 attenuates motor and cognitive deficits following experimental brain injury in the rat. Proc Natl Acad Sci USA. 1996b;93:3428–3433. doi: 10.1073/pnas.93.8.3428. 10.1073/pnas.93.8.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saido TC. Spatial resolution of proteolytic reactions in brain ischemia and Alzheimer's disease. Seikagaku. 1996;68:1507–1522. [PubMed] [Google Scholar]
- Saido TC, Nagao S, Shiramine M, et al. Autolytic transition of µ-calpain upon activation as resolved by antibodies distinguishing between pre- and post-autolysis forms. J Biochem. 1992;111:81–86. doi: 10.1093/oxfordjournals.jbchem.a123723. [DOI] [PubMed] [Google Scholar]
- Saido TC, Yokota M, Nagao S, et al. Spatial resolution of fodrin proteolysis in postischemic brain. J Biol Chem. 1993;268:25239–25243. [PubMed] [Google Scholar]
- Saido TC, Sorimachi H, Suzuki K. Calpain: new perspectives in molecular diversity and physiological-pathological involvement. FASEB J. 1994;8:814–822. [PubMed] [Google Scholar]
- Saito K, Elce JS, Hamos JE, Nixon RA. Widespread activation of calcium-activated neutral proteinase (calpain) in the brain in Alzheimer disease: a potential molecular basis for neuronal degeneration. Proc Natl Acad Sci USA. 1993;90:2628–2632. doi: 10.1073/pnas.90.7.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson J, Marcantonio JM, Duncan G. Calcium ionophore induced proteolysis and cataract: inhibition by cell permeable calpain antagonists. Biochem Biophys Res Commun. 1996;218:893–901. doi: 10.1006/bbrc.1996.0159. 10.1006/bbrc.1996.0159. [DOI] [PubMed] [Google Scholar]
- Sato M, Tani E, Matsumoto T, Fujikawa H, Imajoh Ohmi S. Generation of the catalytic fragment of protein kinase C alpha in spastic canine basilar artery. J Neurosurg. 1997;87:752–756. doi: 10.3171/jns.1997.87.5.0752. [DOI] [PubMed] [Google Scholar]
- Selznick LA, Zheng TS, Flavell RA, Rakic P, Roth KA. Amyloid beta-induced neuronal death is bax-dependent but caspase-independent. J Neuropathol Exp Neurol. 2000;59:271–279. doi: 10.1093/jnen/59.4.271. [DOI] [PubMed] [Google Scholar]
- Seubert P, Baudry M, Dudek S, Lynch G. Calmodulin stimulates the degradation of brain spectrin by calpain. Synapse. 1987;1:20–24. doi: 10.1002/syn.890010105. [DOI] [PubMed] [Google Scholar]
- Seubert P, Ivy G, Larson J, et al. Lesions of entorhinal cortex produce a calpain-mediated degradation of brain spectrin in dentate gyrus. I. Biochemical studies. Brain Res. 1988a;459:226–232. doi: 10.1016/0006-8993(88)90638-5. [DOI] [PubMed] [Google Scholar]
- Seubert P, Larson J, Oliver M, Jung MW, Baudry M, Lynch G. Stimulation of NMDA receptors induces proteolysis of spectrin in hippocampus. Brain Res. 1988b;460:189–194. doi: 10.1016/0006-8993(88)91222-x. [DOI] [PubMed] [Google Scholar]
- Seubert P, Lee K, Lynch G. Ischemia triggers NMDA receptor-linked cytoskeletal proteolysis in hippocampus. Brain Res. 1989a;492:366–370. doi: 10.1016/0006-8993(89)90921-9. [DOI] [PubMed] [Google Scholar]
- Seubert P, Nakagawa Y, Ivy G, Vanderklish P, Baudry M, Lynch G. Intrahippocampal colchicine injection results in spectrin proteolysis. Neuroscience. 1989b;31:195–202. doi: 10.1016/0306-4522(89)90041-9. [DOI] [PubMed] [Google Scholar]
- Seubert P, Peterson C, Vanderklish P, Cotman C, Lynch G. Increased spectrin proteolysis in the brindled mouse mutation. Neurosci Lett. 1990;108:303–308. doi: 10.1016/0304-3940(90)90658-v. [DOI] [PubMed] [Google Scholar]
- Shea TB. Restriction of microM-calcium-requiring calpain activation to the plasma membrane in human neuroblastoma cells: evidence for regionalized influence of a calpain activator protein. J Neurosci Res. 1997a;48:543–550. 10.1002/(sici)1097-4547(19970615)48:6<543::aid-jnr7b3.3.co;2-z. [PubMed] [Google Scholar]
- Shea TB. Phospholipids alter tau conformation, phosphorylation, proteolysis, and association with microtubules: implication for tau function under normal and degenerative conditions. J Neurosci Res. 1997b;50:114–122. doi: 10.1002/(SICI)1097-4547(19971001)50:1<114::AID-JNR12>3.0.CO;2-B. 10.1002/(sici)1097-4547(19971001)50:1<114::aid-jnr12b3.3.co;2-i. [DOI] [PubMed] [Google Scholar]
- Shea TB, Spencer MJ, Beermann ML, Cressmanc M, Nixon RA. Calcium influx into human neuroblastoma cells induces ALZ-50 immunoreactivity: involvement of calpain-mediated hydrolysis of protein kinase C. J Neurochem. 1996;66:1539–15349. doi: 10.1046/j.1471-4159.1996.66041539.x. [DOI] [PubMed] [Google Scholar]
- Shiba E, Kim S, Fujitani M, et al. Possible involvement of calpain in the growth of estrogen receptor positive breast cancer cells. Anticancer Res. 1996;16:773–777. [PubMed] [Google Scholar]
- Shields DC, Banik NL. Putative role of calpain in the pathophysiology of experimental optic neuritis. Exp Eye Res. 1998;67:403–410. doi: 10.1006/exer.1998.0537. 10.1006/exer.1998.0537. [DOI] [PubMed] [Google Scholar]
- Shields DC, Tyor WR, Deibler GE, Hogan EL, Banik NL. Increased calpain expression in activated glial and inflammatory cells in experimental allergic encephalomyelitis. Proc Natl Acad Sci USA. 1998;95:5768–5772. doi: 10.1073/pnas.95.10.5768. 10.1073/pnas.95.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields DC, Schaecher KE, Saido TC, Banik NL. A putative mechanism of demyelination in multiple sclerosis by a proteolytic enzyme, calpain. Proc Natl Acad Sci USA. 1999;96:11486–11491. doi: 10.1073/pnas.96.20.11486. 10.1073/pnas.96.20.11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siman R, Noszak JC. Excitatory amino acids activate calpain I and induce structural protein breakdown in vivo. Neuron. 1988;1:279–287. doi: 10.1016/0896-6273(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Siman R, Baudry M, Lynch G. Brain fodrin: Substrate for calpain I, an endogenous calcium-activated protease. Proc Natl Acad Sci USA. 1984;81:3572–3576. doi: 10.1073/pnas.81.11.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siman R, Noszek JC, Kegerise C. Calpain I activation is specifically related to excitatory amino acid induction of hippocampal damage. J Neurosci. 1989;9:1579–1590. doi: 10.1523/JNEUROSCI.09-05-01579.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siman R, Card JP, Davis LG. Proteolytic processing of beta-amyloid precursor by calpain I. J Neurosci. 1990;10:2400–2411. doi: 10.1523/JNEUROSCI.10-07-02400.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer MJ, Croall DE, Tidball JG. Calpains are activated in necrotic fibers from mdx dystrophic mice. J Biol Chem. 1995;270:10909–10914. doi: 10.1074/jbc.270.18.10909. [DOI] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Meth. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Sutula T, Harrison C, Steward O. Chronic epileptogenesis induced by kindling of the entorhinal cortex: the role of the dentate gyrus. Brain Res. 1986;385:291–299. doi: 10.1016/0006-8993(86)91075-9. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Tsuji S, Kubota S, Kimura Y, Imahori K. Limited autolysis of Ca2+-activated neutral protease (CANP) changes its sensitivity to Ca2+ ions. J Biochem. 1981;90:275–278. doi: 10.1093/oxfordjournals.jbchem.a133463. [DOI] [PubMed] [Google Scholar]
- Takahashi K. Calpain substrate specificity. In: Mellgren RL, Murachi T, editors. Intracellular calcium-dependent proteolysis. Boca Raton: CRC Press, Inc; 1990. pp. 55–74. [Google Scholar]
- Takaoka S, Bart RD, Pearlstein R, Brinkhous A, Warner DS. Neuroprotective effect of NMDA receptor glycine recognition site antagonism persists when brain temperature is controlled. J Cereb Blood Flow Metab. 1997;17:161–167. doi: 10.1097/00004647-199702000-00005. [DOI] [PubMed] [Google Scholar]
- Takeyama Y, Nakanishi H, Uratsuji Y, Kishimoto A, Nishizuka Y. A calcium-protease activator associated with brain microsomal-insoluble elements. FEBS Lett. 1986;94:110–114. doi: 10.1016/0014-5793(86)80060-6. [DOI] [PubMed] [Google Scholar]
- Tijsen MJ, Peters SM, Bindels RJ, van-Os CH, Wetzels JF. Glycine protection against hypoxic injury in isolated rat proximal tubules: the role of proteases. Nephrol Dial Transplant. 1997;12:2549–2556. doi: 10.1093/ndt/12.12.2549. 10.1093/ndt/12.12.2549. [DOI] [PubMed] [Google Scholar]
- Tolnadi S, Korecky B. Calcium dependent proteolysis and its inhibition in ischemic rat myocardium. Can J Cardiol. 1986;2:442–447. [PubMed] [Google Scholar]
- Tsuji T, Shimohama S, Kimura J, Shimizu K. m-Calpain (calcium-activated neutral proteinase) in Alzheimer's disease brains. Neurosci Lett. 1998;248:109–112. doi: 10.1016/s0304-3940(98)00348-6. 10.1016/s0304-3940(98)00348-6. [DOI] [PubMed] [Google Scholar]
- Vanderklish P, Saido TC, Gall C, Lynch G. Proteolysis of spectrin by calpain accompanies theta-burst stimulation in cultured hippocampal slices. Mol Brain Res. 1995;32:25–35. doi: 10.1016/0169-328x(95)00057-y. 10.1016/0169-328x(95)00057-y. [DOI] [PubMed] [Google Scholar]
- Vanderklish PW, Bednarski E, Lynch G. Translational suppression of calpain blocks long-term potentiation. Learning Memory. 1996;3:209–217. doi: 10.1101/lm.3.2-3.209. [DOI] [PubMed] [Google Scholar]
- Vanderklish PW, Krushel LA, Holst BH, Gally JA, Crossin KL, Edelman GM. Marking synaptic activity in dendritic spines with a calpain substrateexhibiting fluorescence resonance energy transfer. Proc Natl Acad Sci USA. 2000;97:2253–2258. doi: 10.1073/pnas.040565597. 10.1073/pnas.040565597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vornov JJ, Tasker RC, Coyle JT. Direct observation of the agonist-specific regional vulnerability to glutamate, NMDA, and kainate neurotoxicity in organotypic hippocampal cultures. Exp Neurol. 1991;114:11–22. doi: 10.1016/0014-4886(91)90079-r. [DOI] [PubMed] [Google Scholar]
- Vornov JJ, Tasker RC, Park J. Neurotoxicity of acute glutamate transport blockage depends on coactivation of both NMDA and AMPA/kainate receptors in organotypic hippocampal cultures. Exp Neurol. 1995;133:7–17. doi: 10.1006/exnr.1995.1002. 10.1006/exnr.1995.1002. [DOI] [PubMed] [Google Scholar]
- Wang KK.W. Calpain and caspase: can you tell the difference? Trends Neurosci. 2000;23:20–26. doi: 10.1016/s0166-2236(99)01536-2. 10.1016/s0166-2236(99)01479-4. [DOI] [PubMed] [Google Scholar]
- Wang L, Bhatnagar A, Ansari NH, Dhir P, Srivastava SK. Mechanism of calcium-induced disintegrative globulization of rat lens fiber cells. Invest Ophthalmol Vis Sci. 1996;37:915–922. [PubMed] [Google Scholar]
- Wolf BB, Goldstein JC, Stennicke HR, et al. Calpain functions in a caspase-independent manner to promote apoptosis-like events during platelet activation. Blood. 1999;94:1683–1692. [PubMed] [Google Scholar]
- Wood DE, Newcomb EW. Caspase-dependent activation of calpain during drug-induced apoptosis. J Biol Chem. 1999;274:8309–8315. doi: 10.1074/jbc.274.12.8309. [DOI] [PubMed] [Google Scholar]
- Xie HQ, Johnson GV. Calcineurin inhibition prevents calpain-mediated proteolysis of tau in differentiated PC12 cells. J Neurosci Res. 1998;53:153–164. doi: 10.1002/(SICI)1097-4547(19980715)53:2<153::AID-JNR4>3.0.CO;2-6. 10.1002/(sici)1097-4547(19980715)53:2<153::aid-jnr4b3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Yamaura I, Tani E, Saido TC, Suzuki K, Minami N, Maeda Y. Calpain-calpastatin system of canine basilar artery in vasospasm. J Neurosurg. 1993;79:537–543. doi: 10.3171/jns.1993.79.4.0537. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Ihara Y. Effects of specific protease inhibitors on amyloid beta-protein 42 secretion. Neurobiol Aging. 1998;19:S77–S79. doi: 10.1016/s0197-4580(98)00031-1. 10.1016/s0197-4580(98)00031-1. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Haass C, Saido TC, Omura S, Ihara Y. Specific increase in amyloid beta-protein 42 secretion ratio by calpain inhibition. Biochemistry. 1997;36:8377–8383. doi: 10.1021/bi970209y. [DOI] [PubMed] [Google Scholar]
- Yang LS, Ksiezak-Reding H. Calpain-induced proteolysis of normal human tau and tau associated with paired helical filaments. Eur J Biochem. 1995;233:9–17. doi: 10.1111/j.1432-1033.1995.009_1.x. [DOI] [PubMed] [Google Scholar]
- Yang LS, Gordon-Krajcer W, Ksiezak-Reding H. Tau released from paired helical filaments with formic acid or guanidine is susceptible to calpain-mediated proteolysis. JNeurochem. 1997;69:1548–1558. doi: 10.1046/j.1471-4159.1997.69041548.x. [DOI] [PubMed] [Google Scholar]
- Yao H, Ginsberg MD, Eveleth DD, et al. Local cerebral glucose utilization and cytoskeletal proteolysis as indices of evolving focal ischemic injury in core and penumbra. J Cereb Blood Flow Metab. 1995;15:398–408. doi: 10.1038/jcbfm.1995.50. [DOI] [PubMed] [Google Scholar]
- Yokota M, Tani E, Tsubuki S, et al. Calpain inhibitor entrapped in liposome rescues ischemic neuronal damage. Brain Res. 1999;819:8–14. doi: 10.1016/s0006-8993(98)01334-1. 10.1016/s0006-8993(98)01334-1. [DOI] [PubMed] [Google Scholar]
- Yuen PW, Wang KW. Therapeutic potential of calpain inhibitors in neurodegenerative disorders. Exp Opin Invest Drugs. 1996;5:1291–1304. [Google Scholar]
- Zhao X, Pike BR, Newcomb JK, Wang KK, Posmantur RM, Hayes RL. Maitotoxin induces calpain but not caspase-3 activation and necrotic cell death in primary septo-hippocampal cultures. Neurochem Res. 1999;24:371–382. doi: 10.1023/a:1020933616351. [DOI] [PubMed] [Google Scholar]