Abstract
Membrane fusion is a ubiquitous event that occurs in a wide range of biological processes. While intracellular membrane fusion mediating organelle trafficking is well understood, much less is known about cell–cell fusion mediating sperm cell–oocyte, myoblast–myoblast and macrophage–macrophage fusion. In the case of mononuclear phagocytes, their fusion is not only associated with the differentiation of osteoclasts, cells which play a key role in the pathogenesis of osteoporosis, but also of giant cells that are present in chronic inflammatory reactions and in tumours. Despite the biological and pathophysiological importance of intercellular fusion events, the actual molecular mechanism of macrophage fusion is still unclear. One of the main research themes in my laboratory has been to investigate the molecular mechanism of mononuclear phagocyte fusion. Our hypothesis has been that macrophage–macrophage fusion, similar to virus–cell fusion, is mediated by specific cell surface proteins. But, in contrast with myoblasts and sperm cells, macrophage fusion is a rare event that occurs in specific instances. To test our hypothesis, we established an in vitro cell–cell fusion assay as a model system which uses alveolar macrophages. Upon multinucleation, these macrophages acquire the osteoclast phenotype. This indicates that multinucleation of macrophages leads to a specific and novel functional phenotype in macrophages. To identify the components of the fusion machinery, we generated four monoclonal antibodies (mAbs) which block the fusion of alveolar macrophages and purified the unique antigen recognized by these mAbs. This led us to the cloning of MFR (Macrophage Fusion Receptor). MFR was cloned simultaneously as P84/SHPS-1/SIRPα/BIT by other laboratories. We subsequently showed that the recombinant extracellular domain of MFR blocks fusion. Most recently, we identified a lower molecular weight form of MFR that is missing two extracellular immunoglobulin (Ig) C domains. Shortly after we cloned MFR, CD47 was reported to be a ligand for P84/SIRPα. We have since generated preliminary results which suggest that CD47 interacts with MFR during adhesion/fusion and is a member of the fusion machinery. We also identified CD44 as a plasma membrane protein which, like MFR, is highly expressed at the onset of fusion. The recombinant soluble extracellular domain of CD44 blocks fusion by interacting with a cell-surface binding site. We now propose a model in which both forms of MFR, CD44, and CD47 mediate macrophage adhesion/fusion and therefore the differentiation of osteoclasts and giant cells.
Osteoclasts and giant cells are characterized by multinucleation and a powerful ability to resorb the substrate onto which they adhere. While osteoclasts and giant cells play an important role in tissue remodelling and defence, respectively, they are also associated with bone loss, granulomatous diseases and tumours. It is well established that although distinct, both cells share the same functional markers, and both differentiate by fusion of precursor cells that belong to the monocyte–macrophage lineage. Indeed, fusion is an obligatory step in the structural and functional differentiation of these cells. Over the last decade, my laboratory has focused on understanding the molecular mechanism by which fusion of macrophages occurs, a prerequisite to controlling the differentiation of osteoclasts and giant cells.
Virus–cell binding and fusion mechanism binding and fusion mechanism
Our initial understanding of membrane fusion was gained through the study of viral fusion reactions (Hernandez et al. 1996). Studies of viruses, especially influenza and human immunodeficiency virus (HIV), have provided strong evidence that viral fusion is mediated by both viral and cellular membrane proteins whose function is to help overcome the repulsive forces that inhibit fusion and/or promote the hydrophobic forces that favour fusion (Wilschut & Hoekstra 1991). The mechanism by which enveloped viruses enter cells has very well defined molecular basis (Hernandez et al. 1996). It is now established that binding and fusion of viruses with host cells is mediated by viral proteins and host cell surface molecules that are used as viral receptors. Virus and host cell plasma membrane binding is mediated by a ‘receptor–ligand’ type of interaction. For instance, the human immunodeficiency virus (HIV), which causes AIDS, binds CD4 on T lymphocytes and macrophages; rhinoviruses bind ICAM-1; haemagglutinin influenza virus binds specific sialic acid residues. The binding of viruses to host cells is mediated by a viral membrane protein which acts as a ‘ligand’ for the host cell receptor. Such ligands include the HIVgp120 surface protein which binds CD4 and the HA1 of influenza virus which binds sialic acid residues.
Most viral proteins which are responsible for binding to host cells are also responsible for fusion. These fusion proteins are integral membrane glycoproteins that exist as oligomers on the virion surface. Most viral fusion proteins contain a stretch of hydrophobic amino acids known as a ‘fusion peptide’, located either at the amino terminal end or within the polypeptide. Several of these proteins are synthesized as precursors that are cleaved post-translationally into two subunits. For instance, the cleavage of the precursor molecule HIVgp160 leads to the formation of gp120 and gp41 (Figure 1). Cleavage of this class of proteins is essential for fusion activity.
Figure 1.
Subunit organization of the fusion glycoproteins of influenza Sendai, human immunodeficiency, and Rous Sarcoma viruses. Hatched boxes represent fusion peptides, filled black boxes represent transmembrane domains. Redrawn after Hernandez et al. (1996).
The viral fusion protein is thought to penetrate the host cell, to destabilize the host cell lipid bilayer and to trigger membrane fusion (Figure 2). HA2 and HIVgp41 viral fusion proteins are two such examples. Virus–cell fusion therefore requires both a ‘ligand–receptor’ type of interaction, which presumably brings the two membranes in close proximity, and a fusion protein.
Figure 2.
A schematic hypothetical model of the postulated fusion site created during influenza virus HA-mediated fusion. Left, the association of several unfolded HA trimers is proposed to dehydrate the intermediate space, thereby forming an intermembrane intermediate. Right, rupture of the intermembrane intermediate perpendicular to the plane of the membranes would create a small pore, or interlamellar attachment site, thereby causing bilayer fusion. Reprinted after Hernandez et al. (1996).
Intracellular membrane fusion during exocytosis
Much progress has been made in recent years regarding intracellular membrane fusion. Intracellular trafficking of molecules from one compartment to another, such as from the endoplasmic reticulum to the golgi, from the golgi to the plasma membrane, requires a donor vesicle and an acceptor target membrane. Fusion of an intracellular vesicle with its target membrane is known to be mediated by a set of conserved proteins collectively referred to as SNAREs (soluble NSF-attachment protein (SNAP) receptors; NSF is N-ethylmaleimide-sensitive fusion protein). Many (vesicle) v- and (target) t-SNAREs have now been characterized in yeast, plants, and animals (Rothman 1994; Bennett 1995; Linial 1997). In exocytosis of synaptic vesicles, for instance, a well-studied membrane fusion reaction, these SNAREs include the plasma-membrane-associated proteins syntaxin and SNAP-25 (synaptosome-associated protein of relative molecular mass 25 kDa), and the vesicular protein synaptobrevin (Figure 3). Although the function of the SNAREs in membrane fusion is not fully understood, it must be fundamental since proteolytic cleavage of the SNAREs by clostridial neurotoxins inhibits neurotransmission. Spontaneous assembly of the SNARE proteins into a ternary complex is followed by the fusion of the vesicle with its target membrane, and release of neurotransmitter into the synaptic cleft (Sutton et al. 1998). This suggests that SNARE proteins are the minimal machinery for cellular membrane fusion. Since SNARE proteins mediate recognition/binding of specific membranes, SNAREs may also mediate fusion, thereby combining the two machineries into one (Figure 4). This model revealed that intracellular membrane fusion may not require the presence of a hydrophobic stretch in the cytoplasmic domain of SNAREs. Importantly, this is a scenario similar to that of viral–cell fusion in which viral proteins bind a cell surface receptor to mediate fusion. Thus, it appears that both viral–cell fusion and intracellular membrane fusion utilize one system that combines two functions, attachment and fusion (Weber et al. 1998). Both fusion mechanisms involve receptor–ligand interactions.
Figure 3.

Hypothetical model of the synaptic fusion complex as it joins two membranes. Reprinted after Sutton et al. (1998).
Figure 4.
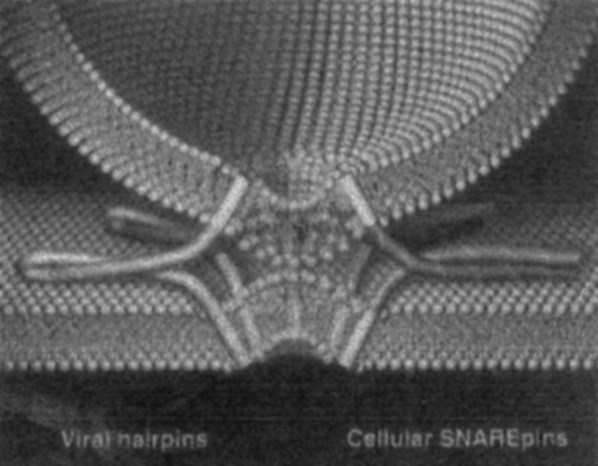
Hypothetical model of the synaptic fusion complex as it joins two membranes. In contrast to viral hairpins (fusion proteins, left), cellular SNAREpins (right) are formed from separate polypeptides that reside in different membranes before fusion. The similarity between SNAREpins (in which pins represent the hydrophobic domain of the polypeptide) and viral hairpins suggests that they all employ a fundamentally similar mechanism to coalesce lipid bilayers. Reprinted after Weber et al. (1998).
Sperm–oocyte, myoblast and trophoblast fusion
It is possible that common mechanisms exist among all fusion events, including cell–cell fusion events. Indeed, ‘fusion proteins’ structurally similar to viral proteins have been identified in sperm cells, myoblasts and trophoblasts.
A putative guinea pig sperm fusion protein, PH-30, had been cloned and characterized (Blobel et al. 1992). PH-30, then called fertilin, was originally identified by a monoclonal antibody that blocks sperm–oocyte fusion. It is an integral membrane glycoprotein located in the posterior head of the mature sperm and is composed of two subunits. Processing of the two subunits is developmentally regulated and occurs in a region of the epididymis where sperm cells become fertilization-competent. Interestingly, processing exposes two new epitopes on fertilin, one of which is recognized by the fusion-inhibitory monoclonal antibody. This antibody, however, does not prevent sperm from binding to the egg plasma membrane. The sequence of the cloned fertilin indicates the presence of a potential fusion peptide and an integrin ligand domain (Blobel et al. 1992). The recent functional analysis of fertilin using gene knockout technology has revealed that fertilin is required for fertilization, but its role in fusion remains uncertain (Cho et al. 1998). Most recently, CD9 was reported as essential for fertilization (Le Naour et al. 2000; Miyado et al. 2000) but its actual role in the fusion mechanism remains unsettled.
A homologue of fertilin called meltrin had been cloned from fusing myoblasts (Yagami-Hiromasa et al. 1995). Meltrin α shares structural homology with fertilinα and was thought to participate in myoblast fusion. Since no further indication on the role of meltrin in myoblast fusion has been reported in the literature, the role played by meltrin α in fusion remains to be investigated. It appears that the extent to which fertilin and meltrin are involved in the fusion event remains unclear.
Most recently, syncytin was cloned from a human testis library and shown to be identical to the human retrovirus HERV-W (Mi et al. 2000). Syncytin transcripts are abundantly expressed in syncytiotrophoblasts, multinucleated cells that originate from the fusion of fetal trophoblasts. The expression of syncytin by testis, the only other tissue in which it is expressed, suggests a possible role of syncytin in fertilization.
Together, our understanding of membrane fusion required for viral–cell fusion or intracellular trafficking converge to a model in which proteins from opposite membranes entertain a ligand–receptor type of interaction. These proteins constitute the minimal fusion machinery for membrane fusion, a model that may apply to the fusion of mononuclear phagocytes.
Role of associated proteins in virus–cell and cell–cell fusion
Virus–cell and cell–cell fusion is a more complicated scenario than originally thought and appears to involve regulatory molecules. While it had been thought that HIV needed only the T lymphocyte receptor CD4 to infect cells, several chemokines have now been demonstrated to slow the growth of HIV in cultures. It has been determined that the chemokine family of G-protein coupled receptors, most notably CXCR4 and CCR5, are involved in HIV infection (Alkhatib et al. 1996; Deng et al. 1996; Dragic et al. 1996; Feng et al. 1996). There are now at least 10 chemokine receptors identified as HIV coreceptors (Dimitrov 1997). Furthermore, the interaction between the adhesion molecules leucocyte function-associated antigen-1 (LFA-1) and intercellular adhesion molecule-1 (ICAM-1) has been described with respect to both virus–cell and cell–cell fusion events. HIV-induced syncytium formation is blocked by a monoclonal antibody directed against the α subunit of LFA-1 (Hildreth & Orentas 1989).
Recent evidence has been reported indicating that cytokine-induced giant cell formation in peripheral blood monocyte cultures (Most et al. 1990; Kazazi et al. 1994) as well as osteoclast development (Kurachi et al. 1993) are inhibited by antibodies directed against LFA-1 and ICAM-1. Members of the cadherin family of homophilic cell adhesion molecules have also been implicated in cell–cell fusion events. While N-cadherin mediated adhesion appears necessary for myoblast fusion (Mege et al. 1992), inhibition of E-cadherin function prevents the differentiation of osteoclasts in a bone marrow culture (Mbalaviele et al. 1995).
It is important to stress here that the assays used in the above mentioned experiments require a cell proliferation/substrate adhesion step in order to generate multinucleated osteoclasts and giant cells. These experiments were not designed to specifically analyse the fusion event, but rather the overall proliferation-substrate adhesion-multinucleation event. So LFA-1, ICAM-1 and E-cadherin are likely to be involved at a step earlier in fusion, such as cell-substrate adhesion that accompanies proliferation.
Another protein thought to participate in macrophage fusion is the purinergic receptor P2Z/P2X7 that binds extracellular ATP. Although J774 cell clones that express this pore-forming receptor at very high levels, as well as HEK293 cells stably transfected with P2X7 cDNA, demonstrate some level of multinucleation when grown to confluence (Chiozzi et al. 1997), oxidized ATP inhibits giant cell formation from Con A and interferon γ-stimulated monocytes (Falzoni et al. 1995). In such cells, however, multinucleation is accompanied by cell death. So while the purinergic receptor P2Z/P2X may be involved at some stage in the cell–cell interaction process, it may not mediate the actual fusion event.
Most recently, a set of proteins thought to enhance or induce cell fusion, initially termed FRP-1 and FRP-2 and now known to be CD98 and integrin α3, respectively (Ohta et al. 1994; Ohgimoto et al. 1995; Higuchi et al. 1998), have been identified in a number of cell lines infected with several different viruses as well as on the surface of monocytes and macrophages. Monoclonal antibodies directed against these proteins stimulate polykaryocyte formation in CD4+ U937 cells transfected with the HIV gp160 gene (Ohta et al. 1994) and in HeLa and FL cells infected with Newcastle disease virus (Ito et al. 1992). In addition, anti-FRP antibodies inhibit giant cell formation in cultures of peripheral blood monocytes (Tabata et al. 1994). Again, these molecules may regulate rather than mediate cell–cell fusion.
While none of the above mentioned proteins appear as actual fusion proteins and may not therefore mediate the actual fusion event, together they suggest that the fusion mechanism of viruses and mammalian cells involves both adhesion molecules and regulatory proteins.
Mononuclear phagocyte multinucleation: osteoclasts and giant cells
Mononuclear phagocytes are unique cells which are distributed ubiquitously in tissues and can be programmed, in specific instances, to fuse in order to differentiate into osteoclasts or multinucleated giant cells. Osteoclasts differentiate on bone which they resorb and therefore play a key role in the pathogenesis of osteoporosis, bone tumours and bone transplant rejection. Giant cells differentiate primarily in chronic inflammatory sites in response to bacterial invasion and foreign bodies such as implants, as well as in tumours. Mononuclear phagocytes are multifaceted cells that can be viewed as the ‘health care’ system for tissues. There, they fulfil immune and non-immune functions, ingest and digest intracellularly, and signal other cells locally for ‘action’. In addition, macrophages can evolve from a mononucleated status in which they can ingest bacteria, foreign material, to a multinucleated status in which they resorb larger components. By virtue of fusing with one another, macrophages acquire the capability to digest larger components. When such components are too large to be endocytosed, macrophages reverse their vectorial destructive ability, and deploy a sophisticated protein machinery that allows them to resorb extracellularly. In short, osteoclasts and giant cells strongly adhere to the substrate that they resorb via a sealing zone, which delineates a domain located extracellularly, and in which lysosomal enzymes and protons are secreted (see Baron 1995 for review). This creates an extracellular lysosome. This allows for the powerful and targeted resorption of extracellularly located material, whether it is aggregated bacteria, implants or bone. Fusion therefore appears as a late, but a key event in the differentiation of multinucleated osteoclasts and giant cells. It has therefore become clear that improving our understanding of the mechanism underlying fusion is essential to design and engineer molecules that control osteoclast and giant cell differentiation.
Role of the extracellular environment in macrophage fusion
Mononuclear phagocytes fuse only in specific microenvironments, i.e. bone surface for osteoclasts and chronic inflammatory sites for giant cells (Langhans 1947; Virchow 1948). It is clear that in order to fuse, these cells must reach a ‘critical density’ (cell–cell contact) and are therefore dependent on the activity of local factors, either growth factors, such as M-CSF, or chemotactic factors. In this respect, we have shown that the cytokine interferon γ is a strong inducer of osteoclast-like cell differentiation in vivo (Vignery et al. 1990) suggesting that it may regulate the production of local factors. Most recently, osteoprotegerin (OPG) (Simonet et al. 1997) and osteoprotegerin ligand (OPGL) (Lacey et al. 1998) have been shown to inhibit and stimulate, respectively, osteoclast-like cell differentiation in spleen cell and bone marrow cultures. Of interest, OPGL is identical to ODF (osteoclast differentiation factor) as well as to TRANCE/RANKL, which is a tumour necrosis factor-related cytokine (Anderson et al. 1997; Wong et al. 1997; Yasuda et al. 1998). OPG and OPGL appear to control osteoclast differentiation from arthroplasty membrane derived macrophages, the very cells that form giant cells (Itonaga et al. 2000). Additional factors may include l,25α(OH)2D3, a potent stimulator of bone resorption and a well-known macrophage and osteoclast-like cell differentiation factor (Abe et al. 1983; Shiina et al. 1986). In the case of cell–cell fusion and membrane fusion between cell compartments, it has been suggested that an acidic environment can induce or trigger these events (Franklin 1958; Hernandez et al. 1996). Fusion of mononuclear phagocytes has been induced in vitro by short exposure of the cell cultures to a low pH (Franklin 1958). The conditions mentioned above as being able to alter cell fusion may be present in chronic inflammatory areas where osteoclasts and giant cells are often observed, as well as in some types of tumours and around grafted materials and implants (Langhans 1947). Much less is known about the normal bone-remodelling microenvironment (Baron et al. 1983; Baron et al. 1986).
Macrophage fusion mechanism: hypothesis
Over the last decade, our laboratory has been studying the mechanism of homotypic fusion of cells belonging to the mononuclear phagocyte lineage. Our hypothesis has been that a specific set of proteins mediates macrophage–macrophage adhesion/fusion, and that these proteins resemble those used by viruses to infect cells. But, in contrast with viruses, myoblasts and sperm cells, which must fuse to allow for infection, striated muscle cell differentiation and fertilization, respectively, macrophages reside in tissues as mononucleated and fuse only in specific instances. Therefore we hypothesized that, in response to local stimuli, macrophages express a fusion machinery that is regulated, i.e. induced in specific instances, and repressed when fusion/multinucleation is complete, and therefore transient.
Osteoclasts and giant cells: sisters or cousins?
While it is generally agreed that osteoclasts differ from giant cells, it is also agreed that both cells originate from the fusion of mononucleated precursors that belong to the mononuclear phagocyte lineage. Over the last decade, in order to investigate the fusion mechanism of macrophages, we have been culturing fusing macrophages that originate from different species (rat, mouse, chick) collected from different tissues (peritoneal cavity, lungs, etc.), as well as generating multinucleated macrophages induced in vivo in muscle, in skin and in the peritoneal cavity (on implanted coverslips, bone particles). Regardless of their origin (species, organ, tissue), all multinucleated macrophages that we generated in vitro and in vivo expressed the functional markers that characterize osteoclasts (Vignery et al. 1989; Vignery et al. 1991a; Vignery et al. 1991b; Vignery et al. 1991c; Baron 1995). Such functional markers include a high and polarized expression of Na, K-ATPases, H+-ATPases, receptor for calcitonin, as well as Tartrate Resistant Acid Phosphatase (TRAP) (unpublished data). At this point in time, we have not been able to identify a molecule whose expression is specific and restricted to either osteoclasts, or to giant cells. This does not imply that these cells are identical. Indeed, they are not. It is likely that multinucleated macrophages acquire a tissue-specific molecular specialization, like macrophages do. By following this reasoning, it is logical to assume that the bone microenvironment differs from that of the lung, the skin, or the peritoneal cavity, and that of all tissues in general. But regardless of the tissue in which macrophages differentiate and reside, multinucleation endows them with a common functionality that is designed to amplify their resorptive ability. So we have been postulating that in order to become multinucleated, macrophages have evolved a machinery that is shared by macrophages in all tissues, including bone.
How do mononucleated precursors accumulate before fusing?
Although osteoclasts and giant cells differentiate by fusion of mononucleated precursor cells, the actual mechanism by which their mononucleated precursors accumulate in a specific site remains unclear. One possibility is that macrophages immigrate one at a time from the blood stream to reach their final destination, in which they fuse with one another. Another possibility is that only one, or relatively few precursors, leave the blood stream to reach their target site of differentiation, and replicate on site in response to a local growth factor, such as M-CSF. That growth factor would be responsible for the clonal expansion of one parent macrophage, whose progeny, the daughter cells, fuse to differentiate into a giant cell or an osteoclast. At this point in time, the mechanism by which macrophages reach a critical concentration that allows for fusion remains unclear. But the possibility of a targeted clonal expansion to a specific site is attractive because it is simple, safe and efficient. One must consider the fact that, in general, giant cells are larger than osteoclasts, i.e. contain hundreds of nuclei, while osteoclasts usually contain only a few, except in diseases such as Paget's disease, and in response to bone implants on which osteoclast-like cells/giant cells differentiate. The reality of the matter is that giant cells differentiate in chronic inflammatory sites, and stand as the landmark of granulomas, while osteoclasts differentiate in the bone compartment, in the apparent absence of inflammation. But one could argue that bone remodelling results from a microinflammatory reaction, the result of which is the replacement of old, unfunctional bone, by newly synthesized bone. Therefore the differentiation of giant cells may result from both a massive migration of monocytes towards the site of aggression (implant, infectious agents) and a local clonal expansion. But given their paucity (0.5% of the bone surface is covered by osteoclasts) and their limited (2–6) number of nuclei, osteoclasts are more likely to differentiate via local, targeted clonal expansion. and this expansion must be regulated since it is limited. But again, the actual cut-off line that discriminates between an osteoclast and a giant cell remains controversial.
Fusing rat alveolar macrophages: a model system
To test our hypothesis, we established a highly pure and efficient in vitro fusion assay that uses alveolar macrophages, the fusion of which initiates hours after plating and is complete within 3 days (Figure 5). This macrophage fusion model system leads to the differentiation of multinucleated cells that express osteoclast markers, and allows for the analysis of late events that accompany cell–cell fusion and immediately precede multinucleation, indicating that macrophage multinucleation is not tissue specific and supporting the concept that osteoclasts and giant cells share a functional goal: to resorb the substrate onto which they adhere. Alveolar macrophages come extremely pure from lung lavages, and have an extraordinary ability to fuse very efficiently, and very quickly in vitro. So this assay differs from assays reported in the literature that mostly generate osteoclast-like cells from bone marrow macrophages (Udagawa et al. 1990). In contrast to most assays, ours does not require cell proliferation or extracellular factors such as M-CSF, and RANKL. This is because most assays use mixed cell populations in which the concentration of macrophages is relatively low to start with. Such cultures require supplementation with first a growth factor, such as M-CSF, to induce the proliferation of mononuclear phagocytes, then with a differentiation factor such as OPGL/RANKL to induce their multinucleation (Lacey et al. 1998). In contrast, our assay is composed of a highly pure population of alveolar macrophages (over 99%) that are postmitotic and initiate fusion within hours after plating. So our model system is specifically designed to investigate macrophage fusion leading to multinucleation (Figure 5).
Figure 5.
Macrophage Fusion Model System. Rat alveolar macrophages plated at high density on day 0, and on day 3, when fusion is 99% complete (c). While the expression of osteoclast markers increases with multinucleation (a), that of MFR and CD44 decreases (b).
Identification of MFR, CD44 and CD47 as possible members of the fusion machinery
To identify the cell surface proteins that mediate fusion, we hypothesized that macrophages have developed a protein machinery similar to that used by viruses to fuse with host cells to inject DNA or RNA. As a result of our working hypothesis, we first identified a 115-kDa plasma membrane protein and a 160-kDa soluble protein that were expressed by multinucleated mouse macrophages generated in vivo on coverslips implanted intraperitoneally. These proteins were not detected in resident peritoneal macrophages from implanted mice, nor from control mice (Vignery 1989). That was the first indication that multinucleation was accompanied by the expression of novel proteins, including a plasma membrane one. However, at that time the yield of mouse macrophages was too low to attempt to purify these proteins, and we needed to identify proteins associated with a function, i.e. macrophage fusion. We then came across a paper published by Sone et al. (1981), who reported the use of rat alveolar macrophages to generate giant cells. So we immediately repeated Sone's experiments, and were astonished by the fusion rate of these cells. In addition, these cells provided a major improvement in macrophage yield, allowing us for the first time to consider raising monoclonal antibodies directed against fusing macrophages and purifying the antigen recognized by such antibodies. But before doing so, we ensured that these multinucleated macrophages expressed the canonical osteoclast markers, such as the Na,K-ATPase, and receptors for calcitonin (Vignery et al. 1989; Vignery et al. 1991a).
Role of MFR in macrophage fusion
To identify the components of the putative fusion machinery, we generated monoclonal antibodies (mAbs) that we selected for (i) altering the fusion of rat alveolar macrophages in vitro; (ii) recognizing a surface determinant on fusing macrophages and; (iii) being of the IgG isotype. To generate mAbs that alter the fusion of macrophages, we immunized mice with rat alveolar macrophages induced to fuse in vitro. Of interest, the rat alveolar macrophages that were used as immunogen were plated onto coverslips that were implanted intraperitoneally in mice. The cells adherent to the coverslips were used either live, or fixed with either paraformaldehyde or glutaraldehyde, as previously described (Vignery 1989). This technique ensures that the antigenic response of the mice is directed to cell surface determinants, which include the fusion protein machinery. We selected four mAbs, 10B11, 10C4, 10C5 and 12D6, that block fusion but not aggregation of alveolar macrophages in vitro, and that recognize a surface determinant on fusing macrophages. We did not identify mAbs that stimulate fusion. The antigens recognized by all four mAbs were (i) detected on the surface of fusing macrophages; (ii) transiently expressed at the onset of fusion, and; (iii) expressed with a molecular weight of 150 kDa (Saginario et al. 1995). We subsequently affinity purified the antigen recognized by mAb10C4. At that time, several of our peptides matched with sequences found in the EST database (Saginario et al. 1998). These EST sequences were used to design and generate probes, using as template cDNA synthesized from RNA isolated from fusing rat alveolar macrophage. These probes were used to screen a fusing rat peritoneal macrophage cDNA library that we constructed. We identified and sequenced several clones, one of which, clone 8.1, contained an open reading frame encoding a putative transmembrane protein of 509 amino acids. We subsequently demonstrated that clone 8.1 codes for 10C4 antigen as its translation product is recognized by mAb10C4 in transiently transfected COS cells. We also generated evidence that the coding sequence of 10C4 antigen is identical in alveolar and peritoneal macrophages, suggesting that the difference in molecular mass between both forms of 10C4 antigen, i.e. 150 kDa vs. 120 kDa, is likely due to different post-translational modifications. We called the 10C4 antigen Macrophage Fusion Receptor (MFR) (Saginario et al. 1998).
MFR is a type I transmembrane protein which belongs to the superfamily of immunoglobulins (Ig) (Figure 6). MFR contains three Ig loops in its extracellular domain, and closely resembles CD4, suggesting that it interacts with a cell surface ligand.
Figure 6.
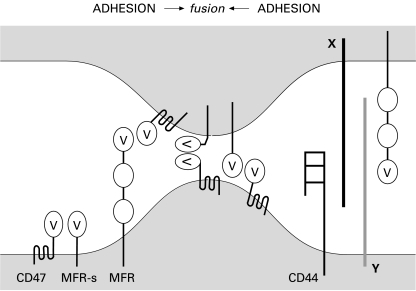
Hypothetical Macrophage Fusion Mechanism. Macrophage–macrophage adhesion is secured by MFR and CD44, interacting either directly or indirectly, via other unknown ligands (X and Y). The stepwise association between the long form of MFR with CD47, followed by the short form of MFR with CD47 could reduce the distance between the cells down to 5–10 nm. That distance may be further reduced if MFR and CD47 bend upon binding. In parallel, it is conceivable that the association between MFRs and CD47 in the same cell secures the mononucleated state of macrophages.
Soon after we deposited the MFR sequence in GenBank, several sequences identical to ours were reported in the literature. They encode a protein called P84/SHPS-1/SIRPα/BIT (Comu et al. 1997; Fujioka et al. 1997; Kharitonenkov et al. 1997; Sano et al. 1997). SHPS-1 was identified as a substrate for the intracellular phosphatases containing SH2 domains, SHP-1 and SHP-2. MFR contains four putative tyrosine phosphorylation sites to which SHP-1 and SHP-2 bins via their SH2 domains. These tyrosines belong to two Immunoreceptor tyrosine-based inhibitory motif (ITIM (V/I/T)XYXX(V/L)). The intracellular domain of MFR has recently been shown to be highly conserved across species and a substrate for SHP-1 in macrophages (Timms et al. 1998; Veillette et al. 1998).
Several isoforms of SHPS-1 have been reported (Timms et al. 1998; Sano et al. 1999). This heterogeneity was partly due to differential glycosylation of SHPS-1, and to the production of an alternatively spliced transcript that lacks the two extracellular immunoglobulin C (IgC) domains (Figure 6). We had also identified a clone (clone 2.1) that lacked the IgC domains. We have recently identified a lower molecular weight form of MFR that we named MFR short (MFRs), and that migrates on SDS-PAGE gels as a 90-kDa protein. Although that 90 kDa form is significantly less abundant than the 150 kDa, it is also induced at the onset of fusion (unpublished observations).
Most recently, we engineered a fusion protein that contains the extracellular IgV domain of MFR (GST-MFRev), thus a domain shared by both forms of MFR, that is recognized by our antifusion mAbs, and blocks fusion (Han et al. 2000). This indicates that our mAbs recognize the IgV domain of MFR and that this domain is likely to play a role in cell–cell adhesion leading to fusion. This is in agreement with the fact that our mAbs are specific for rat MFR, and do not cross-react with either mouse or human MFR. Together, these observations led us to hypothesize that the two IgC domains present in the long form of MFR may be interacting with additional ligand(s).
To investigate whether osteoclasts express MFR, we used as template a human osteoclastoma and a human osteoclast-like cell cDNA library to perform PCR. Both libraries contained cDNAs that coded for human MFR, the sequence of which is highly conserved between rat and human (Saginario et al. 1998; Yamao et al. 1997; Sano et al. 1999). This indicated that MFR gene is expressed by human osteoclasts.
To analyse the role of MFR in macrophage fusion, we engineered recombinant fusion proteins that contain the full length extracellular domain of MFR, i.e. GST-MFRe and Myc-His-MFRe. In parallel, we engineered control fusion proteins using the extracellular domain of rat CD4 (CD4e) and generated GST-CD4e and Myc-His-CD4e fusion proteins. As we detailed in Saginario et al. (1998), GST-MFRe inhibits fusion in a concentration-dependent manner by virtue of binding to a specific site on macrophages. It appears from these experiments that MFR does not require glycosylation to bind fusing macrophages (Saginario et al. 1998).
Role of CD47 in macrophage fusion
Soon after we published MFR, two independent groups reported that Integrin Associated Protein (IAP)/CD47 is a ligand for P84 (Jiang et al. 1999) and a counterreceptor for human SIRPα/MFR (Seiffert et al. 1999). CD47 was identified using expression cloning and competition binding of mAbs, respectively. CD47 is a 50-kDa membrane protein that belongs, like MFR, to the superfamily of immunoglobulins (Lindberg et al. 1993). CD47 traverses the plasma membrane 5 times with its C-terminal domain located intracellularly (Figure 6). Its N-terminal extracellular domain shares considerable similarity to immunoglobulin variable regions. Of significance to our hypothesis, CD47 shares 28% identity and similar hydrophobic profiles with vaccinia virus A38L protein (Parkinson et al. 1995). Although A38L is not known as the actual fusion protein, like IAP/CD47, A38L promotes the entry of Ca++ into cells possibly by forming a pore (Sanderson et al. 1996; Nishiyama et al. 1997). Indeed, pore formation is a classic tactic used by parasites to enter host cells (Kirby et al. 1998). The overexpression of the pore-forming P2Z/P2X7 receptor for ATP leads to cell–cell fusion, followed by cell death. Indeed, overexpression of CD47 or A38L also leads to cell death (Sanderson et al. 1996; Nishiyama et al. 1997).
CD47 is also a receptor for the C-terminal domain of thrombospondin (Fujioka et al. 1997). The thrombospondins (TSP) are a family of extracellular calcium-binding proteins that modulate cellular phenotype. TSP-1 and -2 are both expressed in centres of intramembranous bone formation during development (Tooney et al. 1998), and mice that are deficient in TSP-1 and TSP-2 display an increased cortical thickness (TSP-1) and a mild and variable lordotic curvature of the spine that is apparent at birth (TSP-2) (Kyriakides et al. 1998; Lawler et al. 1998). Together, these observations led us to hypothesize that CD47 expressed by macrophages participates in cell–cell adhesion/fusion by virtue of interacting with MFR.
At this point in time, we have generated data indicating that MFR and CD47 interact via their immunoglobulin V (IgV) domain during fusion (Han et al. 2000). The high expression level of the long form of MFR, which contains two IgC domains, together with the fact that mice deficient in CD47 develop normally (Lindberg et al. 1996) suggests that these domains interact with an additional cell surface ligand, other than CD47. Whether MFR–CD47 interaction plays an important regulatory role or is essential for multinucleation remains unclear.
Role of CD44 in macrophage fusion
CD44 is an integral membrane glycoprotein which plays an important role in both cell–cell and cell–substrate adhesion (Belitsos et al. 1990; Stamenkovic et al. 1991; Sy et al. 1991). CD44 exists as different isoforms in a wide variety of cells and tissues due to post-translational modifications and alternative splicing (Tolg et al. 1993; Naor et al. 1997).
CD44 is involved in cell migration, lymphopoiesis and lymphocyte homing, in which it mediates cell–cell and cell–substrate interactions. A substantial body of data has been published suggesting that certain molecules involved in cell–cell adhesion, such as CD4 and LFA-1, play an important role in the infectivity and cytopathicity of viruses. (Pantaleo et al. 1991). CD44 recognizes and binds to extracellular matrix elements such as hyaluronate (Lesley et al. 1990; Miyake et al. 1990), collagen type I, fibronectin (Jalkanen & Jalkanen 1992) and osteopontin (Weber et al. 1996). The interaction of CD44 with extracellular components, in addition to cell surface molecules, could provide an additional regulatory mechanism to control multinucleation in macrophages. The 100 kDa form of CD44, the most common so-called ‘standard form’ expressed by haematopoietic cells, has been demonstrated to be involved not only in the attachment of poliovirus to HeLa cells (Shepley & Racaniello 1994) but also in the infection of mononuclear phagocytes by HIV (Rivandeneira et al. 1995). CD44 does not, however, act as a viral receptor in either of these two instances. Lastly, Nakamura et al. (1995) reported that osteoclasts express CD44 which is confined to the basolateral membrane. We therefore set out to investigate whether CD44 plays a role in macrophage fusion multinucleation.
We have generated evidence that (i) alveolar macrophages express the standard and short form of CD44, i.e. CD44H (haematopoietic); (ii) CD44 expression by macrophages is highly and transiently induced at the onset of fusion; (iii) CD44 extracellular ligands prevent multinucleation and; (iv) the recombinant soluble extracellular domain of CD44 (GST-CD44e) binds macrophages and prevents multinucleation in vitro. Together, our results suggest that CD44 and its putative cell-surface ligand participate in the cell–cell adhesion/fusion event of macrophages (Sterling et al. 1998) (Figure 6). Because of their concomitant high expression at the onset of fusion, the question as to whether CD44 and MFR bind to, or interact with, each other or with separate determinants remains open.
Macrophage adhesion/fusion model
The identification and characterization of a very efficient macrophage fusion model system has allowed us to identify the putative components of the macrophage fusion machinery, MFR and CD44, the expression of which is highly induced at the onset of fusion in macrophages. We have also obtained preliminary data indicating that CD47, a ligand for P84/SIRPα/MFR, is expressed by fusing macrophages (Han et al. 2000). Together, our efforts have come to fruition and allow us to propose a macrophage fusion model in which MFR and CD47 interact with each other in fusing cells via their IgV domain, as coreceptors, suggesting that MFR and CD47 entertain a receptor–ligand type of interaction during cell–cell adhesion/fusion. This model considers possible additional ligands for MFR and CD44 (Figure 6) (Han et al. 2000). We propose that cell–cell fusion utilize one system that combines two functions, attachment and fusion. Accordingly, MFR and CD47 may constitute the minimal fusion machinery proposed by Weber et al. (1998) for intracellular membrane fusion. In parallel, MFR and CD47 may interact within the same cell plasma membrane to secure mononucleation.
Future goals
While significant progress has been made in attempting to decipher the mechanism by which macrophages interact with one another at the onset of fusion, our pioneering data provide more questions than answers. At this time, it appears critical to identify the cell surface ligands for MFR and CD44. Both forms of MFR, and CD44, are induced at the onset of fusion, and appear to participate in fusion/multinucleation by interacting with a cell surface ligand. While both forms of MFR interact with CD47, it is possible that the two IgC domains present in the long form of MFR confer additional ligand-binding properties, and interact with another ligand. The identification of such ligands is critical to reconstitute the macrophage fusion machinery in vitro. Indeed, this is one of the key questions raised by our work. To investigate whether MFR, CD44 and their ligand(s) mediate fusion, these molecules could be expressed in cell lines subjected to cell–cell adhesion and fusion studies. This would allow for the mapping of the domains that are important for fusion by, for instance, performing structure-function analyses studies using mutated forms of these molecules. The question of whether MFR, CD44 and their ligand(s) mediate fusion/multinucleation in vivo remains open. Such studies could take advantage of the fusion-blocking reagents that we generated, such as mAbs and fusion proteins, to investigate whether they also prevent giant cell/osteoclast-like differentiation in vivo. A strong approach would be to analyse/phenotype mice deficient in MFR, by knocking out the MFR gene in a tissue-specific manner.
It is our hope that investigators from both the ‘giant cell’ and the ‘osteoclast’ fields join their efforts to decipher the mechanism utilized by mononuclear phagocytes to become multinucleated.
Acknowledgments
I would like to take this opportunity to thank Prof Paul Kaye for inviting me to formally discuss our work on macrophage multinucleation at the British Society for Immunology Congress, that was held in the Yorkshire town of Harrogate last December. I am grateful to Prof David R. Katz for inviting me to write this review for the International Journal of Experimental Pathology, of which he is the Editor. This has provided me with a unique opportunity to discuss our current thoughts and ideas related to the field of osteoclast and giant cell differentiation. I also would like to thank my colleagues, postdoctoral associates and students who all, over the years, contributed to this work and made it happen. My special thanks go to my long-term colleagues and close friends Drs Elisabetta Ullu and Christian Tschudi for their invaluable scientific and moral support. This work would not have seen light without the support of our granting agency, the National Institute of Health. Finally, I would like to dedicate this review to my son, Philippe Baron.
References
- Abe E, Miyaura C, Tanaka H, et al. 1α,25-Dihydroxyvitamin D3 promotes fusion of mouse alveolar macrophages both by a direct mechanism and by a spleen cell-mediated indirect mechanism. Proc Natl Acad Sci USA. 1983;80:5907–5911. doi: 10.1073/pnas.80.18.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhatib G, Combadiere C, Broder C, et al. CCCKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- Anderson DM, Maraskovsky E, Billingsley WL, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–179. doi: 10.1038/36593. 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- Baron R. Molecular mechanisms of bone resorption: therapeutic implications. Acta Orthop Scand Supplement. 1995;266:66–70. [PubMed] [Google Scholar]
- Baron R, Neff L, Tran Van P, Vignery A. Cytochemical identification, kinetics and differentiation of osteoclastic precursors in the rat. Am J Pathol. 1986;122:363–378. [PMC free article] [PubMed] [Google Scholar]
- Baron R, Vignery A, Horowitz M. Lymphocytes, macrophages and the regulation of bone remodeling. In: Peck WA, editor. Bone and Mineral Research. New York: Elsevier Science Publications; 1983. pp. 175–243. [Google Scholar]
- Belitsos PC, Hildreth JE, August JT. Homotypic cell aggregation induced by anti-CD44 (Pgp-1) monoclonal antibodies and related to CD44 (Pgp-1) expression. J Immunol. 1990;144:1661–1670. [PubMed] [Google Scholar]
- Bennett MK. SNAREs and the specificity of transport vesicle targeting. Curr Opin Cell Biol. 1995;7:581–586. doi: 10.1016/0955-0674(95)80016-6. [DOI] [PubMed] [Google Scholar]
- Blobel CP, Wolfsberg TG, Turk CW, Myles D, Primakoff P, White JM. A potential fusion peptide and an integrin ligand domain in a protein active in sperm–egg fusion. Nature. 1992;356:248–252. doi: 10.1038/356248a0. [DOI] [PubMed] [Google Scholar]
- Chiozzi P, Sanz J, Ferrari D, et al. Spontaneous cell fusion in macrophage cultures expressing high levels of the P2Z/P2X7 receptor. J Cell Biol. 1997;138:697–706. doi: 10.1083/jcb.138.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho C, Bunch DO, Faure JE, et al. Fertilization defects in sperm from mice lacking fertilin beta. Science. 1998;281:1857–1859. doi: 10.1126/science.281.5384.1857. [DOI] [PubMed] [Google Scholar]
- Comu S, Weng W, Olinsky S, et al. The Murine P84 Neural Adhesion Molecule is SHPS-1, a Member of the Phosphatase-Binding Protein Family. J Neurosci. 1997;17(22):8702–8710. doi: 10.1523/JNEUROSCI.17-22-08702.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, Liu R, Ellmeier W, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- Dimitrov D. How do viruses enter cells? The HIV coreceptors teach us a lesson in complexity. Cell. 1997;91:721–730. doi: 10.1016/s0092-8674(00)80460-2. [DOI] [PubMed] [Google Scholar]
- Dragic T, Litwin V, Allaway G, et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- Falzoni S, Munerati M, Ferrari D, Spisani S, Moretti S, Divirgilio F. The purinergic P2Z receptor of human macrophage cells. Characterization and possible physiological role. J Clin Invest. 1995;95:1207–1216. doi: 10.1172/JCI117770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Broder C, Kennedy P, Berger E. HIV-1 entry: functional cDNA cloning of a seven transmembrane, G-protein coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- Franklin RM. Some observations on the formation of giant cells in tissue cultures of chicken macrophages. Z Natur Forsch. 1958;13:213. [Google Scholar]
- Fujioka Y, Matozaki T, Noguchi T, et al. A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Mol Cell Biol. 1997;16:6887–6899. doi: 10.1128/mcb.16.12.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Sterling H, Chen Y‐M, et al. CD47, a ligand for the Macrophage Fusion Receptor, participates in macrophage multinucliation. J Biol Chem. in press. [DOI] [PubMed]
- Hernandez L, Hoffman L, Wolfsberg T, White J. Virus–cell and cell–cell fusion. Annu Rev Cell Dev Biol. 1996;12:627–661. doi: 10.1146/annurev.cellbio.12.1.627. [DOI] [PubMed] [Google Scholar]
- Higuchi S, Tabata N, Tajima M, et al. Induction of human osteoclast-like cells by treatment of blood monocytes with anti-fusion regulatory protein-1/CD98 monoclonal antibodies. J Bone Min Res. 1998;13:44–49. doi: 10.1359/jbmr.1998.13.1.44. [DOI] [PubMed] [Google Scholar]
- Hildreth J, Orentas R. Involvement of a leukocyte adhesion receptor (LFA-1) in HIV-induced syncyium formation. Science. 1989;244:1075–1078. doi: 10.1126/science.2543075. [DOI] [PubMed] [Google Scholar]
- Ito Y, Komada H, Kusagawa S, et al. Fusion regulation proteins on the cell surface: Isolation and characterization of monoclonal antibodies which enhance giant polykaryocyte formation in Newcastle Disease virus- infected cell lines of human origin. J Virol. 1992;66:5999–6007. doi: 10.1128/jvi.66.10.5999-6007.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itonaga I, Sabokbar A, Murray DW, Athanasou NA. Effect of osteoprotegerin and osteoprotegerin ligand on osteoclast formation by arthroplasty membrane derived macrophages. Ann Rheum Dis. 2000;59:26–31. doi: 10.1136/ard.59.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkanen S, Jalkanen M. Lymphocyte CD44 binds the COOH terminal heparin-binding domain of fibronectin. J Cell Biol. 1992;116:817–825. doi: 10.1083/jcb.116.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P, Lagenaur CF, Narayanan V. Integrin-associated protein is a ligand for the p84 neural adhesion molecule. J Biol Chem. 1999;274:559–562. doi: 10.1074/jbc.274.2.559. [DOI] [PubMed] [Google Scholar]
- Kazazi F, Chang J, Lopez A, Vaas M, Cunningham A. Interleukin 4 and human immunodeficiency virus stimulate LFA-1-ICAM-1-mediate aggregation of monocytes and subsequent giant cell formation. J Gen Vir. 1994;75:2795–2802. doi: 10.1099/0022-1317-75-10-2795. [DOI] [PubMed] [Google Scholar]
- Kharitonenkov A, Chen Z, Sures I, Wang H, Schilling J, Ullrich A. A family of proteins that inhibit signaling through tyrosine kinase receptors. Nature. 1997;386:181–186. doi: 10.1038/386181a0. [DOI] [PubMed] [Google Scholar]
- Kirby JE, Vogel JP, Andrews HL, Isberg RR. Evidence for pore-forming ability by legionella pneumophilia. Mol Microbiol. 1998;27:323–336. doi: 10.1046/j.1365-2958.1998.00680.x. 10.1046/j.1365-2958.1998.00680.x. [DOI] [PubMed] [Google Scholar]
- Kurachi T, Morita I, Murota S. Involvement of adhesion molecules LFA-1 and ICAM-1 in osteoclast development. Biochem Biophys Acta. 1993;1178:259–266. doi: 10.1016/0167-4889(93)90202-z. [DOI] [PubMed] [Google Scholar]
- Kyriakides TR, Zhu YH, Smith LT, et al. Mice that lack Thrombospondin 2 display connestive tissue abnormalities that are asociated with disordered fibrillogenesis, an increased vascular density, and a bleeding diathesis. J Cell Biol. 1998;140:419–430. doi: 10.1083/jcb.140.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- Langhans T. Uber reizenzellen mit wandstandingen kernen in tuberkeln und die fibrose forms des tuberkels. Arch Pathol Anat. 1947;42:332. [Google Scholar]
- Lawler J, Sunday M, Thibert V, et al. Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. J Clin Invest. 1998;101:982–992. doi: 10.1172/JCI1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Naour F, Rubinstein E, Jasmin C, Prenant M, Boucheix C. Severely reduced female fertility in CD9-deficient mice. Science. 2000;287:319–321. doi: 10.1126/science.287.5451.319. 10.1126/science.287.5451.319. [DOI] [PubMed] [Google Scholar]
- Lesley J, Schulte R, Hyman R. Binding of hyaluronic acid to lymphoid cell lines is inhibited by monoclonal antibodies against Pgp-1. Exp Cell Res. 1990;187:224–233. doi: 10.1016/0014-4827(90)90085-o. [DOI] [PubMed] [Google Scholar]
- Lindberg FP, Bullard DC, Caver TE, Gresham HD, Beaudet AL, Brown E. Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science. 1996;274:795–798. doi: 10.1126/science.274.5288.795. 10.1126/science.274.5288.795. [DOI] [PubMed] [Google Scholar]
- Lindberg FP, Gresham HD, Schwarz E, Brown EJ. Molecular cloning of integrin-associate protein: an immunoglobulin family member with multiple membrane-spanning domains implicated in αvβ 3-dependent ligand binding. J Cell Biol. 1993;123:485–496. doi: 10.1083/jcb.123.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linial M. SNARE proteins − why so many, why so few? J Neurochem. 1997;69:1781–1792. doi: 10.1046/j.1471-4159.1997.69051781.x. [DOI] [PubMed] [Google Scholar]
- Mbalaviele G, Chen H, Boyce B, Mundy G, Yoneda T. The role of cadherin in the generation of multinucleated osteoclasts from mononuclear precursors in murine marrow. J Clin Invest. 1995;95:2757–2765. doi: 10.1172/JCI117979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mege R, Goudou D, Diaz C, et al. N-cadherin and N-CAM in myoblast fusion: compared localisation and effect of blockade by peptides and antibodies. J Cell Sci. 1992;103:897–906. doi: 10.1242/jcs.103.4.897. [DOI] [PubMed] [Google Scholar]
- Mi S, Lee X, Li X-P, et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature. 2000;403:785–789. doi: 10.1038/35001608. 10.1038/35001608. [DOI] [PubMed] [Google Scholar]
- Miyado K, Yamada G, Yamada S, et al. Requirement of CD9 on the egg plasma membrane for fertilization. Science. 2000;287:321–324. doi: 10.1126/science.287.5451.321. 10.1126/science.287.5451.321. [DOI] [PubMed] [Google Scholar]
- Miyake K, Underhill C, Lesley J, Kincade R. Hyaluronate can function as a cell adhesion molecule and CD44 participates in hyaluronate recognition. J Exp Cell Med. 1990;172:69–75. doi: 10.1084/jem.172.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Most J, Neumayer H, Dieric M. Cytokine-induced generation of multinucleated giant cells in vitro requires interferon-γ and expression of LFA-1. Eur J Immunol. 1990;20:1661–1667. doi: 10.1002/eji.1830200807. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kenmotsu S-I, Sakai H, Ozawa H. Localization of CD44, the hyaluronate receptor, on the plasma membrane of osteocytes and osteoclasts in rat tibiae. J Bone Mineral Res. 1995;280:225–233. doi: 10.1007/BF00307793. [DOI] [PubMed] [Google Scholar]
- Naor D, Sionov VR, Ish-Shalom D. CD44: structure function and association with the malignant process. Adv Can Res. 1997;71:241–319. doi: 10.1016/s0065-230x(08)60101-3. [DOI] [PubMed] [Google Scholar]
- Nishiyama Y, Tanaka T, Naitoh H, et al. Overexpression of integrin-associated protein (CD47) in rat kidney treated with a renal carcinogen, ferric nitrilotriacetate. Jpn J cancer Res. 1997;88:120–128. doi: 10.1111/j.1349-7006.1997.tb00356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgimoto S, Tabata N, Suga S, et al. Molecular characterization of fusion regulatory protein-1 (FRP-1) that induces multinucleate giant cell formation of monocytes and HIV gp-160 mediated cell fusion. J Immunol. 1995;155:3585–3592. [PubMed] [Google Scholar]
- Ohta H, Tsurudome M, Matsumura H, et al. Molecular and biological characterization of fusion regulatory proteins (FRPs): anti-FRP mAbs induced HIV mediated cell fusion via an integrin system. EMBO J. 1994;13:2044–2055. doi: 10.1002/j.1460-2075.1994.tb06479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G, Butini L, Graziosi C, et al. Human Immunodeficiency Virus (HIV) infection in CD4+ T Lymphocytes genetically deficient in LFA-1: LFA-1 is required for HIV-mediated cell fusion but not for viral transmission. J Ex Med. 1991;173:511–514. doi: 10.1084/jem.173.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JE, Sanderson CM, Smith GL. The vaccinia virus A38L gene product is a 33 kD integral membrane glycoprotein. Virology. 1995;214:177–188. doi: 10.1006/viro.1995.9942. 10.1006/viro.1995.9942. [DOI] [PubMed] [Google Scholar]
- Rivandeneira E, Sauls D, Yu Y, Haynes B, Weinberg J. Inhibition of HIV type I infection of mononuclear phagocytes by anti-CD44 antibodies. AIDS Res Hum Ret. 1995;11:541–546. doi: 10.1089/aid.1995.11.541. [DOI] [PubMed] [Google Scholar]
- Rothman JE. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Saginario C, Qian H-Y, Vignery A. Identification of an inducible surface molecule specific to fusing macrophages. Proc Natl Acad Sci, USA. 1995;92:12210–12214. doi: 10.1073/pnas.92.26.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saginario C, Sterling H, Beckers C, et al. MFR, a putative receptor mediating the fusion of macrophages. Mol Cell Biol. 1998;18:6213–6223. doi: 10.1128/mcb.18.11.6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson CM, Parkinson JE, Hollinshead M, Smith GL. Overexpression of the vaccinia virus A38L integral membrane protein promotes Ca++ influx into infected cells. J Virol. 1996;70:905–914. doi: 10.1128/jvi.70.2.905-914.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano S, Ohnishi H, Omori A, Hasegawa J, Kubota M. BIT, an immune antigen receptor-like molecule in the brain. Fed Eur Biochem Soc. 1997;411:327–334. doi: 10.1016/s0014-5793(97)00724-2. [DOI] [PubMed] [Google Scholar]
- Sano S-I, Ohnishi H, Kubota M. Gene structure of mouse BIT/SHPS-1. Biochem J. 1999;344:667–675. 10.1042/0264-6021:3440667. [PMC free article] [PubMed] [Google Scholar]
- Seiffert M, Cant C, Chen Z, et al. Human signal regulatory protein is expressed on normal, but not on subsets of leukemic myeloid cells and mediates cellular adhesion involving its counterreceptor CD47. Blood. 1999;94:3633–3643. [PubMed] [Google Scholar]
- Shepley M, Racaniello VR. A monoclonal antibody that blocks polovirus attachment recognizes the lymphocyte homing receptor CD44. J Virol. 1994;68:1301–1308. doi: 10.1128/jvi.68.3.1301-1308.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiina Y, Yamaguchi A, Yamana H, Abe E, Yoshiki S, Suda T. Comparison of the mechanism of bone resorption induced by 1 alpha,25-dihydroxyvitamin D3 and lipopolysaccharides. Calcif Tissue Intern. 1986;39:28–34. doi: 10.1007/BF02555737. [DOI] [PubMed] [Google Scholar]
- Simonet WS, Lacey DL, Dunstan CR, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- Sone S, Bucana C, Hoyer LC, Fidler IJ. Kinetics and ultrastructural studies of the induction of rat alveolar macrophage fusion by mediators released from mitogen-stimulated lymphocytes. Am J Pathol. pp. 234–246. [PMC free article] [PubMed]
- Stamenkovic I, Aruffo A, Amiot M, Seed B. The hematopoietic and epithelial forms of CD44 are distinct polypeptides with different adhesion potentials for hyaluronate bearing cells. EMBO J. 1991;10:343–348. doi: 10.1002/j.1460-2075.1991.tb07955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling H, Saginario C, Vignery A. CD44 occupancy prevents the fusion of macrophages. J Cell Biol. 1998;143:1–11. doi: 10.1083/jcb.143.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RB, Fashauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4A Resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- Sy MS, Guo YJ, Stamenkovic I. Distinct effects of two CD44 isoforms on tumor cell growth in vivo. J Exp Med. 1991;174:859–866. doi: 10.1084/jem.174.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata N, Ito M, Shimokata K, et al. Expression of fusion regulatory proteins (FRPs) on human peripheral blood monocytes. J Immunol. 1994;153:3256–3266. [PubMed] [Google Scholar]
- Timms JF, Carlberg K, Gu H, et al. Identification of major binding proteins and substrates for the SH2-Containing protein tyrosine phosphatase SHP-1 in macrophages. Mol Cell Biol. 1998;18(7):3838–3850. doi: 10.1128/mcb.18.7.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolg C, Hofmann M, Herrlich P, Ponta H. Splicing choice from ten variant exons establishes CD44 variability. Nucl Acids Res. 1993;21:1225–1229. doi: 10.1093/nar/21.5.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooney PA, Sakai T, Sakai K, Aeschlimann D, Mosher DF. Restricted localization of thrombospondin-2 protein during mouse embryogenesis: comparison to thrombospondin-1. Matrix Biol. 1998;17:131–143. doi: 10.1016/s0945-053x(98)90026-9. [DOI] [PubMed] [Google Scholar]
- Udagawa N, Takahashi N, Akatsu T, et al. Differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci. 1990;87:7260–7264. doi: 10.1073/pnas.87.18.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillette A, Thibaudeau E, Latour S. High expression of inhibitory receptor SHPS-1 and its association with protein-tyrosine phosphatase SHP-1 in macrophages. J Biol Chem. 1998;273(35):22719–22728. doi: 10.1074/jbc.273.35.22719. [DOI] [PubMed] [Google Scholar]
- Vignery A, Niven-Fairchild T, Ingbar D, Caplan M. Polarized distribution of (Na+,K+) ATPase in giant cells elicitied in vivo and in vitro. J Histochem Cytochem. 1989;37:1265–1271. doi: 10.1177/37.8.2546991. [DOI] [PubMed] [Google Scholar]
- Vignery A, Niven-Fairchild T, Shepard M. Recombinant Murine Interferon-Γ inhibits the fusion of mouse alveolar macrophages in vitro but stimulates the formation of osteoclast-like cells on implanted syngeneic bone particles in vivo. J Bone Miner Res. 1990;5(6):637–644. doi: 10.1002/jbmr.5650050613. [DOI] [PubMed] [Google Scholar]
- Vignery A, Raymond M, Qian H-Y, Wang F, Rosenzweig SA. Multinucleated rat alveolar macrophages express functional receptors for calcitonin. Am J Physiol. 1991a;261:F1026–F1032. doi: 10.1152/ajprenal.1991.261.6.F1026. [DOI] [PubMed] [Google Scholar]
- Vignery A, Wang F, Qian H-Y, Benz Jr E, Gilmore-Hebert M. Detection of the Na,K-ATPase α3 isoform in multinucleated macrophages. Am J Physiol. 1991b;260:F704–F709. doi: 10.1152/ajprenal.1991.260.5.F704. [DOI] [PubMed] [Google Scholar]
- Vignery A, Wang F, Ganz MB. Macrophages express functional receptors for calcitonin gene related peptide. J Cellular Physiology. 1991c;149:301–306. doi: 10.1002/jcp.1041490217. [DOI] [PubMed] [Google Scholar]
- Vignery A. Macrophage multinucleation is accompanied by the expression of new soluble and membrane antigens in mice. Am J Pathol. 1989;135:565–570. [PMC free article] [PubMed] [Google Scholar]
- Virchow R. Reizung und reizbarkeit. Virchows Arch B. 1948;14:1. [Google Scholar]
- Weber G, Ashkar S, Glimcher M, Cantor H. Receptor–ligand interaction between CD44 and osteopontin (Eta-1) Science. 1996;271:509–512. doi: 10.1126/science.271.5248.509. [DOI] [PubMed] [Google Scholar]
- Weber T, Zemelman BV, McNew JA, et al. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- Wilschut J, Hoekstra D. Membrane Fusion. New York: Marcel Dekker, Inc.; 1991. pp. 91–97. [Google Scholar]
- Wong BR, Rho J, Arron J, et al. TRANCE Is a novel ligand of the tumor necrosis factor receptor family that activates C-Jun N-terminal kinase in T Cells. J Biol Chem. 1997;272(40):25190–25194. doi: 10.1074/jbc.272.40.25190. [DOI] [PubMed] [Google Scholar]
- Yagami-Hiromasa T, Sato T, Kurisaki T, Kamijo K, Nabeshima Y-I, Fusisawa-Sehara A. A metalloprotease-disintegrin participating in myoblast fusion. Nature. 1995;377:652–656. doi: 10.1038/377652a0. [DOI] [PubMed] [Google Scholar]
- Yamao T, Matozaki T, Amano K, et al. Mouse and human SHPS-1: molecular cloning of cDNAs and chromosomal localization of genes. Biochem Biophys Res Com. 1997;231:61–67. doi: 10.1006/bbrc.1996.6047. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]