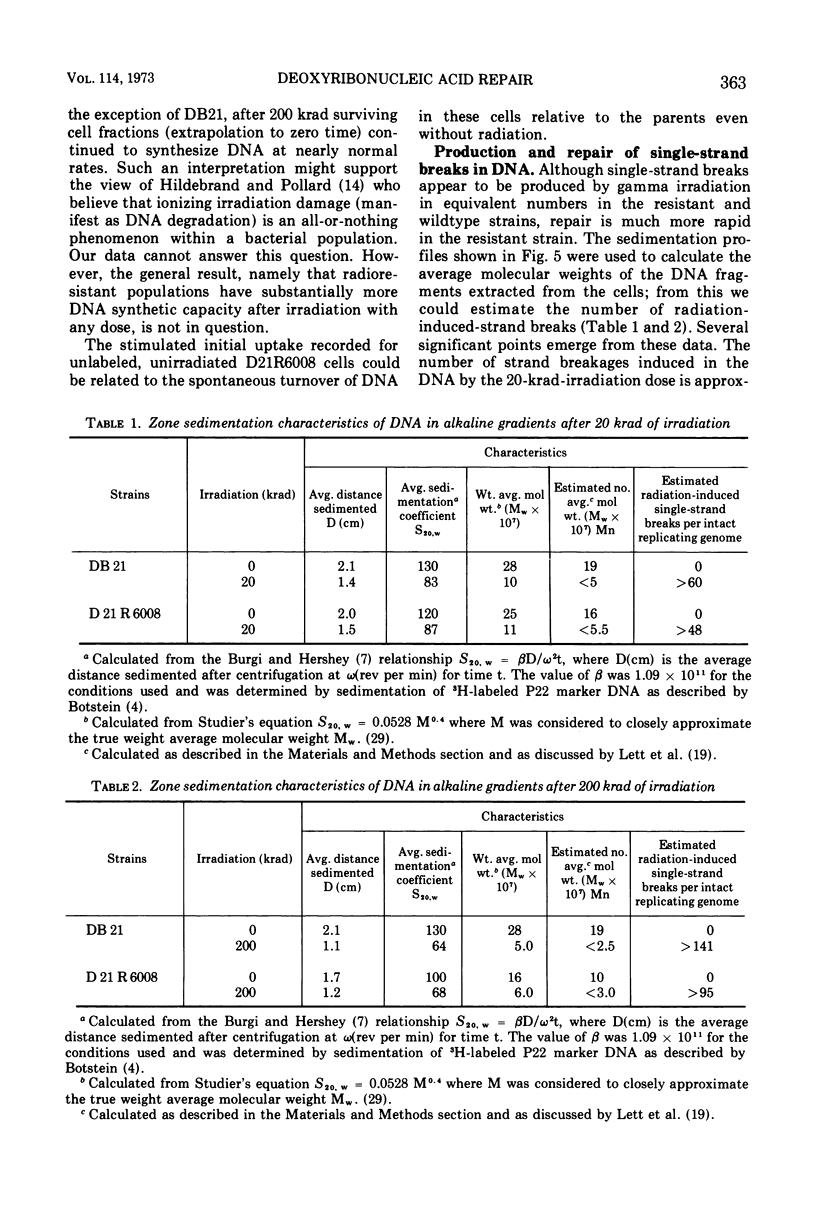
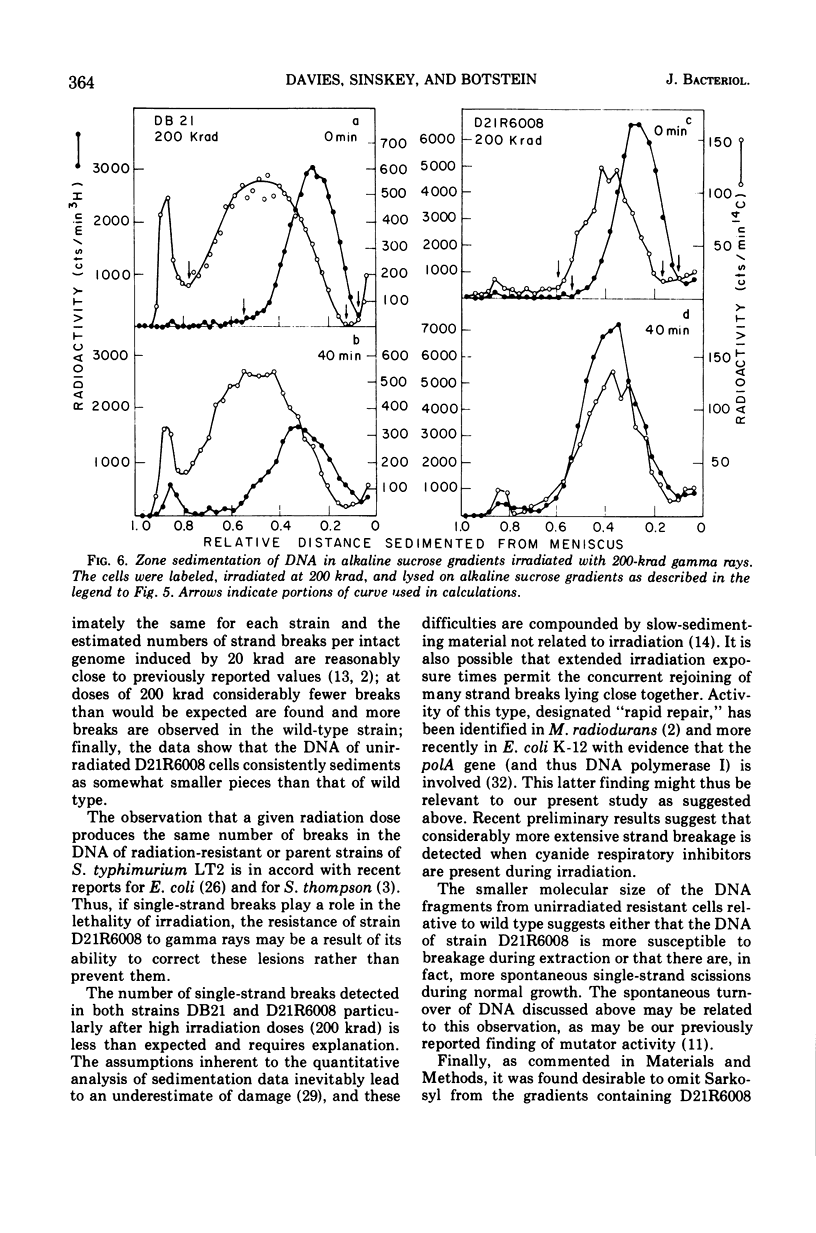
Abstract
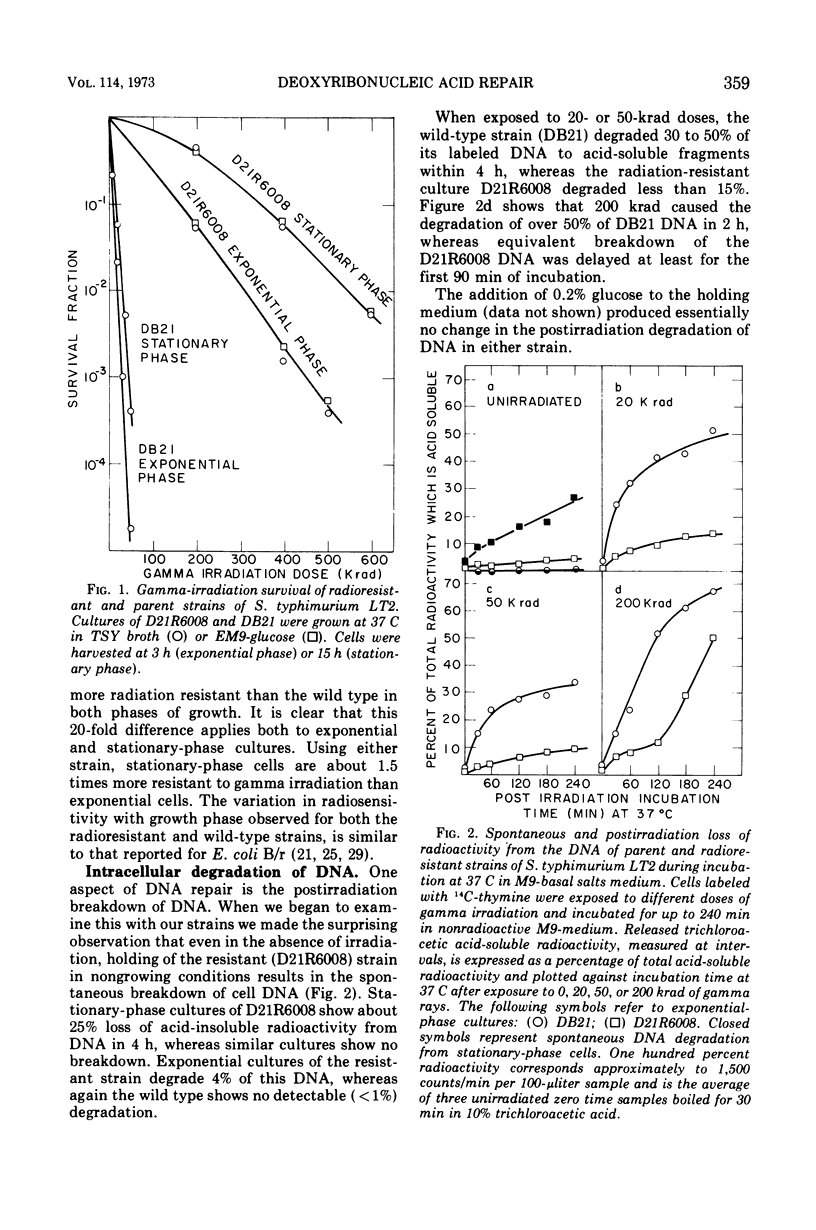
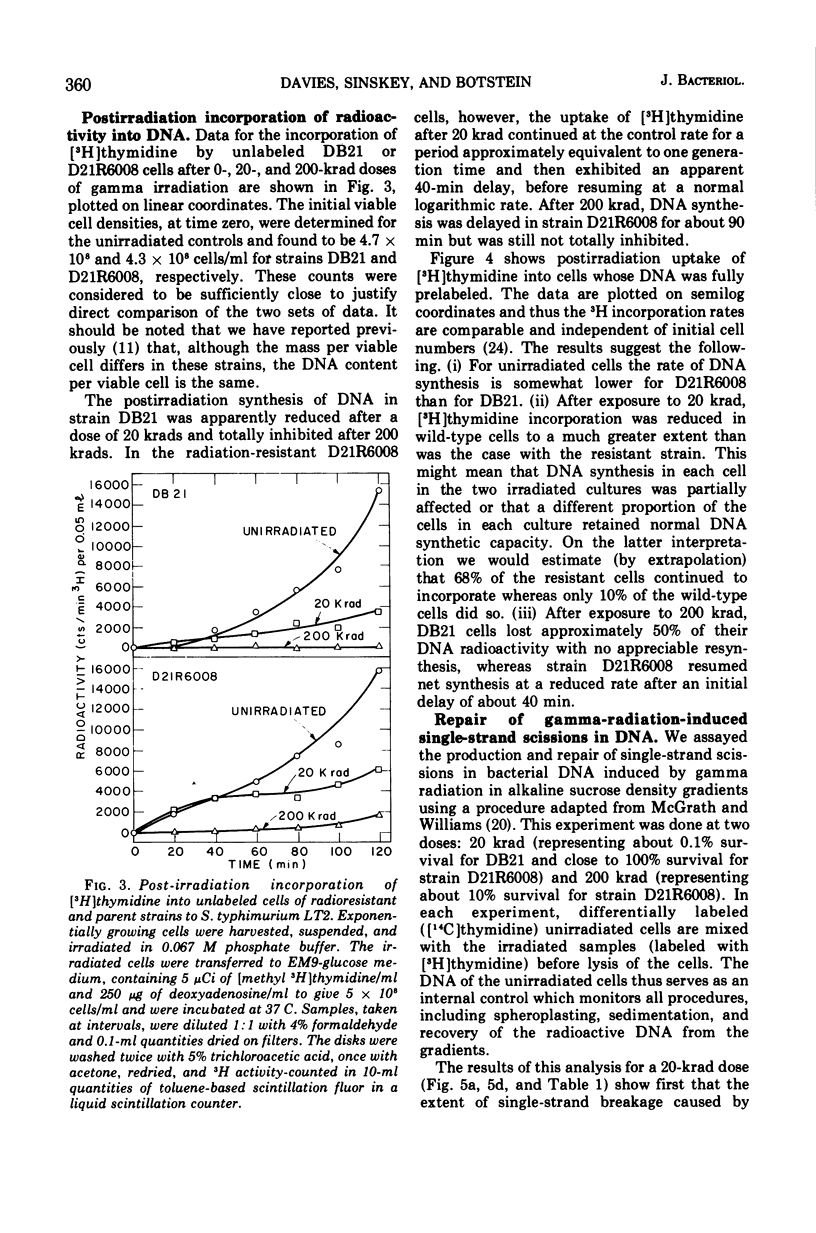
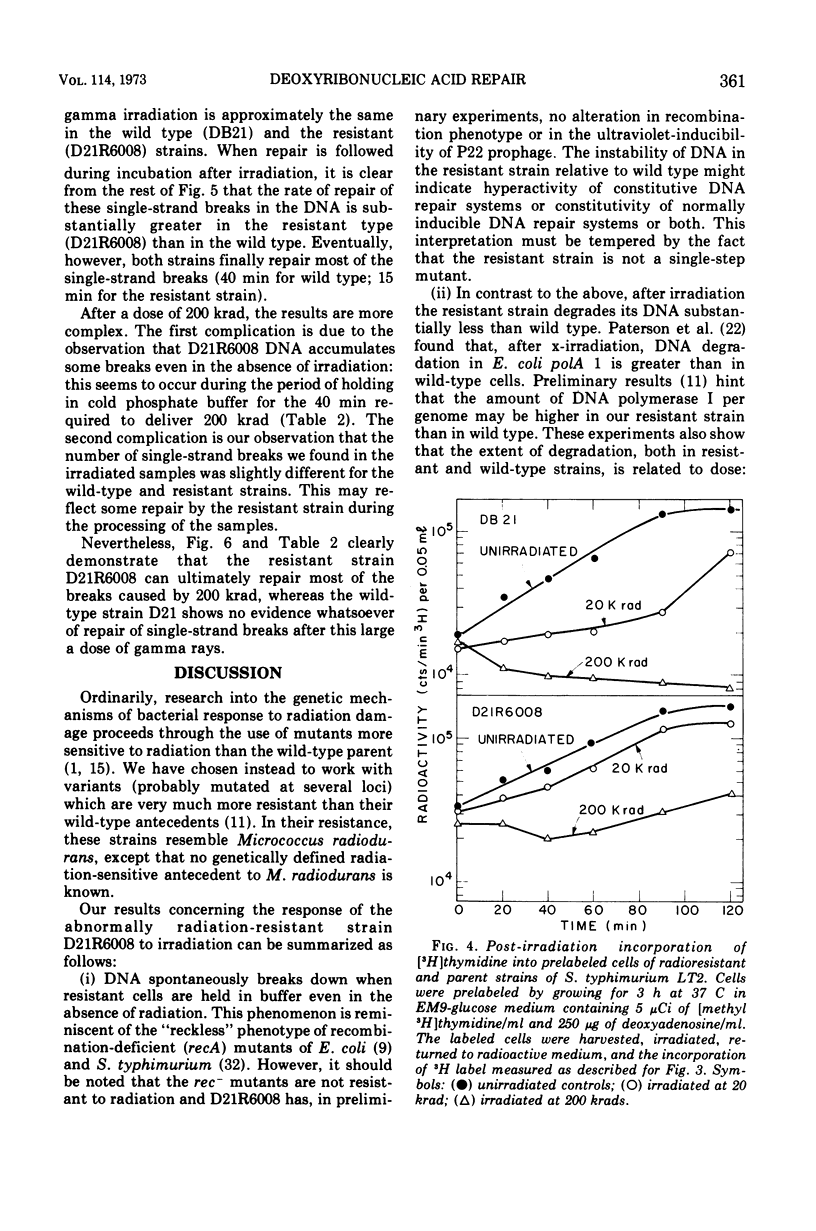
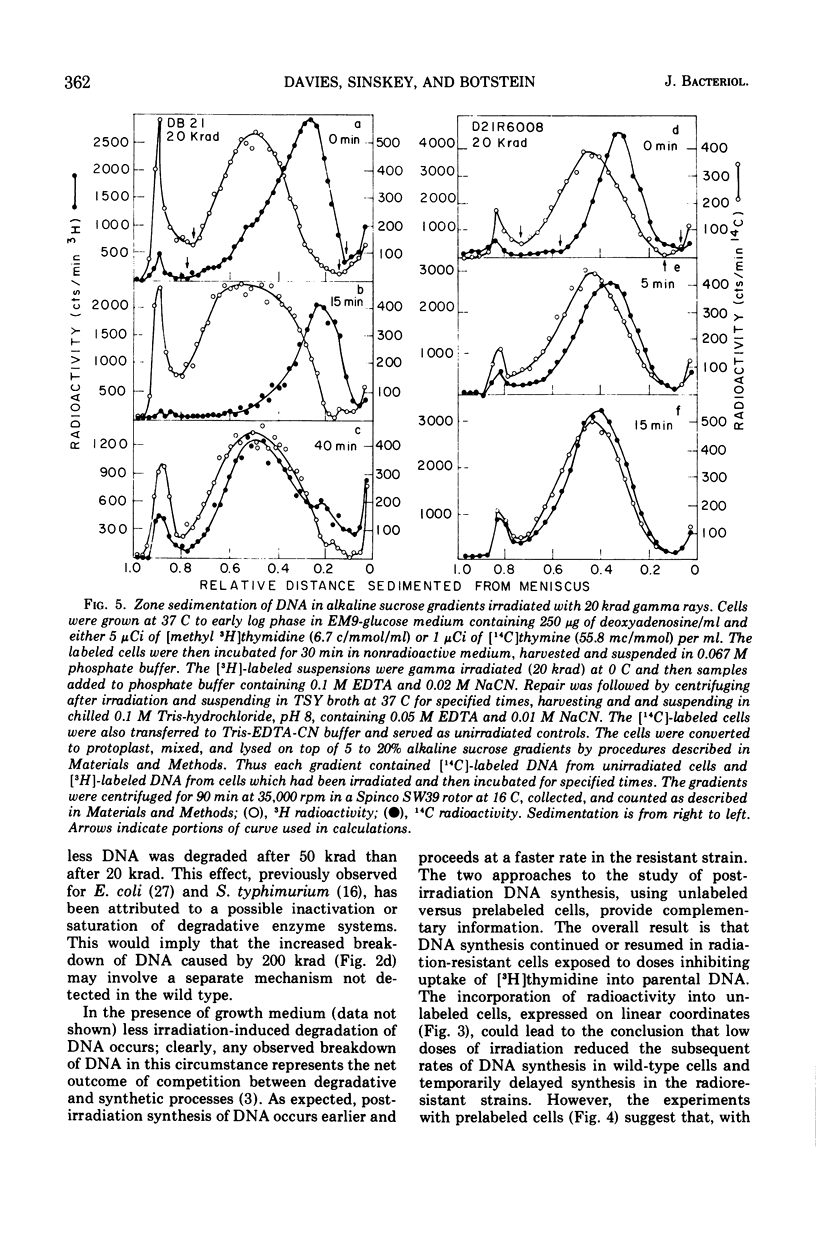
Deoxyribonucleic acid repair was studied in gamma-irradiated wild-type Salmonella typhimurium and in a radiation-resistant derivative 20 times more resistant than wild type. After exposure to 20 or 50 krad, the wild-type strain (DB21) degraded 30 to 50% of its prelabeled DNA into acid-soluble fragments, whereas the radioresistant strain degraded less than 15% after 4 h of incubation. Post-irradiation synthesis of DNA in the wild-type strain DB21 was reduced after a dose of 20 krad and totally inhibited after exposure to 200 krad. With radiation-resistant strain, D21R6008, on the other hand, DNA synthesis was delayed after a dose of 200 krad but not inhibited. Doses of 20 and 200 krad produced a similar number of single-strand breaks in the DNA of both strains as determined by zone sedimentation analysis in alkaline sucrose gradients. The radiation-resistant strain D21R6008, on the other hand, DNA synthesis was strand breaks in its DNA and repairs these damages more rapidly than wild-type Salmonella.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allwood M. C., Jordan D. C. Apparent absence of relationship between repair of single strand breaks in DNA and gamma radiation resistance in Salmonella thompson. Arch Mikrobiol. 1970;70(2):161–166. doi: 10.1007/BF00412207. [DOI] [PubMed] [Google Scholar]
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein D. Synthesis and maturation of phage P22 DNA. I. Identification of intermediates. J Mol Biol. 1968 Jun 28;34(3):621–641. doi: 10.1016/0022-2836(68)90185-x. [DOI] [PubMed] [Google Scholar]
- CAIRNS J. The bacterial chromosome and its manner of replication as seen by autoradiography. J Mol Biol. 1963 Mar;6:208–213. doi: 10.1016/s0022-2836(63)80070-4. [DOI] [PubMed] [Google Scholar]
- COLOBERT L. Etude de la lyse de salmonelles pathogènes provoquée par le lysozyme, après délipidation partielle de la paroi externe. Ann Inst Pasteur (Paris) 1958 Aug;95(2):156–167. [PubMed] [Google Scholar]
- Clark A. J., Chamberlin M., Boyce R. P., Howard-Flanders P. Abnormal metabolic response to ultraviolet light of a recombination deficient mutant of Escherichia coli K12. J Mol Biol. 1966 Aug;19(2):442–454. doi: 10.1016/s0022-2836(66)80015-3. [DOI] [PubMed] [Google Scholar]
- Davies R., Sinskey A. J. Radiation-resistant mutants of Salmonella typhimurium LT2: development and characterization. J Bacteriol. 1973 Jan;113(1):133–144. doi: 10.1128/jb.113.1.133-144.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driedger A. A. Are there multiple attachments between bacterial DNA and the cell membrane? Can J Microbiol. 1970 Sep;16(9):881–882. doi: 10.1139/m70-149. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Rate of production of single-strand breaks in DNA by x-irradiation in situ. J Mol Biol. 1968 Jul 28;35(2):303–309. doi: 10.1016/s0022-2836(68)80026-9. [DOI] [PubMed] [Google Scholar]
- Hildebrand C. E., Pollard E. C. The study of ionizing radiation effects on Escherichia coli by density gradient sedimentation. Biophys J. 1969 Nov;9(11):1312–1322. doi: 10.1016/S0006-3495(69)86453-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P. Genes that control DNA repair and genetic recombination in Escherichia coli. Adv Biol Med Phys. 1968;12:299–317. doi: 10.1016/b978-1-4831-9928-3.50016-3. [DOI] [PubMed] [Google Scholar]
- Hudnik-Plevnik T. A., Djordjević N. Survival, deoxyribonucleic acid breakdown, and synthesis in Salmonella typhimurium as compared with Escherichia coli B strains. J Bacteriol. 1970 Aug;103(2):342–347. doi: 10.1128/jb.103.2.342-347.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IDZIAK E. S., THATCHER F. S. SOME PHYSIOLOGICAL ASPECTS OF MUTANTS OF ESCHERICHIA COLI RESISTANT TO GAMMA IRRADIATION. Can J Microbiol. 1964 Oct;10:683–697. doi: 10.1139/m64-088. [DOI] [PubMed] [Google Scholar]
- Kapp D. S., Smith K. C. Repair of radiation-induced damage in Escherichia coli. II. Effect of rec and uvr mutations on radiosensitivity, and repair of x-ray-induced single-strand breaks in deoxyribonucleic acid. J Bacteriol. 1970 Jul;103(1):49–54. doi: 10.1128/jb.103.1.49-54.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lett J. T., Caldwell I., Little J. G. Repair of x-ray damage to the DNA in Micrococcus radiodurans: the effect of 5-bromodeoxyuridine. J Mol Biol. 1970 Mar;48(3):395–408. doi: 10.1016/0022-2836(70)90053-7. [DOI] [PubMed] [Google Scholar]
- McGrath R. A., Williams R. W. Reconstruction in vivo of irradiated Escherichia coli deoxyribonucleic acid; the rejoining of broken pieces. Nature. 1966 Oct 29;212(5061):534–535. doi: 10.1038/212534a0. [DOI] [PubMed] [Google Scholar]
- Morton R. A., Haynes R. H. Changes in the ultraviolet sensitivity of Escherichia coli during growth in batch cultures. J Bacteriol. 1969 Mar;97(3):1379–1385. doi: 10.1128/jb.97.3.1379-1385.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson M. C., Boyle J. M., Setlow R. B. Ultraviolet- and X-ray-induced responses of a deoxyribonucleic acid polymerase-deficient mutant of Escherichia coli. J Bacteriol. 1971 Jul;107(1):61–67. doi: 10.1128/jb.107.1.61-67.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAPLETON G. E. Variations in the sensitivity of escherichia coli to ionizing radiations during the growth cycle. J Bacteriol. 1955 Oct;70(4):357–362. doi: 10.1128/jb.70.4.357-362.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Smith K. C. DNA synthesis in sensitive and resistant mutants of Escherichia coli B after ultraviolet irradiation. Mutat Res. 1969 Nov-Dec;8(3):481–495. doi: 10.1016/0027-5107(69)90065-7. [DOI] [PubMed] [Google Scholar]
- Smith K. C., O'Leary M. E. The pitfalls of measuring DNA synthesis kinetics as exemplifed in ultraciolet radiation studies. Biochim Biophys Acta. 1968 Dec 17;169(2):430–438. doi: 10.1016/0005-2787(68)90051-8. [DOI] [PubMed] [Google Scholar]
- Stavrić S., Dighton M., Dickie N. DNA-strand breaks in two strains of Escherichia coli after exposure to -rays. Int J Radiat Biol Relat Stud Phys Chem Med. 1971;20(2):101–110. doi: 10.1080/09553007114550951. [DOI] [PubMed] [Google Scholar]
- Town C. D., Smith K. C., Kaplan H. S. DNA polymerase required for rapid repair of x-ray--induced DNA strand breaks in vivo. Science. 1971 May 21;172(3985):851–854. doi: 10.1126/science.172.3985.851. [DOI] [PubMed] [Google Scholar]
- Town C. D., Smith K. C., Kaplan H. S. Production and repair of radiochemical damage in Escherichia coli deoxyribonucleic acid; its modification by culture conditions and relation to survival. J Bacteriol. 1971 Jan;105(1):127–135. doi: 10.1128/jb.105.1.127-135.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay G. Y., Daniels M. J., Schaechter M. Isolation of a cell membrane-DNA-nascent RNA complex from bacteria. J Mol Biol. 1969 Feb 28;40(1):65–76. doi: 10.1016/0022-2836(69)90296-4. [DOI] [PubMed] [Google Scholar]
- Wing J. P., Levine M., Smith H. O. Recombination-deficient mutant of Salmonella typhimurium. J Bacteriol. 1968 May;95(5):1828–1834. doi: 10.1128/jb.95.5.1828-1834.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


