Abstract
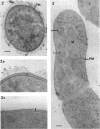
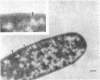
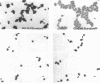
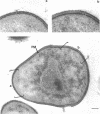
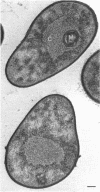
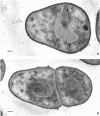
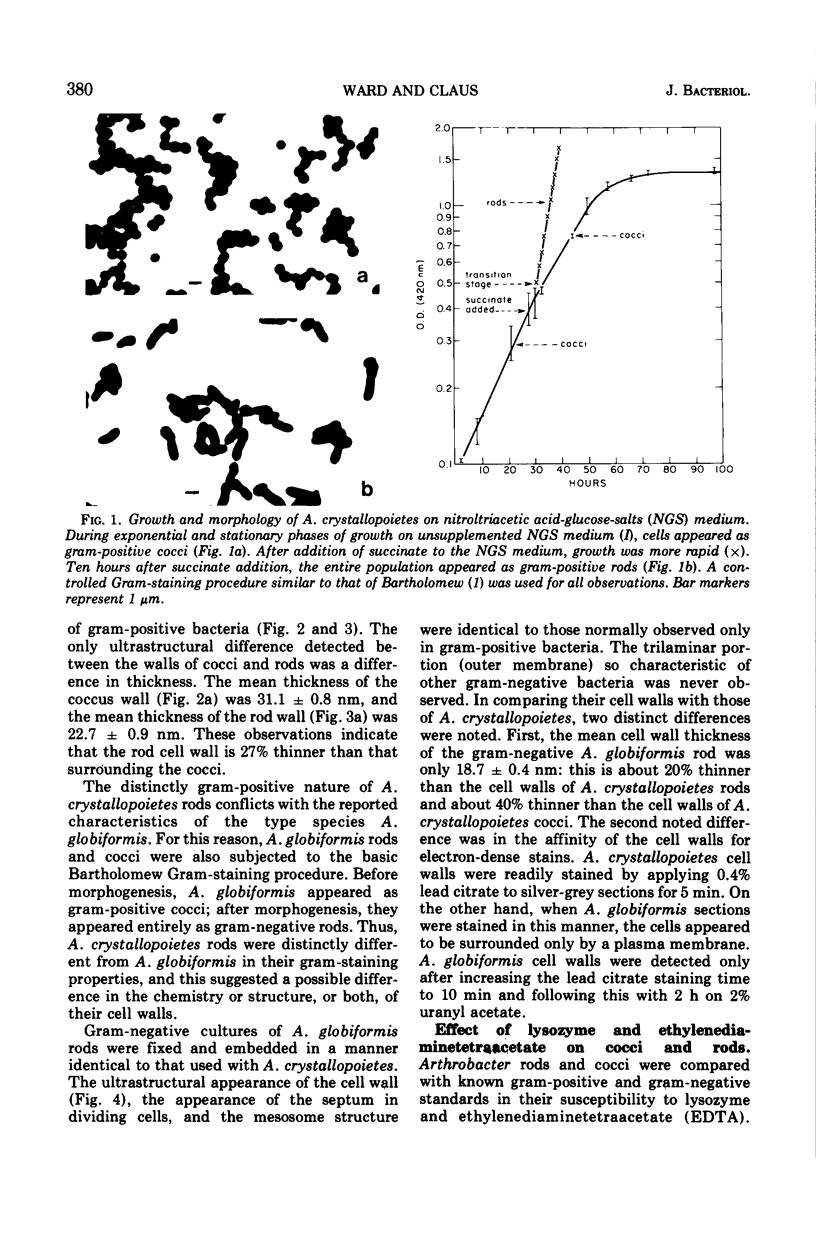
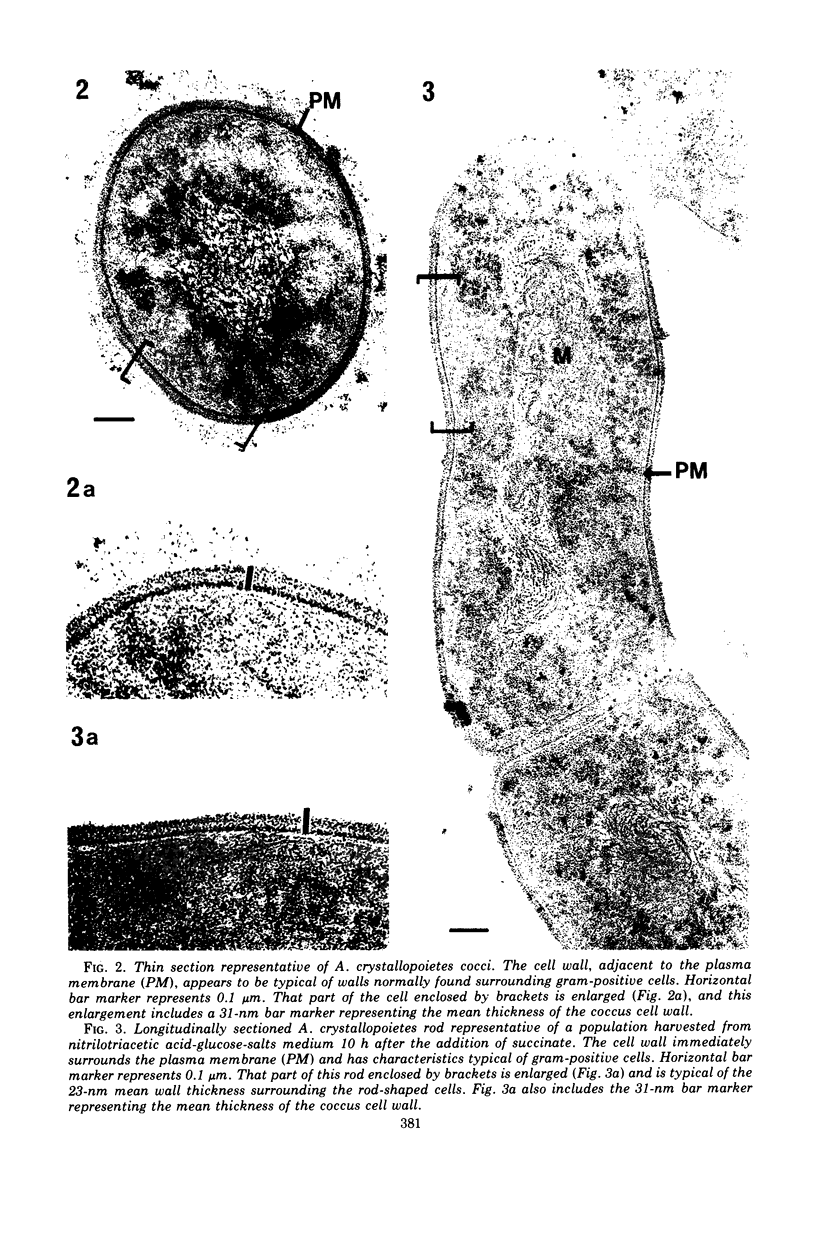
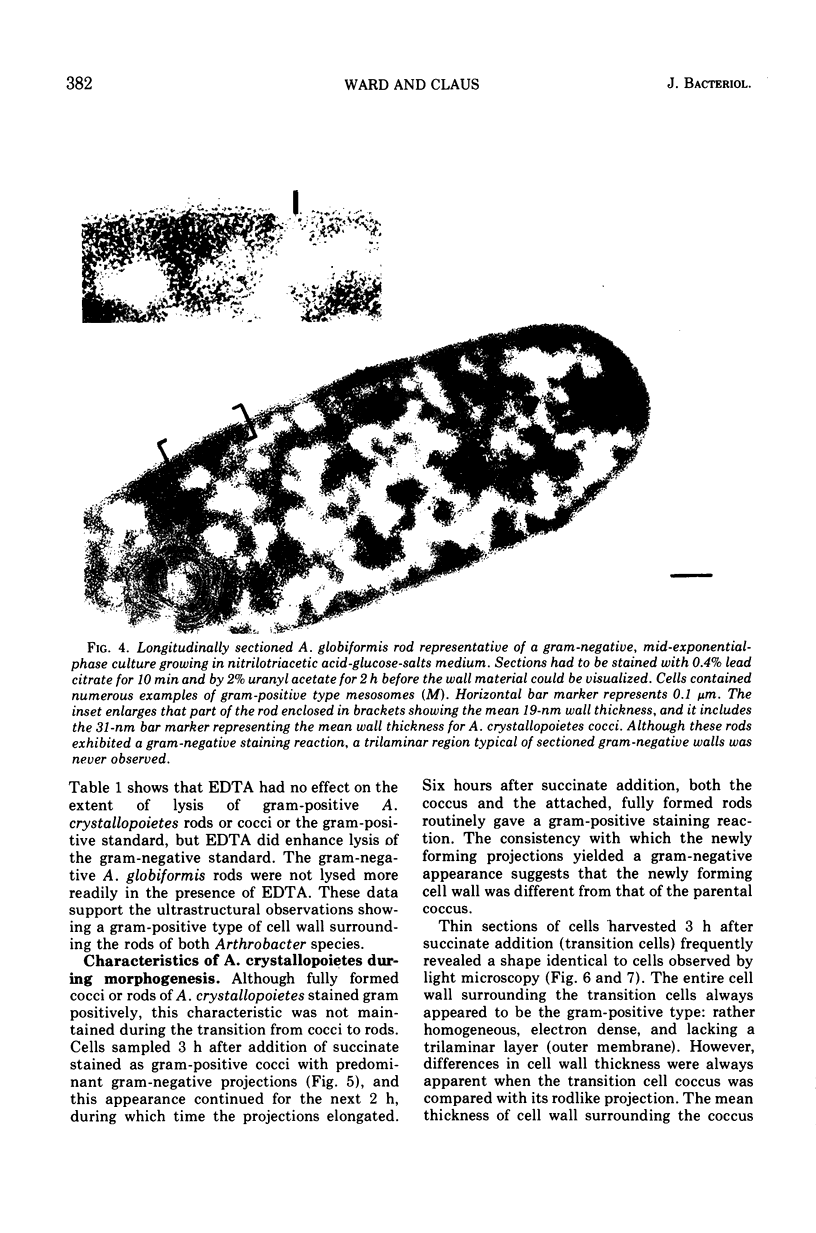
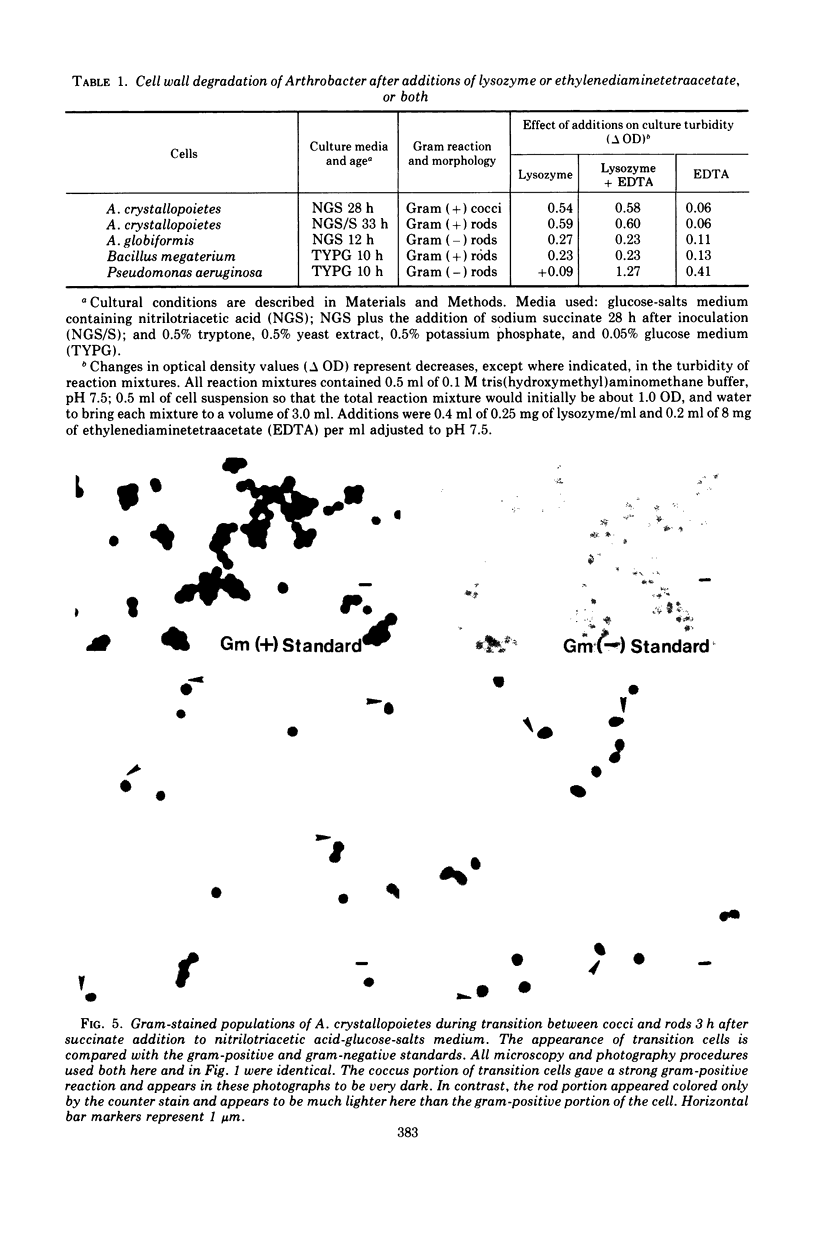
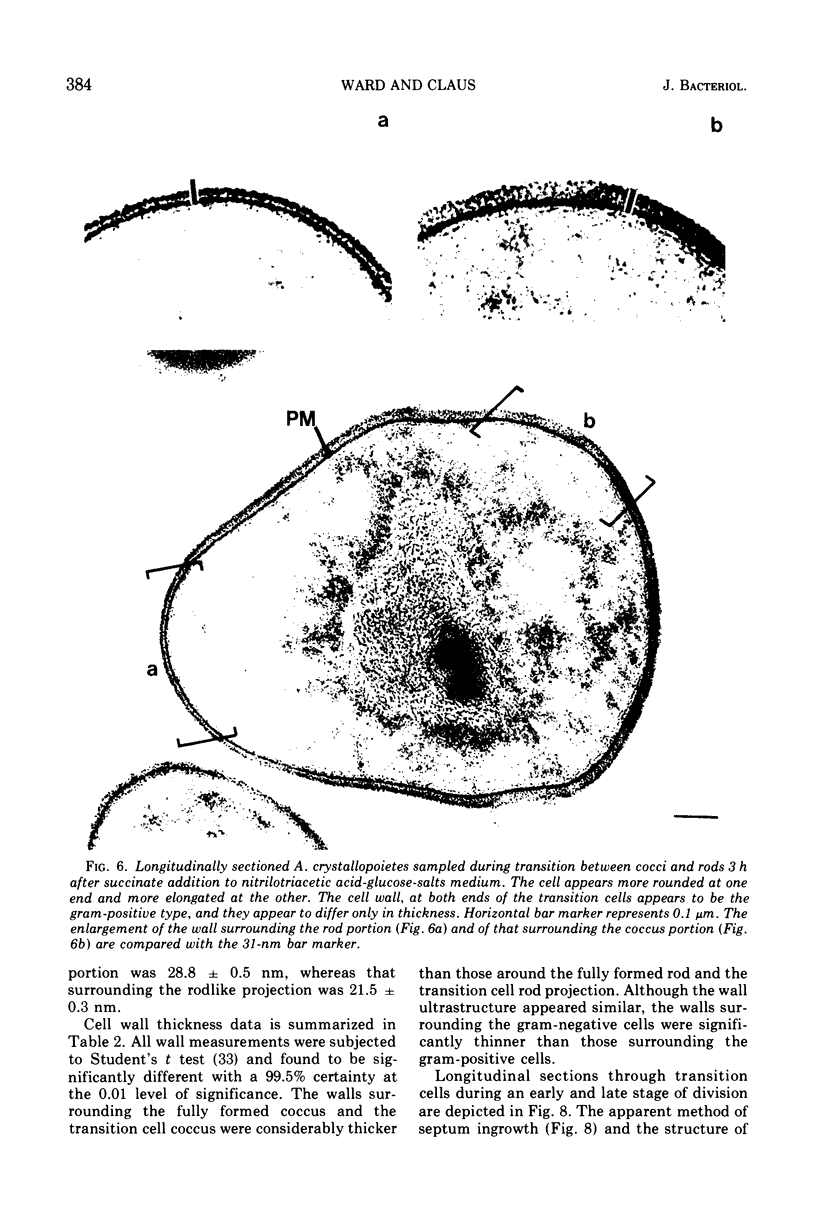
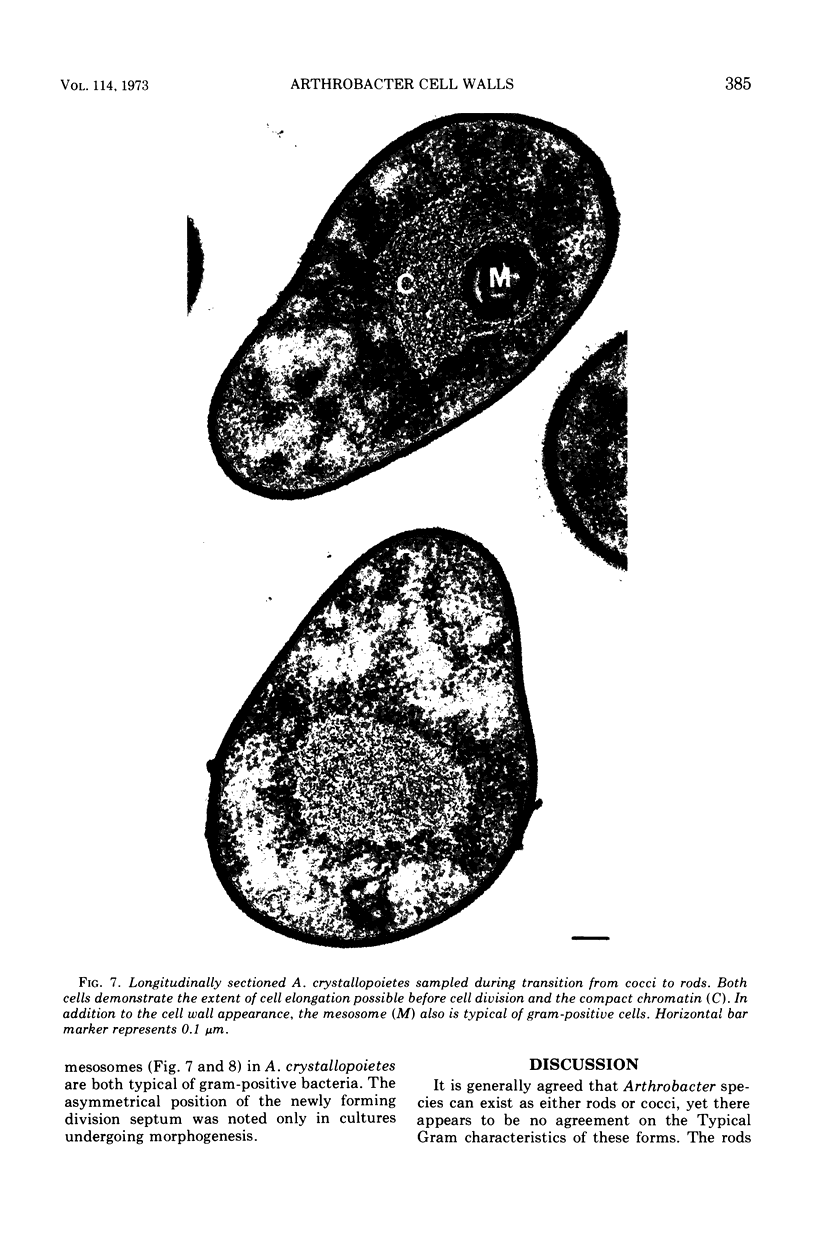
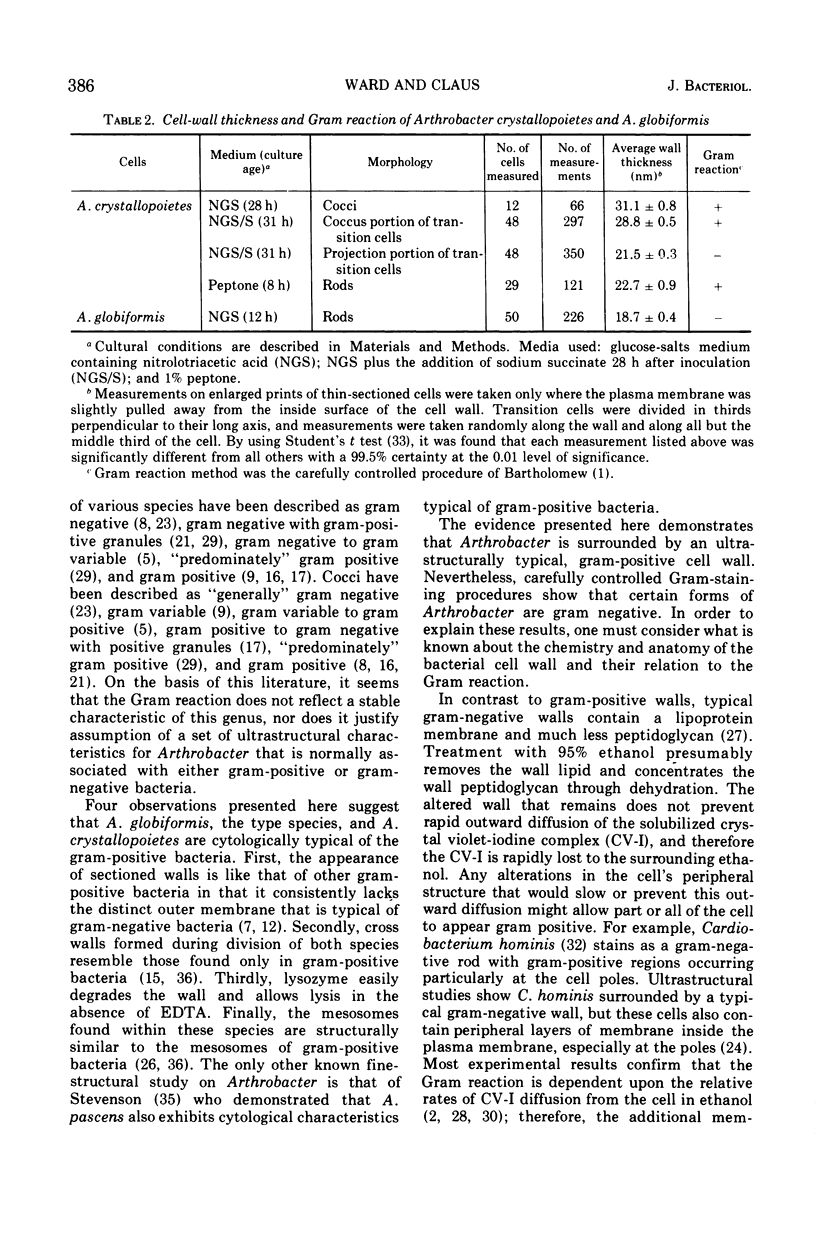
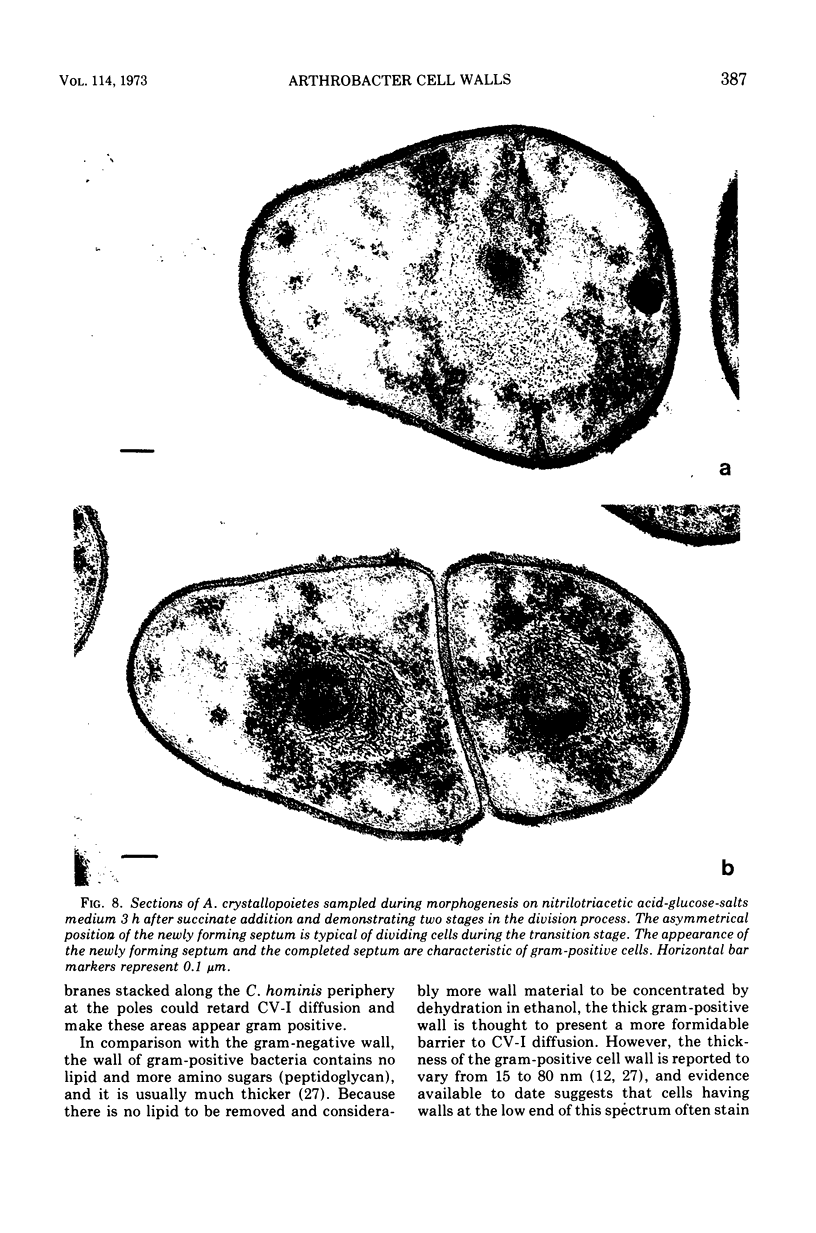
Arthrobacter crystallopoietes growing exponentially as cocci were changed to rods by adding succinate to the medium. Cells were sampled before, during, and after this transition for Gram-staining and ultrastructural studies. Cells were Gram stained by the standardized method of Bartholomew, and all samples were fixed and prepared for thin sectioning in an identical manner. Cocci were gram positive, and thin sections demonstrated a gram-positive type of cell wall having an average thickness of 31 nm. Cells sampled during morphogenesis appeared as cocci with most having a single rodlike projection. The coccus portion of these transition cells was gram positive and bound by a gram-positive type of wall having an average thickness of 29 nm. The rodlike projection of the transition cells appeared to be gram negative; it was also surrounded by a gram-positive type of wall, but its average thickness was only 22 nm. Gram-negative rods of the type species, Arthrobacter globiformis, were also examined and found to produce a gram-positive type of wall with a 19-nm average thickness. Evidence for the trilaminar region, characteristic of most gram-negative bacterial cell walls, was totally lacking in both species. These results suggest that variations in cell wall thickness may be an important contributing factor to the variable Gram-staining characteristics of this genus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLAN Grant Lochhead: an appreciation. Can J Microbiol. 1957 Mar;3(2):103–103. [PubMed] [Google Scholar]
- BARTHOLOMEW J. W., MITTWER T. The Gram stain. Bacteriol Rev. 1952 Mar;16(1):1–29. doi: 10.1128/br.16.1.1-29.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTHOLOMEW J. W. Variables influencing results, and the precise definition of steps in gram staining as a means of standardizing the results obtained. Stain Technol. 1962 May;37:139–155. doi: 10.3109/10520296209117723. [DOI] [PubMed] [Google Scholar]
- Batzing B. L., Claus G. W. Biphasic growth of Acetobacter suboxydans on a glycerol-limiting medium. J Bacteriol. 1971 Oct;108(1):592–595. doi: 10.1128/jb.108.1.592-595.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAUS G. W., ROTH L. E. FINE STRUCTURE OF THE GRAM-NEGATIVE BACTERIUM ACETOBACTER SUBOXYDANS. J Cell Biol. 1964 Feb;20:217–233. doi: 10.1083/jcb.20.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn H. J., Dimmick I. Soil Bacteria Similar in Morphology to Mycobacterium and Corynebacterium. J Bacteriol. 1947 Sep;54(3):291–303. doi: 10.1128/jb.54.3.291-303.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENSIGN J. C., RITTENBERG S. C. A CRYSTALLINE PIGMENT PRODUCED FROM 2-HYDROXYPYRIDINE BY ARTHROBACTER CRYSTALLOPOIETES N.SP. Arch Mikrobiol. 1963 Dec 10;47:137–153. doi: 10.1007/BF00422519. [DOI] [PubMed] [Google Scholar]
- ENSIGN J. C., WOLFE R. S. NUTRITIONAL CONTROL OF MORPHOGENESIS IN ARTHROBACTER CRYSTALLOPIETES. J Bacteriol. 1964 Apr;87:924–932. doi: 10.1128/jb.87.4.924-932.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauert A. M., Thornley M. J. The topography of the bacterial cell wall. Annu Rev Microbiol. 1969;23:159–198. doi: 10.1146/annurev.mi.23.100169.001111. [DOI] [PubMed] [Google Scholar]
- Greenfield S., Claus G. W. Isocitrate dehydrogenase and glutamate synthesis in Acetobacter suboxydans. J Bacteriol. 1969 Dec;100(3):1264–1270. doi: 10.1128/jb.100.3.1264-1270.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Model for cell wall growth of Streptococcus faecalis. J Bacteriol. 1970 Feb;101(2):643–648. doi: 10.1128/jb.101.2.643-648.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Procaryotic cell division with respect to wall and membranes. CRC Crit Rev Microbiol. 1971 May;1(1):29–72. doi: 10.3109/10408417109104477. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUHN D. A., STARR M. P. Arthrobacter atrocyaneus, n. sp., and its blue pigment. Arch Mikrobiol. 1960;36:175–181. doi: 10.1007/BF00412285. [DOI] [PubMed] [Google Scholar]
- Krulwich T. A., Ensign J. C., Tipper D. J., Strominger J. L. Sphere-rod morphogenesis in Arthrobacter crystallopoietes. I. Cell wall composition and polysaccharides of the peptidoglycan. J Bacteriol. 1967 Sep;94(3):734–740. doi: 10.1128/jb.94.3.734-740.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Ensign J. C., Tipper D. J., Strominger J. L. Sphere-rod morphogenesis in Arthrobacter crystallopoietes. II. Peptides of the cell wall peptidoglycan. J Bacteriol. 1967 Sep;94(3):741–750. doi: 10.1128/jb.94.3.741-750.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyn A., Birch-Andersen A., Lapage S. P. An electron microscope study of thin sections of Haemophilus vaginalis (Gardner and Dukes) and some possibly related species. Can J Microbiol. 1966 Dec;12(6):1125–1136. doi: 10.1139/m66-154. [DOI] [PubMed] [Google Scholar]
- Reyn A., Birch-Andersen A., Murray R. G. The fine structure of Cardiobacterium hominis. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(1):51–60. doi: 10.1111/j.1699-0463.1971.tb00032.x. [DOI] [PubMed] [Google Scholar]
- Ryter A. Association of the nucleus and the membrane of bacteria: a morphological study. Bacteriol Rev. 1968 Mar;32(1):39–54. doi: 10.1128/br.32.1.39-54.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALTON M. R. The relationship between the nature of the cell wall and the Gram stain. J Gen Microbiol. 1963 Feb;30:223–235. doi: 10.1099/00221287-30-2-223. [DOI] [PubMed] [Google Scholar]
- SHUGAR D., BARANOWSKA J. Quantitative gram-staining with labelled iodine. Nature. 1958 Feb 1;181(4605):357–358. doi: 10.1038/181357a0. [DOI] [PubMed] [Google Scholar]
- SLOTNICK I. J., DOUGHERTY M. FURTHER CHARACTERIZATION OF AN UNCLASSIFIED GROUP OF BACTERIA CAUSING ENDOCARDITIS IN MAN: CARDIOBACTERIUM HOMINIS GEN. ET SP. N. Antonie Van Leeuwenhoek. 1964;30:261–272. doi: 10.1007/BF02046732. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Stevenson I. L. The fine structure of Arthrobacter pascens and the development of mesosomes during the growth cycle. Can J Microbiol. 1968 Oct;14(10):1029–1034. doi: 10.1139/m68-173. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Iterson W. Symposium on the fine structure and replication of bacteria and their parts. II. Bacterial cytoplasm. Bacteriol Rev. 1965 Sep;29(3):299–325. doi: 10.1128/br.29.3.299-325.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]