Abstract
Malaria infections often cause glomerulonephritis (GN), and multiple factors have been implicated in the pathogenesis of glomerular injury. The role of cytokines in malaria associated glomerulonephritis has not been clearly defined. To study the importance of cytokines in malarial nephritis, we investigated the expression of tumour necrosis factor-alpha (TNF-α), interleukin-1alpha (IL-1α), IL-6, IL-10 and granulocyte macrophage-colony stimulating factor (GM-CSF) in kidneys acutely infected with murine malaria parasite Plasmodium berghei ANKA in C57BL/6 J mice. Groups of six mice sacrificed on days 5, 8–10, 15, and 20 postinfection, and normal controls were used for cytokine analysis. Elevated levels of messenger RNA (mRNA) specific for these cytokines in infected kidneys after day 5 postinfection were demonstrated by reverse transcription-polymerase chain reaction (RT-PCR) analysis. Kidney sections stained with specific antibodies against TNF-α, IL-1α, IL-6, IL-10 and GM-CSF by immunohistochemistry showed that the staining for these cytokines on the glomeruli was positive from day 10 postinfection, and increased progressively, mainly in the infiltrating macrophages and the glomerular mesangium. Strong correlation was found between the expression of TNF-α with IL-6, and IL-1α with IL-6. The expression of TNF-α, IL-1α, IL-6, and IL-10 also strongly correlated with the severity of proteinuria. Our findings show that there is up-regulation of cytokines in the pathogenesis of glomerulonephritis associated with murine malaria infection.
Keywords: glomerulonephritis, murine, malaria, cytokines
Glomerulonephritis (GN) is one of the common manifestations in malaria infections. It is well established that immune complexes initiate glomerular injury both in human and experimental malaria (Boonpucknavig et al. 1973; Boonpucknavig & Sitprija 1979). However, little is known of the immunopathogenic roles of cytokine networks in the pathogenesis of glomerular injury in malarial nephritis. Previous investigators demonstrated that the elevated circulating levels of proinflammatory cytokines, especially tumour necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ), interleukin-1 (IL-1), IL-6, and anti-inflammatory cytokine IL-10, which blocks the production of TNF-α, IL-1, IL-6, are associated with the severity and outcome of the disease in both severe human, and severe murine malaria (Kwiatkowski et al. 1990; Molyneux et al. 1991; Clark et al. 1992; Wenisch et al. 1995). Published data have also indicated that proinflammatory cytokine profiles (e.g. TNF-α, IL-1α, IL-6) and granulocyte macrophage-colony stimulating factor (GM-CSF) play an important role in other forms of glomerulonephritis (Yoshioka et al. 1993; Ryffel et al. 1994; Ortiz et al. 1995; Matsuda et al. 1996; Ueda et al. 1997). Both in vitro and in vivo evidence show that glomerular intrinsic cells can synthesize proinflammatory cytokines (TNF-α, IL-1, IL-6, GM-CSF) under stimulation or in pathological conditions (Baud et al. 1989; Lemay & Singh 1997). Mouse mesangial cells could express IL-10 at both the mRNA and the protein level (Fouqueray et al. 1995; Chadban et al. 1997). The over-production of cytokines has also been documented in various forms of human glomerulonephritis (Tomita et al. 1997). These locally produced cytokines have multiple actions on renal intrinsic cells and infiltrating leucocytes in the glomeruli, by autocrine or paracrine mechanisms.
We have previously shown that the dysregulation of the proinflammatory cytokines TNF-α, IL-1α, IL-6, GM-CSF, and the anti-inflammatory cytokine IL-10 is involved in tubulointerstitial injury in acute murine malaria (Li et al. 1998a). Cellular immune reactions, evidenced by the infiltration of inflammatory cells in the glomeruli, and the up-regulated expression of the major histocompatibility complex (MHC) class I and class II (Ia) antigens, and intercellular adhesion molecule-1(ICAM-1), are also responsible for renal dysfunction in acute malaria (Li et al. 1998b, c). Following these observations, it is shown in the present study that cytokines derived from infiltrating inflammatory cells in the glomeruli and intrinsic glomerular cells may play a central role in the pathogenesis of glomerulonephritis in malaria infections. The expression of TNF-α, IL-1α, IL-6, GM-CSF and IL-10 in kidneys acutely infected with murine malaria parasites was examined by reverse transcription-polymerase chain reaction (RT-PCR) analysis and immunohistochemistry. We demonstrate that there is up-regulation of the cytokines network in the glomeruli in the pathogenesis of glomerulonephritis caused by acute murine malaria infections.
Materials and methods
Parasite infection and assessment of renal dysfunction
A total of 5 × 105 erythrocytes infected with Plasmodium berghei ANKA strain of malaria were inoculated intraperitoneally into seven-week-old C57BL/6 J female mice. The course of infection was followed by calculating the percentage of parasitaemia. Urine samples were collected from individual mice, and the total urinary protein concentration (mg/dl) was measured using a turbidimetry technique (The Boehringer Mannheim Corporation urinary/CSF protein assay; Boehringer Mannheim, Germany).
Collection of kidneys
Groups of six mice were sacrificed by exsanguination under terminal anaesthesia of chloroform on days 5, 8–10, 15, and 20 during the course of infection. Six uninfected mice served as controls. One of the kidneys removed from every killed mouse was snap-frozen in liquid nitrogen and stored at − 80°C for RNA extraction. The other kidney was cut in half longitudinally, half of renal tissues was fixed in 4% paraformaldehyde for 6–8 h, and embedded in paraffin. Three micron thick sections were cut and stained with haematoxylin and eosin (H & E), periodic acid Schiff (PAS), and Masson trichrome stain for histopathological examination. Another half of renal tissues was embedded in OCT compound and snap frozen in liquid nitrogen and stored at − 70°C. Six micron thick cryo-sections were prepared for immunopathology by immunofluorescence microscopy.
Reverse transcription-polymerase chain reaction (RT-PCR)
Messenger RNA was isolated from kidneys by homogenizing samples in 4 m guanidium isothiocyanate under liquid nitrogen, followed by the standard protocol for the QuickPrep Micro mRNA purification kit (Phamarcia Biotech, Uppsala, Sweden). Sample mRNA levels were quantified by reading the absorbance at 260 nm, and 100 ng of mRNA were analysed by RT-PCR using the Access RT-PCR System (Promega, Madison, WI, USA). The following commercial primers were used to assess cytokine gene expression in RT-PCR (Table 1). The housekeeping gene encoding mouse β– actin was used as an internal control for semiquantitative comparison with cytokine transcripts.
Table 1.
Murine primers applied in RT-PCR
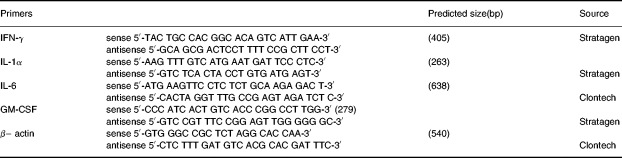
The 50 μl RT-PCR reaction mixtures contained 50 pmol primers, 0.2 mm dNTP mix, 2 mm MgSO4, 5 U AMV reverse transcriptase, 5UTfl DNA polymerase. Cycling parameters were as follows:(1) for cDNA synthesis, 48°C for 45 min, inactivation of AMV at 95°C for 2 min; (2) PCR reactions, denaturation at 94°C for 45 s, annealing at 60 °C for 45 s, extension at 72°C for 2 min up to 35 cycles, followed by a terminal extension at 72°C for seven minutes using PTC-100TM programmable thermal controller (MJ Research, Inc, USA). Negative controls included samples with no RT enzyme, and reaction mixture without mRNA. Reverse transcription-polymerase chain reaction products were examined on 1.2% agarose gels in 1XTAE buffer. Gels were stained with 0.5μg/ml ethidium bromide and photographed under ultraviolet light. Densitometric analysis of stained bands was performed with the ImageMaster VDS (Pharmacia Biotech, Uppsala, Sweden).
Immunohistochemistry
Staining procedure for immunofluorescence: Cryostat sections were fixed in cold acetone (4°C) for 10 min, rinsed in 0.01 m PBS. (1) For immunopathological examination, direct immunofluorecent technique was applied. Kidney sections were incubated with FITC conjugated goat antimouse IgM (1:256; Sigma Immunochemicals, St. Louis, Mo, USA), antimouse IgG (1:128; Sigma), or antimouse IgA (1:16; Sigma) for 30 min at room temperature. (2) For cytokines eximination, indirect immunofluorecent technique was applied. Kidney sections were incubated for 30 min with 10% normal goat serum (Sigma) to block the nonspecific binding sites for TNF-α and IL-1α or normal rabbit serum for IL-6, IL-10 and GM-CSF. Then it was incubated with primary antibodies: polyclonal rabbit antimouse TNF-α or IL-1α (1:100 dilution; Genezyme, Cambridge, MA, USA) for 45 min; or monoclonal rat antimouse IL-6, IL-10, and GM-CSF (1:100 dilution; Genezyme) for 1 h. Subsequent antibody detection was carried out by incubation with biotinylated secondary antibodies for 45 min: goat antirabbit for TNF-α and IL-1α (1:400; Sigma); rabbit antirat (mouse absorbed) for IL-6, IL-10 and GM-CSF (1:200; Vectastain; Vector Laboratories, CA, USA), and then incubated with streptavidin fluorescein (1:100; Amersham, Life Science, UK) for 1 h at room temperature. In between the sections were rinsed in PBS several times. Negative control sections were processed identically except for the sequential omission of the primary antibody, or secondary antibody, or streptavidin fluorescein. The sections were mounted with fluorescent mounting medium (Dako, Carpinteria, CA, USA) and examined under a fluorescence microscope (Zeiss, Germany).
Methods of evaluation
The location, intensity and extent of staining on the glomeruli were evaluated and graded by two authors according to the grading system as applied in previous publications (Li et al. 1998a). Briefly, the intensity was graded as 0 (no staining), 1 + (mild), 2 + (moderate), or 3 + (marked), while the extent of staining was graded as 1 + (1–25% glomeruli positive), 2 + (26–50% positive), 3 + (51–75% positive), or 4 + (> 75% positive). The intensity and distribution were added to obtain the staining score for each case. The scores ranged from 0 (negative staining) to 7 (marked intensity and > 75% areas of glomeruli stained).
Statistical analysis
The Kruskall-Wallis test with multiple comparisons was used to compare the staining scores of sections between different groups of animals postinfection (days 5, 8–10, 15, and 20) and against normal controls. The comparisons between the two groups were tested using the Mann–Whitney-U Wilcoxon Rank Sum W-test. The results of urinary protein tests were analysed with an unpaired two-tailed t-test. A P-value of less than 0.05 was considered significant. Spearman's correlation coefficient (rs) was used to identify: (1) correlation between the development of parasitaemia and the degree of renal dysfunction (proteinuria); (2) correlation between cytokine profiles (TNF-α, IL-1α, IL-6, and IL-10) expression on the glomeruli; (3) expression of cytokines on the glomeruli with the degree of renal dysfunction (proteinuria).
Results
Parasitaemia and kidney dysfunction
All mice developed gradually increasing parasitaemia. Three mice died at days 8–10 postinfection with symptoms of paralysis and black water urine. The excretion of urinary protein in infected groups increased dramatically after day 5 postinfection. The overall comparison between normal control and infected groups showed significant difference (P < 0.001). There was a strong correlation between parasitaemia and proteinuria (rs = 0.82, P < 0.001).
Histopathological changes of glomeruli
Morphological abnormalities were observed during the early stages of infection when parasitaemia was obvious. From day 10 postinfection, increased numbers of cellular infiltrates were seen in the glomeruli. These infiltrates comprised of parasitized erythrocytes, lymphocytes, polymorphonuclear leucocytes, monocytes and pigmented macrophages. There was malarial pigment deposition in the mesangium. At the later stage of the infection, the glomerular mesangial cells were enlarged and proliferated with two to three nuclei in the intercapillary mesangium (Figure 1).
Figure 1.
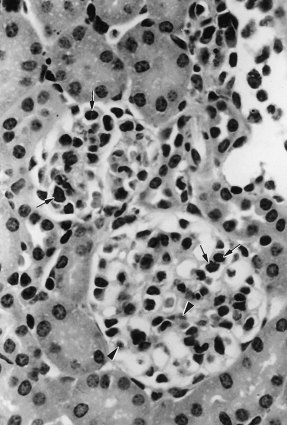
Murine malaria infected glomeruli on day 20. Glomerular hypercellularity with infiltration of pigmented macrophages, lymphocytes, polymorphonuclear leucocytes (arrows); and malaria pigment deposition (arrowhead) in the glomeruli (H & E, ×600).
Reverse transcription-polymerase chain reaction (RT-PCR) analysis of specific mRNA in kidneys
RT-PCR amplification signals of cytokines in the whole kidneys were detected by ethidium bromide staining, both in normal controls and infected kidneys for IL-1α (Figure 2b) and IL-6 (Figure 2c); and the intensity of the ethidium bromide stained specific bands increased after day 5 postinfection; the specific bands for IFN-α and GM-CSF were detected from day 5 and 10 postinfection, respectively (Figure 2a,2c). The mRNA levels of TNF-α and IL-10 were increased after day 5 postinfection as described in previous publication (Li et al. 1998a). The expression of the housekeeping gene β–actin was identical at all time points (Figure 2e). Semiquantitative densitometric analysis on ethidium bromide stained bands of RT-PCR products showed an average increase in TNF-α, IL-6, IL-10 mRNA transcripts by twofold, and IL-1α mRNA by one and half fold over baseline (normal kidney) in infected animals from day 10 and onwards.
Figure 2.

Gel electrophoresis of reverse transcription-ploymerase chain reaction (RT-PCR) amplification products of specific mRNA encoding IFN-α (a), IL-1α (b), IL-6 (c), GM-CSF (d), and the housekeeping gene (– actin (e) in kidneys. The molecular weight was 100 bp DNA ladder. The lanes are: 1, normal kidney; 2, day 5 postinfection (pi); 3, day 10 pi; 4, day 15 pi; 5, day 20 pi; 6, negative control without mRNA. ×600.
Immunopathological observation
Normal and infected kidneys on day 5 showed no staining for IgM, IgG and IgA in the glomeruli. The obvious positive staining in the glomeruli was demonstrated from day 8, 10 and 15 postinfection for IgM, IgG and IgA, respectively, predominantly in the mesangium (Figure 3a).
Figure 3.
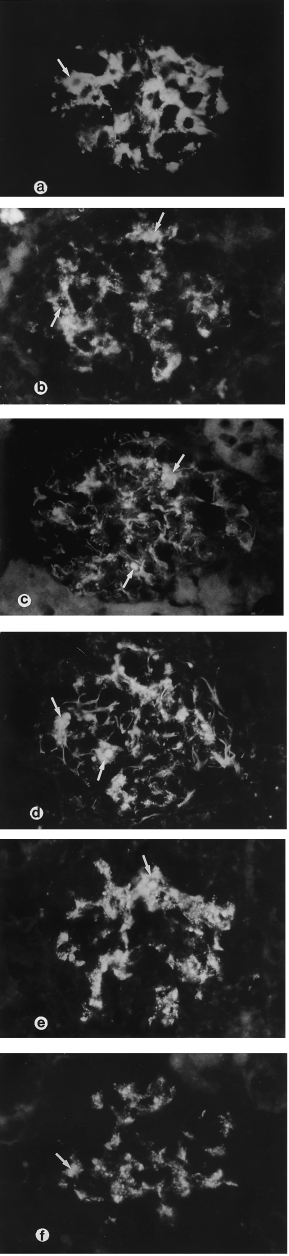
Murine Malaria Infection. Immunopathological lesions in the glomeruli. (a) IgM positive staining in the glomerular mesangium and infiltrating inflammatory cells (arrow) on day 10 postinfection; (b–f) the murine malaria nephritis glomerulus showed positive staining for cytokine markers in the mesangium, and infiltrated inflammatory cells (arrows) on day 20 postinfection; (b) TNF-α (c) IL-1α (d) IL-6 (e) GM-CSF and (f) IL-10 (Immunofluorescence microscopy × 400).
Immunoreaction with proinflammatory cytokines: TNF-α, IL-1α, IL-6 and GM-CSF
In normal and infected kidney sections on day 5, the staining for TNF-α, IL-1α, IL-6, and GM-CSF on glomeruli was negative. The expression of these cytokines in the glomeruli was markedly increased after day 5 postinfection and onwards. The median values of staining scores in the glomeruli for all cases are shown in Figure 4. There were significant differences between normal controls and diseased kidneys after day 5 (P < 0.01), and between days 10–20 postinfection. Strongly positive staining was demonstrated in inflammatory cells infiltrating the glomeruli, particularly macrophages, and weak staining in the mesangium. Mesangial cells also demonstrated positive staining at the later stage of infection (Figure 3b–3e).
Figure 4.
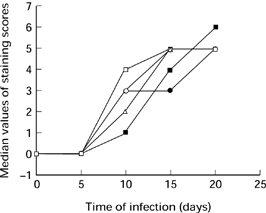
The median values of cytokine staining scores on the glomeruli during the course of infection. ○ TNF-α; • IL-1α; IL-6; □ IL-10; ▪ granulocyte macrophage-colony stimulating factor (GM-CSF).
Immunoreaction with anti-inflammatory cytokine IL-10
In normal kidneys the glomeruli showed negative staining; and in infected kidneys there was positive staining in the mesangium and mesangial cells, and strongly positive staining on the infiltrating inflammatory cells after day 5 postinfection and onwards (Figure 3f). There were significant differences between normal controls and infected groups after day 10, and also between days 10–20 (P < 0.01).
Correlation analysis for cytokine staining scores in the glomeruli with renal dysfunction
There were strong positive correlations between the staining scores of TNF-α, IL-1α and IL-6 in the glomeruli with proteinuria, and weaker correlation between GM-CSF in the glomeruli with proteinuria. IL-10 staining in the glomeruli also showed positive correlation with the degree of proteinuria.
Correlation analysis among cytokines staining scores in the glomeruli
The strongest positive correlation was observed between TNF-α/IL-1α with IL-6 staining in the glomeruli. A strong correlation also was demonstrated between the staining of TNF-α with IL-1α TNF-α with IL-10 and IL-10 with IL-6 in the glomeruli.
Discussion
This is the first study to demonstrate the levels of mRNA transcription for the proinflammatory cytokines INF-γ, IL-1α, IL-6 and GM-CSF in murine malaria nephritis. The investigation also provides immuno-histopathological evidence for the potential role of the proinflammatory cytokines TNF-α, IL-1α, IL-6, GM-CSF and the anti-inflammatory cytokine IL-10 in the pathogenesis of glomerulonephritis associated with malaria infection.
The most important finding in the present study is the in situ up-regulation of the proinflammatory cytokines TNF-α, IL-1α, IL-6 and GM-CSF both at the mRNA and at the protein levels during the course of malarial nephritis. Immunohistochemistry analysis showed that these cytokines were mainly expressed by mesangial cells and infiltrating macrophages in the glomeruli. One possible explanation for the up-regulated expression of these proinflammatory cytokines in infected kidney is that lipopolysaccharide (LPS)-like malaria toxic antigen may induce the production of proinflammatory cytokines TNF-α, IL-1 and IL-6 by macrophages (Bate et al. 1989; Jackobsen et al. 1995). In the present study pigmented macrophages expressing these cytokines were found in the glomeruli after day 5 postinfection. Another possible mechanism for the up-regulation of proinflammatory cytokines may be via locally produced proinflammatory cytokines in the kidneys such as IFN-γ as the circulating level of IFN-γ only increased at the early stage of infection and decreased to normal level after day 10 postinfection (unpublished observation), while the mRNA levels for IFN-γ was elevated in kidneys postinfection. It is possible that the in situ derived IFN-γ from infiltrating macrophages in the glomeruli may modulate the behaviour of intrinsic glomerular cells, and the mesangial cells could be a source of TNF-α, IL-1α, IL-6 and GM-CSF at the late stage of infection. The other possible explanation for the expression of cytokines by glomerular intrinsic cells is that the production of cytokines may be induced by the deposition of immune complexes in the glomeruli (Werber et al. 1987; Van den Dobbelsteen et al. 1993), as we found there was IgM, IgG deposition in the glomeruli from day 8, and day 10 postinfection, respectively.
It is well known that cytokines play an important role in regulating inflammatory responses as autocrine and paracrine signals (Sporn 1997). It has been reported in our previous publication that the circulating levels of IL-1α, IL-6 and GM-CSF were not significantly increased, although the TNF-α level increased at the early stage of infection and gradually decreased from day 10 postinfection (Li et al. 1998a). In the present study, both the mRNA levels and the expression of these proinflammatory cytokines in the glomeruli increased gradually postinfection. It is possible that these endogenous proinflammatory cytokines in the kidneys may modulate the local immune reactions in the glomeruli through cross-talk among intrinsic glomerular cells and the infiltrating inflammatory cells during the course of infection. Previous investigators showed that proinflammatory cytokines had multiple functions on the intrinsic glomerular cells. TNF-α and IL-1 induce the production of IL-6, GM-CSF, leucocyte chemotactic factors such as IL-8, and monocyte chemotactic protein-1 (MCP-1) by cultured mesangial cells (Pirotzky et al. 1990; Zoja et al. 1991). As shown in our model, the staining score for IL-6 strongly correlated with TNF-α and IL-1α in the glomeruli. It is conceivable that the increased synthesis of proinflammatory cytokines, especially GM-CSF and IL-6, may act as comitogenic factors to stimulate the local proliferation and differentiation of mesangial cells as well as their effect on macrophages in the glomeruli. The glomerular hypercellularity, with mesangial cell proliferation and plentiful pigmented macrophages infiltrating the glomeruli, was obvious at the later stage of infection. Studies also demonstrated that enhanced TNF-α and IL-1 gene expression in the renal cortex in murine glomerulonephritis (Boswell et al. 1989), and the administration of TNF-α, IL-1 or IL-6 could aggravate the glomerulonephritis in murine models (Brennan et al. 1989; Mosmann 1994). Proinflammatory cytokines TNF-α, IL-1α and IL-6 were detected both at the mRNA and at the protein levels in glomerular intrinsic cells and infiltrating inflammatory cells in human membranous nephropathy such as lupus nephritis and IgA nephritis (Yasuhiro et al. 1993; Yoshioka et al. 1993; Neale et al. 1995; Tomita et al. 1997). Furthermore, the interdependence of the proinflammatory cytokine networks may activate and affect the secretory phenotype of intrinsic glomerular cells with the up-regulated expression of MHC antigens and adhesion molecules as described in our previous papers (Li et al. 1998b, c), and trigger the cellular immune responses in the glomeruli leading to glomerulonephritis.
The second important finding is the augmented expression of the anti-inflammatory cytokine IL-10 both in the glomerular mesangial cells and in the infiltrating macrophages in diseased kidneys. The increased staining in the mesangium was observed after day 5 postinfection and onwards, maximum on day 15, and slightly decreased at the later stage of infection, which is in keeping with the decrease in mRNA level of IL-10 on day 20 as reported previously (Li et al. 1998a). However, the staining on the infiltrating macrophages increased during the course of infection. It has been reported that proinflammatory stimuli such as TNF-α IL-12, and IL-6 can activate macrophages, T cells and monocytes to produce IL-10 in vitro (Goldman 1988; Mosmann 1994; Daftarian et al. 1996). The expression of the anti-inflammatory cytokine IL-10 in the glomeruli positively correlated with the expression of the proinflammatory cytokines TNF-α, IL-1α and IL-6. Therefore, it is possible that the expression of IL-10 in the glomeruli might be stimulated by proinflammatory cytokines. Another possible explanation for the expression of IL-10 is that immune complexes deposition in the glomeruli may trigger the production of this anti-inflammatory cytokine, as a recent study demonstrated that IgG up-regulated the production of IL-10 in macrophages (Sutterwala et al. 1998). But the potential role of IL-10 expression in the glomeruli in our model has not been defined. Previous reports have found that IL-10 is a growth factor for rat mesangial cells (Chadban et al. 1997), and indicated that the cross-regulation of cytokines is performed by reciprocal inhibitory functions between the proinflammatory and the anti-inflammatory cytokines. Cultured mouse mesangial cells could synthesize and secrete IL-10 with stimulation of lipopolysaccharide (LPS), and the endogenous IL-10 inhibited the generation of TNF-α (Fouqueray et al. 1995). Furthermore, IL-10 could alleviate macrophage dependent glomerulonephritis in rats and crescentic glomerulonephritis in mice (Kitching et al. 1997; Tipping et al. 1997), and decrease the up-regulation of intercellular adhesion molecules in cultured human mesangial cells stimulated with IL-1 (Guo et al. 1997). However, we found coexpression of these cytokines both in the mesangial cells and the infiltrating inflammatory cells. Whether the local production of IL-10 in the glomeruli is beneficial to the host by reducing the local inflammatory response as an anti-inflammatory cytokine, or detrimental by decreasing the cellular defensive response to eradicate malaria parasite, requires further investigation.
In conclusion, the local expression of proinflammatory and anti-inflammatory cytokines in the glomeruli may potentially create a microenvironment leading to immune-mediated glomerulonephritis in acute murine malaria infection.
Acknowledgments
This work was supported by the research grant RP880340 awarded to Professor R. Sinniah and the research grant RP3950327 awarded to Dr A.U. Kara by the National University of Singapore. We wish to thank Mr C.K. Ow and Mr C.Y. Chia for technical assistance and photograph; as well as Ms Rohana bte Din for secretarial assistance.
References
- Bate CAW, Taverne J, Playfair JHL. Soluble malarial antigens are toxic and induce the production of tumour necrosis factor in vivo. Immunol. 1989;66:600–605. [PMC free article] [PubMed] [Google Scholar]
- Baud L, Oudinet JP, Benns M, et al. Production of tumour necrosis factor by rat mesangial cells in response to bacterial lipopolysaccharide. Kidney Int. 1989;35:1111–1118. doi: 10.1038/ki.1989.98. [DOI] [PubMed] [Google Scholar]
- Boonpucknavig V, Boonpucknavig S, Bhamarapravati N. Plasmodium berghei infection in mice: an ultrastructural study of immune complex nephritis. Am. J. Pathol. 1973;70:89–108. [PMC free article] [PubMed] [Google Scholar]
- Boonpucknavig V, Sitprija V. Renal disease in acute Plasmodium falciparum infection in man. Kidney Int. 1979;16:44–52. doi: 10.1038/ki.1979.101. [DOI] [PubMed] [Google Scholar]
- Boswell JM, Yui MA, Burt DW, Kelley VE. Increased tumour necrosis factor-α and IL-1ß gene expression in the kidneys of mice with lupus nephritis. J. Immunol. 1989;141:3050–3054. [PubMed] [Google Scholar]
- Brennan DC, Yui MA, Wuthrich RP, Kelley VE. Tumour necrosis factor and IL-1 in New Zealand black/white mice: enhanced gene expression and acceleration of renal injury. J. Immunol. 1989;143:3470–3475. [PubMed] [Google Scholar]
- Chadban SJ, Tesch GH, Foti R, Atkins RC, Nikolic-Paterson DJ. Interleukin-10 is a mesangial cell growth factor in vitro and in vivo. Lab Invest. 1997;76(5):619–627. [PubMed] [Google Scholar]
- Clark IA, MacMicking ID, Gray KM, Rockett KA, Cowden WB. Malaria mimicry with tumor necrosis factor: contrast between species of murine malaria and plasmodium falciparum. Am. J. Pathol. 1992;140(2):325–336. [PMC free article] [PubMed] [Google Scholar]
- Daftarian PM, Kumar A, Kryworuchko M, Diaz-Mitoma F. IL-10 production is enhanced in human T cells by IL-12 and IL-6 and in monocytes by tumor necrosis factor-α. J. Immunol. 1996;157:12–20. [PubMed] [Google Scholar]
- Fouqueray B, Boutard V, Philippe C, et al. Mesangial cell-derived interleukin-10 modulates mesangial cell response to lipopolysaccharide. Am. J. Pathol. 1995;147(1):176–182. [PMC free article] [PubMed] [Google Scholar]
- Goldman M. Interleukin-10 as an anti-stress cytokine. (abstract) Nephrol. 1988;3(Suppl. 1):S21, P71. [PubMed] [Google Scholar]
- Guo G, Dai ZH, Wang HY. Interleukin-10 inhibits the up-regulation of intercellular adhesion molecule-1 in cultured human mesangial cells induced by interleukin-1. (abstract) Nephrol. 1997;(Suppl. 1):S226, P608. [Google Scholar]
- Jakobsen PH, Bate CAW, Taverne J, Playfair JHL. Malaria: toxins, cytokines and disease. Parasite Immunol. 1995;17:223–231. doi: 10.1111/j.1365-3024.1995.tb01019.x. [DOI] [PubMed] [Google Scholar]
- Kitching AR, Tipping PG, Huang XR, Mutch DA, Holdsworth SR. Interleukin-4 and interleukin-10 attenuate established crescentic glomerulonephritis in mice. Kidney Int. 1997;52:52–59. doi: 10.1038/ki.1997.303. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski D, Hill AVS, Sambou I, et al. TNF concentration in fatal cerebral, nonfatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet. 1990;337:1201–1204. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- Lemay S, Singh AK. Cytokine gene expression in the MRL-lpr-lpr model of murine lupus nephritis. (abstract) Nephrol. 1997;(Suppl. 1):S476, P1603. [Google Scholar]
- Li RM, Kara AU, Sinniah R. Dysregulation of cytokine expression in tubulointerstitial nephritis associated with murine malaria. Kidney Int. 1998a;53(4):845–852. doi: 10.1111/j.1523-1755.1998.00848.x. [DOI] [PubMed] [Google Scholar]
- Li RM, Kara AU, Sinniah R. Upregulation of major histocompatibility complex (MHC) antigen in nephritis associated with murine malaria infection. J. Pathol. 1998b;185(2):212–218. doi: 10.1002/(SICI)1096-9896(199806)185:2<212::AID-PATH61>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Li RM, Kara AU, Sinniah R. In situ analysis of adhesion molecules expression in kidney infected with murine malaria. J. Pathol. 1998c;185(2):219–225. doi: 10.1002/(SICI)1096-9896(199806)185:2<219::AID-PATH77>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Shikata K, Makino H, Sugimoto H, Ota Z. Glomerular expression of macrophage colony-stimulating factor and granulocyte-macrophage colony-stimulating factor in patients with various forms of glomerulonephritis. Lab. Invest. 1996;75(3):403–412. [PubMed] [Google Scholar]
- Molyneux ME, Taylor TE, Wirima JJ, Grau GE. Tumour necrosis factor, interleukin-6, and malaria. Lancet. 1991;337:1098. doi: 10.1016/0140-6736(91)91745-g. [DOI] [PubMed] [Google Scholar]
- Mosmann TR. Properties and function of interleukin-10. Advance Immunol. 1994;56:1–26. [PubMed] [Google Scholar]
- Neale TJ, Ruger BM, Macaulay H, et al. Tumor necrosis factor-α is expressed by glomerular visceral epithelial cells in human membranous nephropathy. Am. J. Pathol. 1995;146(6):1444–1454. [PMC free article] [PubMed] [Google Scholar]
- Ortiz A, Bustos C, Alonso J, et al. Involvement of tumour necrosis factor-α in the pathogenesis of experimental and human glomerulonephritis. Advance Nephrol. 1995;24:53–77. [PubMed] [Google Scholar]
- Pirotzky E, Delattre RM, Hellegouarch A, et al. Interleukin-6 production by tumour necrosis factor and lipopolysaccharide-stimulated rat renal cells. Clin. Immunol. Immunopathol. 1990;56:271–279. doi: 10.1016/0090-1229(90)90148-j. [DOI] [PubMed] [Google Scholar]
- Ryffel B, Car BD, Gunn H, Roman D, Hiestand P, Mihatsch MJ. Interleukin-6 exacerbates glomerulonephritis in (NZB X NZW) F1 mice. Am. J. Pathol. 1994;44(5):927–937. [PMC free article] [PubMed] [Google Scholar]
- Sporn MB. The importance of context in cytokine action. Kidney Int. 1997;51:1352–1354. doi: 10.1038/ki.1997.184. [DOI] [PubMed] [Google Scholar]
- Sutterwala FS, Noel GJ, Salgame P, Mosser DM. Reversal of proinflammatory responses by ligating the macrophage Fc γ receptor type I. J. Exp. Med. 1998;188(1):217–222. doi: 10.1084/jem.188.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipping PG, Huang XR, Holdsworth SR. Interleukin-10 (IL-10) inhibits macrophage dependent glomerulonephritis (GN) in rats. (abstract) Nephrol. 1997;(Suppl. 1):S231, P628. [Google Scholar]
- Tomita S, Ueda Y, Takimoto T, Lee KH, Lidaka K. Detection of cytokines in lupus nephritis. (abstract) Nephrol. 1997;(Suppl. 1):S233, P634. [Google Scholar]
- Ueda Y, Tomita S, Lee KH, Lidaka K. In situ expression of cytokines in membranous glomerulonephritis. (abstract) Nephrol. 1997. p. S233.p. P635.
- Van den Dobbelsteen MEA, Van der Would FJ, Schroejers WEM, Van Es LA, Daha MR. Soluble aggregates of IgG and immune complexes enhanced IL-6 production by renal mesangial cells. Kidney Int. 1993;43:544–553. doi: 10.1038/ki.1993.81. [DOI] [PubMed] [Google Scholar]
- Wenisch C, Parschalk B, Narzt E, Looareesuwan S, Graninger W. Elevated serum levels of IL-10 and IFN-(in patients with acute Plasmodium falciparum malaria. Clin. Immunol. Immunopathol. 1995;74:115–117. doi: 10.1006/clin.1995.1017. [DOI] [PubMed] [Google Scholar]
- Werber B, Emancipator S, Tykocinski M, Sedor JR. The interleukin 1 gene is expressed by rat glomerular mesangial cells and is augmented in immune complex glomerulonephritis. J. Immunol. 1987;138:3207–3212. [PubMed] [Google Scholar]
- Yasuhiro H, Masayuki I, Eiji H, et al. Role of interleukin-6 in the progression of mesangial proliferative glomerulonephritis. Kidney Int. 1993;43(39):S-71–S-75. [PubMed] [Google Scholar]
- Yoshioka K, Takemura T, Murakami K, et al. In situ expression of cytokines in IgA nephritis. Kidney Int. 1993;44:825–833. doi: 10.1038/ki.1993.317. [DOI] [PubMed] [Google Scholar]
- Zoja C, Wang JM, Bettoni S, et al. Interleukin 1 beta and tumour necrosis factor alpha induce gene expression and production of leucocyte chemotactic factors, colony-stimulating factors, and interleukin-6 in human mesangial cells. Am. J. Pathol. 1991;138:991–1003. [PMC free article] [PubMed] [Google Scholar]


