Abstract
Cell suspensions of pseudomonad C, a bacterium capable of growth on methanol as sole carbon source, were able to oxidize methanol, formaldehyde, and formate, although the rates of oxidation for the latter two compounds were much slower. The latter compounds also could not serve as sole carbon sources. Through the use of labeled compounds, it was shown that in the presence of methanol, formaldehyde, formate, and bicarbonate were incorporated into trichloroacetic acid-precipitable material. Hexose phosphate synthetase activity was found, indicating the assimilation of methanol via an allulose pathway. No hydroxypyruvate reductase activity was found, nor was any complex membrane structure observed. Such a combination of characteristics has been observed in an obligate methylotroph (Pseudomonas W1), but pseudomonad C can utilize a variety of non-methyl substrates.
Full text
PDF

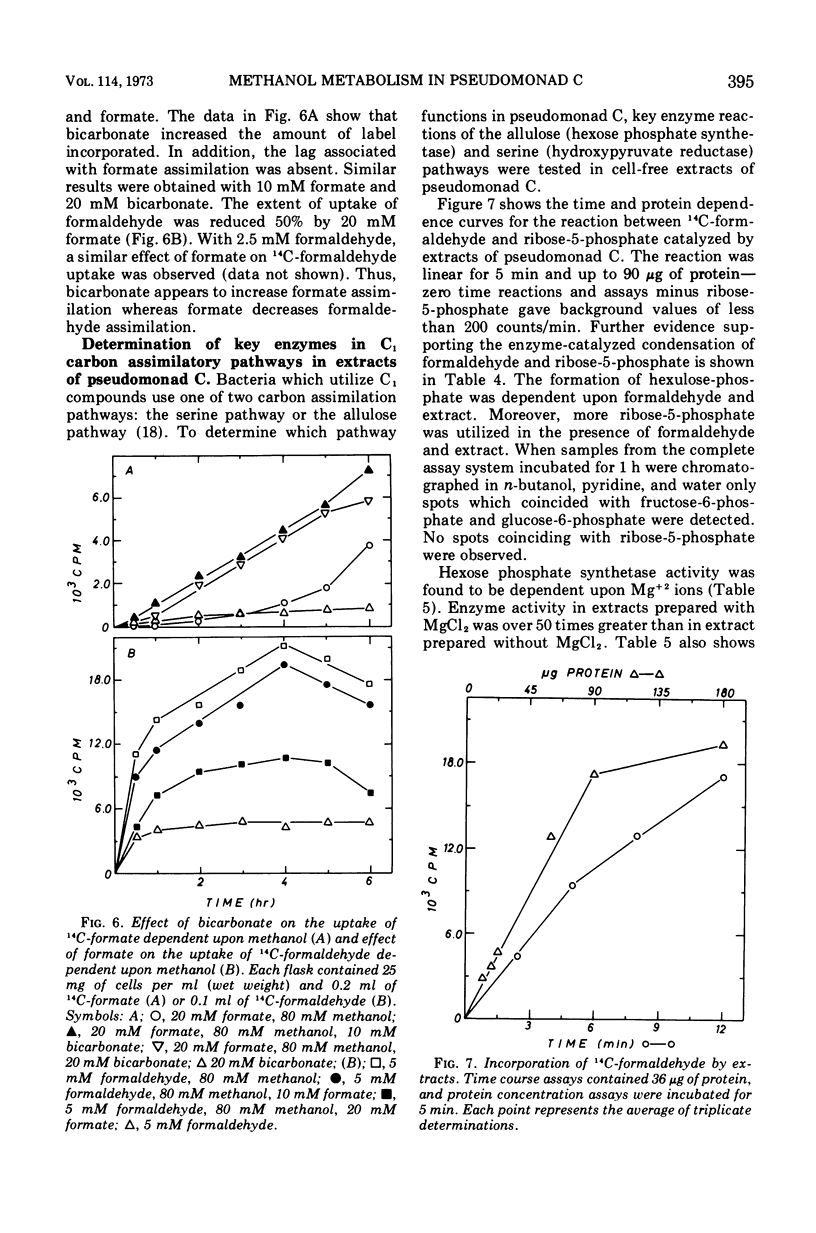
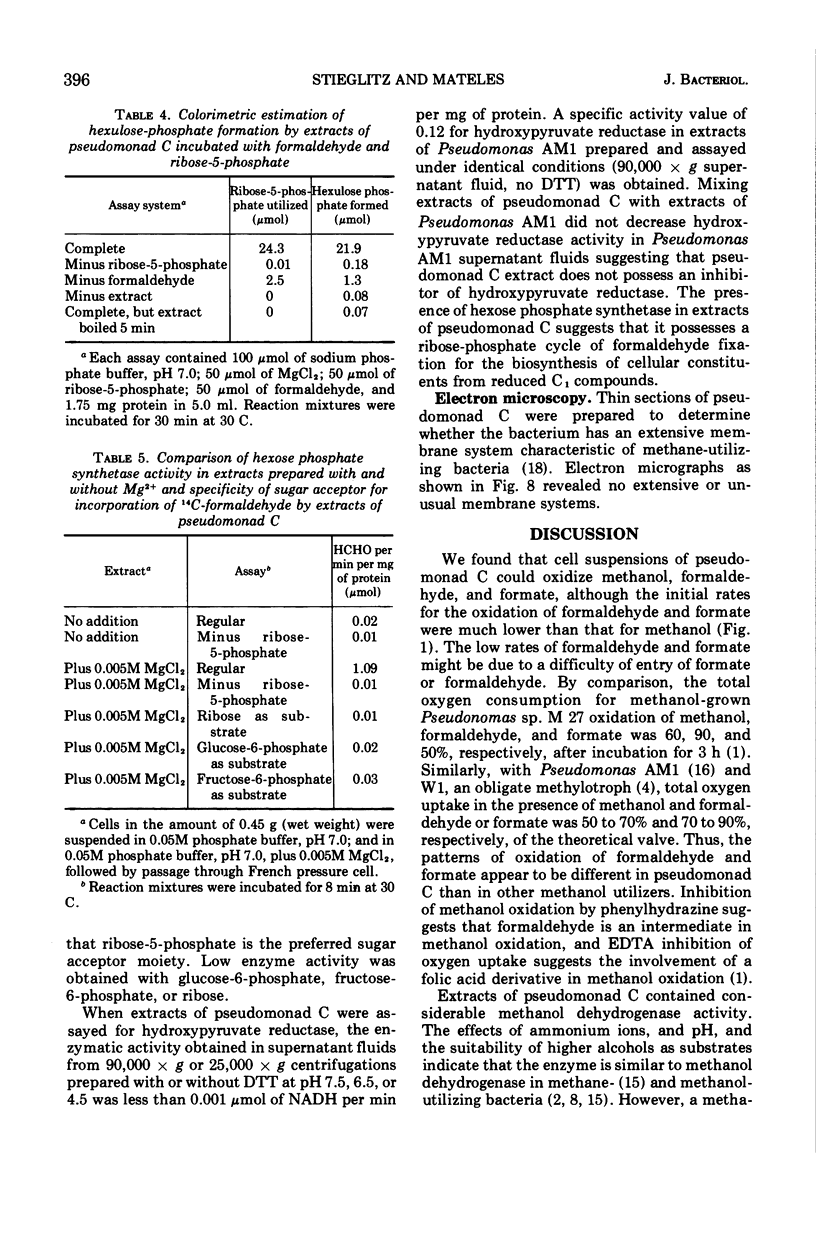
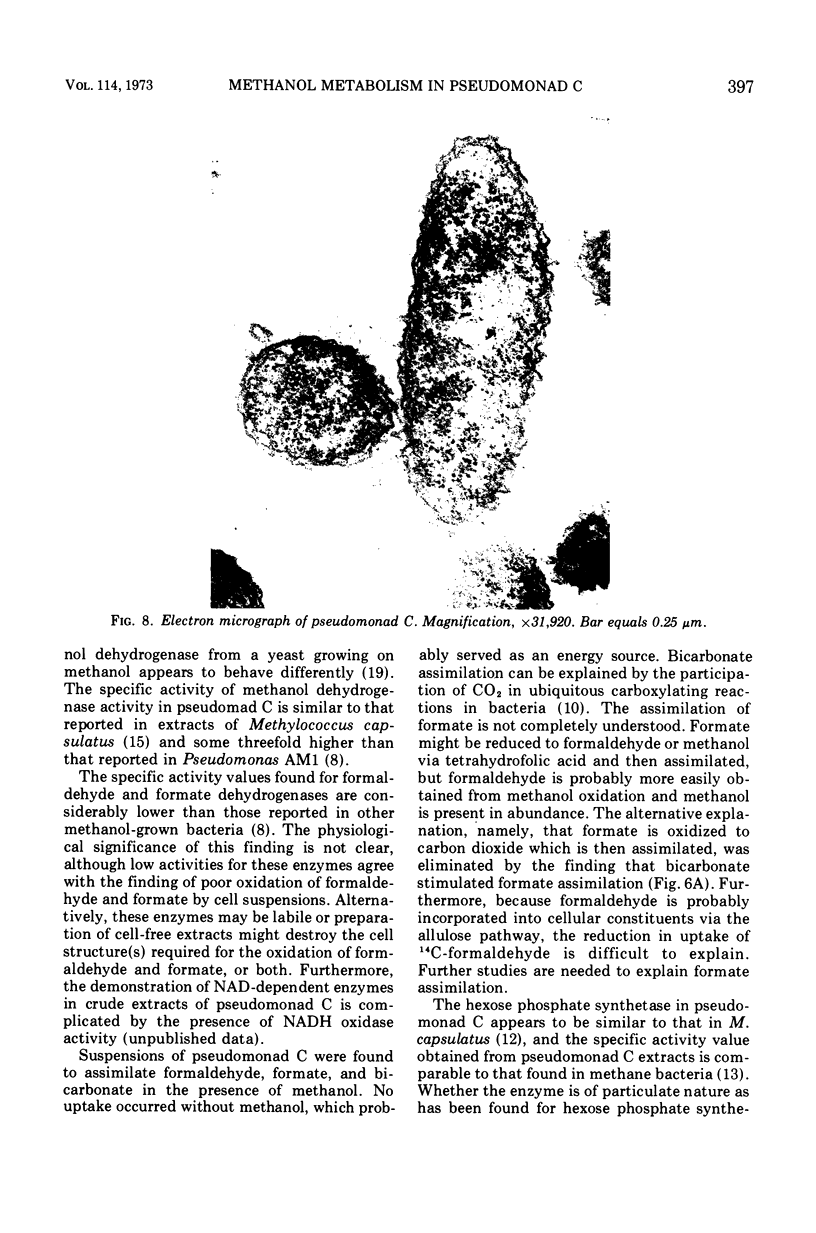

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony C., Zatman L. J. The microbial oxidation of methanol. 2. The methanol-oxidizing enzyme of Pseudomonas sp. M 27. Biochem J. 1964 Sep;92(3):614–621. doi: 10.1042/bj0920614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfan Y., Mateles R. I. New pseudomonad utilizing methanol for growth. Appl Microbiol. 1972 Jan;23(1):135–140. doi: 10.1128/am.23.1.135-140.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISCHE Z., DEVI A. A new colorimetric method for the determination of ketohexoses in presence of aldoses, ketoheptoses and ketopentoses. Biochim Biophys Acta. 1960 Mar 25;39:140–144. doi: 10.1016/0006-3002(60)90129-3. [DOI] [PubMed] [Google Scholar]
- Dahl J. S., Mehta R. J., Hoare D. S. New obligate methylotroph. J Bacteriol. 1972 Feb;109(2):916–921. doi: 10.1128/jb.109.2.916-921.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORECKER B. L., SMYRNIOTIS P. Z., KLENOW H. The formation of sedoheptulose phosphate. J Biol Chem. 1953 Dec;205(2):661–682. [PubMed] [Google Scholar]
- Johnson P. A., Quayle J. R. Microbial growth on C-1 compounds. 6. Oxidation of methanol, formaldehyde and formate by methanol-grown Pseudomonas AM-1. Biochem J. 1964 Nov;93(2):281–290. doi: 10.1042/bj0930281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp M. B., Quayle J. R. Microbial growth on C1 compounds. Incorporation of C1 units into allulose phosphate by extracts of Pseudomonas methanica. Biochem J. 1966 Apr;99(1):41–48. doi: 10.1042/bj0990041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Large P. J., Peel D., Quayle J. R. Microbial growth on C(1) compounds. 4. Carboxylation of phosphoenolpyruvate in methanol-grown Pseudomonas AM1. Biochem J. 1962 Oct;85(1):243–250. doi: 10.1042/bj0850243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large P. J., Quayle J. R. Microbial growth on C(1) compounds. 5. Enzyme activities in extracts of Pseudomonas AM1. Biochem J. 1963 May;87(2):386–396. doi: 10.1042/bj0870386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence A. J., Kemp M. B., Quayle J. R. Synthesis of cell constituents by methane-grown Methylococcus capsulatus and Methanomonas methanooxidans. Biochem J. 1970 Feb;116(4):631–639. doi: 10.1042/bj1160631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence A. J., Quayle J. R. Alternative carbon assimilation pathways in methane-utilizing bacteria. J Gen Microbiol. 1970 Nov;63(3):371–374. doi: 10.1099/00221287-63-3-371. [DOI] [PubMed] [Google Scholar]
- PEEL D., QUAYLE J. R. Microbial growth on C1 compounds. I. Isolation and characterization of Pseudomonas AM 1. Biochem J. 1961 Dec;81:465–469. doi: 10.1042/bj0810465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. N., Hoare D. S. Physiological studies of methane and methanol-oxidizing bacteria: oxidation of C-1 compounds by Methylococcus capsulatus. J Bacteriol. 1971 Jul;107(1):187–192. doi: 10.1128/jb.107.1.187-192.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbons D. W., Harrison J. E., Wadzinski A. M. Metabolism of single carbon compounds. Annu Rev Microbiol. 1970;24:135–158. doi: 10.1146/annurev.mi.24.100170.001031. [DOI] [PubMed] [Google Scholar]