Abstract
The pathogenesis of dengue haemorrhagic fever (DHF) is incompletely understood but it has been suggested that various cytokines may have a role in the process. In this study the profile of the cytokine Transforming Growth Factor-beta 1 (TGF-β1) was investigated in the sera of 79 patients with various grades of dengue illness and in 21 normal healthy controls. Also, TGF-β1-specific mRNA was examined in their peripheral blood mononuclear cells (PBMC). The results showed that neither TGF-β1 protein nor its mRNA were detected in healthy controls. In dengue patients, the TGF-β1 protein and its mRNA were detected in 96%. However, among the patient groups, the levels of TGF-β1 were lowest in patients with dengue fever (DF; mean value 315 ± 95 pg/ml) and were highest in patients with DHF grade IV (mean value 1350 ± 280 pg/ml; P = < 0.001). The cytokine appeared during the first four days of illness (304 ± 90 pg/ml) and gradually increased, reaching peak levels (1050 ± 215 pg/ml) after the 9th day of the illness. Thus TGF-β1 in the sera and TGF-β1-mRNA in the PBMC were present in most of the patients with dengue (96%) but the cytokine levels were highest during the later periods of illness and in patients with DHF grade IV, suggesting a possible role of TGF-β1 in the pathogenesis of DHF.
Keywords: cytokine, pathogenesis, dengue, dengue haemorrhagic fever, TGF-β1
Dengue virus produces either a mild self-limiting acute febrile illness, dengue fever (DF), or a life threatening, severe illness, dengue haemorrhagic fever (DHF). The characteristic pathological features of DHF are increased capillary permeability, cerebral oedema, altered number and functions of leucocytes, increased haematocrit and thrombocytopenia (Bhamarapravati 1993; Chaturvedi et al. 1997). Extensive plasma leakage into various serous cavities of the body may result in profound shock and death. Despite extensive studies, the pathogenesis of DHF is still not fully understood, though various suggestions have been made to explain the plasma leakage (reviewed by Halstead 1993; Kurane & Ennis 1994; Chaturvedi et al. 1997). In a recent study we observed a shift from a Th1-type to a Th2-type cytokine response correlating with increasing severity of the illness, thus indicating a possible role for a Th2-type response in the pathogenesis of DHF (Chaturvedi et al. 1999a). Further, in these patients a striking correlation was observed between increased levels of IL-8 and the severity of DHF, with greater levels in patients with increased grades of the disease and death (Raghupathy et al. 1998). These results suggest that these cytokines may play a role in the pathogenesis of dengue disease and death.
Transforming growth factor-beta (TGF-β) is a multifunctional polypeptide cytokine that is secreted by a variety of cells including macrophages, natural killer cells, Lymphokine-activated killer cells, B cells and both CD4+ and CD8+ T cells. It has multiple immunomodulatory effects on various target cells and tissues. It inhibits proliferation of T and B cells; antagonizes proinflammatory cytokines such as TNF-α and IFN-γ; blocks CTL activity and inhibits induction of receptors for IL-1 and IL-2 rendering cells unresponsive to these cytokines. In vivo, TGF-β inhibits adhesion of T cells and neutrophils to endothelial cells, inhibits activation of macrophages and down regulates MHC class II expression on macrophages. It also controls synthesis and degradation of extracellular matrix in virtually all cell types (reviewed by Hsuan 1989; Ding et al. 1990; Hernandez-Pando 1997). TGF-β1 plays a variety of roles in different conditions therefore it was felt useful to determine the serum levels of TGF-β1 and TGF-β1-mRNA in the peripheral blood mononuclear cells (PBMC) of patients with dengue haemorrhagic fever (DHF). Our results show a direct correlation between the levels of TGF-β1 in serum and the duration as well as the severity of the illness.
Materials and methods
Cases
An extensive epidemic of dengue and dengue haemorrhagic fever occurred in Northern India during August-November 1996. The study population consisted of patients suffering from typical dengue-like illness admitted to the Gandhi Memorial and Associated Hospitals, Lucknow and the Paediatrics Department of the All India Institute of Medical Sciences, New Delhi during this epidemic. All the patients were examined thoroughly by a clinician and laboratory investigations were done. At the time of reporting to the hospital, the clinical presentation of every patient was recorded, Hess test was performed and haematocrit value and platelet counts were measured; the last two tests were repeated daily during the course of their stay in the hospital. In the present study the grade of the illness at the time of admission, when the blood was collected has been taken into consideration. The day of the onset of fever was considered as the day 0 of the illness and thus the day of sample collection was calculated for each patient accordingly. Depending upon the severity of the illness (clinical presentation) and the findings of haematocrit and platelet count, they were classified as DF or DHF grades I, II, III or IV according to the criteria of the World Health Organization (Nimmannitya 1993). A patient was labelled as grade I when his haematocrit values were increased more than 20%; grade II when he had, in addition, spontaneous bleeding in skin or other sites; grade III had hypotension and/or narrowing of pulse pressure to 20 mmHg or less, with cold clammy skin and restlessness (shock); grade IV had undetectable blood pressure or pulse (profound shock). A total of 79 cases were included in the present study. The ages of the patients ranged from 8 months to 55 years, 62% being below 15 years of age. Diagnosis of dengue virus infection was established either by virus isolation (Chaturvedi et al. 1978) or by detection of virus-specific IgM in the sera which was measured using standard protocol (Gentry et al. 1982); in a number of cases dengue IgM capture ELISA was also performed using commercial kits (Pan Bio, East Brisbane, Australia). As controls, 21 normal age-matched healthy individuals, without history of any febrile or other illnesses in the previous three months, were included. Among the patients, 18 were classified as DF, 10 as DHF grade I, 25 as grade II, 13 as grade III and 13 as grade IV. Sera collected from the patients (on the 1st to the 18th day of illness) and the controls were divided into aliquots (to avoid repeated freezing and thawing) and quickly frozen and stored at −60°C. For the determination of cytokine concentration, sera were transported to Kuwait on dry ice and stored at −70°C until tested.
Assay of TGF-β1
Serum TGF-β1 levels were assayed by commercial ELISA kits (purchased from R & D Systems, Minneapolis, USA) according to the instructions of the manufacturer. All the tests were set up in duplicates and the data was analysed by Genesis Windows Software for microplate-based assays (Labsystems, Finland). The minimum detectable concentration by this assay was 7 pg/ml of TGF-β1. The data is presented as mean value ± SD and has been analysed using Students t-test. A P-value of less than 0.05 was considered significant.
Preparation of peripheral blood mononuclear cells, mRNA extraction and RT-PCR
Peripheral venous blood was collected in heparinized tubes. The peripheral blood mononuclear cells (PBMC), monocytes and lymphocytes, were separated from the leucocyte rich plasma on Lymphoprep, density 1.077 g/ml (Nyegaard & Co., Oslo) as described (Agarwal et al. 1998a,1998b). mRNA was extracted from the PBMC of the patients and the controls and used in RT-PCR for human cytokines according to the methods described (Hamida & Mustafa 1998). Briefly, mRNA from the PBMC were extracted by using Quick Prep Micro mRNA purification kit (Pharmacia Biotech, Sweden) according to the manufacturer's instructions. First strand cDNA was synthesized from mRNA by using the first strand cDNA synthesis kit (Pharmacia Biotech, Sweden) according to the protocol of the kit manufacturer. PCR was performed by using the first strand cDNA as the template and the primers specific for the cytokine TGF-β1 (sense-5′ ACCACTGCCGCACAACTCCGGTGAC 3′ and antisense-5′ ATCTATGACAAGTTCAAGCAGAGTA 3′). The primers specific for the house keeping gene, β-actin (sense-5′TGACGGGGTCACCCACACTGTGCCCATCTA 3′ and antisense-5′ CTAGAAGC ATTGCGGTGGACGATGGAGGG 3′) were used as a positive control. The reagents of the DNA PCR kit (Perkin-Elmer, Cetus) were used in amplification reactions according to the manufacturer's instructions. The cycling parameters for PCR were denaturation at 95°C for 15 s, annealing at 60°C for 30 s and extention at 72°C for 45 s. PCR was performed for 35 cycles followed by additional step of extention at 72°C for 7 min and the amplified DNA was analysed by gel electrophoresis. The DNA bands for β-actin (661 bp) and TGF-β1 (268 bp) were size identified by comparing with the bands of molecular weight marker DNA. In all the specimens, the DNA corresponding to β-actin was amplified suggesting that purified mRNA was suitable for RT-PCR. A specimen was considered positive or negative for TGF-β1 mRNA depending upon the presence or absence of a DNA band of 268 bp expected size.
Results
Levels of TGF-β1 in sera
None of the 21 control sera had detectable levels of TGF-β1. Among the 79 patients with dengue, 76 (96%) had TGF-β1 levels above the ‘cut-off’ value. The findings presented in Figure 1 show that as compared to DF patients (mean value of 315 ± 95 pg/ml) significantly higher amounts of TGF-β1 were detected (P = < 0.001) in the sera of patients with all grades of DHF with peak levels found in DHF grade IV patients (mean value of 1350 ± 280 pg/ml). The highest TGF-β1 value (2850 pg/ml) was seen in one patient, aged 5 years, who was bleeding from the nose when the sample was obtained on the 8th day of the illness. Absence of TGF-β1 was seen in three patients of DF; their blood samples were collected within first two days of illness.
Figure 1.
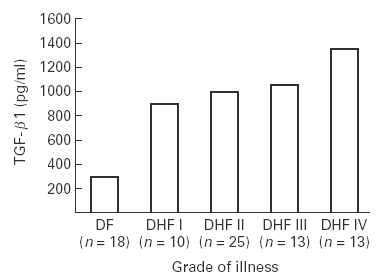
Levels of transforming growth factor-β1 (TGF-β1) in cases of dengue. Sera collected from the patients with various grades of the illness were screened for TGF-β1 concentration by sandwich ELISA using commercial kits. The mean value of the data (pg/ml) have been presented. n= total number of the cases in each group.
Sera obtained from the patients on different days of the illness, were grouped between days 1 and 4, between days 5 and 8 and the day 9 onwards. It was observed that TGF-β1 levels become detectable during the first four days of illness and the mean values were 304 ± 90 pg/ml, a higher level (930 ± 150 pg/ml) was found on days 5–8 and was then raised to 1050 ± 215 pg/ml on day 9 onwards (Figure 2); the difference from the initial period was significant (P = < 0.001). The data was further analysed with respect to the distribution of the patients with various grades of illness according to the day of the illness. The data presented in Figure 3(a) show that during the first four days of the illness the level of TGF-β1 was low in all the patient groups. However, even at these time points, with DF had the lowest levels (225 ± 100 pg/ml) of TGF-β1 while the patients with DHF grades III had the highest (450 ± 150 pg/ml) level (though the number was too small and no patient of DHF grade IV in this group was available). During the 5–8-day period, the levels of TGF-β1 was considerably elevated in all the patient groups with maximum amount of TGF-β1 detected in the sera of the patients with DHF grade IV (1440 ± 320 pg/ml) which were significantly higher (P = < 0.001) than the levels in cases of DF and DHF grades I to III (Figure 3b). From the 9th day onwards, the level of TGF-β1 was significantly reduced in patients with DF (90 ± 30 pg/ml) than the levels found in this category of patients between days 4–8 (650 ± 280 pg/ml; P = < 0.01). Higher levels of TGF-β1 were maintained in the sera of patients with DHF, with peak levels in patients with DHF grades III and IV (Figure 3c).
Figure 2.
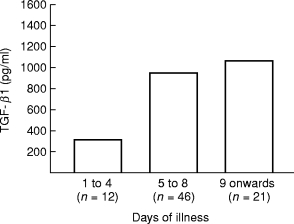
Amount of TGF-β1 in patients sera (pg/ml) as a function of the stage of dengue illness. n = total number of patients in each group.
Figure 3.
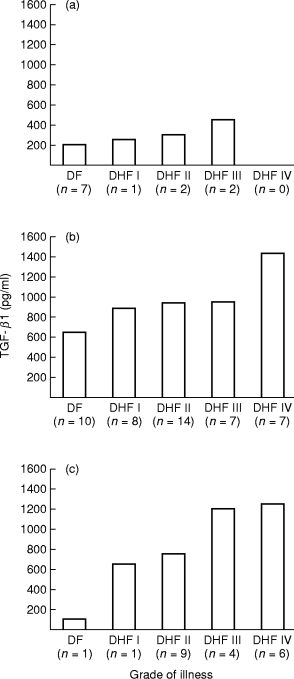
Amount of TGF-β1 in patients sera (pg/ml) as a function of the grade and stage of dengue illness. (a) During days 1–4 of the illness; (b) during days 5–8 of illness; (c) from the day 9 onwards of the illness. n = number of patients in each group.
RT-PCR for TGF-β1 gene expression
TGF-β1 gene expression in PBMC of healthy control subjects, DF and DHF patients was studied by detection of TGF-β1 mRNA using RT-PCR. The results of a representative experiment with controls, DF and DHF grade I, II, III and IV patients are given in Figure 4. The overall results showed that PBMC from none of the healthy control subjects expressed TGF-β1-mRNA, whereas 96% of the dengue patients showed evidence of TGF-β1 gene expression in their PBMC.
Figure 4.
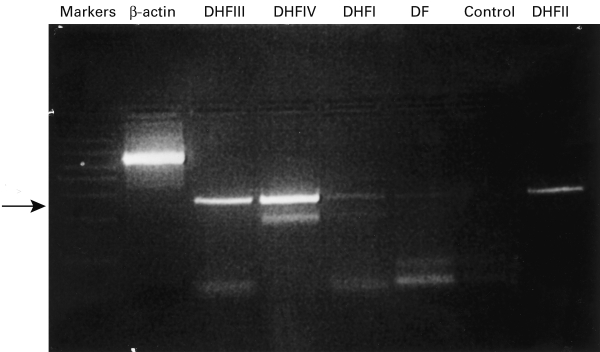
Ethidium bromide-stained agarose gel of PCR products. Detection of the TGF-β1-mRNA (arrow corresponding to 268 bp) amplified from the peripheral blood mononuclear cells (PBMC) of cases of DHF grade IV (Lane 4); grade III (Lane 3); grade II (Lane 8); grade I (Lane 5) and DF (Lanes 6); absence of TGF-β1-mRNA in the PBMC from a normal healthy control (Lane 7); the β-actin (Lane 2) and the TGF-β1 bands were size identified by comparing with the bands of molecular weight marker DNA (Lane 1).
Discussion
This study was undertaken to investigate the possible role of TGF-β1 in the pathogenesis of human dengue disease. TGF-β1 is an important immunoregulatory cytokine but its role in dengue virus infection has not been studied. A number of reports suggest that TGF-β1 has multiple roles to play in the pathogenesis of viral diseases. It contributes to the production of immunosuppression during rat cytomegalovirus (Haagmans et al. 1997) and horse herpes virus type 1 (Charan et al. 1997) infection; regulation of natural killer (NK) cell and T cell proliferation during acute lymphocytic choriomeningitis virus infection (Su et al. 1991); airway inflammation, fibrosis and dysfunction in parainfluenza virus infection (Uhl et al. 1996); pathogenesis of HIV(Li et al. 1998); hepatitis viruses (Tanaka et al. 1996; Yoo et al. 1996; Tsai et al. 1997) and Semliki Forest virus (Morris et al. 1997) infections, etc. but has no significant levels in human influenza A virus infection (Hayden et al. 1998). TGF-β1 may act as a proinflammatory or anti-inflammatory cytokine depending upon its concentration, as in the acute phase it induces secretion of IL-1α and TNF-α thus trying to control the infection (reviewed by Hernandez-Pando et al. 1997). However, TGF-β also decreases the production of free radicals, inhibits receptor expression and functions of IFN-γ, IL-1α, IL-2 and TNF-α, inhibiting Th1-type of cytokines and enhancing production of Th2-type of cytokines such as IL-10 (reviewed by Hernandez-Pando et al. 1997).
In the present study, we have attempted to understand the role of TGF-β1 in the severity and duration of dengue illness examining both cytokine protein and mRNA levels. Patient's specimen 1–4 days, 5–8 days and 9 days after onset of illness were used. To account for TGF-β1 in absence of dengue infection, sera and PBMC from healthy control subjects were included. The results demonstrate that elevated levels of TGF-β1 were detected in 96% of the sera from patients with dengue virus infection but not in the sera of control subjects. The same percentage of the patients with dengue virus infection showed evidence TGF-β1 gene activation, but none of the control subjects. These results suggest activation of TGF-β1 gene by dengue virus infection and secretion of the cytokine. TGF-β1 could be produced by a number of cell types. It is well established that the principal cells to replicate dengue virus are macrophages therefore it is likely that they could be a source of TGF-β1 in the patients with DHF (reviewed by Chaturvedi et al. 1997).
In the patients infected with dengue virus, both the severity of disease and the duration of illness were correlated with the level of TGF-β1, i.e. the maximum levels of TGF-β1 were detected in patients with DHF grade IV and those who had the disease for more than 9 days. Interestingly, in DF patients who have a mild self limiting disease, the level of TGF-β1 was maximum on days 4–8, and it was significantly reduced (P = < 0.001) by day 9 onwards. In more severely sick patients of DHF grade III and IV, the levels of TGF-β1 showed a persistent increase at all time points. There was no significant difference in TGF-β1 levels among the less severely sick patients with DHF grades I and II. These results suggest that TGF-β1 levels correlated with both severity and duration of dengue illness. The only other cytokine to show such high positivity in patients with dengue is the Cytotoxic Factor (hCF) which is also associated with the severity of illness but higher levels are seen during the initial phases (Chaturvedi et al. 1997,1999a,b; Agarwal et al. 1998b)
Studies carried out earlier, on the same group of patients showed a shift from the predominant Th1-type cytokine response observed in cases of DF to the Th2-type in severe cases of DHF grade IV. Increased serum levels of IL-4 and IL-10 were observed mainly in cases of DHF grades III and IV. In contrast, the levels of IFN-γ and IL-2 were highest in cases of DF and were low in DHF grade IV. TNF-α levels did not show a definite associative pattern. The cytokine levels to increase first were IL-2, IFN-γ and TNF-α while IL-4, IL-6, and IL-10 tended to emerge during the 4th to 8th day of the illness. Predominance of the Th1-type response is seen in 66% cases of mild illness (DF) and the Th2-type response in 71% of severe DHF grade IV which has a high fatality rate (Chaturvedi et al. 1999a). Studies carried out in the mouse model supports the above findings (reviewed by Chaturvedi et al. 1997,1999a). In their hypothesis, Kurane & Ennis (1994) had proposed that increase in the levels of TNF-α, IL-1, IL-2, platelet-activating factor, complement activation products C3a and C5a and histamine induce plasma leakage and shock. With the available data we have proposed that dengue virus induces the production of a cytokine cascade that shifts a Th1-dominant response to a Th2-biased response resulting in an exacerbation of dengue disease and death of the patients (Chaturvedi et al. 1997,1999a). The results of this study are consistent with the above hypothesis and with the hypothesis that TGF-β1 plays a role in the complex patho-genesis of severe dengue disease by maintaining the Th2-shift.
Acknowledgments
We are grateful to Professor T.D.Chugh, Chairman, Department of Microbiology, Faculty of Medicine, Kuwait University, Kuwait for constant help and support and to Professor J.C. Coleman for critical appraisal of the manuscript. Thanks are due to DrsA.K.Tripathi and K.L.Srivastava of K.G.Medical College, Lucknow and Professor M.K.Bhan of the All India Institute of Medical Sciences, New Delhi for permission to study their cases. Part of the study was carried out with the financial assistance of the Indian Council of Medical Research, New Delhi and the Kuwait University Research Administration Grant MI085.
References
- 1.Agarwal R, Chaturvedi UC, Misra A, Kapoor S, Nagar R. CD4 positive T cells produce cytotoxic factor in cases of dengue haemorrhagic fever. Curr. Sci. 1998a;74:237–239. [Google Scholar]
- 2.Agarwal R, Chaturvedi UC, Misra A, et al. Production of cytotoxic factor by peripheral blood mononuclear cells (PBMC) of the cases of dengue haemorrhagic fever. Clin. Exp. Immunol. 1998b;112:340–344. doi: 10.1046/j.1365-2249.1998.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhamarapravati N. Pathology of dengue haemorrhagic fever. In: Thongcharoen P, editor. Monograph on Dengue/Dengue Haemorrhagic Fever. New Delhi: WHO-SEARO; 1993. pp. 72–79. No. 22. [Google Scholar]
- 4.Charan S, Palmer K, Chester P, Mire-Sluis AR, Edington N. Transforming growth factor-beta induced by live or ultraviolet-inactivated equid herpes virus type-1 mediates immunosuppression in the horse. Immunology. 1997;90:586–591. doi: 10.1046/j.1365-2567.1997.00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaturvedi UC, Tandon P, Mathur A, Kumar A. Host defence mechanisms against dengue virus infection of mice. J. Gen Virol. 1978;39:293–302. doi: 10.1099/0022-1317-39-2-293. [DOI] [PubMed] [Google Scholar]
- 6.Chaturvedi UC, Dhawan R, Mukerjee R. Immunosuppression and cytotoxicity of dengue infection in the mouse model. In: Gubler DJ, Kuno G, editors. ‘Dengue and Dengue Haemorrhagic Fever. Wallingford: CAB International Press; 1997. pp. 289–309. [Google Scholar]
- 7.Chaturvedi UC, Raghupathy R, Pacsa AS, et al. Shift from a Th1-type response to Th2-type in dengue haemorrhagic fever. Curr. Sci. 1999a;76:63–69. [Google Scholar]
- 8.Chaturvedi UC, Agarwal R, Misra A, Mukerjee R, Kapoor S, Nagar R. Cytotoxic factor in dengue haemorrhagic fever. Med. Principles Pract. 1999b;8:26–31. [Google Scholar]
- 9.Ding A, Nathan CF, Graycar J, Derynck R, Stuehr DJ, Srimal S. Macrophage deactivating factor and transforming growth factors-β1, β2, and β3 inhibit induction of macrophage nitrogen oxide synthesis by IFN-γ. J. Immunol. 1990;145:940–944. [PubMed] [Google Scholar]
- 10.Gentry MK, Henchal EA, McCown JM, Brandt WE, Dalrympl JM. Identification of distinct antigenic determinants on dengue-2 virus using monoclonal antibodies. Am. J. Trop. Med. Hyg. 1982;31:548–555. doi: 10.4269/ajtmh.1982.31.548. [DOI] [PubMed] [Google Scholar]
- 11.Haagmans BL, van den Teerds KJ, van den Eijnden-van Raaij AJ.M, Horzinek MC, Schijns VE. Transforming growth factor beta production during rat cytomegalovirus infection. J. Gen. Virol. 1997;78:205–213. doi: 10.1099/0022-1317-78-1-205. [DOI] [PubMed] [Google Scholar]
- 12.Halstead SB. Pathophysiology and pathogenesis of dengue haemorrhagic fever. In: Thongcharoen P, editor. Monograph on Dengue/Dengue Haemorrhagic Fever. New Delhi: WHO-SEARO; 1993. pp. 80–103. [Google Scholar]
- 13.Hamida N, Mustafa AS. Cytokine gene activation in response to a nonspecific mitogen and specific antigens by peripheral blood mononuclear cells of healthy humans. J. Kuwait Med. Ass. 1998;33:16–21. [Google Scholar]
- 14.Hayden FG, Fritz R, Lobo MC, Alvord W, Strober W, Straus SE. Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defence. J. Clin. Invest. 1998;101:643–649. doi: 10.1172/JCI1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernandez-Pando R, Orozco H, Arriaga EK, Sampieri A, Larriva-Sahd J, Madrid V. Analysis of the local kinetics and localization of interleukin-1α, tumour necrosis factor-α and transforming growth factor-β, during the course of experimental pulmonary tuberculosis. Immunology. 1997;90:607–617. doi: 10.1046/j.1365-2567.1997.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsuan JJ. Transforming growth factors β. Brit. Med. Bull. 1989;45:425–437. doi: 10.1093/oxfordjournals.bmb.a072332. [DOI] [PubMed] [Google Scholar]
- 17.Kurane I, Ennis FA. Cytokines in dengue virus infections: role of cytokines in the pathogenesis of dengue haemorrhagic fever. Semin. Virol. 1994;5:443–448. [Google Scholar]
- 18.Li JM, Shen X, Hu PP, Wang XF. Transforming growth factor beta stimulates the human immunodeficiency virus 1 enhancer and requires NF-kappaB activity. Mol. Cell Biol. 1998;18:110–121. doi: 10.1128/mcb.18.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris MM, Dyson H, Baker D, Harbige LS, Fazakerley JK, Amor S. Characterization of the cellular and cytokine response in the central nervous system following Semliki Forest virus infection. J. Neurol. 1997;74:185–197. doi: 10.1016/s0165-5728(96)00786-2. [DOI] [PubMed] [Google Scholar]
- 20.Nimmannitya S. Clinical manifestations of dengue/dengue haemorrhagic fever. In: Thongcharoen P, editor. Monograph on Dengue/Dengue Haemorrhagic Fever. New Delhi: WHO-SEARO; 1993. pp. 48–54. No. 22. [Google Scholar]
- 21.Raghupathy R, Chaturvedi UC, Al-Sayer H, et al. Elevated levels of IL-8 in dengue haemorrhagic fever. J. Med. Virol. 1998;56:280–285. doi: 10.1002/(sici)1096-9071(199811)56:3<280::aid-jmv18>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 22.Su HC, Leite-Morris KA, Braun L, Biron CA. A role for transforming growth factor-beta 1 in regulating natural killer cell and T lymphocyte proliferative responses during acute infection with lymphocytic choriomeningitis virus. J. Immunol. 1991;147:2717–2727. [PubMed] [Google Scholar]
- 23.Tanaka S, Takenaka K, Matsumata T, Mori R, Sugimachi K. Hepatitis C virus replication is associated with expression of transforming growth factor -alpha and insulin-like growth factor-II in cirrhotic livers. Dig. Dis. Sci. 1996;41:208–215. doi: 10.1007/BF02208606. [DOI] [PubMed] [Google Scholar]
- 24.Tsai JF, Jeng JE, Chuang LY, Chang WY, Tsai JH. Urinary transforming growth factor-beta1 in hepatitis C virus-related chronic liver disease: correlation between high levels and severity of disease. Hepatology. 1997;25:1141–1146. doi: 10.1002/hep.510250516. [DOI] [PubMed] [Google Scholar]
- 25.Uhl EW, Castleman WL, Sorkness RL, Busse WW, Lemanske JR RF, McAllister PK. Parainfluenza virus-induced persistence or airway inflammation, fibrosis, and dysfunction associated with TGF-beta 1 expression in brown Norway rats. Am. J. Respir. Crit. Care Med. 1996;154:1834–1842. doi: 10.1164/ajrccm.154.6.8970378. [DOI] [PubMed] [Google Scholar]
- 26.Yoo YD, Ueda HP, Ark K, Flanders KC, Lee YI, Jay G, Kim SJ. Regulation of transforming growth factor-beta1 expression by the hepatitis B virus (HBV) X transactivator. Role in HBV Pathogenesis. J. Clin. Invest. 1996;97:388–395. doi: 10.1172/JCI118427. [DOI] [PMC free article] [PubMed] [Google Scholar]


