Abstract
Recent epidemiological studies have provided evidence supporting the potential benefits of antioxidants in coronary prevention. We have investigated the effects of vitamin E on platelets, monocytes and endothelial cells in vitro. Pre-incubation of platelets with vitamin E inhibited subsequent thrombin- (P < 0.05, n = 5), collagen- (P < 0.0001, n = 5) and ADP-(P < 0.05, n = 4) induced platelet aggregation measured using a microtitre plate method, or conventional aggregometry. The adhesion of thrombin-activated platelets to collagen was also inhibited by vitamin E (P < 0.05, n = 8), but not by vitamin C (P > 0.05, n = 8); nor was the adhesion of unstimulated platelets significantly affected (P > 0.05, n = 8). Pre-incubation of monocytes with vitamin E inhibited their subsequent adhesion to plastic (P < 0.05, n = 9), and was also associated with an 18% reduction in adhesion to EA.hy 926 endothelial cells (n = 8), although this failed to reach statistical significance. Pre-incubation of the endothelial cells with vitamin E also significantly reduced subsequent mononuclear cell adhesion by 56% (P < 0.05, n = 3).
Keywords: platelets, mononuclear cells, adhesion, aggregation, vitamin E, α–tocopherol, vitamin C
Vitamin E is a potent fat soluble plasma antioxidant (Burton et al. 1983), and it is the predominant antioxidant found in LDL particles. It has been proposed that the oxidative modification of low density lipoprotein (LDL) particles is pivotal in the pathogenesis of atherosclerosis. The ‘lipid oxidation hypothesis’ of Steinberg et al. (1989) has been supported by several recent epidemiological and intervention studies (Rimm et al. 1993; Stampfer et al. 1993; Stephens et al. 1996), and by experimental animal model data (Kita et al. 1987). Vitamin E, particularly its most biologically active isoform α-tocopherol, is the first antioxidant to be depleted during the oxidation of LDL in vitro, indicating its potential importance in preventing LDL oxidation in vivo. Epidemiological studies have shown that the plasma levels of vitamin E are low in patients with vascular disease (Gey & Pushka 1989; Riemersma et al. 1991), and that its dietary levels are predictive of future coronary events (Rimm et al. 1993 Stampfer et al. 1993). Secondary prevention studies, using vitamin E supplements have also demonstrated some benefit (Stephens et al. 1996). However the precise means by which vitamin E exerts its effects are unclear, and there is evidence that it may be acting by a number of different mechanisms unrelated to its antioxidant properties (reviewed by Ferns et al. 1993). These mechanisms may include potentially antiatherogenic effects on several cell types, including monocytes and platelets (Jandak et al. 1989; Salonen et al. 1991; Devaraj et al. 1996). It is possible that lipophilic compounds, such as vitamin E intercalate into the cell membrane, altering its composition and affecting cell responses. In this current study we have investigated the in vitro effects of α-tocopherol on platelet and monocyte function.
Methods
Materials
Eahy cells were a gift from Dr Cora-Jean Edgell, University of North Carolina, USA (Edgell et al. 1983). RPMI-1640, Dulbecco's modified Eagle's medium and foetal calf serum were purchased from Life Technologies Ltd. (Scotland, UK). Absolute ethanol was purchased from Romil Ltd (Cambridge, UK). All other reagents were from Sigma Chemical Co. (Dorset, UK).
Platelet isolation
Whole blood was taken from healthy volunteers (mean age 35 ± 12 years) by venesection from an antecubital vein and anticoagulated with 1/10th volume of 3.8% (w/v) trisodium citrate. The blood was centrifuged at room temperature for 20 min at 200 × g and the upper platelet-rich layer harvested. Apyrase (grade 1) and prostacyclin (PGI2) were added to the platelet-rich plasma at final concentrations of 10 μg/ml and 0.33 μg/ml, respectively. The platelets were pelleted by centrifuging the platelet-rich plasma at 800 × g for 15 min, washed twice in Ca2+-free Tyrode's buffer, and resuspended in Ca2+-free Tyrode's buffer at a concentration of 3 × 1011/l.
Platelet aggregometry
Platelet aggregation was assessed by the method of Fratantoni & Poindexter (1990) using an automated temperature-controlled microtitre plate reader (Anthos HTIII, Labtech International, East Sussex, UK), and was independently validated by comparison with conventional analysis using aggregometry. In brief, the time course of platelet aggregation was monitored in flat-bottomed 96-well microtitre plates (Bibby Sterilin Ltd, Staffordshire, UK) by following the change in optical density. Increasing concentrations of thrombin were added to quadruplicate wells at a final concentration ranging from 0 to 2000 units/l. Calcium chloride was added at to each well at a final concentration of 0.033 μmol/l. Platelet suspension (135 μl) in Ca2+-free Tyrode's buffer at a concentration of 3 × 1011/l was then added to each well, giving a final volume of 150 μl. The initial absorbance of the wells was measured at a wavelength of 405 nm, and subsequent readings were made at one minute intervals over a period of 20 min. The temperature of the reader was maintained at 37 °C throughout the duration of the experiment, and between readings, the plate was shaken at the maximum speed setting. The time taken for the optical density to increase by 0.200 was calculated using Biolize® software (Labtech International, East Sussex, UK). Using this information, a dose–response curve was constructed, and the EC50calculated. The effects of α-tocopherol were examined by preincubating the washed platelets for 1 h at ambient temperature with 1% ethanol (vehicle), or with 100, or 200 μmol/l of α-tocopherol (Sigma Chemical Co., Poole, UK). For standard aggregometry using the method of Born (1962), platelet-rich plasma (500 μl) was preincubated for 5 min at 37 °C with 1% ethanol, or with 100 or 200 μmol/l of α-tocopherol in a glass cuvette within a Payton aggregometer (Ion Trace Inc., Canada). After the incubation period, the platelet suspension was stirred at a constant rate and agonists were added; collagen (2.5–10.0 μg/ml), or adenosine diphosphate (ADP; 2.5–10.0 μmol/l). The aggregatory response was followed for 5 min after the addition of agonist.
Measurement of platelet adhesion
Washed platelets were prepared as described previously, and the concentration adjusted to 1 × 1011/l in Ca2+-free Tyrode's buffer. Platelet adhesion to tissue culture plastic microtitre plates and collagen coated plates was measured using the method of Bellavite et al. (1994). One hundred μl of the platelet suspension was added to uncoated wells and wells coated with collagen (type IV) by incubating with 20μg/ml collagen overnight at 4 °C. The platelets were incubated in the wells for 1 h at 37 °C. Non-adherent platelets were then removed by washing the plates twice with phosphate-buffered saline using an automated plate washer. After washing the plate, 150μl of 0.1 mol/l citrate buffer (pH 5.4) containing 0.1% (v/v) Triton-X-100 and 5 mmol/l p-nitrophenol phosphate was added, and the plate incubated for 1 h at room temperature. The reaction was terminated by the addition of 50 μl of 4 mol/l sodium hydroxide. The absorbance of each well was then measured using an Anthos HTIII plate reader set at a wavelength of 450 nm. The percentage of adherent platelets was calculated using Biolize® software (Labtech International, East Sussex, UK) with reference to a standard curve of known numbers of platelets prepared for each batch of platelets. The effects of α-tocopherol were examined by preincubating the washed platelets for 1 h at ambient temperature with 1% ethanol (vehicle), or with 100, or 200 μmol/l of α-tocopherol. The effects of concurrent stimulation were assessed by adding thrombin (final concentration 250 U/l), or equivalent volume of Tyrode's buffer to the wells. The effects of ascorbate were assessed by incubating washed platelets with 1 and 2 g/l of ascorbic acid (Sigma Chemical Co., Poole, UK), or an equivalent volume of distilled water. For the latter sample, pH was adjusted to the same value as the preparation containing ascorbic acid by the addition of 5 mol/l citric acid.
Monocyte isolation
Whole blood taken from healthy subjects was anticoagulated with trisodium citrate at a final concentration of 0.38% and centrifuged at 200 × g for 20 min. The platelet-rich supernatant was removed and the remaining packed cell layer restored to its original volume with PBS. Five ml aliquots were overlaid onto Histopaque1077 (Sigma Chemical Co., Poole, UK), and mononuclear cells were prepared according to the method of Bøyum (1968). In brief the tubes were centrifuged at 400 × g for 30 min, the mononuclear cell layer was recovered and washed twice in 5 volumes of endotoxin-free PBS. Immediately prior to use, the cells were resuspended in serum-free RPMI 1640 medium (Life Technologies Ltd, Scotland, UK) at a concentration of 6 × 109/l. Cell viability was assessed by trypan-blue exclusion prior to use, and exceeded 95% on all occasions. The platelet contamination of the mononuclear cell preparation was low (the platelet: mononuclear cell ratio was < 1).
EA-hy 926 endothelial cell line culture
EA-hy 926 cells were a gift from Dr C-J Edgell (University of North Carolina, Chapel Hill, USA). They were cultured in Dulbecco's modified Eagle's medium containing 4.5 g/l glucose, 10% fetal calf serum and 100 μmol/l hypoxanthine, 0.4 μmol/l aminopterin and 16 μmol/l thymidine. Cells were grown to confluence for the adhesion assays, and were used at passages 30–40.
Measurement of monocyte adhesion
Aliquots of the suspension of mononuclear cells in serum-free RPMI 1640 medium (6 × 105 cells) were added to 96 well trays containing confluent endothelial cell layers, or cell-free wells. The plates were incubated at 37 °C for 30 min, and nonadherent cells removed by washing twice with PBS. Monocyte-specific adherence was determined by a modification of the method described by Bath et al. (1989). This method relies on the conversion of a colourless substrate, tetramethyl benzidine (TMB) to a blue product by the action of monocyte-specific myeloperoxidase activity. The cells contained in each well were first lysed in 100 μl hexadecyltrimethyl-ammonium bromide (0.5% in PBS; pH 5.0) at 37 °C for 60 min. A freshly made solution of TMB (0.1 mg/ml in 0.05 m phosphate citrate buffer (pH 6.0) containing 0.03% sodium perborate) was added to each well and the plate incubated for 10 min at room temperature. The reaction was stopped by the addition of 50μl 2.5 mol/l sulphuric acid, and the absorbance measured at 450 nm using an Anthos HTIII microplate reader. A standard curve of cell number vs. absorbance was constructed for each batch of mononuclear cells, and the absolute adhesion calculated by reference to this curve. The effects of α-tocopherol were examined by preincubating the EA-hy-926 cells or mononuclear cells for 1 h at ambient temperature with 1% ethanol (vehicle), or with 100, or 200 μmol/l of α-tocopherol.
Statistical analysis
Statistical analysis was performed using Instat software (Graphpad Inc, USA). Significance was assessed using paired t-tests and anova. P < 0.05 was considered statistically significant.
Results
Platelet aggregation
Using the modified method of Fratantoni & Poindexter (1990) and the conditions described above, the intra-assay and interassay variabilities for the determination of thrombin EC50were 6.9% and 15.8%, respectively. Pre-incubation of the washed platelets with α-tocopherol produced a dose-dependent increase in the thrombin EC50 (P < 0.05, anova, n = 5) (Figure 1a), indicating a decrease in platelet sensitivity to thrombin-induced aggregation. Pre-incubation of platelets with α-tocopherol also inhibited the rate (P < 0.0001, anova, n = 5) and extent (P < 0.01, anova, n = 5) of collagen-induced platelet aggregation and the rate of ADP-induced aggregation (P < 0.05, anova, n = 4) assessed using standard aggregometry (Figure 1b-e).
Figure 1.

(a) Effect on thrombin-induced aggregation (measured using the microtitre plate technique) of preincubating platelets with α-tocopherol;  100 μm; ▪ 200μm; □ 1% ethanol. Assays were performed in quadruplicate. Data are expressed as the mean ±SEM (n = 5 donors). *P < 0.05 compared to ethanol control. Ethanol was used at a final concentration of 1%, and at this concentration responses did not differ significantly from control samples without ethanol (data not shown). Effects of preincubating platelets with α-tocopherol (○100 μmol/l; • 200 μmol/l) on the extent (Tmax) and rate (slope) of collagen-(b,c) and ADP-(d, e) induced aggregation using conventional aggregometry. Assays were performed in quadruplicate. Data are expressed as the mean (SEM. *P < 0.05, **P < 0.01 and ***P < 0.001 compared to ethanol control (□).
100 μm; ▪ 200μm; □ 1% ethanol. Assays were performed in quadruplicate. Data are expressed as the mean ±SEM (n = 5 donors). *P < 0.05 compared to ethanol control. Ethanol was used at a final concentration of 1%, and at this concentration responses did not differ significantly from control samples without ethanol (data not shown). Effects of preincubating platelets with α-tocopherol (○100 μmol/l; • 200 μmol/l) on the extent (Tmax) and rate (slope) of collagen-(b,c) and ADP-(d, e) induced aggregation using conventional aggregometry. Assays were performed in quadruplicate. Data are expressed as the mean (SEM. *P < 0.05, **P < 0.01 and ***P < 0.001 compared to ethanol control (□).
Platelet adhesion
The intra- and inter-assay coefficients of variability were 5.4% and 9.4%, respectively, for the measurement of platelet adhesion using the method of Bellavite et al. (1994). The colourimetric assay for platelet acid phosphatase activity was linear up to a concentration of 1 × 1011 platelets/l, and the calibration curve was unaffected by prior activation with thrombin at a concentration of 250 U/l. The adhesion of resting platelets to collagen coated wells was unaffected by preincubation with α-tocopherol at 100 and 200 μmol/l, however, the adhesion of thrombin-stimulated platelets was inhibited by 10% following preincubation with α-tocopherol at 200 μmol/l (P < 0.05, n = 8) (Figure 2a,b). Pre-incubation with 1 and 2 mg with ascorbic acid did not inhibit resting or thrombin-stimulated platelet adhesion (Figure 2c,d).
Figure 2.
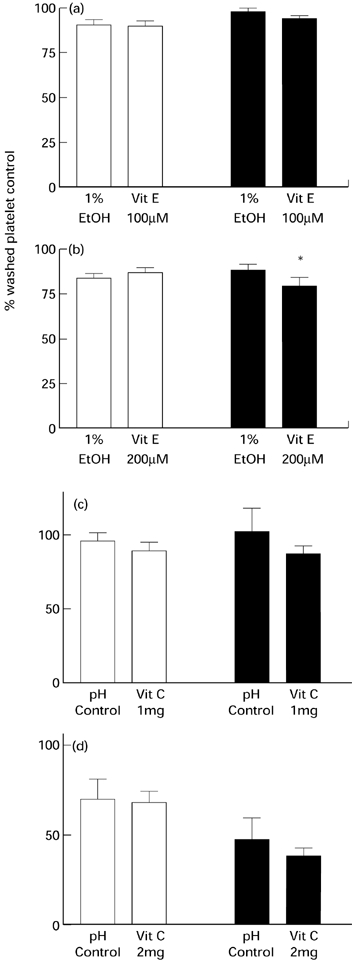
Effects of preincubating platelets with α-tocopherol at (a) 100 μmol/l and (b) 200 μmol/l, or ascorbic acid at (c) 1 g/l and (d) 2 g/l on platelet adhesion. The platelets were either in the quiescent state (□), or stimulated with 250 U/l of thrombin (▪). Assays were performed in quadruplicate. Values are expressed as the mean (SEM. *P < 0.05 compared to ethanol control (n = 8 donors).
Monocyte adhesion
The intra- and inter-assay coefficients of variability were 6.7% and 10.4%, respectively, for the measurement of platelet adhesion using the method of Bath et al. (1989). The degree of monocyte adherence was expressed as a percentage of the total number of cells added to wells, and was calculated by reference to a standard curve produced for each individual batch of mononuclear cells. These standard curves were consistently linear up to the maximum number of monocytes added to each well. Pre-incubation of mononuclear cells with α-tocopherol did not significantly inhibit mononuclear cell adhesion to the endothelial cell line, EA.hy 926, although there was an 18% reduction in mean percentage adhesion (n = 8). However, the preincubation was associated with a significant inhibition of the adhesion to tissue culture plastic (27% reduction; P < 0.05, n = 9) (Figure 3a). Pre-incubation of the endothelial cells with α-tocopherol at 200 μmol/l before the addition of mononuclear cells was also associated with a significant 56% reduction in mononuclear cell adhesion (P < 0.05, n = 3) (Figure 3b).
Figure 3.
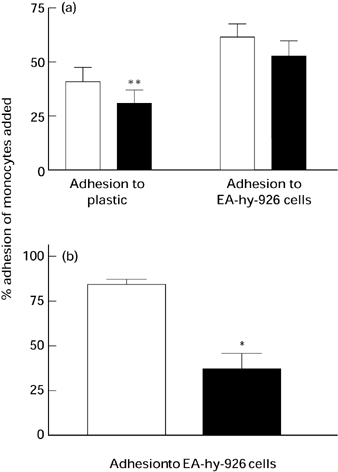
Effects of coincubation of monocytes with α-tocopherol (a) during adhesion to EA.hy 926 endothelial cells (n = 9 donors) and tissue culture plastic (n = 8 donors) and (b) preincubating EA.hy 926 endothelial cells with α-tocopherol on mononuclear cell adhesion. ▪ 200 μmol/l; □ ethanol control. Assays were performed in quadruplicate. Values are expressed as mean (SEM. *P < 0.05 vs. ethanol, **P < 0.01 vs. ethanol.
Discussion
Vitamin E inhibits platelet aggregation and adhesion
We have previously shown that dietary vitamin E supplements inhibit thrombin-induced platelet aggregation ex vivo in patients with hypercholesterolaemia (Williams et al. 1997). Salonen et al. (1991) have also shown that dietary supplementation with a cocktail of antioxidants (vitamins C, E β-carotene and selenium) inhibited ADP-induced platelet aggregation. Steiner (1991) could not demonstrate an effect of vitamin E supplements on platelet aggregation at doses of up to 1200 i.u./day, when used in isolation, although platelet adhesion was shown to be inhibited. More recently, Calzada et al. (1997a) have shown that platelets from healthy individuals given vitamin E supplements were less sensitive to ADP, arachidonic acid and collagen-induced aggregation, whereas dietary supplementation with vitamin C and β-carotene had no significant effect. Products of lipoprotein oxidation, such as hydroperoxyeicosatetranenoic acid (HPETE) have been reported to potentiate aggregation (Calzada et al. 1997b), and decreased levels of vitamin E in platelets, for example found in patients with diabetes mellitus, was associated with enhanced aggregability (Gerster 1993). Gerster proposed that vitamin E may act by modulating the metabolism of arachidonic acid, or by stabilizing the plasma membrane. Indeed it was found that vitamin E inhibited pseudopodia formation, typically associated with platelet activation. These membrane effects may also be expected to affect platelet adhesion, and Steiner (1993) has reported that vitamin E at doses of 400 iu/day inhibits platelet adhesion to a number of matrices by more than 75%. We found that although vitamin E did not affect the adhesion of resting platelets, the adhesion of thrombin-stimulated platelets to collagen was inhibited by vitamin E, but not by ascorbic acid.
Vitamin E alters monocyte adhesion in vitro
Leucocyte penetration into the arterial wall is also thought to be a key event in atherogenesis. Arterial wall macrophages have the ability to oxidize LDL and are a repository of several potent cytokines and growth factors implicated in atherogenesis. Leucocyte adhesion to the vascular endothelium is the critical first step in this process. Effects of vitamins E and C on monocyte function are also indicated by several ex vivo and in vitro studies (Ferns et al. 1993; Faruqi et al. 1994; Devaraj et al. 1996; Weber et al. 1996). In this present study we were unable to demonstrate a significant reduction in monocyte adhesion to the EA.hy 926 endothelial cell line following preincubation with vitamin E. Monocyte adhesion to plastic was inhibited significantly by vitamin E. This finding may be of some importance, as the adhesion of monocytes to plastic is thought to be mediated by the scavenger receptor, the receptor responsible for the uptake of modified LDL. Pre-incubation of the EA.hy 926 cells with vitamin E also inhibited monocyte binding. We (Ferns et al. 1993) have previously shown that dietary antioxidants inhibit monocyte adhesion in vivo, a process that appears to be partially mediated by suppression of NF-kB mobilization within the endothelial cell (Erl et al. 1997). Martin et al. (1997) have also shown that vitamin E reduces endothelial cell IL-1, prostaglandin I2 and intercellular adhesion molecule (ICAM-1) expression following their exposure to LDL.
Conclusions
Pre-incubation of platelets with vitamin E inhibited subsequent thrombin-, collagen- and ADP-induced platelet aggregation. The adhesion of thrombin-activated platelets to collagen was also inhibited by vitamin E, but not by vitamin C; nor was the adhesion of unstimulated platelets significantly affected. Pre-incubation of monocytes with vitamin E inhibited their subsequent adhesion to plastic, and was also associated with a reduction in adhesion to EA.hy 926 endothelial cells, although this failed to reach statistical significance. Pre-incubation of the endothelial cells with vitamin E also significantly reduced subsequent mononuclear cell adhesion. Although the effects of Vitamin E on platelets and mononuclear cells in vitro were modest, they are nevertheless likely to be of pathophysiological significance given the relatively short duration of exposure to vitamin E in vitro, and the fact that we and colleagues have demonstrated effects of vitamin E in vivo following dietary supplementation.
Acknowledgments
This work was supported by grants from the MRC, BHF and University of Leicester.
References
- Bøyum A. Isolation of mononuclear cells and granulocytes from human blood. J. Clin. Invest. 1968;21:77–79. [PubMed] [Google Scholar]
- Bath PM, Booth RFG, Hassall DG. Monocyte-lymphocyte discrimination in a new microtitre-based adhesion assay. J. Immunol. Meth. 1989;118:59–65. doi: 10.1016/0022-1759(89)90053-7. [DOI] [PubMed] [Google Scholar]
- Bellavite P, Andrioli G, Guzzo P, et al. A colorimetric method for the measurement of platelet adhesion in microtitre plates. Anal. Biochem. 1994;216:444–450. doi: 10.1006/abio.1994.1066. [DOI] [PubMed] [Google Scholar]
- Born GVR. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962;194:927–929. doi: 10.1038/194927b0. [DOI] [PubMed] [Google Scholar]
- Burton GW, Joyce A, Ingold KU. Is vitamin E the only lipid-soluble, chain-breaking antioxidant in human blood plasma and erythrocyte membranes? Arch. Biochem. Biophys. 1983;221:281–290. doi: 10.1016/0003-9861(83)90145-5. [DOI] [PubMed] [Google Scholar]
- Calzada C, Bruckdorfer KR, Rice-Evans CA. The influence of antioxidant nutrients on platelet function in healthy volunteers. Atherosclerosis. 1997a;128(1):97–105. doi: 10.1016/s0021-9150(96)05974-6. [DOI] [PubMed] [Google Scholar]
- Calzada C, Vericel E, Lagarde M. Low concentrations of lipid hydroperoxides prime human platelet aggregation specifically via cyclo-oxygenase activation. Biochem. J. 1997b;325(2):495–500. doi: 10.1042/bj3250495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveraj S, Li D, Jialal I. Effects of alpha tocopherol supplementation on monocyte function. Decreased lipid oxidation, interleukin-1β secretion and monocyte adhesion to endothelium. J. Clin. Invest. 1996;98:756–763. doi: 10.1172/JCI118848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgell CJ, MacDonald CC, Graham JB. Permanent cell line expressing human factor VIII related antigen established by hybridization. Proc. Natl. Acad. Sci. USA. 1983;80:3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erl W, Weber C, Wardemann C, Weber PC. α-Tocopheryl succinate inhibits monocytic cell adhesion to endothelial cells by suppressing NF-kappa B mobilization. Am. J. Physiol. 1997;A273:H634–H640. doi: 10.1152/ajpheart.1997.273.2.H634. [DOI] [PubMed] [Google Scholar]
- Faruqi R, De-La-Motte C, Dicorleto PE. α-tocopherol inhibits agonist-induced monocytic cell adhesion to cultured human endothelial cells. J. Clin. Invest. 1994;94(2):592–600. doi: 10.1172/JCI117374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferns GAA, Forster L, Stewart LEEA, Nourooz-Zadeh J, Anggard EE. Probucol inhibits mononuclear cell adhesion to vascular endothelium in the cholesterol-fed rabbit. Atherosclerosis. 1993;100:171–181. doi: 10.1016/0021-9150(93)90203-7. [DOI] [PubMed] [Google Scholar]
- Frantantoni MD, Poindexter BJ. Measuring platelet aggregation with microtitre reader. Am. J. Clin. Path. 1990;94:613–617. doi: 10.1093/ajcp/94.5.613. [DOI] [PubMed] [Google Scholar]
- Gerster H. Prevention of platelet dysfunction by vitamin E in diabetic atherosclerosis. Z-Ernahrungswiss. 1993;32(4):243–261. doi: 10.1007/BF01611163. [DOI] [PubMed] [Google Scholar]
- Gey KF, Pushka P. Plasma vitamin E and A inversely related tomortality from ischaemic heart disease. Ann. New York Acad. Sci., USA. 1989;570:268–282. doi: 10.1111/j.1749-6632.1989.tb14926.x. [DOI] [PubMed] [Google Scholar]
- Jandak J, Steiner M, Richardson PD. Alpha-tocopherol an effective inhibitor of platelet adhesion. Blood. 1989;73:141–149. [PubMed] [Google Scholar]
- Kita T, Nagano Y, Young SG, Witzum JL, Pittman RC, Steinberg D. Probucol prevents the progression of atherosclerosis in Watanabe heritable hyperlipidaemic rabbits, an animal model of familial hypercholesterolaemia. Proc. Natl. Acad. Sci. USA. 1987;84:5928–5931. doi: 10.1073/pnas.84.16.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Foxall T, Blumberg JB, Meydani M. Vitamin E inhibits low density lipoprotein-induced adhesion of monocytes to human aortic endothelial cells in vitro. Arterioscler. Thromb. Vasc. Biol. 1997;17:429–436. doi: 10.1161/01.atv.17.3.429. [DOI] [PubMed] [Google Scholar]
- Riemersma RA, Wood DA, MacIntyre CC, Elton RA, Gey KF, Oliver MF. Risk of angina pectoris and plasma concentrations of vitamin A, C and E and carotene. Lancet. 1991;337:1–5. doi: 10.1016/0140-6736(91)93327-6. [DOI] [PubMed] [Google Scholar]
- Rimm EB, Stampfer MJ, Ascherio A, Giovanniscu E, Colditz GA, Willet WC. Vitamin E consumption and the risk of coronary heart disease in men. New Engl. J. Med. 1993;328:1450–1456. doi: 10.1056/NEJM199305203282004. [DOI] [PubMed] [Google Scholar]
- Salonen JT, Salonen R, Seppanen K, et al. Effects of antioxidant supplementation on platelet function: a randomized pair-matched, placebo-controlled, double-blind trial in men with low antioxidant status. Am. J. Clin. Nutr. 1991;53(5):1222–1229. doi: 10.1093/ajcn/53.5.1222. [DOI] [PubMed] [Google Scholar]
- Stampfer MJ, Hennekens CH, Manson JE, Colditz GA, Rosner B, Willet WC. Vitamin E consumption and risk of coronary heart disease in women. New Engl. J. Med. 1993;328:1444–1449. doi: 10.1056/NEJM199305203282003. [DOI] [PubMed] [Google Scholar]
- Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witzum JL. Beyond cholesterol. Modifications of low density lipoprotein that incresae its atherogenicity. N Engl. J. Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Steiner M. Influence of vitamin E on platelet function in humans. J. Am. Coll Nutr. 1991;10(5):466–473. doi: 10.1080/07315724.1991.10718173. [DOI] [PubMed] [Google Scholar]
- Steiner M. Vitamin E: more than an antioxidant. Clin. Cardiol. 1993;16(4) Suppl. 1:I16–I18. doi: 10.1002/clc.4960161306. [DOI] [PubMed] [Google Scholar]
- Stephens NG, Parsons A, Schofield PM, et al. Randomised controlled trial of vitamin E in patients with coronary disease. Cambridge Heart Antioxidant Study (CHAOS) Lancet. 1996;347:781–786. doi: 10.1016/s0140-6736(96)90866-1. [DOI] [PubMed] [Google Scholar]
- Weber C, Erl W, Weber K, Weber PC. Increased adhesiveness of isolated monocytes to endothelium is prevented by vitamin C intake in smokers. Circulation. 1996;93(8):1488–1492. doi: 10.1161/01.cir.93.8.1488. [DOI] [PubMed] [Google Scholar]
- Williams J, Wong M, Bevan R, Ferns GAA. Dietary vitamin E supplementation inhibits platelet aggregation in patients with hypercholesterolaemia. Int J. Exp. Path. 1997;78:259–266. doi: 10.1046/j.1365-2613.1997.260359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


