Abstract
There has been considerable debate about how copper status may affect the biochemical and cellular processes associated with atherogenesis. We have investigated the effects of graded dietary copper supplementation on processes likely to contribute to atherogenesis, using the cholesterol-fed New Zealand White rabbit model. Rabbits (n = 40) were fed a 0.25–1% cholesterol diet deficient in copper. Animals received either 0, 1, 3 or 20 mg copper/day and were killed after 13 weeks. Plasma cholesterol levels were similar in each dietary group. Aortic concentrations of copper were higher in the 20 mg copper/day animals compared to those receiving 0 mg copper/day (3.70 ± 0.78 vs. 1.33 ± 0.46 µg/g wet tissue; P < 0.05). Aortic superoxide dismutase activity was higher in animals receiving 20 mg copper/day (323 ± 21 IU/mg tissue) compared to the other groups (187 ± 21; 239 ± 53; 201 ± 33 IU/mg tissue) (P > 0.05). En face staining of aortae with oil red O showed that both high copper supplementation (20 mg/day) (67.1 ± 5.5%) and a deficient diet (0 mg/day) (63.1 ± 4.8%) was associated with significantly larger lesions (P < 0.05) compared to moderately supplemented animals (1 mg/day and 3 mg/day) (51.3 ± 6.3 and 42.8 ± 7.9%). These data indicate that in the cholesterol-fed rabbit, there is an optimal dietary copper intake and that dietary copper deficiency or excess are associated with an increased susceptibility to aortic atherosclerosis. Many Western diets contain insufficient copper and these findings indicate that a moderate dietary copper content may confer a degree of cardiac protection to the human population.
Keywords: dietary copper, atherosclerosis, cholesterol-fed rabbit, superoxide dismutase, caeruloplasmin, aortic copper
Introduction
Copper is an essential nutrient in the human diet (Linder 1991), and abnormalities in its body levels have been linked to a number of risk factors for coronary heart disease (CHD). Many Western diets, including those provided in hospitals and other establishments, are reported to be deficient in copper and typically provided 0.3–1.5 mg copper per day. The World Health Organization (WHO) Expert Committee on Trace Elements recommend a daily intake of 33 µg copper/kg body weight, or approximately 2–4 mg copper/day for an adult male (Ferns et al. 1997).
Copper deficiency has been proposed as a risk factor for coronary heart disease because of its association with elevated plasma cholesterol levels (Klevay 1997). There is considerable evidence supporting this hypothesis, which we have recently reviewed (Ferns et al. 1997). However, epidemiological studies also show that elevated plasma copper levels are associated with coronary risk. Prospective studies have shown high serum copper levels are associated with an increased future risk of CHD in Finnish (Salonen et al. 1991; Nyyssönen et al. 1997) and Dutch men (Kok et al. 1998). However the majority of copper is transported in plasma as caeruloplasmin, and serum copper levels are strongly related to levels of caeruloplasmin (Goode et al. 1998). Previous studies have shown that high plasma caeruloplasmin levels are associated with CHD risk in prospective studies in both men and women (Reunanen et al. 1992; Nyyssönen et al. 1997). Caeruloplasmin, however, is an acute phase reactant, and its plasma levels are elevated in infectious and inflammatory states and following traumatic injury (Gutteridge & Stocks 1981). It is therefore unclear whether these epidemiological data reflect a positive association between CHD and copper status per se, or whether raised serum copper levels are an indication of an underlying inflammatory process.
We have recently demonstrated that moderate dietary copper supplementation inhibits atherogenesis in the cholesterol-fed rabbit (Lamb et al. 1999). In the present study, we have examined these apparent discrepancies by investigating the effect of dietary copper deficiency and graded supplementation on atherosclerosis in cholesterol-fed rabbits.
Materials and methods
Materials
Formulated rabbit diets (< 0.5 mg copper/kg) were supplied by Special Diet Services (Witham, Essex, UK). All reagents were analytical grade and supplied by Fisher Scientific (Loughborough, Leicester, UK) unless otherwise stated.
Rabbit colonies
Juvenile New Zealand White rabbits (10-week-old) weighing approximately 2.0 kg were housed in the Experimental Biology Unit at the University of Surrey, Guildford in accordance with Home Office regulations. Food and water were allowed ad libitum.
Dietary groups
Each experimental group consisted of 10 rabbits. All rabbits were fed a copper-deficient food containing 0.25–1% (w/w) cholesterol. Rabbits received either 0, 5, 20 or 140 mg copper acetate monohydrate/L in their drinking water. Water consumption was measured and each rabbit drank between 400 and 500 ml per day throughout the experimental period. This approximates to receiving 0, 1, 3 or 20 mg copper/day. The plasma cholesterol levels were measured at fortnightly intervals. This enabled the modification of the cholesterol content of cholesterol-containing diets to maintain the plasma cholesterol at approximately 20 mmol/L. This was achieved by mixing the copper-deficient 1% cholesterol diets with the corresponding copper-deficient food diets to produce individually tailored diets with different cholesterol content.
Blood sampling
Blood was drawn from the ear vein before the start of the experimental diet and at fortnightly intervals thereafter into heparinized containers and plasma obtained by centrifugation at 4° C. Plasma was stored at − 70° C prior to analysis.
Cholesterol measurement
Plasma cholesterol levels were determined using a cholesterol oxidase colourimetric kit (Boehringer Mannheim, Lewes, East Sussex), and measured on a Cobas Bio analyser (Roche, Lewes, East Sussex).
Plasma ferrioxidase activity
Caeruloplasmin levels were measured by assaying freshly drawn plasma for ferrioxidase activity (Schosinsky et al. 1974). Briefly, 0.05 mL plasma was added in duplicate to 0.75 mL 0.1 m acetate buffer pH 5.0. The substrate, 0.2 mL 7.88 mm o-dianisidine 2HCl (Sigma, Poole, Dorset, UK) was added and the reaction terminated in the first tube after 5 min and after 15 min in the second tube with 2 mL 9 m sulphuric acid. The absorbance after 5 min was measured at 540 nm and subtracted from that after 15 min. The activity was calculated using the molar absorption coefficient of the oxidized substrate (9.6 mL/µmol/cm) and expressed as IU/L.
Animal killing
Thirteen weeks after initiation of the formulated diets, the animals were anaesthetized with xylazine (3.5 mg/kg intramuscularly) (Bayer Plc, Bury St Edmunds, Suffolk, UK) and ketamine (18 mg/kg intramuscularly) (Willows Francis Veterinary, Crawley, West Sussex, UK), heparinized (300IU/kg intravenously) (Sigma, Poole, Dorset, UK) and the abdominal aorta cannulated. Rabbits were then killed with an overdose of pentobarbitone (Rhone Merieux, Harlow, Essex, UK) and the jugular veins transected for perfusion run-off. Rabbits were perfused with isotonic saline and 5 rabbits from each dietary group were perfusion-fixed in situ with 4% paraformaldehyde in isotonic saline as described previously (Rutherford et al. 1997). Following perfusion, the entire thoracic aortae were isolated, cleaned of fascia and divided longitudinally. One half was placed into fresh 4% paraformaldehyde in isotonic saline prior to staining with oil red O for morphometric analysis as previously described (Rutherford et al. 1997). The remaining half was divided into defined segments using the intercostal branches as markers. Segments from perfusion fixed aorta were frozen for measurement of copper content whilst, segments from unfixed aortae were frozen for measurement of superoxide dismutase activities.
Quantification of lesional area
Longitudinal halves of aortae were rinsed in 80% propan-2-ol and stained for 90 secs in 80% propan-2-ol containing 2% (w/v) oil red O (Sigma, Poole, Dorset, UK). Aortae were rinsed in 80% propan-2-ol, restained for a further 90 secs in 80% propan-2-ol containing 2% (w/v) oil red O followed by destaining in 80% propan-2-ol and phosphate buffered saline as described previously (Rutherford et al. 1997). The sections were pinned out on a cork board and en face images acquired using a JVC CCD camera. The area staining positively with oil red O was quantified using QWin 550C image analysis software (Leica Microsystems, Cambridge, Cambridgeshire, UK) and expressed as a percentage of the total area analysed.
Tissue and plasma copper content
All reagents were treated with chelex-100 prior to use. Aortic samples (0.5 g) between the second and third intercostal branches were dissolved in 7 mL 3.5 m nitric acid by heating to 150 C for 30 min in a Techator digester (Perstop Analytical Ltd, Bristol, UK). When cool, 1 mL 11.6 m perchloric acid was added and tubes heated to 150 C for 30 min, 200 C for 15 min and 250 C for 15 min and then allowed to cool. The volume was made up to 10 mL with 1% nitric acid. The copper content was measured by flame atomic absorption spectroscopy in an SP9 atomic absorption spectrophotometer (Pye Unicam, Cambridge, UK) against 0.05–5 µg copper/mL standards (Puchades et al. 1989).
Aortic superoxide dismutase (SOD) activity
Small frozen sections of aorta (5–15 mg) from between the third and fourth intercostal branches were carefully cleaned of adherent fascia, weighed, dipped in liquid nitrogen and crushed in a liquid nitrogen-cooled Mikro-Dismembrator II (B. Braun, Melsungen, Germany). Powdered tissue was dissolved in phosphate buffered saline containing 0.3 m potassium bromide and 3% (v/v) protease inhibitor cocktail (Sigma, Poole, Dorset, UK), sonicated for 5 min at full power in an Ultrawave sonicating water bath (Philip Harris Scientific, London, UK) and the supernatant retained following centrifugation at 6500 × g for 10 min. The protein content of the soluble fraction was determined using a modification of the method of Lowry et al. (1951). The soluble aortic extract was analysed for SOD activity using a Ransod kit (Randox Laboratories, Crumlin, Co. Antrim, N Ireland) according to manufacturers' instructions and a Cobas Mira analyser (Roche, Lewes, East Sussex, UK).
Statistical analysis
Comparisons were performed using the Mann Whitney test for non-parametric data. Statistical significance was assumed with a P-value < 0.05. All analyses were performed using Instat 2 (GraphPad Software Inc, San Diego, CA, USA) software.
Results
Blood chemistry
In the two groups of animals fed cholesterol-enriched diets, plasma cholesterol levels increased to approximately 20 mmol/L approximately 4 weeks after commencing the diets containing 1% cholesterol. The cholesterol composition of the diets was altered as necessary to maintain plasma cholesterol at a level of approximately 20 mmol/L. The mean plasma cholesterol levels over the 13 week experiment were similar in all groups of rabbits (20.9 ± 1.9 mmol/L/week (0 mg/day), 20.7 ± 2.2 mmol/L/week (1 mg/day), 20.2 ± 2.3 mmol/L/week (3 mg/day) and 21.2 ± 1.9 mmol/L/week (20 mg/day)) and shown in Table 1.
Table 1.
Blood samples were drawn every week and plasma levels of cholesterol, total copper and ferrioxidase activities were determined. Each value represents the mean ± SEM of 10 rabbits. Statistical analysis was performed using the Mann Whitney test.
| Daily copper intake | ||||
|---|---|---|---|---|
| 0 mg | 1 mg | 3 mg | 20 mg | |
| Plasma cholesterol(mmol/L/week) | 21.3 ± 1.9 | 20.2 ± 2.3 | 20.7 ± 2.2 | 20.9 ± 1.9 |
| Plasma copper(µmol/L/week) | 11.6 ± 1.1 | 14 ± 1.3 | 12.6 ± 0.7 | 17.9 ± 2.1* |
| Plasma ferrioxidase (IU/l/week) | 38.2 ± 5.1 | 42.4 ± 5.4 | 35.0 ± 3.8 | 57.6 ± 11.8* |
| Plasma copper: Ferrioxidase ratio | 0.30 ± 0.15 | 0.33 ± 0.17 | 0.36 ± 0.18 | 0.31 ± 0.21 |
P < 0.05 (20 mg/day vs. 0, 1 and 3 mg/day).
The mean plasma copper concentration from rabbits supplemented with 20 mg/day copper (17.9 ± 2.1 µmol/L/week) was significantly higher (P < 0.05) than those supplemented with either 3, 1 or 0 mg copper/day (12.6 ± 0.7, 14 ± 1.3 and 11.6 ± 1.1 µmol/L/week, respectively) (Table 1). The mean plasma copper concentrations from rabbits supplemented with 3, 1 or 0 mg copper/day did not differ significantly.
The mean plasma caeruloplasmin concentration, assayed as plasma ferrioxidase activity, was significantly higher (P < 0.05) in rabbits supplemented with 20 mg/day copper (57.6 ± 11.8 IU/l/week) than those supplemented with either 3, 1 or 0 mg copper/day (35.0 ± 3.8, 42.4 ± 5.4 and 38.2 ± 5.1 IU/l/week, respectively) (Table 1). The mean plasma copper concentrations from rabbits supplemented with 3, 1 or 0 mg copper/day did not differ significantly. The ratio of plasma copper to plasma ferrioxidase activity was similar in each group.
Effect of dietary copper on aortic copper concentration
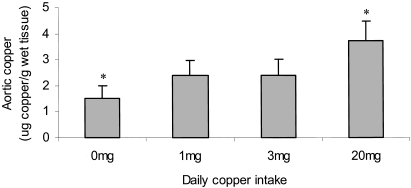
The mean copper content of the thoracic aorta at the level of the second and third intercostal branches was measured by atomic absorption spectroscopy. There was a significant positive association between aortic copper content and the dietary copper intake (1.33 ± 0.46 µg copper/g wet tissue (0 mg/day), 2.42 ± 0.57 µg copper/g wet tissue (1 mg/day), 2.36 ± 0.61 µg copper/g wet tissue (3 mg/day) and 3.70 ± 0.78 µg copper/g wet tissue (20 mg/day)) (P < 0.05, Fig. 1).
Figure 1.
The copper content of the the aortae at the level of the 4th intercostal branch were determined by flame atomic absorption spectroscopy. The values are mean µg copper/g wet weight tissue ± SEM of 10 rabbits. Statistical analysis was performed using the Mann Whitney test. *P < 0.05 (0 mg/day vs. 20 mg/day).
Effect of dietary copper on aortic superoxide dismutase in cholesterol-fed rabbits
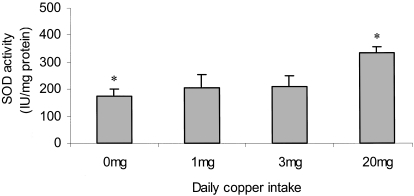
There was a positive association between aortic superoxide dismutase activity and the dietary intake of copper (187 ± 21 IU/mg wet tissue (0 mg/day), 239 ± 53 IU/mg wet tissue (1 mg/day), 201 ± 33 IU/mg wet tissue (3 mg/day) and 323 ± 21 IU/mg wet tissue (20 mg/day)) (Fig. 2). The SOD activity in the animals fed 0 mg copper/day was found to be significantly lower than the group fed 20 mg copper/day (P < 0.05).
Figure 2.
The superoxide dismutase activity of the cytosolic fraction from cells within the aorta was measured. The values are mean IU/mg protein ± SEM of 5 rabbits. Statistical analysis was performed using the Mann Whitney test. *P < 0.05 (0 mg/day vs. 20 mg/day).
Effect of copper supplementation on atherosclerosis in thoracic aorta of cholesterol-fed rabbits
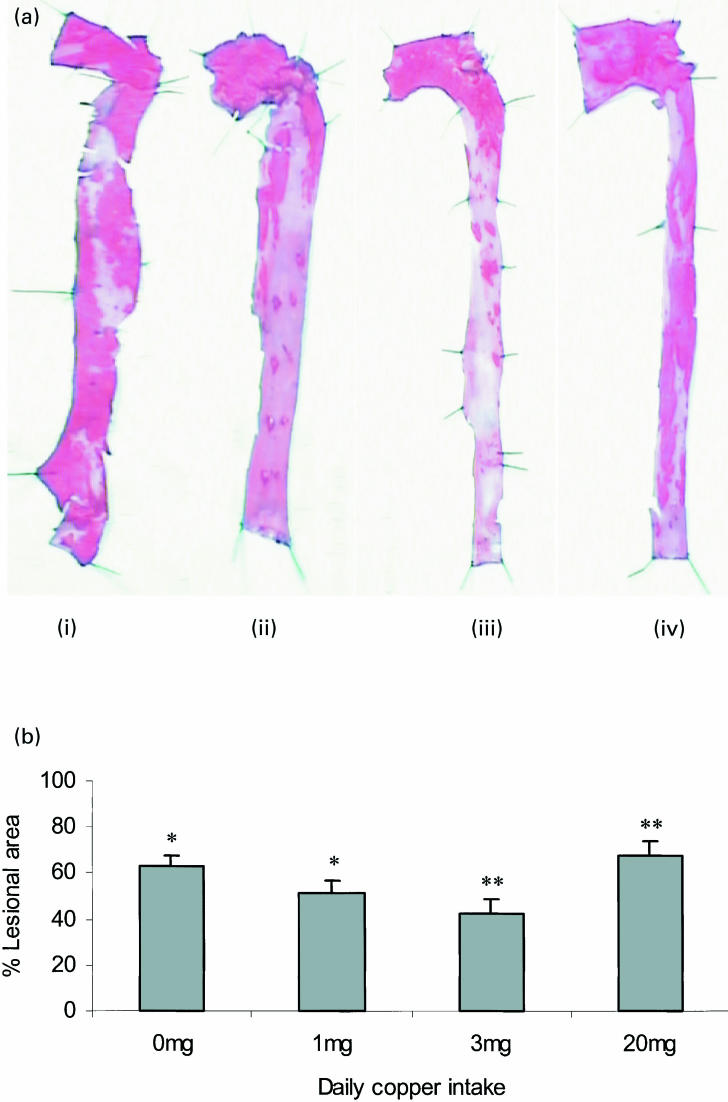
Longitudinal halves of aortae were stained with oil red O for quantification of the area of the aortae that contained macroscopic lesions. Figure 3a shows typical aortae from rabbits fed a copper-deficient cholesterol diet supplemented with 0, 1, 3 or 20 mg copper/day (Fig. 3a(i) (ii) (iii) and (iv), respectively). The percentage of the aorta staining positively for lipid using oil red O was significantly greater in aorta from cholesterol-fed rabbits supplemented with 0 and 20 mg copper/day (63.1 ± 4.8 and 67.1 ± 5.5%, respectively) than those supplemented with 1 and 3 mg copper/day (51.3 ± 6.3 and 42.8 ± 7.9%, respectively) (P < 0.05) (Fig. 3b).
Figure 3.
(a) Oil red O stained sections of aortae from rabbits fed a copper-deficient cholesterol-containing diet supplemented with 0 (i), 1 (ii), 3 (iii) or 20 (iv) mg copper/day are shown. (b) The percentage of the aorta stained positively for oil red O was calculated. Each bar represents the mean ± SEM from 10 rabbits. Statistical analysis was performed using the Mann Whitney test. *P < 0.05 (0 mg/day vs. 1 mg/day). **P < 0.05 (3 mg/day vs. 20 mg/day).
Discussion
Copper deficiency has been proposed as a risk factor for coronary heart disease because of its association with elevated plasma cholesterol levels (Klevay 1997). Epidemiological evidence suggests that elevated plasma copper levels may correlate positively with cardiac risk (reviewed by Ferns et al. 1997). However, we have previously demonstrated that moderate dietary copper supplementation inhibits atherogenesis in the cholesterol-fed rabbit (Lamb et al. 1999). We therefore investigated the effects of graded dietary copper supplementation in the cholesterol-fed rabbit in order to assess the effects of differing copper status on atherogenesis in this model.
Choice of dietary copper supplement levels
Many Western diets are reported to be deficient in copper and typically provide 0.3–1.5 mg copper per day compared to a recommended daily intake of 2–4 mg copper/day. Rabbit food diet typically contains 3 mg copper/kg providing approximately 0.5 mg copper/day. Therefore whilst human and rabbit dietary copper requirements are likely to be different, a rabbit on a standard diet is provided with a similar level of daily copper to that contained within the most deficient Western diets. The diet used in this experiment was formulated to contain 0.5 mg copper/kg, which provides < 0.1 mg copper/day. Copper supplements in the drinking water provided each group with approximately 0, 1, 3, and 20 mg copper/day. These diets therefore model several human diets: (1) a severely copper deficient diet (2) an average Western diet (3) a diet containing the WHO recommended copper intake and (4) an excessive intake of dietary copper.
The effect of dietary copper supplementation on plasma copper levels, plasma ferrioxidase activities and tissue copper levels
Aortic tissue copper concentration was significantly higher in rabbits receiving the highest copper supplement and was associated with both an increase in plasma copper levels and caeruloplasmin concentrations in these animals. It is therefore possible that the copper that accumulates within peripheral tissue is derived from caeruloplasmin plasma copper pool. Increased aortic copper concentrations may impact on several processes relevant to atherogenesis. For example it is possible that increasing tissue copper may increase local production of free radicals and result in oxidation of low density lipoprotein (LDL) within the arterial wall. The oxidation of LDL may be of critical importance in atherosclerosis (Steinberg et al. 1989) and can be catalysed in vitro by copper ions (Lynch & Frei 1993) and caeruloplasmin (Ehrenwald et al. 1994). Indeed, significant quantities of catalytically active copper (0.01–1.8 µg/mL) have been found in human atherosclerotic lesions (Smith et al. 1992). In comparison, aortae from rabbits receiving the highest copper supplement contained 0.8–6.6 µg copper/g tissue. However, it is unknown what proportion of this may be catalytically active.
Dietary copper supplementation increases aortic superoxide dismutase activity
We found increased superoxide dismutase activity in the soluble fraction from aortae of cholesterol-fed rabbits supplemented with 20 mg copper/day compared to both copper deficient rabbits and those receiving moderate copper supplements. It is unclear whether this increased SOD activity is due to de novo protein synthesis or increased activity of existing protein. The expression of Cu/Zn SOD is regulated in part by the CCAAT enhancer binding protein (C/EBP) family of transcription factors. C/EBP are induced in response to oxidative stress (Kishimoto et al. 1994), which can subsequently upregulate the expression of Cu/Zn superoxide dismutase (CuZnSOD) (Seo et al. 1996).
Aortic atherosclerosis is modulated in a biphasic manner by dietary copper content in the cholesterol-fed rabbit
In the present study we have found that a moderate dietary copper intake is associated with a reduced extent of atherosclerosis in the thoracic aorta of the cholesterol-fed rabbit compared to animals receiving a copper deficient diet. This is consistent with our previous findings (Lamb et al. 1999). However, at higher levels of dietary copper content this effect appears to be reversed. Moreover, the effects of dietary copper on atherosclerosis appear to be dependent on the arterial copper concentrations.
Copper may therefore be exerting both protective and detrimental effects in these animals. We have demonstrated that aortic SOD activity increases in association with increasing copper supplementation. This may be induced by local oxidative stress, but may still confer an overall antioxidant protection on the arterial tissue, at least in the groups receiving moderate copper supplements. Copper may also increase the bioavailability of nitric oxide (NO) by accelerating the decomposition of S-nitrosoglutathione (Dicks & Williams 1996), elaborating NO and thereby increasing vasodilatation and reducing blood pressure and shear stress. There is also some evidence that copper activates endothelial NOS (Ohnishi et al. 1997). Exogenous copper ions have been shown to potentiate both NO- and calcium ionophore-mediated relaxation of pre-constricted arteries in organ bath experiments (Plane et al. 1997).
Although a local increase in oxidative stress, mediated by moderately elevated copper levels, may cause the induction of superoxide dismutase and increase nitric oxide bioavailability, further increases in oxidative stress associated with higher aortic copper levels may reverse these potentially beneficial effects. This may then result in an increase in the oxidation of LDL causing an increase in foam cell formation and lesion progression.
Conclusion
Copper is an essential nutrient in the human diet and low body levels have been linked to a number of risk factors for coronary heart disease (CHD). There is currently no recommended daily copper allowance for humans, but the WHO has suggested that 2–3 mg copper per day may be beneficial. However, many Western diets contain considerably less than this. The findings from this study indicate that 1–3 mg dietary copper per day in the cholesterol-fed rabbit is associated with a reduced susceptibility to aortic atherosclerosis. We also find that a copper deficient diet and an excessive daily copper supplement (20 mg copper per day) are associated with increased atherogenesis in the cholesterol-fed rabbit. Despite the caveat that human and rabbit dietary requirements are different, these data indicate that a moderate dietary copper content may confer a degree of cardiac protection to the human population.
Acknowledgments
This work was supported by the British Heart Foundation.
References
- Dicks AP, Williams DL. Generation of nitric oxide from S-nitrosothiols using protein bound Cu2+ sources. Chem. Biol. 1996;3:655–659. doi: 10.1016/s1074-5521(96)90133-7. [DOI] [PubMed] [Google Scholar]
- Ehrenwald E, Chisolm GM, Fox PL. Intact human ceruloplasmin oxidatively modifies low density lipoprotein. J. Clin. Invest. 1994;93:1493–1501. doi: 10.1172/JCI117127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferns GA, Lamb DJ, Taylor T. The possible role of copper in atherogenesis: The Blue Janus. Atherosclerosis. 1997;133:139–152. doi: 10.1016/s0021-9150(97)00130-5. 10.1016/s0021-9150(97)00130-5. [DOI] [PubMed] [Google Scholar]
- Goode CA, Dinh CT, Linder MC. Mechanism of copper transport and delivery in mammals: review and reccent findings. In: Kies C, editor. Copper Bioavailability and Metabolism. Vol. 258. New York: Plenum Press; 1998. pp. 131–144. [DOI] [PubMed] [Google Scholar]
- Gutteridge JM, Stocks J. Caeruloplasmin. physiological and pathological perspectives. Crit. Rev. Clin. Lab Sci. 1981;14:257–329. doi: 10.3109/10408368109105866. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Taga T, Akira S. Cytokine signal transduction. Cell. 1994;76:253–262. doi: 10.1016/0092-8674(94)90333-6. [DOI] [PubMed] [Google Scholar]
- Seo SJ, Kim HT, Cho G, Rho HM, Jung G. Sp1 and C/EBP-related factor regulate the transcription of human Cu/Zn SOD gene. Gene. 1996;178:177–185. doi: 10.1016/0378-1119(96)00383-6. [DOI] [PubMed] [Google Scholar]
- Klevay LM. Coronary heart disease. The zinc/copper hypothesis. Am. J. Clin. Nutr. 1997;28:764–774. doi: 10.1093/ajcn/28.7.764. [DOI] [PubMed] [Google Scholar]
- Kok FJ, Van Duijn CM, Hofman A, et al. Serum copper and zinc and the risk of death from cancer and cardiovascular disease. Am. J. Epidemiol. 1998;128:352–359. doi: 10.1093/oxfordjournals.aje.a114975. [DOI] [PubMed] [Google Scholar]
- Lamb DJ, Reeves GL, Taylor A, Ferns GAA. Dietary copper supplementation reduces atherosclerosis in the cholesterol-fed rabbit. Atherosclerosis. 1999;146:33–43. doi: 10.1016/s0021-9150(99)00123-9. [DOI] [PubMed] [Google Scholar]
- Linder MC. Nutrition and Metabolism of the Trace Elements. In: Linder MC, editor. Nutritional Biochemistry and Metabolism. Amsterdam: Elsevier; 1991. pp. 36–49. [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurements with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–271. [PubMed] [Google Scholar]
- Lynch SM, Frei B. Mechanisms of copper and iron dependent oxidative modification of human low density lipoprotein. J. Lip. Res. 1993;34:1745–1753. [PubMed] [Google Scholar]
- Nyyssönen K, Parviainen MT, Salonen R, Tuomilehto J, Salonen JT. Vitamin C deficiency and risk of myocardial infarction: prospective study of men from eastern Finland. Br. Med. J. 1997;314:634–638. doi: 10.1136/bmj.314.7081.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi T, Ishizaki T, Sasaki F, et al. The effect of Cu2+ on rat pulmonary arterial rings. Eur J. Pharmacol. 1997;319:49–55. doi: 10.1016/s0014-2999(96)00833-3. [DOI] [PubMed] [Google Scholar]
- Plane F, Wigmore S, Angelini GD, Jeremy JY. Effect of copper on nitric oxide synthase and guanylyl cyclase activity in the rat isolated aorta. Br. J. Pharmacol. 1997;121:345–350. doi: 10.1038/sj.bjp.0701144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchades R, Maquieira A, Planta M. Rapid digestion procedure for the determination of lead in vegetable tissues by electrothermal atomisation atomic absorption spectroscopy. Analyst. 1989;114:1397–1399. [Google Scholar]
- Reunanen A, Knekt P, Aaron R-K. Serum ceruloplasmin level and the risk of myocardial infarction and stroke. Am. J. Epidemiol. 1992;136:1082–1090. doi: 10.1093/oxfordjournals.aje.a116573. [DOI] [PubMed] [Google Scholar]
- Rutherford C, Martin W, Carrier M, Anggard EE, Ferns GAA. Endogenously elicited antibodies to platelet derived growth factor-BB and platelet cytosolic protein inhibit aortic lesion development in the cholesterol-fed rabbit. Int J. Exp. Path. 1997;78:21–32. doi: 10.1046/j.1365-2613.1997.d01-237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonen JT, Salonen R, Korpela H, Suntionen S, Tuomilento J. Serum copper and the risk of acute myocardial infarction: a prospective population study in men in Eastern Finland. Am. J. Epid. 1991;134:268–276. doi: 10.1093/oxfordjournals.aje.a116080. [DOI] [PubMed] [Google Scholar]
- Schosinsky KH, Lehmann HP, Beeler M. Measurement of ceruloplasmin from its oxidase activity in serum by use of O-dianisidne dihydrochloride. Clin. Chem. 1974;20:1556–1563. [PubMed] [Google Scholar]
- Smith C, Mitchinson MJ, Aruoma OI, Halliwell B. Stimulation of lipid peroxidation and hydroxyl radical generation by the contents of human atherosclerotic lesions. Biochem. J. 1992;286:901–905. doi: 10.1042/bj2860901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol – modifications of low density lipoprotein that increases its atherogenicity. N Eng. J. Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]