Abstract
Type VIII collagen is upregulated after vessel injury, and this collagen has been implicated in both smooth muscle cell migration and angiogenesis. This study examines the temporal and spatial pattern of expression of type VIII collagen in porcine coronary vessels at specific time points after balloon angioplasty.
In situ hybridization studies demonstrated that collagen VIII messenger ribonucleic acid (mRNA) was markedly elevated in the neoadventitia at 3 days post-angioplasty. By 14 days, elevated collagen VIII message was seen mainly in the neointima and this expression decreased to background levels by 90 days. The distribution of collagen VIII protein, detected using immunohistochemistry, was similar but the up-regulation lagged behind the mRNA increase by a few days. Pre-treatment of sections with pepsin highlighted variations in the organization and appearance of extracellular collagen VIII containing structures in both injured and normal vessels.
New vessel formation was evident in the neoadventitia after 3 days, but there was no colocalization of type VIII collagen immunostaining with that of von Willebrand factor (a marker of endothelial cells) in the neoadventitia.
These data show that up-regulation of collagen VIII in the neoadventitia is an important early marker of the coronary arterial response to injury, and is not associated with new vessel formation.
Keywords: Angioplasty, smooth muscle, coronary circulation, type VIII collagen, angiogenesis
Introduction
Collagen VIII is a non-fibrillar short chain collagen comprising α1(VIII) and α2(VIII) chains. In Descemet's membrane in the eye, collagen VIII is organized into a hexagonal lattice network (Shuttleworth 1997). It was originally found as an endothelial cell product (Sage et al. 1980), is upregulated during angiogenesis and in the heart during morphogenesis (Sage & Iruela–Arispe 1990). In the adult it is found in the subendothelial and medial layers of the arterial wall (Kittelberger et al. 1990). Previously, we have demonstrated that collagen VIII is a constitutive product of vascular smooth muscle cells (VSMCs) in adult vessels and that it is upregulated in foetal cells and arterial lesions (MacBeath et al. 1996; Plenz et al. 1999). Other groups have found that collagen VIII is expressed by VSMCs in response to vascular injury in the rat carotid, is localized to the media and neointima and is associated with cell migration in this model (Bendeck et al. 1996; Sibinga et al. 1997).
Although the rat carotid injury model has been useful in characterizing the development of neointima, this model differs from large animal coronary artery injury in many important respects. These include the type of injury caused by angioplasty (Clowes et al. 1983; Schwartz et al. 1990), the relative importance of vascular remodelling vs. neointimal hyperplasia in determining final lumen size (Post et al. 1994; Mintz et al. 1996), biochemical differences between phenotypically modulated VSMCs (Newman et al. 1995) and the response to treatments designed to treat restenosis (Lam et al. 1992). The porcine coronary artery angioplasty model overcomes many of the limitations of the rat carotid model hyperplasia in determining final lumen size (Post et al. 1994; Mintz et al. 1996), biochemical differences between phenotypically modulated VSMCs (Newman et al. 1995) and the response to treatments designed to treat restenosis (Lam et al. 1992). The porcine coronary artery angioplasty model is thought to be a more accurate reflection of the biological response to coronary angioplasty in humans (Ferrell et al. 1992).
The purpose of this study was to conduct a detailed investigation of the temporal and spatial pattern of expression of type VIII collagen in a porcine model of coronary angioplasty. We demonstrate that type VIII collagen is a marker for the early adventitial proliferative response following coronary artery injury, and that this collagen is associated with smooth muscle cell migration and not angiogenesis in these arteries.
Methods
Animal species and coronary angioplasty
Female juvenile domestic swine (18–25 kg) were used for these studies. All procedures and animal handling were authorized by the Home Office (UK) under the Animals (Scientific Procedures) Act 1986 and the investigation conformed with The guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. The number of animals used was kept to the minimum necessary to demonstrate consistent results.
Each animal was given 150 mg aspirin on the day prior to the procedure and then on alternate days until the end of the experiment. The animals (n = 16) were anaesthetized using a mixture of halothane (2–3%), nitrous oxide and oxygen administered through a tightly fitting face mask. Arterial access was obtained via a cut-down to the left or right carotid artery and following intra-arterial injection of 5000 µ of heparin an 8F right Judkins guide catheter was positioned in the left coronary artery under fluoroscopic guidance (Siemens Siremobil 2). A 3.0-mm angioplasty balloon was advanced into the left anterior descending artery and also into the circumflex artery if this was large enough. Three inflations at 6–10 atmospheres were performed in each vessel for 30 s with a 1 min gap between inflations and the angioplasty balloon was then withdrawn. The right coronary artery was used as an uninjured control. Vessel patency was confirmed post-angioplasty by angiography in all cases. At the end of the procedure the carotid artery was ligated and the skin incision sutured. The animals were allowed to recover from the anaesthetic and a normal diet and full care were provided.
Tissue preparation
At the allotted times for euthanasia, 3 days, 1 week, 2 weeks, 4 weeks and 3 months post angioplasty, the animals were killed with an intravenous injection of pentobarbitone. Immediately after death, the heart was removed and rinsed in sterile, normal saline at 4 C. Three mm sections of the injured vessels and normal right coronary artery controls were taken and snap frozen in isopentane chilled in liquid nitrogen. Frozen sections were embedded in Tissue-Tek embedding medium (Sakura, Tokyo, Japan) and stored at – 70 C until sectioned. Seven µm thick sections were cut on a cryostat and air dried onto superfrost slides (BDH, Poole, UK). Only vessels demonstrating significant balloon injury with fracture of the internal elastic lamina and dissection through at least part of the medial layer were included in the study (total n = 19: 5 at 3 days, 3 at 7 days, 5 at 14 days, 3 at 28 days and 3 at 90 days) along with 4 uninjured control vessels. Thus, all injured vessels had an equivalent degree of injury with dissection through the media.
In situ hybridization
A full length complementary deoxyribonucleic acid (cDNA) clone for the human α1(VIII) chain previously subcloned into pBluescript (MacBeath et al. 1996) (pBlu-HaI8) was used to generate digoxygenin labelled sense and antisense (AS) riboprobes. The specificity of the AS riboprobe was confirmed by Northern blotting and hybridization studies using mRNA extracted from porcine aortic VSMCs. A major 5.5kb transcript was identified by the AS probe but no significant hybridization was found using the sense control riboprobe (data not shown). The plasmid was linearized with ECO RV and T3 RNA polymerase used to generate the sense transcripts while the AS strands were generated using PVU II and T7 polymerase (all enzymes from Boehringer Mannheim, Cologne, Germany). Digoxygenin was incorporated into the RNA transcripts by the addition of digoxygenin-labelled deoxyuridine triphosphate (dUTP) to the in vitro transcription reaction according to the supplier's methods (Boehringer Mannheim, Cologne, Germany). This method yielded digoxygenin labelled sense and AS probes of approximately 500 bases in length.
Frozen sections of injured and normal coronary arteries were air-dried onto Superfrost slides and fixed in 4% paraformaldehyde in phosphate buffered saline. The sections were washed in 0.5 × SSC and then prehybridized at 42 C for 2 h. Prehybridization buffer (rHB2) (Wilcox 1993) consisted of 10 mm dithiothreitol, 0.3 m NaCl, 20 mm Tris, pH 8, 5 mm EDTA, 1 × Denhardt's, 10% dextran sulphate, 50% formamide and salmon sperm DNA (40 µg/mL). Sense or AS riboprobes were added to the prehybridization buffer to a final concentration of 400 ng/mL and hybridized overnight at 42 C. Sections were then washed in increasing stringency washes to a maximum stringency of 0.1 × SSC at 58 C for 30 min and then blocked with 1% blocking reagent (Boehringer Mannheim, Cologne, Germany). Alkaline phosphatase conjugated antidigoxygenin antibody (Boehringer Mannheim, Cologne, Germany) was used at 1 : 400 dilution and the sections were visualized using 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium substrate (Vector Laboratories, Burlingame, CA, USA) and the nuclei counterstained with nuclear fast red (Vector Laboratories, Burlingame, CA, USA).
Immunohistochemistry
Immunohistochemistry was performed using a standard three-stage immunoperoxidase revealing system (Dako, Cambridge, UK). The sections were fixed in acetone and then washed in Tris buffered saline (TBS). All subsequent procedures were performed at room temperature. Some sections were treated with pepsin (1000 µ/mL, Sigma Diagnostics, Poole, UK) for 1 h. The sections were then incubated with 0.6% (v/v) hydrogen peroxide in methanol for 30 min to remove endogenous peroxidase activity. Non-specific epitopes were blocked by incubation with 20% (v/v) normal goat serum (Sigma Diagnostics) in TBS for 10 min in a humidified chamber. Excess serum was removed and primary antibodies diluted in TBS were added for 1 h. The following primary antibodies were used: mouse monoclonal antibody to collagen VIII (1 : 1000, Seikagaku Corporation, Tokyo, Japan) and rabbit polyclonal antibody to Von Willebrand Factor (1 : 5000, Dako). Normal mouse serum (1 : 2000), rabbit immunoglobulins (1 : 200) and guinea pig serum (1 : 1000) were used as negative controls. Sections were washed with TBS containing 0.01% (v/v) Tween-20 for 5 min and then incubated for 30 min with an affinity purified, biotinylated goat antirabbit/mouse antibody (1 : 100, Dako) or an affinity purified, biotinylated goat antiguinea pig antibody (1 : 100, Dako) (as appropriate). The sections were washed as before and incubated with streptavidin-biotin-peroxidase complex (Dako) for 30 min. The sections were developed by incubation with freshly prepared 2% (v/v) diaminobenzidine (BDH-Merck) containing 0.01% (v/v) hydrogen peroxide in TBS for 5 min and the reaction stopped by washing in water. The sections were then counterstained in haemotoxylin and finally dehydrated back to xylene and mounted in XAM neutral medium (BDH, Poole, UK). Immuno-positivity was seen as a brown stain and nuclei were stained blue.
Results
Localization of type VIII collagen mRNA
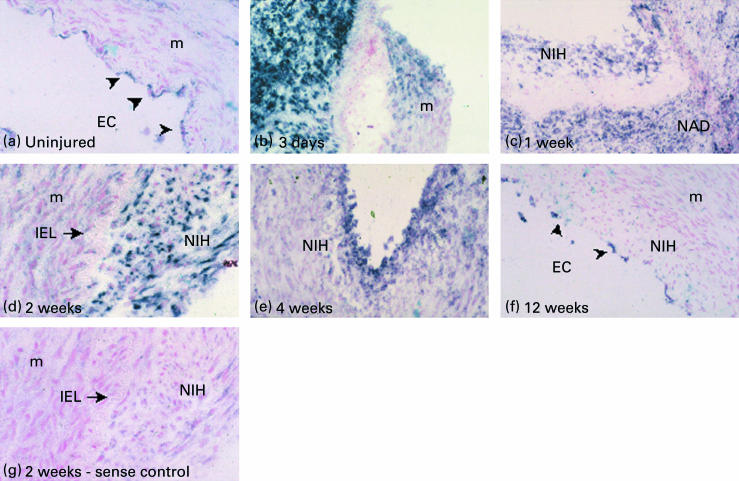
In situ hybridization was performed to investigate the expression of α1(VIII) collagen mRNA in uninjured vessels and at specific time points after angioplasty, as described in Materials and methods (Fig. 1,a-g). Uninjured vessels showed low levels of expression of collagen VIII by some, but not all, endothelial cells (Fig. 1a). There was a dramatic upregulation of collagen VIII expression by 3 days post-angioplasty, predominantly in the neoadventitia, coinciding with a marked increase in the number and density of cells in this area (Fig. 1b). At this time point, collagen VIII mRNA was also expressed by some of the medial cells at the site of injury (Fig. 1b). Between 1 and 2 weeks post-angioplasty, collagen VIII was expressed by both adventitial and neointimal cells but not by adjacent medial cells (Fig. 1c,d). By 4 weeks post-angioplasty, expression had significantly reduced and was mainly restricted to the neointima along its luminal edge (Fig. 1e). Three months after balloon angioplasty, as in the uninjured control vessels, only endothelial cells lining the vessel lumen were found to express mRNA for collagen VIII (Fig. 1f). No staining was seen in the sense control (Fig. 1g).
Figure 1.
In situ hybridization shows dynamic changes in the pattern of collagen VIII mRNA expression. (a) Only EC express collagen VIII in uninjured control vessels. (b) Marked upregulation of mRNA predominantly in the adventitia at 3 days. (c) By 1 week collagen VIII is expressed in both the NIH and in the NAD while expression is predominantly restricted to the NIH by 14 days post-injury (d). (e) Residual expression is only seen in peri-luminal NIH by 4 weeks and only by EC at 12 weeks (f). (g) Sense control hybridization was negative. EC = endothelial cells, M = media, NIH = neointimal hyperplasia, NAD = neoadventitia, IEL = internal elastic lamina. Magnification: a, d, g at × 50, b, c, e, f at × 25. The arrows indicate endothelial cells.
Immunolocalization of type VIII collagen
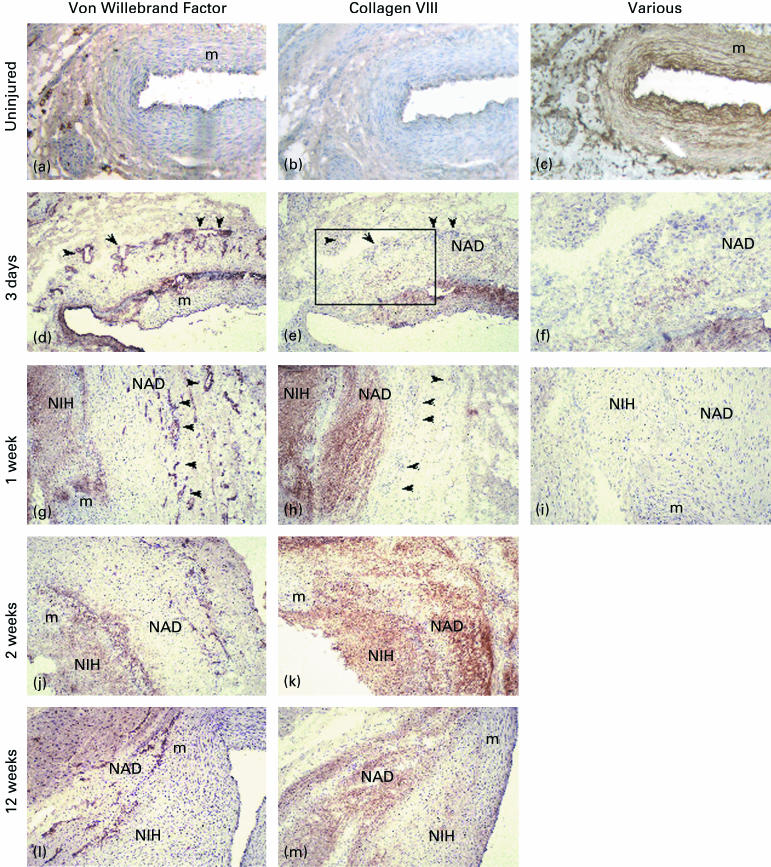
The temporal and spatial pattern of distribution of type VIII collagen in these specimens was investigated by immunohistochemical staining (Fig. 2). In addition, the relationship between type VIII collagen and new blood vessels located in the neoadventitia (identified using antibodies against von Willebrand factor) was investigated.
Figure 2.
Immunohistochemical localization of collagen VIII and von Willebrand factor in adjacent sections. Occasional small vessels were found in the uninjured adventitia (a). Little or no collagen VIII was detectable in the uninjured vessel wall (b) although pepsin pre-treatment (c) revealed significant immunostaining in the subendothelial and medial regions. A significant increase in adventitial small vessel number was detected 3 days post-injury (d) although only minimal collagen VIII was found in the adventitia at this time point (e). There was no association of collagen VIII with small vessels present in the adventitia. The box in panel e is shown at higher magnification in f to demonstrate early adventitial collagen VIII expression. Adventitial vessels are indicated with arrowheads in panels d, e, g and h. Marked neointimal and neoadventitial staining for collagen VIII was seen at 1 week postinjury (h) but these regions did not correspond with areas of high small vessel density in the outer neoadventitia (g). No immunostaining was found on the normal mouse serum negative controls (i). Widespread neointimal and neoadventitial collagen VIII was detected by 2 weeks (k) although small vessel density was noted to be decreasing by this timepoint (j) and beyond (l). Collagen VIII immunostaining decreased in intensity by 12 weeks with no neointimal staining and reduced staining in the neoadventitia (m). Magnification: a-e, g-m × 40. F × 80.
Control, uninjured coronary arteries exhibited specific immunostaining for collagen VIII in the subendothelial layer only (Fig. 2b). However, pre-treatment of the sections with pepsin revealed the presence of significant levels of type VIII collagen that was arranged in a lamellar pattern in the media of these normal arteries (Fig. 2c).
At 3 days post-angioplasty, the vessels showed evidence of dissection through the media, loss of endothelium and part of the subendothelial layer and a marked adventitial reaction consisting of cells, extracellular matrix and new blood vessels (Fig. 2d). Trace amounts of type VIII collagen were found in the neoadventitia at this time point (Fig. 2e,f), and this staining did not correspond with small blood vessels present in the outer neoadventitia (compare Fig. 2d,e). However, by 7 days post-angioplasty, increased immunostaining for type VIII collagen was evident in both the neointima and the neoadventitia (Fig. 2h). Again, this staining did not correspond with areas of high small vessel density in the neoadventitia (Fig. 2g). By 14 days postangioplasty, a significant neointimal mass had developed and the number of small blood vessels in the neoadventitia had decreased (Fig. 2j). At this time point, intense diffuse immunostaining for collagen VIII was present in both neoadventitia and neointima (Fig. 2k). A similar diffuse pattern of staining for type VIII collagen in the neointima and the neoadventitia was also revealed after pre-treatment of these specimens with pepsin (not shown). By 90 days, the number of new blood vessels in the neoadventitia had markedly decreased (Fig. 2l), neointimal and neoadventitial staining for type VIII collagen was markedly reduced, and specific immunostaining was again seen in both old and new subendothelial layers (Fig. 2m). Pre-treatment of these sections with pepsin revealed an organized lamellar distribution of type VIII collagen throughout the vessel wall (results not shown). No staining was seen in control sections (Fig. 2i).
Discussion
We have demonstrated that upregulation of type VIII collagen in the adventitia is an important early marker of the coronary artery response to injury. We demonstrate that collagen VIII is not associated with the formation of new blood vessels in the neoadventitia. These results, and those of colleagues (Bendeck et al. 1996; Sibinga et al. 1997), demonstrate that collagen VIII is closely associated with the proliferating and migrating cells observed following angioplasty of porcine coronary arteries suggesting that the primary role of type VIII collagen is to regulate smooth muscle cell function in large arteries.
Marked upregulation of collagen VIII mRNA was first seen in neoadventitial cells at 3 days post-procedure, which coincides with the early cellular accumulation and proliferation in this region (Scott et al. 1996b; Shi et al. 1996a). Along with the migrating adventitial cells the site of cellular activation of collagen VIII moved to the neointima and then returned to baseline by 90 days post-injury, when only endothelial cells lining the vessel lumen were seen to express collagen VIII. The central role of the adventitia in other aspects of the early response to vessel injury in the coronary circulation has been reported; marked proliferation of adventitial fibroblasts was observed 3 days post-injury with subsequent switch into a myofibroblast phenotype (Scott et al. 1996b; Shi et al. 1996b;). Earliest expression of platelet derived growth factor and TGF-ß1 has been localized to the proliferating adventitial cells which then appeared to migrate into the neointima and contributed, at least in part, to the resulting hyperplastic mass (Scott et al. 1996a, Shi et al. 1996b). Our finding that collagen VIII is also first upregulated in the neoadventitia, confirms the importance of the adventitia as one of the principal sites governing the response to coronary arterial injury and highlights a potential role for collagen VIII as an early matrix marker for vascular injury or disease.
Increased immunohistochemical staining for collagen VIII protein reflected the distribution of mRNA upregulation, although its time course lagged behind that of transcriptional activation. Thus, the protein was first detected in significant quantities at 7 days post-injury in the neoadventitia and was present at high levels in both the neointima and neoadventitia by 14 days. Collagen VIII immunostaining persisted up to 28 days and was only lost at 90 days. By comparison, in the rat carotid balloon injury model, neointimal immunostaining decreased earlier and there was no evidence of adventitial involvement (Bendeck et al. 1996; Sibinga et al. 1997). This may be due to more rapid kinetics of cell proliferation and migration in the rat than in the pig (Clowes et al. 1983; Carter et al. 1994) and the fact that migrating cells originate from the media in the rat carotid as opposed to the adventitia in the porcine coronary artery.
We have investigated a porcine model of coronary artery balloon injury and along with other groups reported the development of a neoadventitia as a potential determinant of vascular remodelling (Sinha & Heagerty 1996; Shi et al. 1996a). Studies using this model have suggested that the initial cellular proliferation after angioplasty occurs in the adventitia and phenotypic modulation and migration of these cells contributes significantly to neointimal hyperplasia (Scott et al. 1996b, Shi et al. 1996b). Recent studies have also highlighted the importance of new vessel formation (angiogenesis) in the development of the neoadventitia and in arterial remodelling after angioplasty (Pels et al. 1999). Interestingly, angiogenesis has also been shown to be a critical determinant of atherosclerotic plaque development (Jeziorska & Woolley 1999a; Jeziorska & Woolley 1999b; McCarthy et al. 1999) and inhibition of angiogenesis has been shown to reduce plaque growth (Moulton et al. 1999).
Balloon angioplasty elicited an angiogenic response in the neoadventitia that was maximal at three days post-injury and subsequently underwent progressive regression. This regression has been shown to coincide with arterial narrowing (Pels et al. 1999). We now demonstrate that type VIII collagen is not associated with the new blood vessels present in the adventitia. This result was surprising as previous studies have shown that type VIII collagen is expressed by cultured endothelial cells, is upregulated during angiogenesis in vitro and is present in embryonic capillaries (Sage et al. 1980; Sage & Ireula–Arispe 1990). Taken together, these data may suggest either that type VIII collagen is not necessary for new blood vessel formation in adult tissues or that this collagen is required for the stabilization of new blood vessels.
Bendeck et al. (1996) hypothesized that collagen VIII forms a provisional matrix that facilitates cell migration and is cleared once the cells arrive in the neointima. In contrast, our immunohistochemical studies following pepsin pre-treatment reveal that considerable collagen VIII is present throughout the vessel at all time points both before and after injury. The presence of significant levels of collagen VIII in the tunica media which was only revealed following pepsin digestion and which had a lamellar distribution has been previously reported (Kittelberger et al. 1990). After injury, newly deposited collagen VIII is detected without pepsin treatment, and this collagen has a diffuse appearance in both the neoadventitia and the neointima. By 90 days post-injury, only subendothelial collagen VIII was seen without pepsin treatment but all layers of the vessel wall were stained after enzyme digestion.
It is unclear what the relationship between the two forms of type VIII collagen is. Does the newly deposited diffuse type VIII collagen become organized into a lamellar structure with time or is it removed by proteolysis? A number of studies have shown upregulation of plasmin and matrix metalloproteinases following arterial injury and suggested that this may provide the mechanism for removal of the provisional matrix (Bendeck et al. 1996, Huo et al. 2000). However, the finding of substantial amounts of type VIII collagen in both normal vessels and 90 days after injury suggests that type VIII collagen is not only a component of the provisional matrix for smooth muscle cell migration, but is also an important component of normal vessels.
It is tempting to speculate that these different properties may reflect differences in chain composition and/or supramolecular organization of type VIII collagen in the artery wall. The chain composition of collagen VIII in vivo is currently unresolved. We have recently shown that stable heterotrimers and homotrimers can be formed in vitro (Illidge et al. 1998). Sibinga et al. (1997) showed upregulation of mRNA for both α1(VIII) and α2(VIII) chains in the rat carotid injury model although there were differences in the time course of expression for the two different chains. It has recently been reported that the two chains of type VIII collagen are not colocalized in bovine aorta (Greenhill et al. 2000). Our study conclusively demonstrates that adventitial up-regulation of collagen VIII is an important part of the early response to coronary arterial injury and highlights the role of collagen VIII as a potential marker for arterial injury. The remodelling of extracellular collagen VIII and masking of reactive epitopes at later time points after injury may have a role in governing VSMC function. Further investigation of the molecular composition of collagen VIII in vivo and the effects of different chain compositions on VSMC phenotype and migration is now warranted.
Acknowledgments
The work presented herein and the authors SS and CMK are supported by the Medical Research Council (UK), grant numbers: G84/4707 and G117/268. We thank the Department of Radiology, University of Manchester, for provision of the image intensifier. We also thank Miss Adele Poole for excellent technical assistance.
References
- Bendeck MP, Regenass S, Tom WD, et al. Differential expression of α1 type VIII collagen in injured platelet-derived growth factor-BB-stimulated rat carotid arteries. Circ. Res. 1996;79:524–531. doi: 10.1161/01.res.79.3.524. [DOI] [PubMed] [Google Scholar]
- Carter AJ, Laird JR, Farb A, Kufs W, Wortham DC, Virmani R. Morphologic characteristics of lesion formation and time course of smooth muscle cell proliferation in a porcine proliferative restenosis model. J. Am. Coll. Cardiol. 1994;24:1398–1405. doi: 10.1016/0735-1097(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Clowes AW, Reidy MA, Clowes MM. Kinetics of cellular proliferation after arterial injury. Lab. Invest. 1983;49:327–333. [PubMed] [Google Scholar]
- Ferrell M, Fuster V, Gold HK, Chesebro JH. A dilemma for the 1990s: Choosing appropriate experimental animal model for the prevention of restenosis. Circulation. 1992;85:1630–1631. doi: 10.1161/01.cir.85.4.1630. [DOI] [PubMed] [Google Scholar]
- Greenhill NS, Ruger BM, Hasan Q, Davis P. The α1(VIII) and α2(VIII) collagen chains form two distinct homotrimeris proteins in vivo. Matrix Biology. 2000;19:19–28. doi: 10.1016/s0945-053x(99)00053-0. 10.1016/s0945-053x(99)00053-0. [DOI] [PubMed] [Google Scholar]
- Huo G, Mulholland D, Gronska MA, Bendeck MP. Type VIII collagen stimulates smooth muscle cell migration and matrix metalloproteinases after arterial injury. Amer. J. Path. 2000;156:467–476. doi: 10.1016/S0002-9440(10)64751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illidge C, Kielty C, Shuttleworth A. The α1 (VIII) and α2 (VIII) chains of type VIII collagen can form stable homotrimeric molecules. J. Biol. Chem. 1998;273:22091–22095. doi: 10.1074/jbc.273.34.22091. 10.1074/jbc.273.34.22091. [DOI] [PubMed] [Google Scholar]
- Jeziorska M, Woolley DE. Local neovascularization and cellular composition within vulnerable regions of atherosclerotic plaques of human carotid arteries. J. Pathol. 1999a;188:189–196. doi: 10.1002/(SICI)1096-9896(199906)188:2<189::AID-PATH336>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Jeziorska M, Woolley DE. Neovascularization in early atherosclerotic lesions of human carotid arteries: its potential contribution to plaque development. Hum. Pathol. 1999b;30:919–925. doi: 10.1016/s0046-8177(99)90245-9. [DOI] [PubMed] [Google Scholar]
- Kittelberger R, Davis PF, Flynn DW, Greenhill NS. Distribution of type VIII collagen in tissues: an immunohistochemical study. Connect. Tissue Res. 1990;24:303–318. doi: 10.3109/03008209009152157. [DOI] [PubMed] [Google Scholar]
- Lam JYT, Lacoste L, Bourassa MG. Cilazapril and early atherosclerotic changes after balloon injury of porcine carotid arteries. Circulation. 1992;85:1542–1547. doi: 10.1161/01.cir.85.4.1542. [DOI] [PubMed] [Google Scholar]
- MacBeath JRE, Kielty CM, Shuttleworth CA. Type VIII collagen is a product of vascular smooth-muscle cells in development and disease. Biochem. J. 1996;319:993–998. doi: 10.1042/bj3190993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MJ, Loftus IM, Thompson MM, et al. Angiogenesis and the atherosclerotic carotid plaque: an association between symptomology and plaque morphology. J. Vasc. Surg. 1999;30:261–268. doi: 10.1016/s0741-5214(99)70136-9. [DOI] [PubMed] [Google Scholar]
- Mintz GS, Popma JJ, Pichard AD, et al. Arterial remodeling after coronary angioplasty: a serial intravascular ultrsound study. Circulation. 1996;94:35–43. doi: 10.1161/01.cir.94.1.35. [DOI] [PubMed] [Google Scholar]
- Moulton KS, Heller E, Konerding MA, Flynn E, Palinski W, Folkman J. Angiogenesis inhibitors endosatin or TNP‐470 reduce intimal neovascularisation and plaque growth in apolipoprotein E‐deficient mice. Circulation. 1999;99:1726–1732. doi: 10.1161/01.cir.99.13.1726. [DOI] [PubMed] [Google Scholar]
- Newman CM, Brunn BC, Porter KE, Mistry PK, Shanahan CM, Weissberg PL. Osteopontin is not a marker for proliferating human vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 1995;15:2010–2018. doi: 10.1161/01.atv.15.11.2010. [DOI] [PubMed] [Google Scholar]
- Pels K, Labinaz M, Hoffest G, O'Brien ER. Advential angiogenesis early after coronary angioplasty. Correlation with arterial remodelling. Arterioscler. Thromb. Vasc. Biol. 1999;19:226–238. doi: 10.1161/01.atv.19.2.229. [DOI] [PubMed] [Google Scholar]
- Plenz G, Dorszewski A, Breithardt G, Robeneck H. Expression of type VIII collagen after cholesterol diet and injury in the rabbit model of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 1999;19:1201–1209. doi: 10.1161/01.atv.19.5.1201. [DOI] [PubMed] [Google Scholar]
- Post MJ, Borst C, Kuntz RE. The relative importance of arterial remodeling compared with intimal hyperplasia in lumen renarrowing after balloon angioplasty: a study in the normal rabbit and the hypercholesterolemic Yucatan micropig. Circulation. 1994;89:2816–2821. doi: 10.1161/01.cir.89.6.2816. [DOI] [PubMed] [Google Scholar]
- Sage H, Iruela-Arispe ML. Type VIII collagen in murine development. Association with capillary formation in vitro. Ann. NY. Acad. Sci. 1990;580:17–31. doi: 10.1111/j.1749-6632.1990.tb17914.x. [DOI] [PubMed] [Google Scholar]
- Sage H, Pritzl P, Bornstein P. A unique pepsin-sensitive collagen synthesized by aortic endothelial cells in culture. Biochemistry. 1980;19:5747–5755. doi: 10.1021/bi00566a013. [DOI] [PubMed] [Google Scholar]
- Schwartz RS, Murphy JG, Edwards WD, Camrud AR, Vlietstra RE, Holmes DR., Jr Restenosis after balloon angioplasty: a practical proliferative model in porcine coronary arteries. Circulation. 1990;82:2190–2200. doi: 10.1161/01.cir.82.6.2190. [DOI] [PubMed] [Google Scholar]
- Scott NA, Cipolla GD, Ross CE, et al. Identification of a potential role for the adventitia in vascular lesion formation after balloon overstretch injury of porcine coronary arteries. Circulation. 1996b;93:2178–2187. doi: 10.1161/01.cir.93.12.2178. [DOI] [PubMed] [Google Scholar]
- Shi Y, Pieniek M, Fard A, O'Brien J, Mannion JD, Zalewski A. Adventitial remodeling after coronary arterial injury. Circulation. 1996a;93:340–348. doi: 10.1161/01.cir.93.2.340. [DOI] [PubMed] [Google Scholar]
- Shi Y, O'Brien JE, Fard A, Mannion JD, Wang D, Zalewski A. Adventitial myofibroblasts contribute to neointimal formation in injured porcine coronary arteries. Circulation. 1996b;94:1655–1664. doi: 10.1161/01.cir.94.7.1655. [DOI] [PubMed] [Google Scholar]
- Shuttleworth CA. Type VIII collagen. Int J. Biochem. Cell Biol. 1997;10:1145–1148. doi: 10.1016/s1357-2725(97)00033-2. [DOI] [PubMed] [Google Scholar]
- Sibinga NES, Foster LC, Hsieh C, et al. Collagen VIII is expressed by vascular smooth muscle cells in response to vascular injury. Circ. Res. 1997;80:532–541. doi: 10.1161/01.res.80.4.532. [DOI] [PubMed] [Google Scholar]
- Sinha S, Heagerty AM. Adventitial changes following coronary artery balloon injury in a porcine model. Eur. Heart J. 1996;17(Suppl.):116. (Abstract) [Google Scholar]
- Wilcox JN. Fundamental principles of in situ hybridisation. J. Histochem. Cytochem. 1993;41:1725–1733. doi: 10.1177/41.12.8245419. [DOI] [PubMed] [Google Scholar]