Abstract
Mice infected with a macrophagotropic strain of Trypanosoma cruzi develop progressive splenomegaly due to reactive hyperplasia with increased number of lymphocytes and macrophages, culminating in parasite disintegration and necrosis of parasitized cells. Necrotic changes have been attributed to the liberation of toxic cytokines, including TNF-α, from parasitized macrophages. In the present study, the presence of TNF‐α was investigated in situ. In addition the participation of destroyed parasites in inducing the liberation of TNF-α was examined in two highly susceptible mice strains (C3H and Swiss) and a more resistant strain (DBA). Swiss (90) C3H/He (83) and DBA (30) mice were infected with the Peruvian strain of T. cruzi. Nineteen infected Swiss mice, and 22 infected C3H/He were treated with Benznidazole (one or two doses, 100 mg/kg bw/day), on the 8th and 9th days after infection. Necrotic splenic lesions occurred in both susceptible and resistant strains of mice. Although differing in degree, lesions were more intense in C3H and Swiss than in DBA mice. Comparing untreated and treated susceptible mice, necrotic lesions were significantly less intense in the latter. By specific monoclonal antibody immunolabelling, TNF-α was demonstrated in the cytoplasm of macrophages and within necrotic areas, from Swiss, C3H/He and DBA mouse spleens. In conclusion, TNF-α, probably synthesized by macrophages, was strongly expressed at the sites of parasite and cell destruction, thus appearing to play a pivotal role in splenic necrotic changes associated with severe experimental T. cruzi infection.
Keywords: Trypanosoma cruzi, spleen necrosis, macrophage parasitism, TNF-α, C3H/He mice, DBA mice, benznidazole
Introduction
Macrophagotropic strains of Trypanosoma cruzi cause intense parasitism of macrophages of both splenic white and red pulps in mice (Andrade 1985a; Andrade et al. 1985c). This is followed by focal necrosis involving intracellular parasites as well as parasitized and nonparasitized macrophages. A sudden drop of parasitaemia is then observed but is, paradoxically, accompanied by high mortality of the animals. The involvement of TNF-α and other cytokines in these events has therefore been suggested (Cordeiro et al. 1997).
Several studies have demonstrated the participation of TNF-α during acute infection due to T. cruzi. Tarleton 1988) showed that T. cruzi is a potent priming agent for TNF-α production by spleen cells stimulated with LPS in vitro. Revelli et al. (1999a) demonstrated that infection of mice with the Tulauhen strain causes a significant serum level increase of TNF-α. Activation of macrophages by the intracellular parasites can stimulate their trypanocidal mechanism, with production of TNF-α and toxic metabolites from oxygen, especially nitric oxide (Gazzinelli et al. 1992; Silva et al. 1995), which ultimately lead to intracellular parasite destruction. The present study investigated whether this same mechanism could also be responsible for the necrosis of the splenic tissue and the macrophages themselves. The in situ presence of TNF-α was investigated, as well as the participation of destroyed parasites in inducing the liberation of that cytokine, by comparison with the sudden intracellular disintegration of parasites induced by chemotherapy (Andrade & Freitas 1987).
Resistance to T. cruzi infection and mortality rates differ between different strains of mice (Andrade et al. 1985b). It has been demonstrated that macrophages of susceptible strains of mice produce higher levels of TNF-α than the macrophages of most resistant strains (Russo et al. 1989; Starobinas et al. 1991). The high mortality of the most susceptible strains of mice is probably dependent on cytokine liberation in the spleen, with extensive tissue necrosis. The Peruvian strain of T. cruzi, used in the present study, is highly virulent. Six inbred mice strains, previously tested in susceptibility to this strain (Andrade et al. 1985b), showed only slight differences on parasitaemia, survival time and intensity of lesions, the DBA being the most resistant.
Considering these observations, three strains of mice were included in this investigation: outbred Swiss mice and inbred C3H/He strain as the most susceptible, and DBA as the most resistant (Andrade et al. 1985b).
Materials and methods
Experimental animals
Ninety Swiss outbtred mice (Group I) and 83 C3H/He outbred mice (Group II), and 30 DBA inbred mice (Group III), weighing 10–20 g were used. The animals were infected with the Peruvian strain of T. cruzi, a macrophagotropic strain (Andrade 1985); 10 Swiss and C3H mice and 5 DBA were left as non-infected controls.
Inoculum
Blood forms obtained from infected mice were intraperitoneally administered (1 × 105 trypomastigotes/10 g of body weight).
Experimental groups
Three groups were considered for each mouse strain
Group I
Ninety Swiss mice: Group Ia: 61 mice infected with the Peruvian strain of T. cruzi; Group Ib: 19 mice infected and treated with Benznidazole; Group Ic: 10 uninfected controls.
Group II
Eighty-three C3H/He mice: Group IIa: 51 mice infected with the Peruvian strain of T. cruzi; Group IIb:22 mice infected and treated with Benznidazole; Group IIc: 10 uninfected controls.
Group III
Thirty-five DBA mice: Group IIIa: 30 mice infected with the Peruvian strain of T.cruzi; Group IIIb: 5 uninfected controls.
Parasitaemia
Parasitaemia was evaluated daily from the third day of infection until death or sacrifice, by counting trypomastigotes in 50 microscopic fields (400x) in the blood taken from the tail and examined between slide and coverslip.
Treatment with Benznidazole
Treatment with Benznidazole was performed in the dose of 100 mg/kg body weight/day. The schedules of treatment for both groups were as follows: 9 Swiss and 10 C3H/He mice were treated with one single dose at the 8th day of infection and sacrificed after 24 or 48 h. Ten Swiss and 10 C3H/He were treated with two doses at the 8th and 9th day, respectively, and sacrificed 24 or 48 h after the second dose.
Histopathology
Infected and control mice were sacrificed on the 7th, 8th, 9th, 10th and 11th days after infection. Perfusion of the spleen was performed by injection of PBS into the left ventricle of the heart, after section of the portal vein. Complete autopsies were done and fragments of several organs were fixed in buffered Milloning Formalin, pH 7.6 and embedded in paraffin; 5 µm-thick sections were stained with Hematoxylin and Eosin (H&E).
Immunohistochemistry
Fragments of the spleen were immediately embedded in tissue-teck (OCT compound-Miles Inc. Diagnostic Division, Elkhart, USA) frozen in liquid Nitrogen and cryopreserved in a freezer at −70 °C.
Sections of 6 µm were cut in a cryostat at −10 °C, placed on slides previously treated with10% Poly L-Lisin (Sigma, St. Louis, MO, USA), fixed in dehydrated Acetone, and treated with PBS containing 0.1% Saponin (Sigma, St. Louis, MO, USA) and 1% Bovin Serum Albumin (BSA) (Sigma St. Louis, MO, USA). For blocking non-specific binding, sections were treated with 30% BSA in PBS for 20 min at room temperature.
Sections were incubated overnight at 4 °C in an humidified chamber with Rat IgG antimouse –TNF-α antiserum (MP6-XT3) (Pharmigen, San Diego, CA, USA) diluted 1 : 50 in PBS pH 7,4/saponin 0,1%/BSA 1%. After washing with PBS and saponin, sections were incubated in normal sheep serum, for 20 min for additional blocking of non-specific binding. The slides were then incubated for 30 min at 37 °C in a humidified chamber with sheep–anti-Rat IgG antibody conjugated to Peroxidase (Boehringer-Mannheim Biochemica, Germany) diluted 1 : 1000 in PBS. Inhibition of the endogenous peroxidase was achieved by a 30 minute incubation in 0.3% H2O2 at room temperature. The colour was developed with 0.06% 3,3′-diaminobenzidine tetrahydrochloride (DAB) (Sigma, St. Louis, MO, USA) and 0.06% H2O2 plus 1% dimethylsulfoxide (Sigma, St. Louis, MO, USA). Sections were counterstained with 1% methyl-green for 2 min, dehydrated and mounted with Permount.
Positive control
Cells of the lineage J774 described as ‘macrophage like’ (Ralph & Nakoinz 1975; Van Furth et al. 1985) were maintained in RPMI cell culture medium with 10% bovine foetal serum, and collected during the exponential phase growth, at a concentration of 106 cells/mL. The cells were stimulated for 24 h with 10 ng/mL Lipopolysacharide (LPS) from the cellular membrane of Escherichia coli (Sigma, St. Louis, MO, USA) in a CO2 chamber, at 37 C. Stimulated cells were centrifuged at 1.500 r.p.m. for 10 min at 4 C, washed with RPMI and centrifuged a second time at 1.500 r.p.m. for 10 min The pellet was suspended in PBS at a concentration of 106 cells/mL, and re-centrifuged at 2.500 r.p.m. for 5 min in a cytocentrifuge (Cytospin-3-Shandon, Life Sciences International, England) for adhesion to slides previously treated with Poly l-lysine.
Negative control
Normal rat serum from a diluted 1 : 3000, was used in substitution of the primary antibody (Rat IgG antimouse TNF-α).
Statistical analysis
For comparative evaluation of the Parasitaemias, of Swiss vs. C3H/He; Swiss vs. DBA and C3H vs. DBA mice, the non-parametric Kruskal–Wallis test was used in Graph pad Software.
Results
Parasitaemia
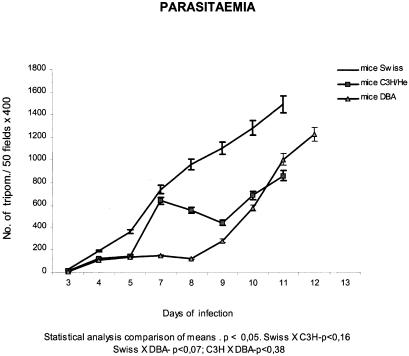
As shown in Fig. 1, parasitaemic levels increased until the 11th day following inoculation, in both groups of Swiss and C3H/He mice and the 12th day in DBA mice. The profiles of Parasitaemia differed between the three strains, showing an early peak (7 days) in the C3H/He mice, progressive increasing in the Swiss mice and the lower levels in the DBA until the 10th day of infection. Statistical analysis did not show significant differences (Fig. 1) between the three groups.
Figure 1.
Parasitaemic profiles from three mouse strains (Swiss,C3H/He and DBA), infected with the Peruvian strain of Trypanosoma cruzi showing the differences in the evolution of the parasitaemia, with low levels for the DBA mice until the 10th day, the progressive increasing for the Swiss mice until the 11τh day, and the early peak at 7 days for the C3H/He.
Mortality
Both groups of infected mice, Swiss and C3H/He, showed increasing mortality rates from the 9th day of infection, reaching 100% cumulative mortality at the 11th day of infection (Figs 2a, b). Mortality rates for the DBA mice were null until the 11th day, increased by the 12th day, and reached 100% on the 14th day (Fig. 2c). Survival time was 11 days for the susceptible strains (Swiss and C3H/He), and 14 days for the most resistant strains (DBA).
Figure 2.
a, b, c: Cumulative mortality for Swiss, C3H/He and DBA mice, infected with the Peruvian strain of T. cruzi. By the 11th day post-infection, mortality-rate reached 100%, for Swiss and C3H/He strains. Survival time was 11 days for the susceptible strains (Swiss and C3H/He) and 14 days for the most resistant (DBA).
Histopathology
The spleen was the most compromised organ in Swiss and C3H/He mice infected with the Peruvian strain of T. cruzi. Massive parasitism of macrophages in both red and white pulps was observed in conjunction with the macrophagotropism of the parasite strain (Figs 3a, b, c). From the 7th day of infection, hyperplasia of lymphoid follicles was observed, with large germinal centres showing vacuolated macrophages containing cellular debris and desintegrated parasites (Fig. 3B). In the red pulp, a large accumulation of parasite-loaded macrophages was observed in samples from both strains of mice (Figs 3a, c). Parasitism of macrophages in the spleen of DBA mice was, on the contrary, very mild. Hyperplasia of the lymphoid follicles was seen from the 7th day of infection, with vacuolated macrophages in the germinal centres containing cellular debris or disintegrating parasites. Macrophages with well preserved amastigotes of T. cruzi were rarely seen both in the white and red pulps until the 12th day of infection. In C3H/He mice, necrosis of parasitized macrophages and parasites, as well as of other splenic cells, was present from the 7th day until the 11th day. These aspects were seen in focal areas of the red pulp (Fig. 3d) and germinal centres (Figs 3e, f). In the Swiss mice these necrotic changes were more evident at the 9th and 10th days of infection. Parasite clearance and subsiding hyperplastic changes occurred earlier for C3H/He mice, coinciding with a decrease in parasitaemia (Fig. 1). In DBA mice, necrotic areas were also present in a small proportion of the cases by the 8th and 9th days of infection, appearing as small foci in the red and white pulps at sites of destroyed macrophages. The areas of necrosis of macrophages, and of other cells, were characterized by cytoplasmic vacuolization, nuclear picnosis and caryolisis, loss of cellular limits, and parasite desintegration. Apoptosis of lymphocytes was also seen in the germinal centres of lymphoid follicles and appeared as cell debris into the cytoplasm of macrophages.
Figure 3.
Several aspects of the spleen of mice infected with T. cruzi, showing the massive parasitism of macrophages, hyperplasia of lymphoid follicles with large germinal centres and necrotic lesions of the red and white pulps: a) in the red pulp, lymphocytic proliferation and macrophages with nests of amastigotes in the cytoplasm on the 7th day of infection, C3H/He mouse, H&E, 1000X; b) amplified germinal centre showing vacuolate macrophages containing cellular debris and parasite remnants; c) red pulp, parasitized macrophages appear clear and vacuolated, in an early phase of cell and parasite disintegration; d) advanced macrophage disintegration, with focal necrosis of the red pulp; e, f) focal areas of necrosis in the germinal centres, with macrophages, lymphocytes and disintegrating parasites, picnotic nuclei and cells debris. b, c, d, e, f) − H&E 400X).
In the liver, focal infiltrates of macrophages containing amastigotes, either well preserved or in desintegration, were seen in the parenchyma. Desintegrated parasites corresponded to necrosis of macrophages and also necrosis of the hepatic cells in the focal areas. These aspects are less marked in the resistant strain of mice.
In mice treated with one dose of benznidazole, moderate hyperplasia of lymphoid follicles was observed 24 h after treatment. Macrophages containing cellular debris, focal necrosis in germinal centres and red pulp in the absence of parasites were also observed (Figs 4a, b). By 48 h after treatment, a clear regression of the alterations was noted, with a remaining moderate hyperplasia of lymphoid follicles, without parasites or necrosis.
Figure 4.
Splenic changes in mice infected with T. cruzi and treated with Benznidazole: a) hyperplastic germinal centres, with focal area of necrosis, and absence of parasites; H&E, 250X; b) focal area of cell necrosis and apoptosis in the red pulp; one macrophage with vacuolated cytoplasm (arrow head), free from parasites; H&E, 400X.
In the mice treated with two doses of Benznidazole, a moderate hyperplasia of the germinal centres, with macrophages containing disintegrating parasites in the absence of necrosis, was observed 24 hrs after the first dose. These alterations subsided almost completely 48 hrs after the second dose.
Immunohistochemistry
As a positive control for TNF-α antibody, J774 cells were stained with the monoclonal anti-TNF-α antibody and were positive (Fig. 5a).
Figure 5.
Immunohistochemical demonstration of TNFα in the necrotic lesions of the spleen of mice infected with T. cruzi: a) positive control: cells J774 stimulated with LPS, 400X; b, c, d, e) positive immunolabelling for TNFα as intracellular deposits in macrophages (arrows) and disintegrated parasites, and interstitial grumous material in necrotic areas; f) TNFα expression in scattered areas of the spleen of an infected mouse treated with Benznidazole (1 dose, 48 h), 400X.
In sections of spleen from Swiss, C3H/He or DBA infected mice, TNF-α was expressed in focal necrotic areas at germinal centres and red pulp. Anti-TNFα staining was visible in intracellular deposits (Figs 5b, d, arrows) together with disintegrating parasites or in extracellular grumous material within areas of necrosis (Figs 5c, d, e). A clear correspondence between TNF-α positive immunolabelled sections and necrotic lesions in H&E stained slides was evident (Figs. 3, 5) being less marked in the spleen of DBA mice.
For mice treated with Benznidazole, the necrotic areas within germinal centres and red pulp seen 24 and 48 h after the first dose also revealed the presence of TNF-α. However the staining was less marked and less extensive than that of samples from untreated mice (Fig. 5f).
Discussion
The present results indicate the involvement of TNF-α in the development of severe splenic lesions occurring at the height of the acute phase of T. cruzi infection in mice. The presence of TNF-α in splenic lesions of mice emphasizes the important role of this cytokine in the destruction of parasitized macrophages and intracellular parasites, probably as part of the innate immune response to control the parasite multiplication, in the initial phase of infection with T. cruzi.
Infection of mice with T. cruzi is known to elicit prominent proliferation of lymphoid cells characteristic of polyclonal activation of B and T cells, associated with immunosuppression (Corsini & Costa 1981a,b; D'Império et al. 1986; Minóprio et al. 1986). These aspects do not preclude the concomitant development of defence mechanisms, initiated by the phagocytosis of parasites by the macrophages, followed by intracellular destruction.
T. cruzi-stimulated T cells are a source of IFNγ and have a prominent influence on the stimulation of macrophages for the production of Type 1 cytokines, such as IL-12, TNF-α and IFNγ that can be interpreted as helpful for the control of parasitaemia but also may induce toxic lesions, leading to death of the animals (Dos Reis 1997). The earlier expression of TNF-α in splenic lesions of C3H/He mice infected with the Peruvian strain compared to infected Swiss mice is consistent with an earlier decreased parasitaemia in C3H/HE mice. On the contrary, although developing reactive hyperplasia of the lymphoid follicles, the more resistant mouse strain (DBA) showed a lower response in the prodution of TNF-α, expressed in situ by the limited necrotic areas seen in the spleen.
It has been demonstrated that macrophages of susceptible mice produce higher levels of TNF-α, than the macrophages of resistant mice (Russo et al. 1989; Starobinas et al. 1991). However, the 100% mortality of the Swiss, C3H and DBA mice, between the 11th and 14th day of infection, confirms previous observations indicating that the patterns of resistance of different mouse strains are also influenced by the parasite strains (Andrade et al. 1985b). Mortality occurred in the same proportion (100%) with slight differences in survival pattern in six inbred mouse strains infected with the Peruvian strain (Andrade et al. 1985b), being the DBA (inbred) strain showing the higher survival time (14 days). The high virulence of the Peruvian strain, as well as the Y strain, used by Cordeiro et al. (1997), may result from their capacity to parasitize macrophages, which stimulate the production of TNF-α (Tarleton 1988). Revelli et al. (1999a) detected a correlation between virulence of the parasite strain and the high levels of serum TNFα in infected mice.
Interestingly, the increased destruction of intracellular parasites by specific treatment with Benznidazole did not enhance the necrotic lesions of macrophages and spleen cells. On the contrary, a clear regression of the necrotic lesions was seen by 48 h after the start of treatment, both with one or two doses. As has been previously demonstrated (Andrade & Freitas 1987), the intracellular action of chemotherapy in the heart leads to the massive death of parasites, with cytoplasmic vacuolization, necrosis of amastigotes and ultrastructural alterations in parasitized cardiomyocites, but without apparent damage to surrounding cells. The direct action of Benznidazole on the cytokine profiles of activated macrophage has been postulated by different authors. Murta et al. (1999), studying in vivo the effect of chemotherapy on the parasite interaction with macrophages, demonstrated that phagocytosis and destruction of parasites were significantly enhanced by drug treatment, with increased production of IL-12 and TNF-α. Revelli et al. (1999b) demonstrated in vitro that Benznidazole treatment of macrophages prestimulated with LPS or IFNγ significantly reduced nitrites, IL-6 and IL-10 and partially inihibited TNF-α production.
The results of the present investigation confirm the production of TNF-α at sites of parasite and tissue destruction in the spleen of mice acutely infected with T. cruzi, predominating in susceptible strains as compared with the resistant DBA strain. An increase in the production of TNF-α was not detected after parasite destruction induced by chemotherapy. The microbicidal mechanisms of macrophages, with prodution of TNF-α and other cytokines (Gazzinelli et al. 1992; Silva et al. 1995; Dos Reis 1997), seem to be primarily responsible for the necrotic lesions of the spleen. High rates of mortality, coincident with decreased tissue parasitism, indicate that the reactive state of macrophages induced during parasite elimination may be deleterious for the host.
Acknowledgments
Thanks are due to Brendan L. Flannery (University of California, Berkeley, CA, USA), for language review and to Edson L. P. Camandaroba (Centro de Pesquisas Gonçalo Moniz/Fiocruz) for statistical analysis of the results.
References
- Andrade SG. Morphological and behavioural characterization of Trypanosoma cruzi strains. Rev. Soc. Bras. Medical Trop. 1985;18:39–46. [Google Scholar]
- Andrade SG, Andrade V, Brodskyn C, Magalhães JB, Barral-Netto M. Immunological response of Swiss mice to infection with three different strains of Trypanosoma cruzi. Ann. Trop. Med. Parasitol. 1985a;79:397–407. doi: 10.1080/00034983.1985.11811938. [DOI] [PubMed] [Google Scholar]
- Andrade V, Barral Netto M, Andrade SG. Patterns of resistance of inbred mice to Trypanosoma cruzi are determined by parasite strain. Braz. J. Med. Biol. Res. 1985b;18:499–506. [PubMed] [Google Scholar]
- Andrade V, Barral-Netto M, Andrade SG, Magalhães JB. Aspéctos imunológicos da infecção de seis linhagens isogênicas de camundongos por três diferentes cepas do Trypanosoma cruzi. Mem. Inst. Oswaldo Cruz. 1985c;80:203–211. doi: 10.1590/s0074-02761985000200011. [DOI] [PubMed] [Google Scholar]
- Andrade SG, Freitas LAR. Trypanosoma cruzi: cardiac myocells alterations due to spontaneous or therapeutically induced intracellular parasite disintegration. Cell. Mol. Biol. 1987;33:797–805. [PubMed] [Google Scholar]
- Cordeiro MS, Dahia ACG, Andrade ZA. kinectics of Trypanosoma cruzi destruction in the mouse spleen. Rev. Soc. Bras. Med. Trop. 1997;30:3–9. doi: 10.1590/s0037-86821997000100002. [DOI] [PubMed] [Google Scholar]
- Corsini AC, Costa MG. Immunosupression in mice infected with Trypanosoma cruzi (Chagas 1909). I. Evidences of polyclonal B cell activation in experimental infections mimicked by extract prepared from circulating trypomastigotes. Rev. Inst. Med. Trop. São Paulo. 1981a;23:114–121. [PubMed] [Google Scholar]
- Corsini AC, Costa MG. Immunosupression in mice infected with Trypanosoma cruzi (Chagas 1909). II. Trypomastigote crude extract (TCE) supress the humoral immune response in mice. Rev. Inst. Med. Trop. São Paulo. 1981b;23:122–126. [PubMed] [Google Scholar]
- D'Império Lima MR, Eisen H, Minoprio P, Joskowicz M, Coutinho A. Persisitence of polyclonal B cell activation with undetectable parasitemia in late stages of experimental Chagas' Disease. J. Immunol. 1986;137:335–336. [PubMed] [Google Scholar]
- Dos Reis G. Cell-mediated immunity in experimental Trypanosoma cruzi infection. Parasitol. Today. 1997;13:335–342. doi: 10.1016/s0169-4758(97)01073-9. [DOI] [PubMed] [Google Scholar]
- Gazzinelli RT, Oswald IP, Hieny S, James SL, Sher A. The microbicidal activity of IFNγ treated macrophage against Trypanosoma cruzi involves an 1-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by lL-10 and TGF-β. Eur. J. Immunol. 1992;22:2501–2506. doi: 10.1002/eji.1830221006. [DOI] [PubMed] [Google Scholar]
- Minóprio P, Eisen H, Forni L, D'Império Lima MR, Joskowicz M, Coutinho A. Polyclonal lympphocyte responses to murine T. cruzi infection. 1. Quantificação of both T and B responses. Scand. J. Immunol. 1986;24:661–668. doi: 10.1111/j.1365-3083.1986.tb02185.x. [DOI] [PubMed] [Google Scholar]
- Murta SMF, Ropert C, Alves RO, Gazzinelli RT, Romanha AJ. In-vivo treatment with benznidazole enhances phagocytosis, parasite destruction and cytokine release by macrophages during infection with a drug-susceptible but not with a drug-resistant Trypanosoma cruzi population. Parasite Immunol. 1999;21:535–544. doi: 10.1046/j.1365-3024.1999.00251.x. [DOI] [PubMed] [Google Scholar]
- Ralph P, Nakoinz I. Phagocytosis and cytolysis by a macrophage tumour and its cloned cell line. Nature. 1975;257:393–394. doi: 10.1038/257393a0. [DOI] [PubMed] [Google Scholar]
- Revelli S, Gómez L, Wietzerbin J, Botasso O, Bassombrio MA. Levels of tumor necrosis factor alpha, gamma interferon, and interleukins 4, 6 and 10 as determined in mice infected with virulent or attenuated strains of Trypanosoma cruzi. Parasitol. Res. 1999a;85:147–50. doi: 10.1007/s004360050524. [DOI] [PubMed] [Google Scholar]
- Revelli S, Le Page C, Piaggio E, Wietzerbin J, Botasso O. Benznidazole, a drug employed in the treatment of Chagas ‘disease, down-regulates the syntesis of nitrite and cytocines by murine stimulated macrophages. Clin. Exp. Immunol. 1999b;118:217–227. doi: 10.1046/j.1365-2249.1999.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo M, Starobinas N, Ribeiro dos Santos R, Minoprio P, Eisen H, Hontebeyrie-Joskowicz M. Susceptible mice present higher macrophage activation than resistant mice during infections with myotropic strains of Trypanosoma cruzi. Parasite Immunol. 1989;11:385–395. doi: 10.1111/j.1365-3024.1989.tb00675.x. [DOI] [PubMed] [Google Scholar]
- Silva JS, Vespa GN, Cardoso MA, Aliberti JC, Cunha FQ. Tumor necrosis factor alpha mediates resistance to Trypanosoma cruzi infection in mice by inducing nitric oxide production in infected gamma interferon-activated macrophages. Infect Immun. 1995;63:4862–4867. doi: 10.1128/iai.63.12.4862-4867.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starobinas N, Russo M, Minoprio P, Hontebeyriejoskowic M. Is TNF alpha involved in early suscetibility of Trypanosoma cruzi – infected C3H/He mice? Res. Immunol. 1991;142:117–122. doi: 10.1016/0923-2494(91)90019-f. [DOI] [PubMed] [Google Scholar]
- Tarleton RL. Tumor necrosis factor (cachectin) production during experimental Chagas’ disease. Clin. Exp. Immunol. 1988;73:186–190. [PMC free article] [PubMed] [Google Scholar]
- Van Furth R, Van Schadewijk-Nieuwstad M, Elzenga-Claasen I, Cornelisse C, Nibbering P. Morphological, cytochemical, functional, and proliferative characteristics of four murine macrophage-like cell Lines. Cell. Immunol. 1985;90:339–357. doi: 10.1016/0008-8749(85)90199-6. [DOI] [PubMed] [Google Scholar]