Abstract
Eps15 is a substrate for the tyrosine kinase of the epidermal growth factor receptor (EGFR) and is characterized by the presence of a novel protein:protein interaction domain, the EH domain. Eps15 also stably binds the clathrin adaptor protein complex AP-2. Previous work demonstrated an essential role for eps15 in receptor-mediated endocytosis. In this study we show that, upon activation of the EGFR kinase, eps15 undergoes dramatic relocalization consisting of 1) initial relocalization to the plasma membrane and 2) subsequent colocalization with the EGFR in various intracellular compartments of the endocytic pathway, with the notable exclusion of coated vesicles. Relocalization of eps15 is independent of its binding to the EGFR or of binding of the receptor to AP-2. Furthermore, eps15 appears to undergo tyrosine phosphorylation both at the plasma membrane and in a nocodazole-sensitive compartment, suggesting sustained phosphorylation in endocytic compartments. Our results are consistent with a model in which eps15 undergoes cycles of association:dissociation with membranes and suggest multiple roles for this protein in the endocytic pathway.
INTRODUCTION
Receptor tyrosine kinases (RTKs) are rapidly internalized, through clathrin-coated pits, following binding to the cognate ligand and activation of their intrinsic kinase activity (reviewed in Sorkin and Waters, 1993). After internalization, RTKs are sorted through vesicular structures into intracellular endosomal compartments where decisions are made regarding whether to recycle the receptor to the cell surface or target it to lysosomes for degradation (Sorkin and Waters, 1993). The epidermal growth factor (EGF) receptor (EGFR) represents one of the best characterized systems. In its intracellular domain, three regions have been defined that contain endocytic codes, i.e., amino acid sequences capable by themselves of sustaining EGFR internalization (Chang et al., 1993). Endocytic codes are thought to be cryptic in the unstimulated EGFR and to be unmasked by conformational changes that follow receptor activation and autophosphorylation (Nesterov et al., 1995a). In other types of receptors, such as cargo receptors, endocytic codes are probably continuously exposed, thus determining constitutive internalization. In many cases endocytic codes contain a critical tyrosine residue, thus being known as “tyrosine-based” signals (reviewed in Mellman, 1996; Marks et al., 1997), and are interchangeable among receptors (Chang et al., 1993). There is ample evidence that tyrosine-based signals, in the EGFR and in other receptors, bind directly to the clathrin adaptor protein complex AP-2 (Glickman et al., 1989; Nesterov et al., 1995b; Sorkin et al., 1996).
AP-2 is a heterotetrameric protein complex that couples the process of coated pit assembly to that of recruitment of membrane receptors, through its ability to bind to receptor endocytic codes, on the one hand, and to clathrin, on the other (reviewed in Robinsons, 1992, 1994; Kirchhausen, 1993). It is unknown whether AP-2 is directed to the plasma membrane solely because of its interaction with receptor endocytic codes; however, this possibility is unlikely, because receptors are present also in compartments to which adaptor complexes are not normally recruited (reviewed in Kirchhausen et al., 1997). In addition, removal of the tyrosine-based signal, responsible for AP-2 binding, in the EGFR did not appreciably affect internalization kinetics (Nesterov et al., 1995b), at least at low levels of receptor expression (Sorkin et al., 1996). Finally, there is evidence of specificity in the endocytic machinery as shown by the findings that EGFRs compete with themselves but not with transferrin receptor (TfR) for endocytosis (Dickson et al., 1983; Hanover et al., 1985; Wiley, 1988; Wiley et al., 1991). Thus, recruitment of AP-2 to the plasma membrane might require a docking apparatus other than the receptors themselves (reviewed in Kirchhausen et al., 1997). One might speculate further that different docking machineries are responsible for endocytic specificity.
A recently discovered family of RTK substrates, comprising the related eps15 and eps15R proteins, is also involved in receptor-mediated endocytosis. Eps15 (Fazioli et al., 1993; Wong et al., 1994) and eps15R (Schumacher et al., 1995; Wong et al., 1995; Coda et al., 1998) are characterized by the presence of three copies of a novel protein:protein interaction domain, the EH domain (Wong et al., 1995; Di Fiore et al., 1997). A number of observations have recently linked eps15, eps15R, and other EH-containing proteins to coated pits-mediated internalization. 1) eps15 (Tebar et al., 1996; Van Delft et al., 1997b) and eps15R (Coda et al., 1998) colocalize with markers of the plasma membrane clathrin-coated pits and vesicles; 2) by electron microscopy, eps15 is found at the rim of budding coated vesicles (Tebar et al., 1996); 3) eps15 and eps15R are constitutively associated with the clathrin adaptor protein complex AP-2 (Benmerah et al., 1995, 1996; Iannolo et al., 1997; Van Delft et al., 1997b; Coda et al., 1998); 4) a putative 160-kDa EH-containing protein is associated with the γ-subunit of the Golgi adaptor complex AP-1 (Robinsons and Page, 1996); 5) End3p, an EH-containing yeast protein, is essential for endocytosis of the α-factor receptor (Benedetti et al., 1994); 6) mutations in the EH domains of another yeast protein, Pan1p, impair endocytosis (Wendland et al., 1996; Tang et al., 1997); in addition, Pan1p functions as an adaptor protein involved in the assembly of protein:protein interactions essential for endocytosis (Wendland and Emr, 1998); 7) the amino acid motif NPF (Asn-ProPhe) is a ligand for the EH domain (Salcini et al., 1997) and functions as an internalization motif in yeast (Tan et al., 1996); 8) microinjection of anti-eps15 or anti-eps15R antibodies inhibits endocytosis of EGF and TfR (Carbone et al., 1997, Benmerah et al., 1998); and 9) overexpression of a dominant negative mutant of eps15, comprising only its AP-2 binding region, is also able to inhibit EGFR internalization (Carbone et al., 1997).
In this study we analyzed the intracellular distribution of eps15 and its association with organelles of the endocytic pathway during internalization of the EGFR. Our observations, viewed in the context of existing knowledge, are compatible with a model in which eps15 might serve, at least in the case of EGFR internalization, as a docking molecule responsible for AP-2 recruitment to the plasma membrane. In addition, we show that eps15 colocalizes with the EGFR, and is phosphorylated by it, in various compartments of the endocytic pathway, raising the possibility of its additional involvement in late steps of the endocytic process.
MATERIALS AND METHODS
Cell lines
NIH-EGFR transfectants have been described previously (Di Fiore et al., 1987). B82 cells, transfected with normal EGFR wild type (WT), mutant kinase-inactive EGFR (Chen et al., 1989), and mutant EGFR lacking the sequence for AP-2 binding (Nesterov et al., 1995b), were kindly provided by Dr. G. N. Gill (University of California, San Diego, CA). All cells were cultured in DMEM supplemented with 10% fetal calf serum and antibiotics. In B82 cell cultures, 2 mM methotrexate was added to the medium
For morphological experiments, cells were serum-starved for 12 h and incubated with EGF (100 ng/ml) (Gibco, Grand Island, NY) and anti-EGFR 108.1 monoclonal antibody (1:100 in PBS) or with PDGF (20 ng/ml) (Upstate Biotechnology, Waltham, MA) at 4°C for 1 h and then warmed to 37°C for an additional 10 or 30 min or kept at 4°C before fixation. Treatment with nocodazole (20 μg/ml) to depolymerize microtubules was performed in NIH-EGFR cells for 1 h at 37°C before EGF addition and prolonged during incubations with EGF as above. To label early endosomes, cells depleted of serum for 12 h were incubated in medium containing 18 nm BSA–colloidal gold particles for 10 min at 37°C to allow their internalization in early endocytic compartment before fixation.
Immunofluorescence and Confocal Microscopy
Cells, grown on coverslips, were fixed in methanol at −20°C for 4 min. After washing in PBS, cells were incubated for 1 h at 25°C with an anti-eps15 affinity-purified polyclonal antibody (1 μg/ml in PBS, described below). The bound antibodies were visualized with anti-rabbit IgG FITC (1:100 in PBS, 30 min at 25°C) (Cappel Research Products, Durham, NC). For microtubule staining, cells were successively incubated with anti-tubulin monoclonal antibody (1:100 in PBS, 1 h at 25°C) (Sigma, St. Louis, MO) and visualized with anti-mouse IgG TRITC (1:10 in PBS, 1 h at 25°C) (Cappel) after appropriate washing in PBS. For Golgi apparatus identification in immunofluorescence, NIH-EGFR cells were incubated with the lectin HPA-FITC (1:10 in PBS, 1 h at 25°C) (Sigma). For AP-2 staining, cells were incubated with anti-AP-2 monoclonal antibody (mAb) (1:100 in PBS, 1 h at 25°C) (AP.6 mAb, kindly provided by Dr. Alexander Sorkin, University of Colorado). For double immunofluorescence, the anti-eps15 affinity-purified polyclonal antibody was visualized with anti-rabbit IgG Texas Red (1:50 in PBS) (Jackson ImmunoResearch, West Grove, PA) and anti-EGFR monoclonal antibody or anti-AP-2 monoclonal antibody was detected with anti-mouse IgG FITC (1:10 in PBS, 30 min at 25°C) (Cappel). Colocalization of the two fluorescence signals was analyzed by a Zeiss Invert Laser Scan Microscope (Zeiss, Oberkochen, Germany). To follow the internalization of PDGFR, NIH-EGFR cells were treated with PDGF and fixed as described above and incubated with anti-PDGFR-A C20 polyclonal antibody (1:50 in PBS, 1 h at 25°C) (Santa Cruz Biotechnology, Santa Cruz, CA), and the bound antibodies were visualized with anti-rabbit IgG FITC (1:100 in PBS, 1 h at 25°C) (Cappel).
The anti-eps15 antibody used in this study was raised against the full-length murine eps15, recombinantly produced as a GST-fusion protein (GST/eps15–2-907; the numbers refer to the amino acid positions of the eps15 moiety present in the GST fusion), by immunizing New Zealand rabbits. The total serum was immunopurified by a two-step procedure involving first depletion of the anti-GST component by three cycles of affinity chromatography on agarose-immobilized GST and then by affinity purification onto agarose-immobilized GST/eps15–2-907. The anti-eps15 serum was described in Coda et al. (1998) and shown not to cross-react with eps15R, the other member of the eps15 family of proteins.
Immunoelectron Microscopy
Cells untreated or treated with EGF as above, and cells incubated with BSA–gold (18 nm), were processed for postembedding immunocytochemistry. Briefly, cells were fixed with 0.5% glutaraldehyde in PBS for 1 h at 25°C, partially dehydrated in ethanol, and embedded in LR White resin. Thin sections were collected on nickel grids and immunolabeled with the anti-eps15 affinity-purified antibody antibodies (10 μg/ml in Tris, for 1 h at 25°C) followed by protein A colloidal gold (18 nm) prepared by the citrate method (Slot and Geuze, 1981). In double-labeling experiments, the sections were first incubated alternatively with anticathepsin D polyclonal antibodies (1:100 in Tris, for 1 h at 25°C) (kindly provided by Dr. Ciro Isidoro, University of Torino, Torino, Italy), with anti-αC subunit of AP-2, Ab31 polyclonal antibodies (1:5 in Tris, 1 h at 25°C) (kindly provided by Dr. Alexander Sorkin, University of Colorado, Denver, CO) or with anti-EGFR 528 monoclonal antibody (1:5 in Tris, 1 h at 25°C) (Santa Cruz Biotechnology), followed by 10 nm protein-A colloidal gold conjugates (British BioCell International, Cardiff, UK) and then with the anti-eps15 affinity-purified antibody followed by 18 nm protein A gold particles. Control experiments were performed 1) by omission of the primary antibody or 2) by incubation of the sections with anti-eps15 polyclonal antibody previously adsorbed with the full-length eps15 protein (for 1 h at 25°C). All sections were finally stained with uranyl acetate and lead hydroxide.
In preembedding experiments, B82 cells, treated as described above with EGF and anti-EGFR monoclonal antibody for 1 h at 4°C and fixed or warmed to 37°C for 5 min before fixation in 0.5% glutaraldehyde (for 30 min at 4°C), were incubated with goat anti-mouse IgG (1:10 in PBS, for 1 h at 4°C) (Cappel), and finally labeled with 18 nm protein-A gold particles. Cells were then processed for conventional thin sections electron microscopy (post-fixed in 1% osmium tetroxide, stained with uranyl acetate 5 mg/ml, dehydrated in acetone, and finally embedded in Epon 812).
Density of the immunogold labeling, determined as gold particles/micrometer of membrane length, and the statistical analysis were evaluated using a Sigma Scan Measurement System (Jandel Scientific, Corte Madera, CA).
Protein Studies
Immunoprecipitation, immunoblotting, and coimmunoprecipitations were performed as described previously (Fazioli et al., 1993; Coda et al., 1998). Typically, we used 50–100 μg of total cellular proteins for direct immunoblot analysis and 3–5 mg of total cellular proteins for immunoprecipitation/immunoblotting experiments. Immunoblots were decorated with the appropriate primary antibody (see below) and detection was with horseradish peroxidase conjugated with specific secondary antisera followed by enhanced chemiluminescence reaction.
For coimmunoprecipitation studies, cells were lysed with a buffer containing 1% Triton X-100 (Pierce, Rockford, IL), 50 mM HEPES, pH 7.5, 150 mM NaCl, 10% glycerol, 1.5 mM MgCl2, 5 mM EGTA, protease inhibitors (4 mM phenyl methylsulfonylfluoride and 100 mg/ml aprotinin), and phosphatase inhibitors (10 mM sodium orthovanadate and 20 mM sodium pyrophosphate); cell lysates were used immediately, without freeze/thawing. Immunoprecipitations and coimmunoprecipitations were performed for 1 h, and immune complexes were recovered by adsorption to Gamma Bind G-agarose (Pharmacia, Piscataway, NJ).
Antibodies used were 1) the described affinity-purified anti-eps15 antibody (Coda et al., 1998), 2) a commercial antiphosphotyrosine monoclonal antibody (Upstate Biotech.), 3) a monoclonal anti-α-adaptin recognizing both αA and αC adaptins (Sigma), 4) commercial anti-shc antibodies (Santa Cruz Biotechnology), and 5) the E7 anti-EGFR peptide serum (Di Fiore et al., 1990).
RESULTS
Intracellular Localization of Eps15
As a preliminary step, we analyzed the intracellular localization of eps15 in different cell lines. Because one of the research goals was to investigate modifications of the distribution of eps15 upon RTK activation, we focused our attention on cell lines expressing EGFR, which is able to phosphorylate eps15 efficiently (Fazioli et al., 1993). In particular, two models of rodent fibroblasts, NIH-EGFR (Di Fiore et al., 1987) and B82-EGFR (Chen et al., 1989) cells, were used. These lines were genetically engineered to express elevated levels of the EGFR, in the order of 0.5–1.0 × 106 receptors/cell.
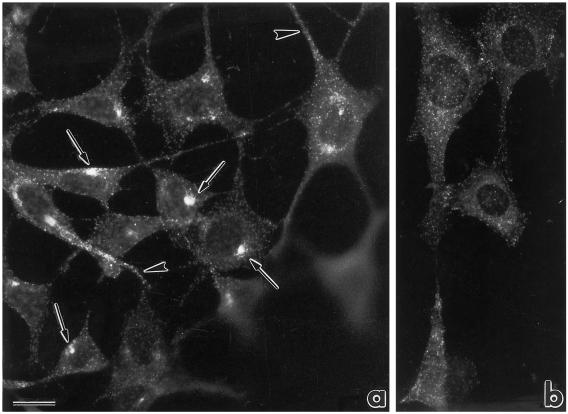
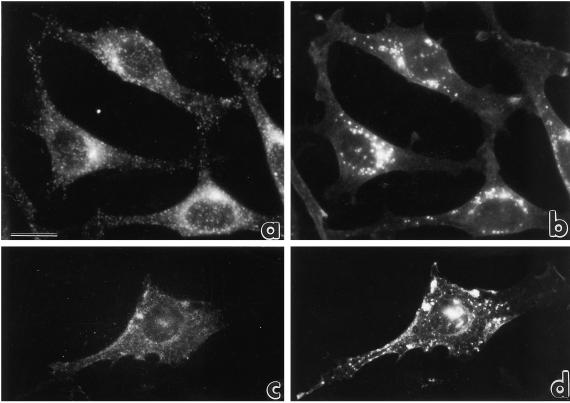
The intracellular localization of eps15 protein was analyzed by indirect immunofluorescence in NIH-EGFR cells, using a polyclonal antibody directed against the full-length eps15 protein. In logarithmically growing NIH-EGFRs, the signal appeared punctate and dispersed throughout the cell. Signals were present at the cell periphery and along cell projections (Figure 1a, arrowheads) or concentrated in the perinuclear Golgi region (Figure 1a, arrows). Nocodazole treatment, which induces dispersion and fragmentation of the Golgi apparatus by microtubule depolymerization, also induced a dispersion of the eps15 perinuclear concentration (Figure 1b). Similar results were obtained in B82-EGFR (Figure 3a1) and in A172 glioblastoma, HeLa cervical carcinoma, and CV1 monkey kidney cell lines (our unpublished results).
Figure 1.
Immunofluorescence analysis of the intracellular localization of eps15 in NIH-EGFR transfectants. (a) The pattern of staining with anti-eps15 antibody is punctate and the small dots are localized either at the cell periphery and along cell projections (arrowheads) or concentrated in the perinuclear Golgi area (arrows). (b) After treatment with nocodazole, resulting in microtubule depolymerization and subsequent Golgi fragmentation, the dots appear dispersed from the perinuclear cluster. The disrupting effect of nocodazole on microtubules was established by double staining with anti-tubulin antibodies. Bar, 5 μm. The anti-eps15 staining in this and all subsequent experiments was obtained with the described affinity-purified anti-eps15 antibody. In addition, comparable results were obtained using anti-eps15 monoclonal antibodies).
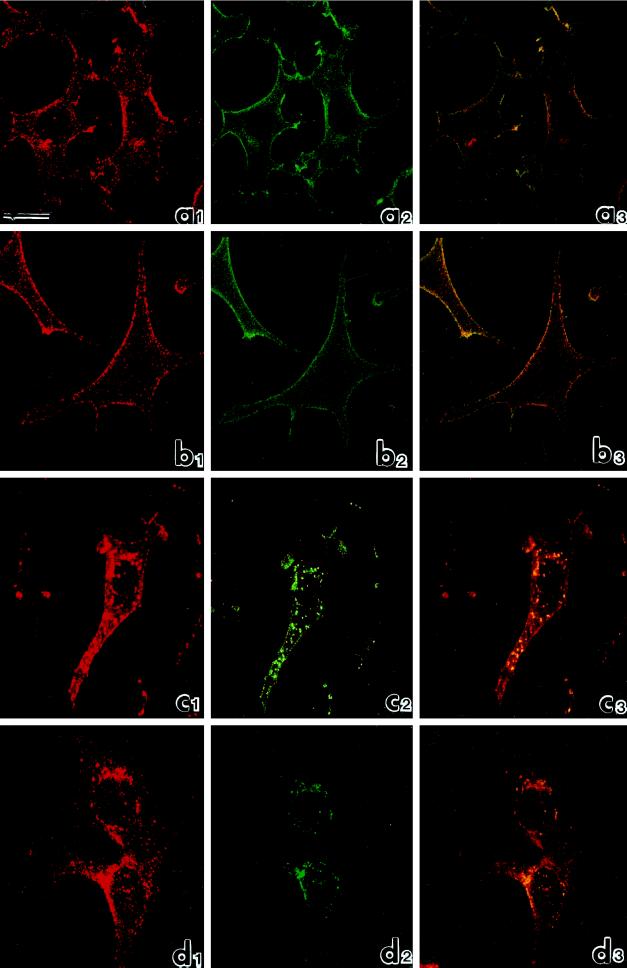
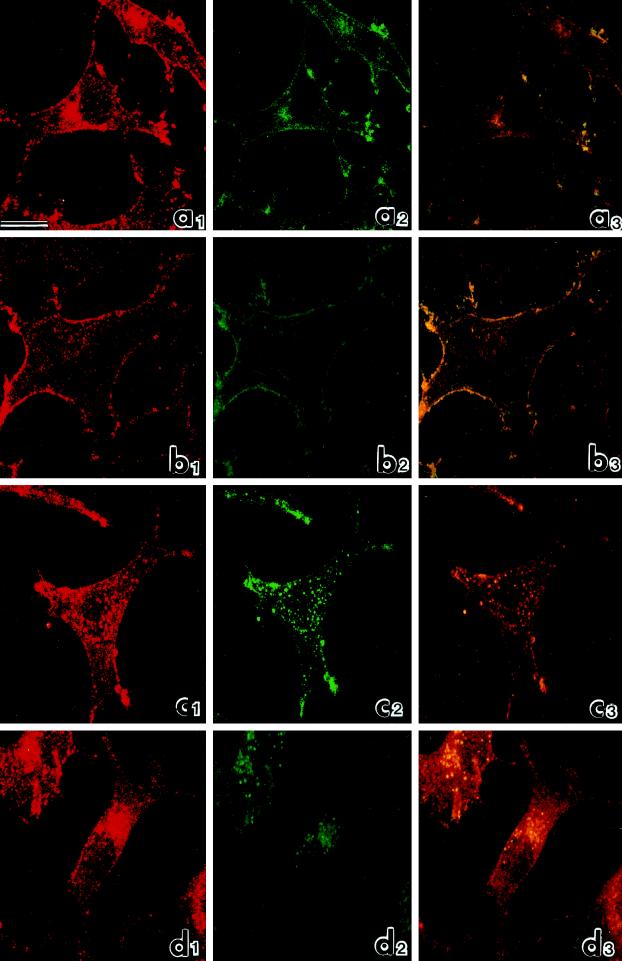
Figure 3.
Confocal microscopic analysis of the distribution of eps15 (red: rhodamine staining) and EGFR (green: fluorescein staining) in B82 cells expressing EGFR WT. (a) Untreated cells; (b) cells treated with EGF for 1 h at 4°C; (c) cells treated with EGF for 1 h at 4°C and warmed to 37°C for 10 min; (d) cells treated with EGF for 1 h at 4°C and warmed to 37°C for 30 min. The extent of colocalization, assessed by superimposing red and green signals, is shown in yellow (panels labeled 3). Bar, 10 μm.
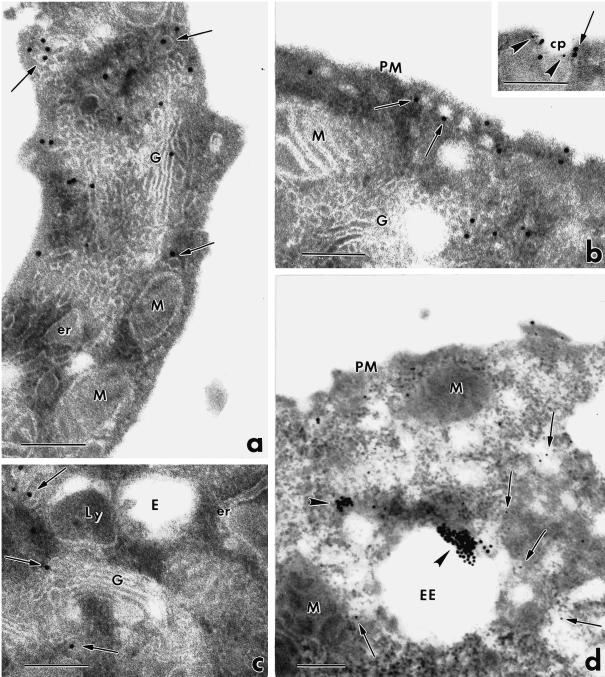
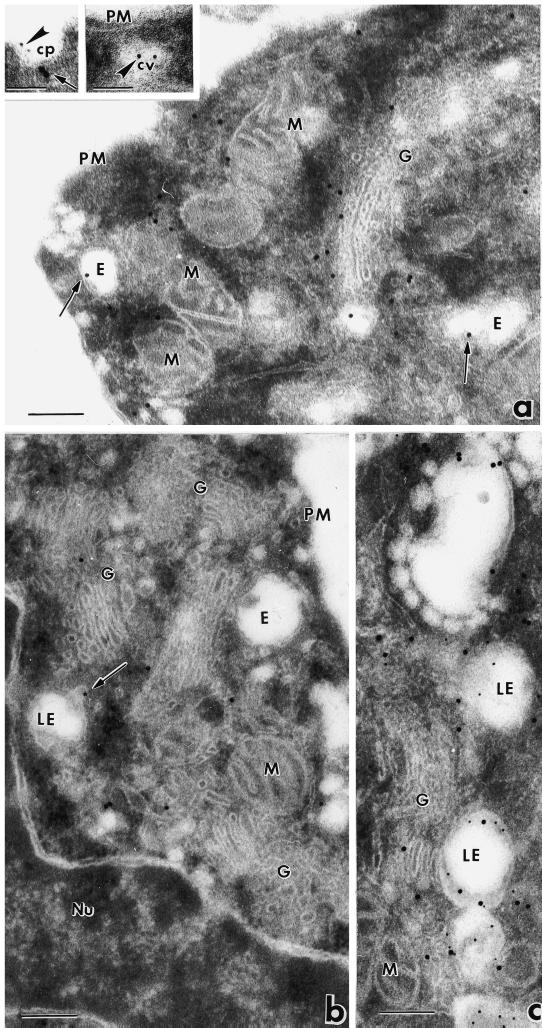
To identify the intracellular sites and structures of eps15 localization, we performed immunoelectron microscopy with anti-eps15 antibodies and protein A–colloidal golds on thin sections of resin-embedded NIH-EGFR cells. In logarithmically growing cells, immunolabeling was mostly present on small vesicles (50–60 nm in diameter, measured using both the magnification scale bar and the 18 nm gold particles as an internal size marker) (Figure 2, arrows) distributed either at the cell periphery and in close proximity to the plasma membrane (Figure 2b), or in the Golgi perinuclear area (Figure 2, a and c), juxtaposed to Golgi cisternae. Eps15 appeared also associated with clathrin-coated pits at the cell plasma membrane, as shown previously (Tebar et al., 1996). Double labeling with anti–α-adaptin antibodies indicated colocalization of eps15 and α-adaptin, as reported (Tebar et al., 1996; Van Delft et al., 1997b) (Figure 2b, inset). Labeled vesicles were also frequently observed near endosomal structures (Figure 2, c and d, arrows), identified as early endosomes by the presence inside their lumen of internalized BSA–gold particles (15 min of internalization at 37°C) (Figure 2d, arrowheads). Immunolabeling of eps15 was not observed on the plasma membranes (Figure 2, a, b, and d), on Golgi cisternae (Figure 2, a and c) and on endosomal or lysosomal membranes (Figure 2, c and d). In control experiments, gold immunolabeling was drastically reduced by incubation of the cell sections with anti-eps15 antibody in the presence of the competing full-length eps15 protein (100 μg/ml). Thus, eps15 appears mostly associated with the membranes of small vesicles located in peripheral as well as perinuclear regions. The intracellular distribution of these vesicles, frequently observed in the proximity of plasma membranes, Golgi cisternae, and endosomes, suggests that they may represent transport carriers of the endocytic pathway.
Figure 2.
Immunoelectron microscopic analysis of eps15 localization. NIH-EGFR cells were resin-embedded, and thin sections were immunolabeled with anti-eps15 antibody and protein A–gold. (a–c) Gold particles (18 nm) are associated with small vesicles located either at the cell periphery and close to the plasma membrane (b, arrows) or in proximity to Golgi complexes (a and c, arrows) and to endosomal and lysosomal structures (c). Immunolabeling for eps15 appears also present at the cell surface in clathrin-coated pits, identified by double labeling with antibody to the α subunit of AP-2 (detail in b, eps15: large golds; AP-2: small golds). (d) Immunogold particles corresponding to eps15 (arrows point to small golds) are frequently seen on vesicles located close to early endosomes identified by internalized BSA–gold (arrowheads point to large golds). er, Endoplasmic reticulum; M, mitochondrion; PM, plasma membrane; G, Golgi complex; cp, clathrin-coated pit; E, endosome; Ly, lysosome; EE, early endosome. Bars, 0.2 μm.
Eps15 is redistributed during EGFR and PDGFR endocytosis: redistribution depends on kinase activity of the receptors and is controlled by microtubules
Eps15 is a substrate for activated RTKs, including EGFR; however, the subcellular localization of eps15 is not modified by EGF treatment (Tebar et al., 1996; Van Delft et al., 1997b; our unpublished results), even when receptor-saturating doses (100 ng/ml, equivalent to 17 nM) of the ligand are used. This result somehow conflicts with the postulated role of eps15 in receptor-mediated endocytosis (Carbone et al., 1997; Benmerah et al., 1998): a dynamic process involving rapid and progressive sorting of molecules in various cellular compartments. One possible explanation is that the various pools of eps15 (coated pits-associated, small vesicle-associated, trans-Golgi network-associated) evidenced in immunoelectron microscopy (Figure 2) are in a dynamic equilibrium, possibly controlled by rapidly reversible posttranslational modifications of eps15. If this were the case, redistribution of eps15 should become evident upon induction of a synchronous wave of receptor-mediated endocytosis. In an attempt to achieve this, we treated NIH-EGFR or B82-EGFR cells with receptor-saturating doses of EGF (100 ng/ml) at 4°C, a temperature that is nonpermissive for EGFR internalization, albeit allowing its catalytic activation. After receptor loading, cells were shifted to 37°C for different time points to permit endocytosis. Under these various conditions we analyzed, by double immunofluorescence, the localization of eps15 and EGFR. To selectively follow internalizing receptors, cells were incubated simultaneously with EGF and a monoclonal antibody directed against the EGFR extracellular portion, which does not compete for EGF binding and does not induce receptor cross-linking and internalization.
Figure 3 shows a confocal analysis of B82-EGFR cells under various conditions. In logarithmically growing cells, as expected, the eps15 signal (red) appeared punctate and distributed throughout the cell, with only marginal enrichment at the cell periphery (Figure 3a1). The EGFR signal (green) appeared mostly associated with the plasma membrane (Figure 3a2), and only a modest amount of colocalization was visible by superimposition of the red and green signals in the three-dimensional reconstruction (colocalized eps15 and EGFR appear in yellow) (Figure 3a3). Treatment with EGF for 1 h at 4°C dramatically induced recruitment of eps15 to the plasma membrane and substantially increased colocalization of eps15 and EGFR at the cell surfaces (Figure 3b). Subsequent warming to 37°C for 10 min induced redistribution of eps15 toward central areas of the cells (Figure 3c1), where the eps15 punctate signal colocalized with dots corresponding to internalized EGFR (Figure 3c2). After 30 min of warming, both eps15 (Figure 3d1) and EGFR (Figure 3d2) signals appeared concentrated in the perinuclear area. Thus activation of the EGFR kinase induced recruitment of eps15 at the plasma membrane, and synchronous triggering of endocytosis provoked redistribution of eps15 to the perinuclear area. In both cases eps15 signals colocalized with those of EGFR, indicating the presence of eps15 in the intracellular sites of trafficking of the receptor. Of note, redistribution and colocalization of eps15 with the EGFR involved a sizable fraction of the eps15 pool, but not its totality.
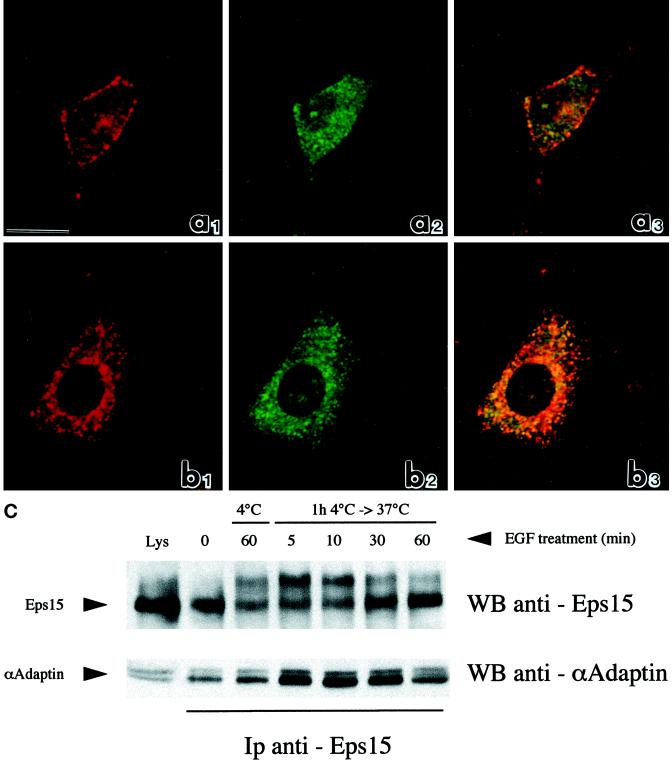
Parallel confocal analysis of B82-EGFR cells treated with EGF as above and double-labeled with anti–α-adaptin (green signal) and anti-eps15 (red signal) antibodies indicated colocalization of eps15 and α-adaptin (yellow signal) mostly at the cell plasma membrane after treatment with EGF for 1 h at 4°C (Figure 4a1–3) and in the perinuclear area after an additional 30 min of warming at 37°C (Figure 4b1–3). Similarly coimmunoprecipitation between α-adaptin and eps15 was not affected by treatment with EGF (Figure 4C). Thus, colocalization and physical association of eps15 and AP-2, which has been already demonstrated in EGF untreated cells (Tebar et al., 1996; Van Delft et al., 1997b), persists during redistribution of eps15 toward the plasma membrane at 4°C of EGF treatment and toward the perinuclear area after EGFR internalization.
Figure 4.
Association between eps15 and AP-2 in various conditions of EGF treatment. (a, b) Confocal microscopic analysis of the distribution of eps15 (red: rhodamine staining) and AP-2 (green: fluorescein staining) in B82 cells expressing EGFR WT. (a) Cells treated with EGF for 1 h at 4°C; (b) cells treated with EGF for 1 h at 4°C and warmed to 37°C for 30 min. Colocalization of the two signals is shown in yellow (panels labeled 3). Bar, 10 μm. (c) Coimmunoprecipitation of eps15 and α-adaptin. B82 cells expressing wild-type EGFR were serum-starved for 24 hours and then treated with EGF (100 ng/ml) for 1 h at 4°C (lane labeled 1h 4°C). Cells were then shifted to 37°C for the indicated lengths of time (lanes labeled 4°C → 37°C). Total cellular lysates (4 mg) were immunoprecipitated with anti-eps15 antibody and subjected to immunoblots with anti-eps15 (top) or anti–α-adaptin (bottom). The lanes labeled Lys were loaded with 100 μg of total lysates to serve as a reference. Positions of eps15 and α-adaptin are indicated by arrows. The shift in mobility of eps15, upon EGF treatment, visible in the top panel, has been described and is probably caused by monoubiquitination.
Redistribution of eps15 during endocytosis was totally dependent on kinase activity of the EGFR. We used a kinase-defective EGFR mutant, which does not internalize in response to the ligand (Chen et al., 1989; Wiley et al., 1991). The results revealed 1) no redistribution of eps15 after treatment with EGF either at 4°C or during subsequent warming to 37°C, 2) unperturbed distribution of EGFR kinase-defective mutant, as expected (Wiley et al., 1991), and 3) low levels of colocalization of eps15 and EGFR kinase-defective mutant at all time points.
Similar results were obtained in NIH-EGFR cells that were subjected to the same protocol of treatment with either EGF or PDGF (our unpublished results). In addition, treatment with nocodazole for 1 h at 37°C before EGF binding and warming to 37°C for 30 min prevented eps15 (Figure 5c) and EGFR (Figure 5d) redistribution toward the juxtanuclear region of the cell. Because depolymerization of microtubules prevents late steps of EGFR endocytosis (Gruenberg and Maxfield, 1995), it appears that eps15 colocalizes with EGFR along the entire pathway of receptor internalization.
Figure 5.
Immunofluorescence analysis of the redistribution of eps15 during EGFR endocytosis in NIH-EGFR cells treated with nocodazole. Double staining of eps15 (left panels) and of EGFR (right panels) on NIH-EGFR cells treated with EGF and anti-EGFR mAb for 1 h at 4°C and then warmed for 30 min at 37°C, in the absence (a and b) or presence (c and d) of nocodazole. Treatment with EGF at 4°C followed by incubation for 30 min at 37°C induces concentration of the eps15 and EGFR signals in the perinuclear area (a and b, respectively). Treatment with nocodazole blocks the redistribution of both eps15 and EGFR toward the central portion of the cell, keeping the signals at the cell periphery (c and d). Bar, 5 μm.
Eps15 is localized first on early and then on late endosomes during EGFR endocytosis
To identify the intracellular structures involved in eps15 redistribution during EGFR endocytosis, we performed immunolabeling with anti-eps15 monoclonal antibody (mAb) and protein A–colloidal gold conjugates of thin sections of NIH-EGFR resin-embedded cells incubated with EGF at 4°C and fixed or warmed to 37°C for 10 or 30 min, to allow receptor internalization before fixation. Double immunolabeling of EGFR and eps15 was also performed. After treatment with EGF at 4°C, immunogold labeling of eps15 appeared to be present mostly on small vesicles closely apposed to the plasma membrane or associated with surface pits, as expected (Table 1). Warming to 37°C for 10 min induced immunogold association with endosomal structures (Figure 6a, arrows, and Table 1) and with vesicles mostly located close to the Golgi complex (Figure 6a). Colocalization of eps15 (large golds) and EGFR (small golds) was observed on clathrin-coated pits at the cell surface (Figure 6a, left inset), on small noncoated vesicles, and on early endosomes (our unpublished results). Interestingly, clathrin-coated vesicles juxtaposed to the plasma membrane were positively labeled only for EGFR, appearing negative for eps15 (Figure 6a, right inset). After 30 min of warming, gold labeling of eps15 appeared confined to the perinuclear area (Figure 6b), frequently associated with the membranes of endosomes (Table 1) identified as late endosomes by morphological criteria (Figure 6b), and in double-labeling experiments by positive reaction to cathepsin D, a marker of late endosomes and lysosomes (Figure 6c). At the 30-min time point of warming, endosomes and vesicles at the cell periphery appeared unlabeled (Figure 6b). Similar results were obtained in B82-EGFR cells. Thus, eps15 appears to associate with endosomal membranes after EGFR endocytosis and down-regulation.
Table 1.
Quantitation of eps 15 gold immunolabeling associated with the plasma membrane and endosomal membranes in NIH-EGFR cells after EGF binding and internalization
| Plasma membrane (golds/μm ± SEM) | Early endosomes (golds/μm ± SEM) | Late endosomes (golds/μm ± SEM) | |
|---|---|---|---|
| No EGF | 0.7 ± 0.1 | 0.2 ± 0.1 | 0 |
| EGF 1 h 4°C | 1.5 ± 0.5 | 0.2 ± 0.4 | 0.1 ± 0.5 |
| EGF 1 h 4°C + 10 min 37°C | 0.3 ± 0.1 | 2.1 ± 0.3 | 0.5 ± 0.2 |
| EGF 1 h 4°C + 30 min 37°C | 0.4 ± 0.1 | 0.7 ± 1.7 | 2.3 ± 0.2 |
Immunolabeling density was determined by counting the number of gold particles per micrometer length of plasma membrane and of early or late endosome membranes. For each time point, 15 images of plasma membrane profiles and of single organelle membranes were analyzed.
Figure 6.
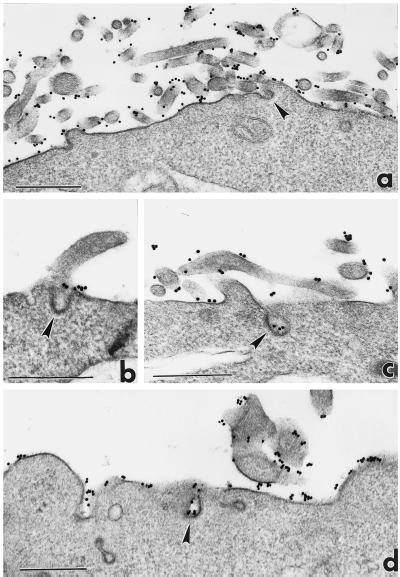
Immunoelectron microscopic analysis of eps15 redistribution during EGFR endocytosis. NIH-EGFR cells were treated with EGF for 1 h at 4°C and warmed to 37°C for 10 min (a) or 30 min (b and c). After 10 min of warming, gold immunolabeling appears associated with endosomal membranes (a, arrows) and vesicles mostly localized in proximity to Golgi cisternae (a). Colocalization of eps15 (large golds) and EGFR (small golds) is observed on clathrin-coated pits at the cell surface (Figure 5a, left inset; eps15: arrow, EGFR: arrowhead), whereas clathrin-coated vesicles juxtaposed to the plasma membrane appear positively labeled only for EGFR (Figure 5a, right inset, arrowhead). At 30 min of warming, gold particles are present in the central perinuclear region of the cell (b and c), close to Golgi complexes (c), and around late endosomes (b and c) positive for cathepsin D (small golds in c). In b, the arrow points to a vesicle positively labeled for eps15 in the process of fusion with a late endosome. Nu, Nucleus; LE, late endosome; cv, clathrin-coated vesicle. Bars, 0.2 μm; insets in a, 0.1 μm.
Biochemical Events Involved in Eps15 Redistribution and Colocalization with EGFR during Endocytosis
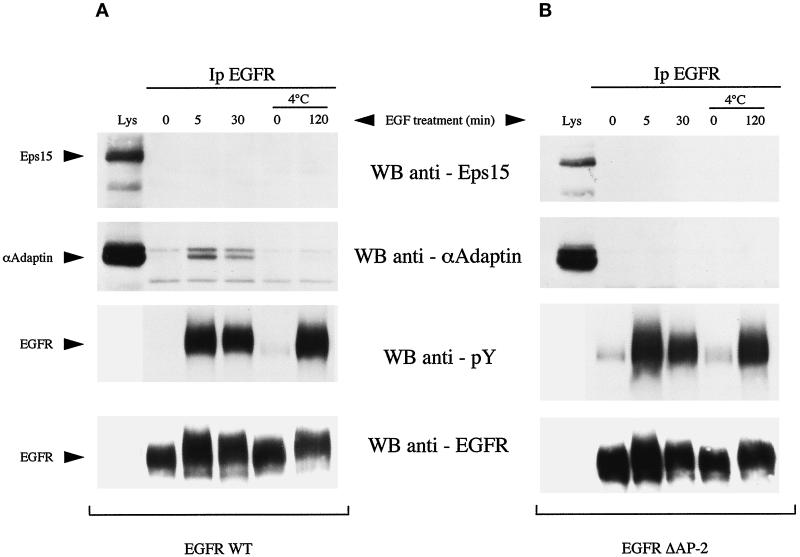
A simple mechanism to account for recruitment of eps15 to the plasma membrane upon EGFR activation is binding to the activated EGFR. There is discordance in the literature as to whether eps15 can associate, directly or indirectly, with the EGFR in vivo (Fazioli et al., 1993; Van Delft et al., 1997b). In B82-EGFR (Figure 7A) or in NIH-EGFR cells (our unpublished results), we were consistently unable to evidence coimmunoprecipitation of the EGFR with eps15. This was true also in the case in which cells were treated with 100 ng/ml EGF for 2 h at 4°C (Figure 7A), a condition that maximizes association of eps15 with the plasma membrane (Figure 3b1). Of note, under the same conditions of coimmunoprecipitation, EGF-dependent association of the α subunit of the AP-2 clathrin adaptor complex protein with EGFR was readily detectable (Figure 7A). EGFR/AP-2 association was evident when stimulation with EGF was performed at 37°C, but not at 4°C, as reported previously (Sorkin and Carpenter, 1993), because this latter condition prevents recruitment of activated EGFR into coated pits, where interaction with AP-2 takes place. In addition, under the same conditions of coimmunoprecipitation, we could readily detect association of eps15 with several binding partners, such as AP-2, NUMB, and RAB/Rip (Iannolo et al., 1997; Salcini et al., 1997; Coda et al., 1998). Thus we favor the hypothesis that recruitment of eps15 to the plasma membrane is not caused by a stable detergent-resistant association between eps15 and the EGFR.
Figure 7.
Association of AP-2 or eps15 with EGFR WT or a mutant deficient in AP-2 binding (EGFRΔAP-2). B82 cells expressing wild-type EGFR (A) or the AP-2–binding mutant EGFR (B) were serum-starved for 24 hours (SF) and then treated with EGF (100 ng/ml) for the indicated times. In some case, as indicated, EGF treatment was performed at 4°C after 24 h of serum starvation at 37°C, followed by 2 h of serum starvation at 4°C. Total cellular lysates were then immunoprecipitated with the anti-EGFR antibody E7 and subjected to immunoblots with (from top to bottom): anti-eps15 (5 mg of lysates), anti–α-adaptin (5 mg of lysates), anti-pTyr (1 mg of lysate), anti-EGFR (1 mg of lysate). The lanes labeled Lys were loaded with 50 μg of total lysates to serve as a reference. The positions of eps15, α-adaptin, and EGFR are indicated by arrows.
A hypothetical association between EGFR and eps15, however, could be indirect and mediated by adaptor molecule(s). Under this scenario it might also be more difficult to detect significant coimmunoprecipitation between EGFR and eps15. The best candidate for such an adaptor role is AP-2. Eps15 is constitutively associated with AP-2 (Benmerah et al., 1995, 1996; Iannolo et al., 1997; Van Delft et al., 1997b). AP-2 in turn binds to EGFR upon EGF-induced activation of the receptor (Sorkin and Carpenter, 1993). To investigate the role of the constitutive interaction of eps15 with AP-2 in determining redistribution of eps15 and colocalization of eps15 with EGFR during receptor internalization, we used B82 cells expressing a mutant form of EGFR deficient in AP-2 binding (Nesterov et al., 1995b). This mutant (EGFR958f993–1186, henceforth referred to as EGFRΔAP-2) bears an interstitial deletion between amino acids 959 and 992 that removes the binding site for AP-2 and does not coimmunoprecipitate with AP-2 (Nesterov et al., 1995b) (Figure 6B).
Double immunofluorescence and confocal analysis were performed to evaluate eps15 redistribution and colocalization with the EGFRΔAP-2 mutant. A remarkably similar behavior of eps15 redistribution and EGFR internalization, as well as extent of colocalization, was observed in the cells expressing WT (Figure 3) or mutant (Figure 8) receptors. In the absence of EGF treatment, the signal corresponding to eps15 in B82-EGFRΔAP-2 cells (Figure 8a1) revealed the same intracellular localization described for untreated B82-EGFR cells (Figure 3a1), and the EGFRΔAP-2 appeared localized to the plasma membranes as expected (Figure 8a2). Treatment with EGF for 1 h at 4°C induced recruitment of eps15 to the plasma membranes and increased colocalization of eps15 and EGFRΔAP-2 at the cell surfaces (Figure 8b1–3). Warming to 37°C for 10 min (Figure 8c) or for 30 min (Figure 8d) resulted in eps15 redistribution and EGFRΔAP-2 endocytosis, as described above for B82-EGFR cells (Figure 3). At both time points of warming, high levels of colocalization of the two signals were evident in correspondence of the intracellular sites of trafficking of EGFR (Figure 8, c3 and d3). Therefore, eps15 colocalization with EGFR is not dependent on AP-2 binding to the receptors.
Figure 8.
Confocal microscopy of B82 cells expressing a mutant EGFR deficient in AP-2 binding (EGFRΔAP-2). Legend as in Figure 3.
To ascertain whether the observed EGFR internalization process was occurring through clathrin-coated pits, also in the absence of AP-2 interaction with the receptors, we performed immunoelectron microscopy on B82 cells expressing EGFR WT or mutant EGFRΔAP-2. Cells were treated with EGF and anti-EGFR mAb at 4°C as above and then warmed to 37°C for 5 min to allow receptor clustering into coated pits at the plasma membrane. In cells expressing both types of receptors, immunogold labeling of EGFR appeared localized in clathrin-coated pits upon warming to 37°C (Figure 9 and Table 2). This observation implies that EGFR may enter into clathrin-coated pits independently on AP-2 binding and suggest that eps15, which colocalizes with EGFR also independently on AP-2, may play a role in this first step of internalization. Interestingly the EGFRΔAP-2 is competent for eps15 tyrosine phosphorylation, with a kinetics virtually indistinguishable from wild-type EGFR (Figure 10A), indicating that a hypothetical recruitment of eps15 to the EGFR through AP-2 is not necessary for eps15 to serve efficiently as a kinase substrate.
Figure 9.
Surface immunogold labeling with anti-EGFR mAb of B82 cells expressing EGFR WT (a–c) or EGFRΔAP-2 mutant (d). After incubation with EGF for 1 h at 4°C (a), EGFRs are distributed over the cell plasma membranes, preferentially associated with the microvilli, and excluded by clathrin-coated pits (a, arrowhead). After warming to 37°C for 5 min, either WT EGFRs (b and c) or mutant EGFRs (d) appear clustered over the nonvillous portions of the plasma membrane (b) and inside clathrin-coated pits (c and d, arrowheads). Bars, 0.5 μm.
Table 2.
Quantitative distribution of EGFR immunogold labeling on the surface of B82 cells expressing EGFR and EGFRΔAP-2 after EGF binding and internalization
| Total μm length of plasma membrane analyzed | Total number of gold particles counted | Gold particles/μm ± SEM | Gold particles/μm over villous surface ± SEM | Gold particles/μm over nonvillous surface ± SEM | Gold particles/μm in clathrin-coated pits | |
|---|---|---|---|---|---|---|
| EGFR (EGF 1 h 4°C) | 183.9 | 1425 | 7.9 ± 0.8 | 11.8 ± 0.5 | 5.5 ± 1.0 | 0.3 ± 0.3 |
| EGFRΔAP-2 (EGF 1 h 4°C) | 138.1 | 1283 | 9.9 ± 1.2 | 13.8 ± 1.4 | 6.4 ± 1.0 | 0.7 ± 0.4 |
| EGFR (EGF 1 h 4°C + 5 min 37°C) | 223.1 | 846 | 3.8 ± 0.3 | 3.1 ± 0.4 | 4.3 ± 0.3 | 2.7 ± 0.8 |
| EGFRΔAP-2 (EGF 1 h 4°C + 5 min 37°C) | 182.5 | 789 | 4.2 ± 0.5 | 2.9 ± 0.4 | 4.7 ± 0.7 | 3.4 ± 0.7 |
For each time point of EGF treatment, 10 plasma membrane profiles and 20 clathrin-coated pits, randomly photographed from two different experiments, were analyzed.
Figure 10.
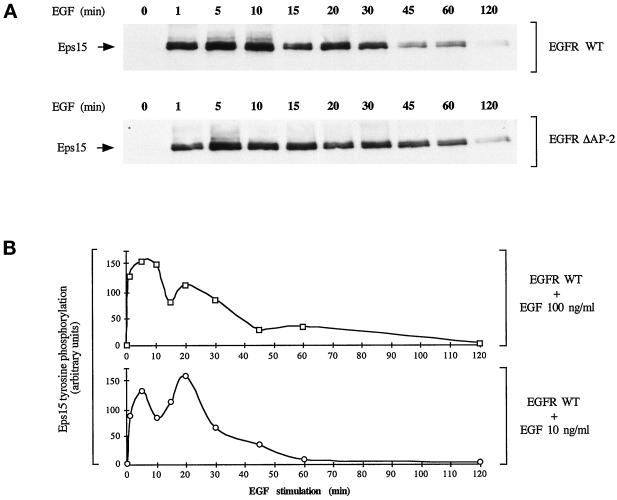
Tyrosine phosphorylation of eps15 by EGFR WT or by EGFRΔAP-2. (A) B82 cells expressing wild-type EGFR (top) or the AP-2–binding mutant EGFR (bottom) were serum-starved for 24 h and then treated with EGF (100 ng/ml) for the indicated times. Total cellular lysates (2 mg) were then immunoprecipitated with the anti-eps15 antibody and subjected to immunoblots with anti-pTyr. The position of eps15 is indicated by arrows. (B) Time kinetics of eps15 tyrosine phosphorylation in B82-EGFR cells treated with various concentrations of EGF. pTyr-containing eps15 was assessed by immunoprecipitation/blotting experiments, as in A. Densitometric scans of the blot were obtained, and results are reported after correction for the amount of eps15 immunoprecipitated revealed by stripping of the blots and decoration with an anti-eps15 antibody.
Tyrosine Phosphorylation of Eps15 during Receptor Internalization
The observation that eps15 colocalizes with the EGFR at various steps in the internalization route raises the question of whether eps15 can be phosphorylated by the receptor at locations other than the plasma membrane. This possibility is indirectly supported by the fact that the time-dependent kinetics of eps15 tyrosine phosphorylation by EGFR (Figure 10B) shows frequently a biphasic behavior with two peaks of phosphorylation, one around 5 min and a second between 20 and 30 min of EGF addition. This behavior is even more pronounced if EGF is added at nonreceptor saturating doses (10 ng/ml equal to 1.7 nM) (Figure 10B). Thus the apparent biphasic time kinetics could be the result of prolonged phosphorylation occurring in some endosomal compartment.
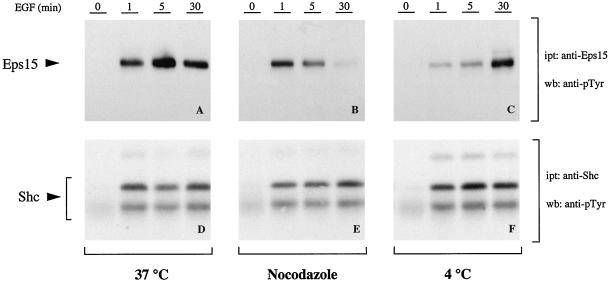
To gain insight into this issue we analyzed the phosphotyrosine (pTyr) content of eps15 in cells stimulated with EGF in the presence or absence of nocodazole. In parallel, under the same conditions, we analyzed the pTyr content of shc, an EGFR substrate whose phosphorylation is likely to occur exclusively at the plasma membrane, because its localization is not altered under conditions designed to inhibit EGFR endocytosis (Lotti et al., 1996). As shown in Figure 11, a and b, nocodazole treatment did not affect the initial phases of eps15 phosphorylation (1 min) but severely decreased the pTyr content at 5 and 30 min. As expected, shc tyrosine phosphorylation was unaffected by nocodazole treatment at all time points (Figures 11, d and e). A simple model to explain these results is that eps15 is subjected to a first wave of phosphorylation by the EGFR concomitantly to its recruitment to the plasma membrane, a step that should be nocodazole-insensitive. Subsequently, eps15 might be subject to further phosphorylation by the receptors, even in the late phases of the endocytic process, which are nocodazole-sensitive.
Figure 11.
Tyrosine phosphorylation of eps15 and shc under various conditions of EGF treatment. B82-EGFR cells were serum-starved for 24 hours (0) and then treated with EGF (100 ng/ml) for the indicated times. In A and D, cells were treated at 37°C after serum starvation at 37°C; in B and E, cells were treated at 37°C after serum starvation at 37°C and treatment in the presence of nocodazole (20 μg/ml for 1 h; nocodazole was kept also during EGF stimulation). In C and F, cells were serum-starved at 37°C and for an additional 2 h at 4°C, followed by treatment with EGF at 4°C. Total cellular lysates (2 mg) were immunoprecipitated with either an anti-eps15 (A–C) or an anti-shc (D–F) antibody and then immunoblotted with anti-pTyr. The positions of eps15 and shc are indicated by arrows.
Interestingly, when EGF treatment was performed at 4°C, eps15 was still tyrosine-phosphorylated (Figure 11C), albeit with a delayed kinetics, with respect to shc (Figure 11D). A quantitative assessment of these latter results is impossible, because too many variables are unaccounted for, including kinase efficiency of the EGFR and phosphatase(s) activity in vivo at 4°C versus 37°C; however, it appears as if phosphorylation of eps15 by the EGFR at the plasma membrane does not require recruitment of the receptor into coated pits, this phenomenon being inhibited at 4°C.
DISCUSSION
The endocytic pathway followed by RTKs, and in particular by EGFR, has been widely studied (reviewed in Sorkin and Waters, 1993). Upon ligand binding, EGFR enters clathrin-coated pits at the cell surfaces, is internalized by clathrin-coated vesicles, and reaches early endosomes where sorting occurs to the plasma membrane for receptor recycling or to late endosomes and lysosomes for down-regulation. Several lines of evidence (reviewed in Di Fiore et al., 1997) suggest that eps15 plays a role in the control of endocytosis, a possibility that is antagonized, however, by the observation that eps15 distribution does not change appreciably upon ligand-induced endocytosis of the EGFR (Tebar et al., 1996; Van Delft et al., 1997b). We were conversely able to show that the eps15 undergoes dramatic relocalization during EGFR endocytosis, by first being recruited to the plasma membrane and then colocalizing with the EGFR in early and late endosomes.
In our opinion, the initial relocalization of eps15 to the plasma membrane deserves particular attention. Two major models for RTK-mediated internalization have been proposed. In the first, ligand-bound receptors enter coated pits simply by virtue of their random lateral mobility in the plasma membrane. Once they reach the pit, they are retained by specific components of the pit machinery. The best candidate for such a role is the clathrin adaptor complex protein AP-2. In the EGFR system, AP-2 binds in a ligand-dependent manner (Sorkin and Carpenter, 1993) to a tyrosine-based signal, which is part of one of the three endocytic codes present in the C-terminal portion of the EGFR. Binding of AP-2 to the EGFR requires activation of the receptor kinase activity, autophosphorylation, and conformational changes that probably unmask the binding site; however, endocytosis of EGFR is a second-order saturable process, likely involving competition among EGFRs for a downstream component of the endocytic machinery (Lund et al., 1990). Quantitative considerations (reviewed in Nesterov et al., 1995b) militate against AP-2 being such a component. In addition, AP-2-binding mutants of the EGFR display unperturbed internalization kinetics (Nesterov et al., 1995b), at least at low levels of receptor expression (Sorkin et al., 1996). In addition, evidence has been provided that a tyrosine kinase substrate, different from EGFR itself, is needed for efficient recruitment into coated pits of ligand-activated EGFR (Lamaze and Schmid, 1995).
In a second model, a membrane-bound docking apparatus (or several receptor-specific apparatuses) might exist that facilitates targeting of receptors to coated pits. The docking molecule (or a component of the docking apparatus) might coincide with the aforementioned tyrosine kinase substrate, other than the EGFR. Eps15 possesses several of the characteristics that fit this role: 1) it is an EGFR substrate (Fazioli et al., 1993); 2) it is an essential component of the endocytic pathway (Carbone et al., 1997); 3) it localizes to the plasma membrane and colocalizes with the EGFR after ligand stimulation (this study); and 4) it is constitutively bound to AP-2 (Benmerah et al., 1995). One might thus postulate that constitutive nonplasma membrane-bound eps15/AP-2 complexes are recruited to the plasma membrane by EGFR-induced posttranslational modifications of eps15. Tyrosine phosphorylation is the most obvious candidate for such a modification, albeit other modifications, such as the recently shown monoubiquitination of eps15 (Van Delft et al., 1997a), cannot be excluded at this stage. The relocalization of AP-2 to the plasma membrane can then trigger assembly of the clathrin lattice and receptor retention into the pit through various mechanisms (reviewed in Kirchhausen et al., 1997). At this stage eps15 might dissociate from AP-2, as indicated by recent findings of Cupers et al. (1998).
The above model would predict that plasma membrane relocalization and tyrosine phosphorylation of eps15 are independent of binding of AP-2 to the receptor: a prediction that was experimentally validated in this study. In addition, this model would easily accommodate experimental findings such as the preferential localization of eps15 at the rims of the coated pits (Tebar et al., 1996). As originally pointed out by Tebar et al. (1996), the localization of eps15 at the rims of coated pits is surprising, because AP-2, the binding partner, is localized throughout the coat profile. This observation led Kirchhausen et al. (1997) to postulate that eps15 might undergo cycles of binding to and release from AP-2 during coat assembly. In our proposed model, the location of eps15 at the rim, which is likely the growing part of a forming pit, would be self-explanatory, if eps15 were to function as a docking/recruiting molecule.
Recruitment of eps15 to the plasma membrane does not require a stable detergent-resistant physical interaction (direct or indirect) between eps15 and the EGFR (this study). In addition, a stoichiometrically significant interaction between eps15 and EGFR could not be immediately reconciled with the absence of eps15 from the deeper parts of coated pits (Tebar et al., 1996) where EGFR and AP-2 are abundant. Indeed, there is discordance in the literature as to whether eps15 is complexed to the EGFR in vivo (Fazioli et al., 1993; Van Delft et al., 1997b; this study). Part of the differences can be cell-specific because in a survey of several cell lines expressing the EGFR we identified some cell lines in which eps15 and EGFR can indeed be coimmunoprecipitated (our unpublished results), albeit with rather low stoichiometry: <1% of the eps15 pool. The lack of universality of the phenomenon and its quantitative limitedness appear to militate against its relevance; however, one can envision a transitory, unstable interaction between eps15 and EGFR at the recruiting edge of the pit, which might account for all of the above observations.
An interesting finding is that in addition to the expected association with coated pits at the cell surface, eps15 is localized on intracellular vesicles not only peripheral, but also perinuclear. EGFR activation and endocytosis determines first a recruitment of eps15 at the cell surface and later a redistribution toward the central area of the cell. This phenomenon, although not involving the total eps15 pool, clearly reflects the behavior of a large amount of eps15 molecules. In addition, eps15 colocalizes with the EGFR during endocytosis. Thus, our findings pose the question as to whether eps15 is “trafficked” with the EGFR from the plasma membrane to the endosomes or whether it is “targeted” to endosomal membranes. We note that although we were able to readily detect association of eps15 with coated pits and early and late endosomes, we could not detect its presence in clathrin-coated vesicles. This result is consistent with those reported by Cupers et al. (1998), who showed that purified coated vesicles contain negligible amounts of eps15 and that eps15 is lost during coat assembly in vitro. Thus, we favor the possibility that multiple cycles of association:dissociation of eps15 with membranes occur as the EGFR moves forward in the endocytic pathway. The molecular determinants involved in the “retargeting” of eps15 to endosomes are unknown; however, the kinetics of eps15 tyrosine phosphorylation correlates with its reassociation to endosomes. A late peak of eps15 tyrosine phosphorylation occurs, in fact, in a nocodazole-sensitive compartment. Thus, in analogy with what we propose for eps15 recruitment to the plasma membrane, the EGFR might be able to phosphorylate eps15 in the endosomal compartment, thereby determining its association with endosomal membranes.
Also somewhat surprising is our finding that eps15 colocalizes with α-adaptin not only at the plasma membrane, in cells treated with EGF at 4°C, but also in perinuclear structures after warming at 37°C. We do not know whether these structures also contain internalized EGFR, thus representing bona fide early or late endosomes. On the basis of current knowledge, this possibility appears unlikely. These structures, however, might represent intermediate carrier vesicles, other than endosomes, involved in the receptor transport and/or sorting along the endocytic pathway.
The retargeting of eps15 to endosomes, after its dissociation from coated vesicles, strongly suggests that eps15 plays a role in the late steps of endocytosis, different from that exerted at the plasma membrane level in the formation of coated pits. Indeed some molecular evidence already exists that these two putative functions of eps15 can be dissociated. We have shown previously that microinjection of anti-eps15 antibodies efficiently inhibits internalization of EGFR (Carbone et al., 1997). In addition, overexpression of a dominant negative mutant comprising only the AP-2 binding region of eps15 (L2 region) inhibited internalization of EGFR and establishment of successful infection by Sindbis virus, which is known to require coated pits-mediated internalization of the virus (reviewed in Marsh and Helenius, 1989). Surprisingly, an eps15 mutant encompassing only its three EH domains had no effect on EGFR internalization but completely inhibited Sindbis infection (Carbone et al., 1997; our unpublished observations). One possible explanation for this discrepancy is that the inhibition by EH domains is exerted at a step in the endocytic pathway other than internalization, as for example in the transport from early endosomes to the more acidic late endosomes, a step required for viral fusion and infection (Marsh and Helenius, 1989). If this were true, it would direct the attention regarding a possible role of eps15 in late endocytosis to the protein:protein interaction abilities of the EH domain, an intriguing possibility that warrants further investigation.
ACKNOWLEDGMENTS
We are very grateful to Dr. Gordon N. Gill for providing B82 cells expressing EGFR WT and mutants. We thank Dr. Maria Gianmatteo for help in the confocal analysis, Mr. Ilio Piras for excellent photographic work, and Mr. Giuseppe Lucania and Ms. Lucia Cutini for excellent technical assistance. This work was partially supported by grants from Ministero Università Ricerca Scientifica Tecnologica, from Associazione Italiana per la Ricerca sul Cancro (AIRC), from Consiglio Nazionale delle Ricerche (Target Project on “Biotechnology”), from the European Community (BIOMED-2 Program), from the Armenise-Harvard Foundation, and from the Fondazione Ferrero.
REFERENCES
- Benedetti H, Raths S, Crausaz F, Riezman H. The END3 gene encodes a protein that is required for the internalization step of endocytosis and for actin cytoskeleton organization in yeast. Mol Biol Cell. 1994;5:1023–1037. doi: 10.1091/mbc.5.9.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmerah A, Bègue B, Dautry-Varsat A, Cerf-Bensussan N. The ear of α-adaptin interacts with the COOH-terminal domain of the eps15 protein. J Biol Chem. 1996;271:12111–12116. doi: 10.1074/jbc.271.20.12111. [DOI] [PubMed] [Google Scholar]
- Benmerah A, Gagnon J, Mégarbané B, Dautry-Varsat A, Cerf-Bensussan N. The tyrosine kinase substrate eps15 is constitutively associated with the plasma membrane adaptor AP-2. J Cell Biol. 1995;131:1831–1838. doi: 10.1083/jcb.131.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmerah A, Lamaze C, Begue B, Schmid SL, Dautry-Varsat A, Cerf-Bensussan N. AP-2/eps15 interaction is required for receptor-mediated endocytosis. J Cell Biol. 1998;140:1055–1062. doi: 10.1083/jcb.140.5.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone R, Fré S, Iannolo G, Belleudi F, Mancini P, Pelicci PG, Torrisi MR, Di Fiore PP. Eps15 and eps15R are essential components of the endocytic pathway. Cancer Res. 1997;57:5498–5504. [PubMed] [Google Scholar]
- Chang CP, et al. Ligand-induced internalization of the epidermal growth factor is mediated by multiple endocytic codes analogous to the tyrosine motif found in constitutively internalized receptors. J Biol Chem. 1993;268:19312–19320. [PubMed] [Google Scholar]
- Chen WS, et al. Functional independence of the epidermal growth factor receptor from a domain required for ligand-induced internalization and calcium regulation. Cell. 1989;59:33–43. doi: 10.1016/0092-8674(89)90867-2. [DOI] [PubMed] [Google Scholar]
- Coda L, Salcini AE, Confalonieri S, Pelicci G, Sorkina T, Sorkin A, Pelicci PG, Di Fiore PP. Eps15R is a tyrosine kinase substrate with characteristics of a docking protein possibly involved in coated pits-mediated internalization. J Biol Chem. 1998;273:3003–3012. doi: 10.1074/jbc.273.5.3003. [DOI] [PubMed] [Google Scholar]
- Cupers P, Jadhav AP, Kirchhausen T. Assembly of clathrin coats disrupts the association between eps15 and AP-2. J Biol Chem. 1998;272:1847–1850. doi: 10.1074/jbc.273.4.1847. [DOI] [PubMed] [Google Scholar]
- Dickson RB, Hanover JA, Willingham MC, Pastan I. Prelysosomal divergence of transferrin and epidermal growth factor during receptor-mediated endocytosis. Biochemistry. 1983;22:5667–5674. doi: 10.1021/bi00293a033. [DOI] [PubMed] [Google Scholar]
- Di Fiore PP, Pelicci PG, Sorkin A. EH: a novel protein-protein interaction domain potentially involved in intracellular sorting. Trends Biochem Sci. 1997;22:411–413. doi: 10.1016/s0968-0004(97)01127-4. [DOI] [PubMed] [Google Scholar]
- Di Fiore PP, Pierce JH, Fleming TP, Hazan R, Ullrich A, King CR, Schlessinger J, Aaronson SA. Overexpression of the human EGF receptor confers an EGF-dependent transformed phenotype to NIH 3T3 cells. Cell. 1987;51:1063–1070. doi: 10.1016/0092-8674(87)90592-7. [DOI] [PubMed] [Google Scholar]
- Di Fiore PP, Segatto O, Lonardo F, Fazioli F, Pierce JH, Aaronson SA. The carboxy-terminal domains of erbB-2 and epidermal growth factor exert different regulatory effects on intrinsic receptor tyrosine function and transforming activity. Mol Cell Biol. 1990;10:2749–2756. doi: 10.1128/mcb.10.6.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazioli F, Minichiello L, Matoskova B, Wong WT, Di Fiore PP. Eps15, a novel tyrosine kinase substrate, exhibits transforming activity. Mol Cell Biol. 1993;13:5814–5828. doi: 10.1128/mcb.13.9.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannolo G, Salcini AE, Gaidarov I, Goodman OB, Jr, Baulida J, Carpenter G, Pelicci PG, Di Fiore PP, Keen JH. Mapping of the molecular determinants involved in the interaction between eps15 and AP-2. Cancer Res. 1997;57:240–245. [PubMed] [Google Scholar]
- Glickman JN, Conibera E, Pearse BM. Specificity of binding of clathrin adaptors to sorting signals on the mannose-6-phosphate/insulin-like growth factor II receptor. EMBO J. 1989;8:1041–1047. doi: 10.1002/j.1460-2075.1989.tb03471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J, Maxfield FR. Membrane transport in the endocytic pathway. Curr Opin Cell Biol. 1995;7:552–563. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- Hanover JA, Beguinot L, Willingham MC, Pastan IH. Transit of receptor for epidermal growth factor and transferrin through clathrin-coated pits. Analysis of the kinetics of receptor entry. J Biol Chem. 1985;260:15938–15945. [PubMed] [Google Scholar]
- Kirchhausen T. Coated pits and vesicles—sorting it all out. Curr Opin Struct Biol. 1993;3:182–188. [Google Scholar]
- Kirchhausen T, Bonifacino JS, Reizman H. Linking cargo to vesicle formation: receptor tail interactions with coat proteins. Curr Opin Cell Biol. 1997;9:488–495. doi: 10.1016/s0955-0674(97)80024-5. [DOI] [PubMed] [Google Scholar]
- Lamaze C, Schmid S. Recruitment of epidermal growth factor receptors into coated pits requires their activated tyrosine kinase. J Cell Biol. 1995;129:47–54. doi: 10.1083/jcb.129.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotti LV, Lanfrancone L, Migliaccio E, Zompetta C, Pelicci G, Salcini AE, Falini B, Pelicci PG, Torrisi MR. Shc proteins are localized on endoplasmic reticulum membranes and are redistributed after tyrosine kinase receptor activation. Mol Cell Biol. 1996;16:1946–1954. doi: 10.1128/mcb.16.5.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund KA, Opresko LK, Starbuck C, Walsh BJ, Wiley HS. Quantitative analysis of the endocytic system involved in hormone-induced receptor internalization. J Biol Chem. 1990;265:15713–15723. [PubMed] [Google Scholar]
- Marks MS, Ohno H, Kirchhausen T, Bonifacino JS. Protein sorting by tyrosine-based signals: adapting to the Ys and wherefores. Trends Cell Biol. 1997;7:124–128. doi: 10.1016/S0962-8924(96)10057-X. [DOI] [PubMed] [Google Scholar]
- Marsh M, Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107–151. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- Nesterov A, Kurten RC, Gill GN. Association of epidermal growth factor receptors with coated pit adaptins via a tyrosine phosphorylation-regulated mechanism. J Biol Chem. 1995a;270:6320–6327. doi: 10.1074/jbc.270.11.6320. [DOI] [PubMed] [Google Scholar]
- Nesterov A, Wiley HS, Gill GN. Ligand-induced endocytosis of epidermal growth factor receptors that are defective in binding adaptor proteins. Proc Natl Acad Sci USA. 1995b;92:8719–8723. doi: 10.1073/pnas.92.19.8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinsons MS. Adaptins. Trends Cell Biol. 1992;2:293–297. doi: 10.1016/0962-8924(92)90118-7. [DOI] [PubMed] [Google Scholar]
- Robinsons MS. The role of clathrin, adaptors and dynamin in endocytosis. Curr Opin Cell Biol. 1994;6:538–544. doi: 10.1016/0955-0674(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Robinsons MS, Page LJ. Identification of novel proteins that interact with γ-adaptin. Mol Biol Cell. 1996;7:168a. (abstract 977). [Google Scholar]
- Salcini AE, Confalonieri S, Doria M, Santolini E, Tassi E, Minenkova O, Cesareni G, Pelicci PG, Di Fiore PP. Binding specificity and in vivo targets of the EH domain, a novel protein-protein interaction module. Genes Dev. 1997;11:2239–2249. doi: 10.1101/gad.11.17.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher C, Knudsen BS, Ohuci T, Di Fiore PP, Glasman RH, Hanafusa H. The SH3 domain of Crk binds specifically to a conserved proline-rich motif in Eps15 and Eps15R. J Biol Chem. 1995;270:15341–15347. doi: 10.1074/jbc.270.25.15341. [DOI] [PubMed] [Google Scholar]
- Slot JW, Geuze HJ. Sizing of protein A-colloidal gold probes for immunoelectron microscopy. J Cell Biol. 1981;90:553–536. doi: 10.1083/jcb.90.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A, Carpenter G. Interaction of activated EGF receptors with coated pit adaptins. Science. 1993;261:612–615. doi: 10.1126/science.8342026. [DOI] [PubMed] [Google Scholar]
- Sorkin A, Waters CM. Endocytosis of growth factor receptors. BioEssays. 1993;15:375–382. doi: 10.1002/bies.950150603. [DOI] [PubMed] [Google Scholar]
- Sorkin A, Mazzotti M, Sorkina T, Scotto L, Beguinot L. Epidermal growth factor receptor interaction with clathrin adaptors is mediated by the Tyr974-containing internalization motif. J Biol Chem. 1996;271:13377–13384. doi: 10.1074/jbc.271.23.13377. [DOI] [PubMed] [Google Scholar]
- Tan PK, Howard JP, Payne GS. The sequence NPFXD defines a new class of endocytosis signal in Saccharomyces cerevisiae. J Cell Biol. 1996;135:1789–1800. doi: 10.1083/jcb.135.6.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang HY, Munn A, Cai M. EH domain proteins Pan1p and End3p are components of a complex that plays a dual role in organization of the cortical actin cytoskeleton and endocytosis in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17:4294–4304. doi: 10.1128/mcb.17.8.4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebar F, Sorkina T, Sorkin A, Ericsson M, Kirchhausen T. Eps15 is a component of clathrin-coated pits and vesicles and is located at the rim of coated pits. J Biol Chem. 1996;271:28727–28730. doi: 10.1074/jbc.271.46.28727. [DOI] [PubMed] [Google Scholar]
- Van Delft S, Govers R, Stous GJ, Verkleij AJ, Bergen en Henegouwen PMP. Epidermal growth factor induces ubiquitination of eps15. J Biol Chem. 1997a;272:14013–14016. doi: 10.1074/jbc.272.22.14013. [DOI] [PubMed] [Google Scholar]
- Van Delft S, Schumacher C, Hage W, Verkleij AJ, Bergen en Henegouwen PMP. Association and colocalization of eps15 with adaptor protein-2 and clathrin. J Cell Biol. 1997b;136:811–821. doi: 10.1083/jcb.136.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland B, Emr SD. Pan1p, yeast eps15, functions as a multivalent adaptor that coordinates protein-protein interactions essential for endocytosis. J Cell Biol. 1998;141:71–84. doi: 10.1083/jcb.141.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland B, Mc Caffery JM, Xiao Q, Emr SD. A novel fluorescence-activated cell sorter-based screen for yeast endocytosis mutants identifies a yeast homologue of mammalian eps15. J Cell Biol. 1996;135:1485–1500. doi: 10.1083/jcb.135.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley HS. Anomalous binding of epidermal growth factor to A431 is due to the effect of high receptor densities and saturable endocytic system. J Cell Biol. 1988;107:801–810. doi: 10.1083/jcb.107.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley HS, Herbst JJ, Walsh BJ, Lauffenburger DA, Rosenfeld MG, Gill GN. The role of tyrosine kinase activity in endocytosis, compartmentation, and down-regulation of the epidermal growth factor receptor. J Biol Chem. 1991;266:11083–11094. [PubMed] [Google Scholar]
- Wong WT, Kraus MH, Carlomagno F, Zelano A, Druck T, Croce CM, Huebner K, Di Fiore PP. The human eps15 gene, encoding a tyrosine kinase substrate, is conserved in evolution and maps to 1p31–p32. Oncogene. 1994;9:1591–1597. [PubMed] [Google Scholar]
- Wong WT, Schumacher C, Salcini AE, Romano A, Castagnino P, Pelicci PG, Di Fiore PP. A protein-binding domain, EH, identified in the receptor tyrosine kinase substrate eps15 and conserved in evolution. Proc Natl Acad Sci USA. 1995;92:9530–9534. doi: 10.1073/pnas.92.21.9530. [DOI] [PMC free article] [PubMed] [Google Scholar]