Abstract
The conditions known as ‘hereditary stomatocytosis and allied syndromes’ comprise a group of dominantly inherited human haemolytic anaemias characterized by a plasma membrane ‘leak’ to the univalent cations Na and K, an example of a small but growing group of diseases where pathology can be directly attributed to abnormal membrane transport. A number of case reports in the different variants have alluded to temperature-related phenomena, including loss of K on storage at room temperature (giving ‘pseudohyperkalaemia’) and lysis of cells when stored in the cold (‘cryohydrocytosis’). This review collects together published studies of these temperature effects, which show very major differences in the ‘leak’ K transport. Two main variations on normal emerge: a ‘shallow slope’ type, in which the flux shows an abnormally low dependence on temperature in the range 37–20°C, and ‘high minimum’, in which the minimum in this flux, which occurs in normal cells at 8°C, is shifted up to 23°C. These temperature studies provide a powerful method for phenotypic characterization.
Keywords: stomatocytosis, K transport, membrane transport, ion leak, temperature
The generic term ‘hereditary stomatocytosis and allied syndromes’ was coined by Dacie (1985) to encompass a class of dominantly inherited human red cell conditions in which some kind of plasma membrane ‘leak’ to the univalent cations sodium and potassium is central to the pathophysiology and diagnosis (Stewart 1993; Lux & Palek 1995). The name ‘stomatocyte’ was coined to describe the erythrocyte morphology of the first case of these diseases (Lock et al. 1961) (Figure 1). The ion leak was discovered in a similar case by American workers (Zarkowsky et al. 1968). Many subsequent families with haematological disease of widely varying severity have now been described, all with some kind of cation leak. Although stomatocytes are seen in some other congenital haemolytic anaemias in which no ion leak is present, we will not consider these here. In the UK, we are aware of 17 families that fall into 7 distinguishable groups. The aim of this review is to emphasize temperature effects, which have come to represent an important phenotypic discriminator among the cases that we see. It is first necessary briefly to consider the movements of Na and K across the normal red cell membrane.
Figure 1.
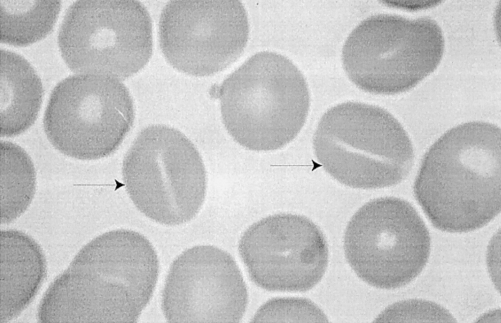
Blood film from patient with hereditary stomatocytosis (Family E), showing stomatocytes: red cells with slit-like central pallor (arrowed). A dried film was stained with May Grunwald Giemsa. Magnification, × 4000.
Na and K transport in normal human red cells
The steady state intracellular Na and K concentrations within the human red cell are governed by the balance between the energy consuming, ouabain-inhibited NaK pump and a so-called ‘passive leak’ (Ellory et al. 1998). For K at least, this ‘passive leak’ can be experimentally estimated as that isotopic flux which persists in the presence of the two inhibitors ouabain (for the NaK pump) and bumetanide (for the NaK2CL cotransport system). The molecular nature of this flux is not clear but in normal cells it is largely composed of a process of passive diffusion, showing linear, Fick-type, activation by external [K] (Stewart 1993). In the stomatocytic cells, it seems that this process is simply magnified.
This review largely concerns the temperature dependence of these fluxes. While all of the other transport systems show the expected monotonic dependence on temperature in the range 37–0°C, this passive leak process, even in normal cells, shows a paradoxical effect. As the temperature falls (Figure 2), the rate of the passive leak decreases more or less linearly down to 20°C on the logarithmic plot, with a Q1037–27 (i.e. the ratio of flux at 37°C to flux at 27°C) of about 2.2. At 20°C, the curve begins to flatten and there is a minimum at about 8°C, the explanation for which is unclear (Stewart et al. 1980). This minimum is found in monkey (Stewart et al. 1989) and dog (Elford 1975) red cells but not in other species, including hibernators (Hall & Willis 1986).
Figure 2.
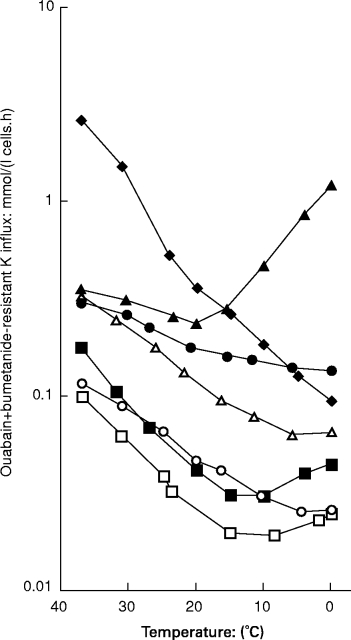
Comparison of ‘leak’ tracer fluxes across the red cell membrane as a function of temperature in different kindreds. The degree of haemolysis is roughly proportional to the ‘leak’ at 37°C. The normal temperature profile (□) shows a minimum at about 10°C. Families A (○) and B (•) show a ‘shallow slope’ variant such that the the slope in the interval 37–20°C is less steep than normal. While Family A shows negligible haemolysis, family B has frank haemolytic anaemia, proportionate to the abnormality in leak at 37°C. Family C (cryohydrocytosis, ▴) shows a ‘high minimum’ effect such that the minimum which occurs in normal cells at 10°C is shifted up to 23°C. Families D (dehydrated HSt, ▪) and F (Woking, ▵) shows a profile that is simply parallel to normal. Family E (overhydrated HSt, ♦) shows a monotonic profile with no minumum.Red cells were washed in 150 mm NaCl, 15 mm MOPS (pH 7.4 at 23°C), 5 mm glucose and suspended to about 5% haematocrit in this solution with 0.1 mm ouabain and bumetanide. K(86Rb) was added to a final concentration of 5 mm and the cells were incubated at the temperatures shown for 30–120 min, before being washed free of extracellular isotope prior to lysis, deproteinization and scintillation counting for trapped radioactivity (Coles et al. 1999b). Data is redrawn from the following sources: Family A (Stewart & Ellory 1985); B (Coles et al. 1999b); C (Coles et al. 1999a); D & E (Stewart 1993); F, unpublished.
Pathophysiological background
In the overtly haemolytic cases, the steady state intracellular levels of Na and K inside the cell are abnormal, and the rates of isotopic tracer fluxes of both the ‘leak’ and NaK pump fluxes (the balance between which governs the intracellular Na and K concentrations: see below) are increased. The data can simply be interpreted as evidence for a primary ‘leak’ with a compensatory increase in NaK pump activity which is only partly adequate. This excessive ‘leak’ compromises the osmotic integrity of the cell, which lyses prematurely. Note that although the intracellular [Na] and [K] inside the red cell can be grossly abnormal in these conditions, the plasma [Na] and [K] are always normal in fresh blood. The abnormal values reflect an abnormal intracellular steady state; the kidney and other homeostatic mechanisms maintain the plasma homeostasis.
The basic cause of these diseases remains unclear. We have recently mapped several families with two forms of the anaemia to a locus on chromosome 16 (Carella et al. 1998; Iolascon et al. 1999). Although other ‘leaky channel’ diseases have been shown to be directly due to mutations in the ion channels themselves (Caldwell & Schaller 1992), biochemical evidence in the HSts points to a fundamental defect in the organization of the lipid bilayer. According to the bilayer couple hypothesis of Sheetz & Singer (1974) ‘stomatocytosis’ itself almost certainly implies excessive packing of the inner leaflet of the bilayer itself. Although a large amount of circumstantial evidence in normal cells supports the substance of the bilayer couple hypothesis, it has not been possible to verify this experimentally in the stomatocytoses themselves. Secondly, one variant (dehydrated HSt, see below) shows frank abnormalities in lipid composition (Clark et al. 1993) with an abnormality in lipid metabolism (Shohet et al. 1971). Thirdly, in yet another form of the disease (overhydrated HSt, see below), a membrane protein, called ‘stomatin’, is missing from the membrane. Its sequence is known (Stewart et al. 1992), but no mutation is present in the gene in these patients (Wang et al. 1992). Nevertheless we presume that this protein is in some way relevant and evidence points to a role for this protein in lipid organization: it is related indirectly by sequence homology to a nematode protein with predicted lipid handling properties (Barnes et al. 1996); and it has been found to bind labelled phosphatidylethanolamine on labelling studies (Desneves et al. 1996).
Clinically, these patients show haemolytic anaemias ranging in severity from negligible to moderately severe. Easily the most troublesome clinical complication is a tendency to thrombosis, manifest after splenectomy, a procedure which should be severely avoided in all of these conditions (Stewart et al. 1996). This is almost certainly the result of the red cell pathology itself and does not reflect an abnormality in any other cell type, all of which seem to be normal in most families, although neonatal ascites does occur in some families which must reflect a non-red-cell abnormality (Grootenboer et al. 1998).
Previous clinical classifications have rested on severity, on the state of cellular hydration, on plasma membrane lipid composition and on the presence or absence of the membrane protein, stomatin (Stewart 1993). Over the years, case reports on different families (alluded to below) have pointed to different temperature effects, evident when the red cells are manipulated in the laboratory. These fall into two forms: ‘pseudohyperkalaemia’, artefactually high measurements of plasma [K] secondary to temperature-dependent loss of K from red cells on storage at room temperature; and ‘cryohydrocytosis’, swelling and lysis of red cells stored at refrigerator temperatures. We have recently made a number of studies of these temperature effects in British families, which are collected together here for the first time. These studies show that all of these temperature effects can be related to the temperature dependence of the so-called ‘passive leak’ to K and Na, which can show a very wide variation in behaviour in different families. The temperature dependence of the leak can explain not only the cellular effects, but also form a basis for clinical classification and differentiation.
Different variants of hereditary stomatocytoses
Figure 2 shows a comparison of ouabain-plus-bumetanide resistant (‘leak’) K influx in 6 British families, whose haematological features are collected in Table 1.
Table 1.
Comparison of haematological, cation flux and structural data in different hereditary stomatocytosis variants. Intracellular electrolytes were measured by flame photometry and K influx by using 86 Rb as a tracer, as described (Coles et al. 1999b). The presence or absence of the Band 7.2b protein (‘stomatin’) was determined by SDS polyacrylamide gel electrophoresis and both Coomassie staining and by western blotting with a specific antibody [Stewart 1992, 673]. Membrane lipid composition was determined by proton NMR (Adosraku et al. 1994). The thermotropic data are derived from Fig. 1.
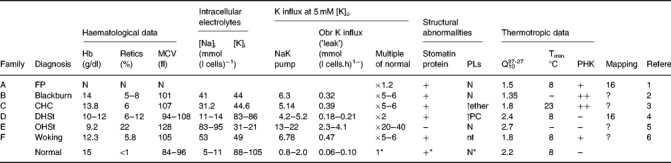
FP, familial pseudohyperkalaemia; CHC, cryohydrocytosis; DHSt, dehydrated hereditary stomatocytosis; OHSt, overhydrated hereditary stomatocytosis; retics, reticulocyte count; obr, ouabain-plus-bumetanide-resistant; PHK, denotes the presence or absence of pseudohyperkalaemia. N, normal; MCV, mean cell volume; PLs, phospholipids; ether, ether phospholipids; PC, phosphatidylcholine; Q1037-27, ratio of flux rate between 37 and 27°C; Tmin, temperature of minimum K influx; mapping, chromosome to which family maps in genetic studies; nt, not tested.
References: 1, Stewart et al. 1979; Stewart & Ellory 1985; 2, Coles et al. 1999a; 3, Coles et al. 1999b; 4, Carella et al. 1998; 5, Meadow 1967; 6, unpublished. * by definition.
Familial pseudohyperkalaemia (Edinburgh, Family A)
Studies of these temperature effects were first prompted by the identification of a mild, haematologically trivial, condition, ‘familial pseudohyperkalaemia’ (familial pseudohyperkalaemia: family A) (Stewart et al. 1979), in which there is a compensated haemolytic state but no frank anaemia, while the intracellular [Na] and [K] levels in fresh cells are virtually normal. These patients presented through the chemical pathology service with ‘pseudohyperkalaemia’: i.e. elevated plasma potassium levels (up to 8 mmol/l after 6 h storage) due to net loss of K from red cells on storage. We found that this loss on storage was a simple temperature effect, the result of cooling to room temperature (Stewart & Ellory 1985). These cases show a flux which is minimally increased at 37°C (Figure 2), consistent with the mild haemolytic state, but which shows a shallow slope in the interval 37–20°C, such that the flux at 20°C is markedly different from normal. The Q1037–27 is 1.5 and a minimum is seen at about 10°C. The NaK pump (not shown here), by contrast, shows a normal temperature dependence: on cooling to 20°C, the steady state preserving the intracellular [K] at 37°C is lost and K appears in the plasma (Stewart & Ellory 1985). We have recently mapped a genetic locus for this family to chromosome 16q24-ter (Iolascon et al. 1999).
Further such cases with the label ‘familial pseudohyperkalaemia’ have been described (Luciani et al. 1980; Leadbetter & O'Dowd 1982; Meenaghan et al. 1985; Vantyghen et al. 1991), who show the same combination of pseudohyperkalaemia due to a red cell temperature effect with normal or virtually normal haematology, but few have been characterized in this way. One at least (Meenaghan et al. 1985) is different and will be considered below under ‘cryohydrocytosis’.
HSt Blackburn (Family B)
Alani et al. (1994) described a British family with frank haemolytic anaemia (originally labelled as ‘hereditary spherocytosis’) associated with very marked pseudohyperkalaemia (up to 12 mmol/l): we have further investigated this family (denoted ‘B’ here) and it emerges that they have a novel form of stomatocytosis (Coles et al. 1999b), with high intracellular [Na] and low [K]. Flux rates at 37°C (Figure 2) are about 5 times normal. On cooling, these cells show the same shallow slope effect as seen in familial pseudohyperkalaemia, family A, but since the cells are leaky at 37°C there is frank haemolytic anaemia with reticulocytosis and jaundice. The Q1037–27 is even lower in this family than in family A at 1.35 and there is no definite minimum, although the curve does flatten as it approaches 0°C. A recently described French family may be similar (Grootenboer et al. 1998).
Cryohydrocytosis (Families C)
In ‘cryohydrocytosis’ (Miller et al. 1965), there is a mild-to-moderate stomatocytic anaemia with cells which are leaky at 37°C, but which show a remarkable cold sensitivity, showing markedly increased autohaemolysis on storage at 4 or 0°C, associated with gain of Na and loss of K at this temperature (Mentzer & Lande 1980). While normal red cells show very little lysis after 48 h under these conditions–perhaps 1 or 2%–these cells show more than 50% lysis. We have found that these patients also show pseudohyperkalaemia, and the temperature dependence of the ouabain-plus-bumetanide resistant K flux (Figure 2) shows a most remarkable pattern, with the minimum shifted up from 8°C to 23°C such that the flux at 0°C exceeds that at 37°C (Coles et al. 1999a). As in familial pseudohyperkalaemia (family A), and family B (Blackburn), the NaK pump (not shown) shows a typical monotonic temperature dependence and is virtually nonfunctional at 0°C. Thus simply cooling these cells to 0°C exposes a very marked leak with no compensatory NaK pump activity. The cell swells and bursts. The pseudohyperkalaemia results from the pump-leak imbalance at 20°C, exactly as in familial pseudohyperkalaemia and HSt Blackburn. Structurally, we find in both families that these cells show a slight excess of ether lipids in the membrane, which we are investigating. We know of two further families like this in the UK.
The Scottish family originally described by Meenaghan et al. (1985), which showed the familial pseudohyperkalaemia phenotype of nearly normal haematology with temperature dependent hyperkalaemia, may be a mild variant of this ‘high-minimum’ abnormality: in this family, the fluxes were nearly normal at 37°C and, as in family A, frank haemolysis was not present. The family described by Dagher et al. (1988) probably shows a similar effect but it is not identical.
It is interesting that virtually identical high-minimum effects can be elicited in normal human red cells by replacement of external Na in the cell suspension medium by an organic cation such as choline or N-methyl-d-glucamine (Blackstock & Stewart 1986), or by replacement of Cl by an organic anion such as salicylate or thiocyanate (Wieth 1970). These effects can be interpreted in terms of the lyotropic series of ions (Hunter 1986). Thus these patients have a genetic defect which in some way renders the cell resistant to the stabilizing effect of high-lyotropic-series ions (small, highly charged: Na, Cl) at low temperatures.
Dehydrated HSt (hereditary xerocytosis; hereditary hyperphosphatidylcholine haemolytic anaemia: Family D)
Clinically, the commonest form of HSt is ‘dehydrated HSt’ (Glader et al. 1974) or ‘hereditary xerocytosis’, in which the membrane leak is less severe than in families B and C. We know of 4 families with this variant in the UK. It is now recognized that dehydrated HSt is indistinguishable on present diagnostic criteria from that condition which Jaffe and Gottfried described as ‘hereditary hyperphosphatidylcholine haemolytic anaemia’ (Jaffe & Gottfried 1968; Clark et al. 1993). We have recently mapped a locus for this condition to chromosome 16 (Carella et al. 1998). These cells show a flux increase of 2–3 fold compared to normal (Figure 2), and the temperature curve in these cells is parallel to normal but simply shifted up the y-axis. The Q1037–27 is 2.4, slightly greater than normal and there is a minimum at 10°C.
Overhydrated HSt
This is the original ‘hereditary stomatocytosis’ condition, first described by Lock and colleagues (Lock et al. 1961) is now referred to as ‘overhydrated HSt’ or ‘hereditary hydrocytosis’ (Family E, Table 1). This condition is both the most haematologically severe and the most dramatically abnormal in ion transport terms: the intracellular Na and K levels are grossly abnormal, showing a reversal of the normal internal [Na]:[K] ratio (Zarkowsky et al. 1968) with flux rates over 20 times normal. In addition, these cells are deficient in the Band 7.2b membrane protein, ‘stomatin’ (Lande et al. 1982; Eber et al. 1989), whose sequence is known but whose function remains obscure (Hiebl-Dirschmied et al. 1991; Stewart et al. 1992). There is no obvious mutation in the gene for this protein in these cases (Wang et al. 1992). (This protein is present in all other families described here). The flux at 37°C in these cases is very high and the flux shows a simple monotonic temperature dependence with no minimum (Figure 2, diamonds). The Q1037–27 is 2.7, greater than normal.
HSt Woking (Family F)
In this 6th family (unpublished), flux rates are about 5–6 times normal at 37°C (Figure 2), comparable with families B and C, but the temperature dependence is parallel to normal and the cells do not show pseudohyperkalaemia or low temperature lysis.
Summary of temperature results
The leak K fluxes in the six stomatocytic families considered here all show different temperature profiles, which can be classified into 4 qualitatively similar patterns. These patterns can be distinguished by the Q1037–27 and by the position of the flux minimum, as follows:
Type 1: parallel to normal (Figure 2) but displaced upwards, as seen in dehydrated HSt (families D, F);
Type 2: with a shallow slope in the range 37–20°C (Figure 2) seen in families A (familial pseudohyperkalaemia), and B (Blackburn).
Type 3: purely monotonic with a steep slope (Figure 2), in family E, overhydrated HSt;
Type 4: with a displaced minimum at 23°C rather than 8°C (Figure 2), seen in family E (cryohydrocytosis), showing a marked rise at lower temperatures such that the flux at zero degrees exceeds that at 37°C.
Interpretation
These patterns of temperature dependence must be related to the fundamental physical abnormalities which underlie these disorders. The membranes are not simply more or less leaky: there are qualitative differences in addition to the quantitative. The differences in Q1037–27 over the interval 37–20°C in families A and B invite an interpretation in terms of Arrhenius activation energies in those cases where the plot is roughly linear in this range (i.e. all families except C, cryohydrocytosis). The lower values of Q1037–27 could imply a lower ‘activation energy’, a reduced energy barrier for transport (Atkins 1978), but the physical meaning of this is hard to conceive in a complex biological situation involving a number of possible energy barriers. The nonlinear behaviour of the plot below this temperature in normal cells makes such interpretation doubly difficult, for presumably there must be occurring some progressive temperature-dependent change in the state of the membrane which causes this paradoxical behaviour, and this questions the assumption that the ‘activation energy’ is a constant above this temperature.
It is tempting to try to interpret these effect in terms of lipid behaviour. As pointed out in the Introduction, there are disturbances of lipid handling in some these conditions and these probably underlie the ion leak, although this is not yet clear. In cryohydrocytosis, an abnormality in the susceptibility of phosphatidylcholine to phospholipase at low temperatures, possibly reflecting abnormal ‘packing’ at low temperatures, has been described (Schwartz et al. 1985). In poikilotherms, adaptive changes in phospholipid acyl chain composition occur with changing ambient temperature (Tiku et al. 1996), reflecting the importance of lipid composition in temperature effects, and while the red cells of a homeotherm could not possibly be expected to show such adaptive reconstruction, these studies do illustrate the importance in biology of lipid acyl chain composition in the context of temperature. In pure lipid systems, it is well known that permeability to many chemical species including monovalent cations shows a maximum around phase transitions (Papahadjopoulos et al. 1973), possibly due to packing defects at interfaces between phases (Corvera et al. 1992), such that, above the transition, permeability can increase with decreasing temperature, and this kind of effect could well be operative in the cryohydrocytosis cells, although one might expect a generalized increase in permeability rather than a specific leak to the univalent cations. Such phase behaviour in lipid systems can be influenced by electrolytes of the lyotropic series (Hunter 1986). While the complex, cholesterol-containing, red cell membrane does not show clear calorimetric transitions (Gottlieb & Eanes 1974), it is nevertheless possible that small, laterally localized domains, analogous to ‘rafts’ (Simons & Ikonen 1997) may show such behaviour and be related to the leak, either directly or because an ion channel is caught up in it. There is some evidence for domain formation in normal human red cell ghosts (Rodgers & Glaser 1991, 1993) and new methods of Fourier transform infra red spectroscopy, which employ deuterated phospholipids (Moore et al. 1996), may be able to identify such transitions in these abnormal red cells.
In conclusion, these comparative temperature studies of different variants of the stomatocytic anaemias show widely varying patterns of temperature dependence of ion fluxes which must now represent an essential element of the complete phenotypic characterization of these conditions. While previous classification criteria (e.g. cell hydration) remain relevant, these thermotropic differences can distinguish phenotypes which are otherwise quite similar and illustrate the wide spectrum of mutations which must underlie these conditions.
Acknowledgments
We thank Action Research and the Wellcome Trust for support. We thank E.J. Blackstock and M.C. Chetty for technical assistance. The cogent advice of Profs W.B. Gratzer and J.C. Ellory is appreciated. Dr May-Jean King has kindly referred patients. We thank Drs J.A.L. Amess, Helen Kelsey, Anne Miller, P. Hill, J. R. Trounce and D.A. Newsome for permission to report their patients.
References
- Alani FSS, Dyer T, Hindle E, et al. Pseudohyperkalaemia associated with hereditary spherocytosis in four members of a family. Postgrad. Med. J. 1994;70:749–751. doi: 10.1136/pgmj.70.828.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins P. Physical Chemistry. Oxford: University Press; 1978. [Google Scholar]
- Barnes TM, Jin Y, Horvitz HR, Ruvkun G, Hekimi S. The Caenorhabditis elegans behavioral gene unc-24 encodes a novel bipartite protein similar to both erythrocyte protein Band 7.2 (stomatin) and nonspecific lipid transfer protein. J. Neurochem. 1996;67:46–57. doi: 10.1046/j.1471-4159.1996.67010046.x. [DOI] [PubMed] [Google Scholar]
- Blackstock EJ, Stewart GW. The dependence on external cation of sodium and potassium fluxes across the human red cell membrane at low temperatures. J. Physiol. 1986;375:403–420. doi: 10.1113/jphysiol.1986.sp016124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell JH, Schaller KL. Opening the gates on ion channel diseases. Nat. Genet. 1992;2:87–89. doi: 10.1038/ng1092-87. [DOI] [PubMed] [Google Scholar]
- Carella M, Stewart GW, Ajetunmobi JF, et al. Genomewide search for dehydrated hereditary stomatocytosis (hereditary xerocytosis): Mapping of locus to chromosome 16 (q23-qter) Am. J. Hum. Genet. 1998;63:810–816. doi: 10.1086/302024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MR, Shohet SB, Gottfried EL. Hereditary hemolytic disease with increased red blood cell phosphatidylcholine and dehydration: one, two, or many disorders? Am. J. Hematol. 1993;42:25–30. doi: 10.1002/ajh.2830420107. [DOI] [PubMed] [Google Scholar]
- Coles SE, Chetty MC, Ho MM, et al. Two British families with variants on the ‘cryohydrocytosis’ form of hereditary stomatocytosis. Br. J. Haematol. 1999a;105:1055–1065. doi: 10.1046/j.1365-2141.1999.01444.x. [DOI] [PubMed] [Google Scholar]
- Coles SE, Ho MM, Chetty MC, Nicolaou A, Stewart GW. Hereditary stomatocytosis with marked pseudohyperkalaemia. Br. J. Haematol. 1999b;104:275–283. doi: 10.1046/j.1365-2141.1999.01191.x. [DOI] [PubMed] [Google Scholar]
- Corvera E, Mouritsen OG, Singer MA, Zuckermann MJ. The permeability and the effect of acyl-chain length for phospholipid bilayers containing cholesterol: theory and experiment. Biochim. Biophys. Acta. 1992;1107:261–270. doi: 10.1016/0005-2736(92)90413-g. [DOI] [PubMed] [Google Scholar]
- Dacie JV. The Haemolytic Anaemias. Vol. 1. Churchill: Edinburgh; 1985. The Hereditary Haemolytic Anaemias, Part 1; pp. 258–291. [Google Scholar]
- Dagher G, Vantyghem MC, Doise B, et al. Altered erythrocyte cation permeability in familial pseudohyperkalaemia. Clin. Sci. 1988;77:213–216. doi: 10.1042/cs0770213. [DOI] [PubMed] [Google Scholar]
- Desneves J, Berman A, Dynon K, et al. Human erythrocyte band 7.2b is preferentially labeled by a photoreactive phospholipid. Biochem. Biophys. Res. Comm. 1996;224:108–114. doi: 10.1006/bbrc.1996.0992. [DOI] [PubMed] [Google Scholar]
- Eber SW, Lande WM, Iarocci TA, et al. Hereditary stomatocytosis: consistent association with an integral membrane protein deficiency. Br. J. Haematol. 1989;72:452–455. doi: 10.1111/j.1365-2141.1989.tb07731.x. [DOI] [PubMed] [Google Scholar]
- Elford BC. Interactions between temperature and tonicity on cation transport in dog red cells. J. Physiol. 1975;246:371–395. doi: 10.1113/jphysiol.1975.sp010895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellory JC, Gibson JC, Stewart GW. Pathophysiology of abnormal cell Volume in human red cells. In: Lang F, editor. Cell Volume Regulation. Basel: Karger; 1998. pp. 120–239. [DOI] [PubMed] [Google Scholar]
- Glader BE, Fortier N, Albala MM, Nathan DG. Congenital hemolytic anemia associated with dehydrated erythrocytes and increased potassium loss. N. Eng. J. Med. 1974;291:491–496. doi: 10.1056/NEJM197409052911003. [DOI] [PubMed] [Google Scholar]
- Gottlieb MH, Eanes ED. On phase transitions in erythrocyte membranes and extracted membrane lipids. Biochim. Biophys. Acta. 1974;373:519–522. doi: 10.1016/0005-2736(74)90033-9. [DOI] [PubMed] [Google Scholar]
- Grootenboer S, Schischmanoff PO, Cynober T, et al. A genetic syndrome associating dehydrated hereditary stomatocytosis, pseudohyperkalaemia and perinatal oedema. Br. J. Haematol. 1998;103:383–386. doi: 10.1046/j.1365-2141.1998.00992.x. [DOI] [PubMed] [Google Scholar]
- Hall AC, Willis JS. The temperature dependence of passive potassium permeability in mammalian erythrocytes. Cryobiology. 1986;23:395–405. doi: 10.1016/0011-2240(86)90024-6. [DOI] [PubMed] [Google Scholar]
- Hiebl-Dirschmied CM, Entler B, Glotzmann C, et al. Cloning and nucleotide sequence of cDNA encoding human erythrocyte band 7 integral membrane protein. Biochim. Biophys. Acta. 1991;1090:123–124. doi: 10.1016/0167-4781(91)90047-p. [DOI] [PubMed] [Google Scholar]
- Hunter R. Foundations of Colloid Science. Oxford: Clarendon Press; 1986. [Google Scholar]
- Iolascon A, Stewart GW, Ajetunmobi JF, et al. Familial pseudohyperkalemia maps to the same locus as dehydrated hereditary stomatocytosis (hereditary xerocytosis) Blood. 1999;93:3120–3123. [PubMed] [Google Scholar]
- Jaffe ER, Gottfried EL. Hereditary nonspherocytic hemolytic disease associated with an altered phospholipid composition of the erythrocytes. J. Clin. Invest. 1968;47:1375–1388. doi: 10.1172/JCI105829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande WM, Thiemann PW, Mentzer WM. Missing band 7 membrane protein in two patients with high Na, low K erythrocytes. J. Clin. Invest. 1982;70:1273–1280. doi: 10.1172/JCI110726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leadbetter S, O'Dowd TC. Possible screening test for familial pseudohyperkalaemia. Lancet. 1982;ii:103–104. doi: 10.1016/s0140-6736(82)91724-x. [DOI] [PubMed] [Google Scholar]
- Lock SP, Sephton Smith R, Hardisty RM. Stomatocytosis: a hereditary haemolytic anomaly associated with haemolytic anaemia. Br. J. Haematol. 1961;7:303–314. doi: 10.1111/j.1365-2141.1961.tb00341.x. [DOI] [PubMed] [Google Scholar]
- Luciani J-C, Lavabre-Bertand T, Fourcade J, et al. Familial pseudohyperkalaemia. Lancet. 1980;i:491. doi: 10.1016/s0140-6736(80)91043-0. [DOI] [PubMed] [Google Scholar]
- Lux S, Palek J. Disorders of the red cell membrane. In: Handin R, Lux S, Stossel T, editors. Blood: Principles and Practice of Hematology. Philadelphia: JB Lippincott; 1995. pp. 1701–1818. [Google Scholar]
- Meenaghan M, Follett GF, Brophy PJ. Temperature sensitivity of potassium flux into red blood cells in the familial pseudohyperkalaemia syndrome. Biochim. Biophys. Acta. 1985;821:72–78. doi: 10.1016/0005-2736(85)90155-5. [DOI] [PubMed] [Google Scholar]
- Mentzer WC, Lande WM. Hemolytic anaemia resulting from abnormal red cell membrane cation permeability — hydrocytosis and cryocytosis. In: Srivastava S, editor. Red Blood Cell and Lens Metabolism. Amsterdam: Elsevier; 1980. pp. 311–314. [Google Scholar]
- Miller G, Townes PL, Macwhinney JB. A new congenital hemolytic anaemia with deformed erythrocytes (? ‘stomatocytes’) and remarkable susceptibility of erythrocytes to cold hemolysis in vitro. Pediatrics. 1965;35:906–915. [PubMed] [Google Scholar]
- Moore DJ, Sills RH, Patel N, Mendelsohn R. Conformational order of phospholipids incorporated into human erythrocytes: an FTIR spectroscopy study. Biochem. 1996;35:229–235. doi: 10.1021/bi951692k. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D, Jacobson K, Nir S, Isac T. Phase transitions in phospholipid vesicles. Fluorescence polarization and permeability measurements concerning the effect of temperature and cholesterol. Biochim. Biophys. Acta. 1973;311:330–348. doi: 10.1016/0005-2736(73)90314-3. [DOI] [PubMed] [Google Scholar]
- Rodgers W, Glaser M. Characterization of lipid domains in erythrocyte membranes. Proc. Natl. Acad. Sci. USA. 1991;88:1364–1368. doi: 10.1073/pnas.88.4.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers W, Glaser M. Distribution of proteins and lipids in the erythrocyte membrane. Biochem. 1993;32:12592–12598. doi: 10.1021/bi00210a007. [DOI] [PubMed] [Google Scholar]
- Schwartz RS, Chiu DTY, Lubin B. Plasma membrane phospholipid organisation in human erythrocytes. Curr. Top. Haematol. 1985;5:64–112. [PubMed] [Google Scholar]
- Sheetz M, Singer SJ. Biological membranes as bilayer couples: a molecular mechanism of drug–erythrcoyte interactions. Proc. Natl. Acad. Sci. (USA) 1974;71:4457. doi: 10.1073/pnas.71.11.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohet SB, Livermore BM, Nathan DG, Jaffe ER. Hereditary hemolytic anemia associated with abnormal membrane lipids: mechanism of accumulation of phospahtidylcholine. Blood. 1971;38:445–456. [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Stewart GW, Corrall RJM, Fyffe JA, Stockdill GM, Strong JA. Familial pseudohyperkalaemia. A new syndrome. Lancet. 1979;ii:175–177. doi: 10.1016/s0140-6736(79)91437-5. [DOI] [PubMed] [Google Scholar]
- Stewart GW, Ellory JC, Klein RA. Increased human red cell cation passive permeability below 12°C. Nature. 1980;286:403–404. doi: 10.1038/286403a0. [DOI] [PubMed] [Google Scholar]
- Stewart GW, Ellory JC. A family with mild xerocytosis showing increased cation permeability at low temperatures. Clin. Sci. 1985;69:309–319. doi: 10.1042/cs0690309. [DOI] [PubMed] [Google Scholar]
- Stewart GW, Blackstock EJ, Hall AC, Ellory JC. Potassium transport in monkey erythrocytes. Exp. Biol. 1989;48:167–172. [PubMed] [Google Scholar]
- Stewart GW, Hepworth-Jones BE, Keen JN, et al. Isolation of cDNA coding for a ubiquitous membrane protein deficient in high Na, low K stomatocytic erythrocytes. Blood. 1992;79:1593–1601. [PubMed] [Google Scholar]
- Stewart GW. The membrane defect in hereditary stomatocytosis. In: Tanner MJA, Anstee DJ, editors. Red Cell Membrane Antigens. London: Bailliere-Tyndall; 1993. pp. 371–400. [DOI] [PubMed] [Google Scholar]
- Stewart GW, Amess JAL, Eber S, et al. Thrombo-embolic disease after splenectomy for hereditary stomatocytosis. Br. J. Haematol. 1996;93:303–310. doi: 10.1046/j.1365-2141.1996.4881033.x. [DOI] [PubMed] [Google Scholar]
- Tiku PE, Gracey AY, Macartney AI, Beynon RJ, Cossins AR. Cold-induced expression of delta 9-desaturase in carp by transcriptional and posttranslational mechanisms. Science. 1996;271:815–818. doi: 10.1126/science.271.5250.815. [DOI] [PubMed] [Google Scholar]
- Vantyghen MC, Dagher G, Doise B, et al. Pseudo-hyperkaliemie. A Propos d'une observation familiale. Ann. Endocrinol. 1991;52:104–108. [PubMed] [Google Scholar]
- Wang D, Turetsky T, Perrine S, Johnson RM, Mentzer WC. Further studies on RBC membrane protein 7.2B deficiency in hereditary stomatocytosis. Blood. 1992;80(Suppl. 1):275a. [Google Scholar]
- Wieth JO. Paradoxical temperature dependence of sodium and potassium fluxes in human red cells. J. Physiol. 1970;207:563–580. doi: 10.1113/jphysiol.1970.sp009081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarkowsky HS, Oski FA, Shaafi R, Shohet SB, Nathan DG. Congenital hemolytic anemia with high sodium, low potassium red cells. Studies of membrane permeability. N. Eng. J. Med. 1968;278:573–581. doi: 10.1056/NEJM196803142781101. [DOI] [PubMed] [Google Scholar]


