Abstract
The distribution of the myoepithelial cells during regeneration of the rat parotid gland after atrophy induced by one week of parotid duct ligation was investigated by immunohistochemistry for actin and transmission electron microscopy (TEM). Immunohistochemically, residual ducts were surrounded by actin-positive cells when clips were removed from the duct. Three days later, most of the newly formed acini originating from the residual ducts were also embraced by actin-positive cells. After 10 days, actin-positivity tended to be seen as dots around acini that decreased in number day by day. On day 21 actin-positive cells mainly surrounded intercalated ducts with only a few positive reactions identified at the acinar periphery. Electron microscopically, residual ducts and newly formed acini were peripherally embraced by myoepithelial cells before day 5. After day 7, shift of myoepithelial cells from the periphery of acini to the duct-acinar junctional region was identified. Then few myoepithelial cells were identified at the periphery of acini. These observations indicate that myoepithelial cells migrate from the acinar periphery to the duct-acinar junctional region during rat parotid regeneration, and that such behaviour is closely related to that seen during rat parotid development.
Keywords: myoepithelial cell, distribution, parotid gland, regeneration, duct ligation
Atrophy of salivary glands induced by duct obstruction and subsequent regeneration after recanalization of the duct is of clinical relevance and has been repeatedly examined experimentally. During atrophy duct cells persist in contrast to the disappearance of all or most acinar cells (Standish & Shafer 1957; Bhasker et al. 1966; Tamarin 1971a, b; Walker & Gobé 1987). Formerly it was believed that disappearance of acinar cells was due to their de-differentiation to form duct cells (Standish & Shafer 1957; Bhasker et al. 1966; Tamarin 1971a, b), however, it is now understood that the acinar cells are deleted by apoptosis (Walker & Gobé 1987). After recanalization of the duct, acinar cells reappear and the structure of glandular tissue recovers (Bhasker et al. 1966; Tamarin 1971a, b; Burford-Mason et al. 1993; Cummins et al. 1994; Takahashi et al. 1998). It was recently demonstrated that new acinar cells derive from the proliferation and differentiation of residual duct cells and the number of acinar cells increases quickly by active proliferation of the newly formed acinar cells (Takahashi et al. 1998) or of original acinar cells remaining under conditions of probable incomplete obstruction (Burford-Mason et al. 1993; Cummins et al. 1994). These findings are relevant to the histogenesis of salivary gland tumours.
Myoepithelial cells are also speculated to participate in the histogenesis of several kinds of salivary gland tumours (Batsakis et al. 1983; Dardick et al. 1987; Morinaga et al. 1987). Nevertheless there are a few investigations of myoepithelial cells in atrophic and regenerating salivary gland. Recently Burgess et al. (1996) demonstrated proliferation of myoepithelial cells during both atrophy and regeneration of the rat parotid after duct ligation. In the same experimental model, parenchymal cell population changes including that of myoepithelial cells were calculated (Burgess & Dardick 1998), however, it remains unclear how myoepithelial cells behave and their distribution changes during the regenerative phase. To clarify this, we used actin immunohistochemistry and transmission electron microscopy (TEM) to examine myoepithelial cell changes during parotid gland regeneration after complete duct obstruction induced by double ligation.
Materials and methods
Experimental Procedures
Sixty-six male Wistar rats weighing 190–210 g were divided into experimental and control groups. In the experimental animals, the right parotid duct was doublely ligated with metal clips as described previously (Takahashi et al. 1998). The clips were removed one week later and the animals killed at 0, 1, 3, 5, 7, 10, 12, 14, 17 and 21 days. The control animals were not operated at all.
All animal experimentation followed the Guide for the Care and Use of Laboratory Animals, Hokkaido University School of Dentistry.
Immunohistochemistry
Four experimental animals at each time point, and four control animals were killed by deep inhalation of ether for immunohistochemistry. The fresh right parotid glands were removed and immediately frozen in liquid nitrogen. Frozen sections were cut with a cryostat, air-dried and immersed in 10% neutral buffered formalin solution for 2 min. After rinsing with phosphate-buffered saline (PBS), the sections were incubated with mouse anti-muscle actin monoclonal antibody HHF35 (DAKO) not recognizing non-muscle actin, biotinylated anti-mouse rabbit polyclonal antibody (DAKO) and streptavidin-biotin horse-radish peroxidase complex (DAKO) in turn. Peroxidase activity was visualized by 3,3′-diaminobenzidine method and the immunostained sections were lightly counterstained with Mayer's haematoxylin.
Normal mouse serum was substituted for the primary antibody in the negative control sections.
Quantification
Four immunostained sections from each animal were used. In each section the number of acini and those in which an actin-positive reaction was identified at the periphery were counted in randomly chosen fields at a magnification of ×200 (OLYMPUS, Tokyo, BH-2 microscope). Approximately 500 acini were counted and the percentage labelled were calculated. The mean of the four sections was taken as representative value of that animal. Then the mean and standard deviation (SD) were calculated for the 4 experimental animals at each time point and 4 control animals.
Transmission electron microscopy (TEM)
The experimental and control animals for TEM were perfused with 2% paraformaldehyde-1.25% glutaraldehyde buffered at pH 7.4 with 0.05 m sodium cacodylate under pentobarbital general anaesthesia. The right parotid glands were immersed in the same solution for 4 h and rinsed in 0.05 m cacodylate buffer. Tissue was then postfixed in 1% osmium tetroxide for 2 h, dehydrated, and embedded in Epon 812. Semithin sections stained with methylene blue-azure II were examined by light microscopy, and ultrathin sections of chosen areas double-stained with uranyl acetate and lead citrate examined by transmission electron microscopy (HITACHI, Tokyo, H-7000 electron microsope).
Results
Details of histological changes during parotid gland regeneration after duct recanalization have been described previously (Takahashi et al. 1998). In summary, experimental parotid glands showed marked atrophy one week after ligation (day 0). Acinar cells had disappeared and parenchyma comprised residual ducts only. On day 3 newly formed acini arising from residual ducts were observed. Thereafter acini increased in number and maturation such that by day 10 the histology of experimental parotid glands was indistinguishable from that of controls.
Immunohistochemical observations
In control parotid glands, actin-positive cells were identified at the periphery of the intercalated ducts. Only 9.3% of acini were surrounded by one or more actin-positive cells, and there was no reaction in striated and excretory ducts. In the stroma, blood vessels showed positivity to anti-actin antibody (Figure 1a).
Figure 1.
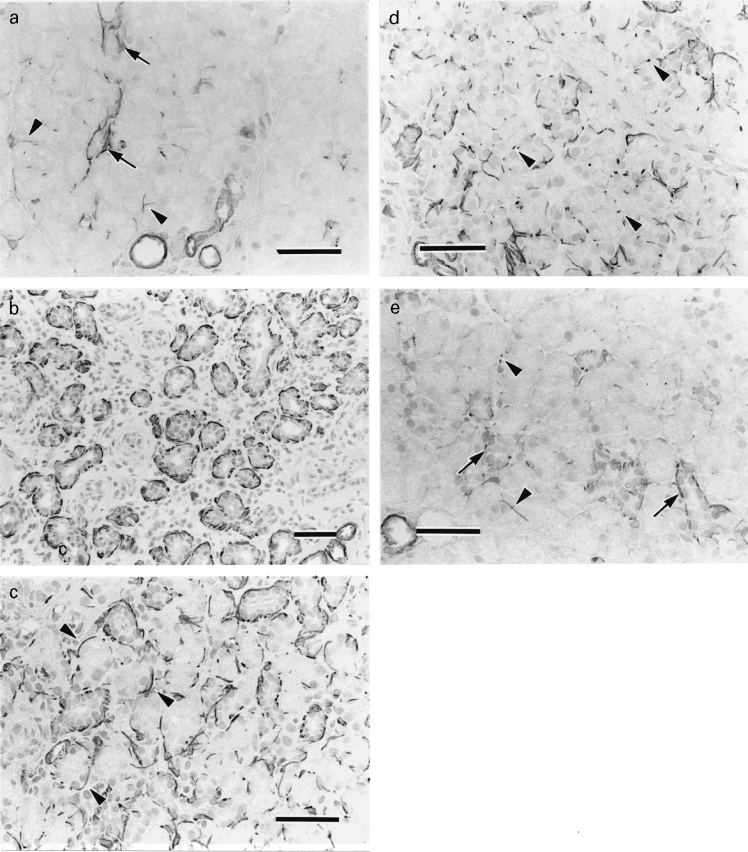
Actin immunohistochemistry of a, the control and b–e, experimental glands. a, Actin-positive reaction is observed at the periphery of the intercalated ducts (arrows) and a few acini (arrowheads). Blood vessels are also positive. b, Day 0. Actin-positive reactions are identified around many residual ducts. c, Day 3. Newly formed acini are embraced by actin-positive cells (arrowheads). d, Day 10. Actin-positive reactions tend to be dots (arrowheads) around acini. e, Day 21. The positivity is observed at the periphery of intercalated ducts (arrows) and a few acini (arrowheads) (bar = 50 μm for all parts).
In experimental parotid glands on day 0, there was a higher concentration of actin-positive cells in the atrophic glandular tissue than in the control. Many residual ducts were surrounded by actin-positive cells. At the periphery of ducts corresponding to striated and excretory ducts, a positive reaction to antiactin antibody was not identified (Figure 1b). Between days 3 and 7, newly formed acini were embraced by actin-positive cells (Figure 1c). After day 10, actin-positive reactions tended to be dots around acini and decreased in number day by day (Figure 1d). By day 21 actin-positive cells mainly surrounded intercalated ducts with few positive reactions at the periphery of acini identified. The pattern of immunostaining in experimental glands was similar to that in controls (Figure 1e).
Figure 2 shows changes in the percentage of acini with actin-positive reactions at the periphery in experimental glands at each time point. On day 3 and 5 the value was highest at approximately 80%, and thereafter declined. The decine was particularly abrupt between days 12 and 17. From day 17 the percentage approached control levels.
Figure 2.
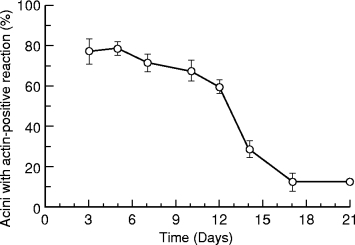
Changes in percentage of acini with actin-positive reactions at the periphery in experimental parotid glands after removal of clips. The highest is 78.4% on day 5 and the lowest is 12.5% on day 17 and 21. The mean in controls is 9.3% (not shown in the graph). (n = 4 for experimental animals at each time point; results expressed as mean ± SD)
TEM observations
Flat or spindled myoepithelial cells were located at the periphery of residual ducts one week after ligation. The myoepithelial cells showed normal characteristics, including numerous microfilaments sometimes associated with dense bodies and caveolae along the basal plasma membrane (Figure 3a). On day 3, most new acini differentiated from residual ducts were peripherally surrounded by flat or spindled myoepithelial cells (Figure 3b). After day 7, the location of myoepithelial cells varied. There were not only myoepithelial cells located at the periphery of acini but also partially (Figure 3c) or mostly (Figure 3d) within the acinus. These myoepithelial cells retained microfilaments with dense bodies and caveolae at the basal plasma membrane (Figure 3e). In addition, some myoepithelial cells were located between duct and acinar cells (Figure 3f). Day by day myoepithelial cells around acini decreased in number becoming most frequent around intercalated ducts.
Figure 3.
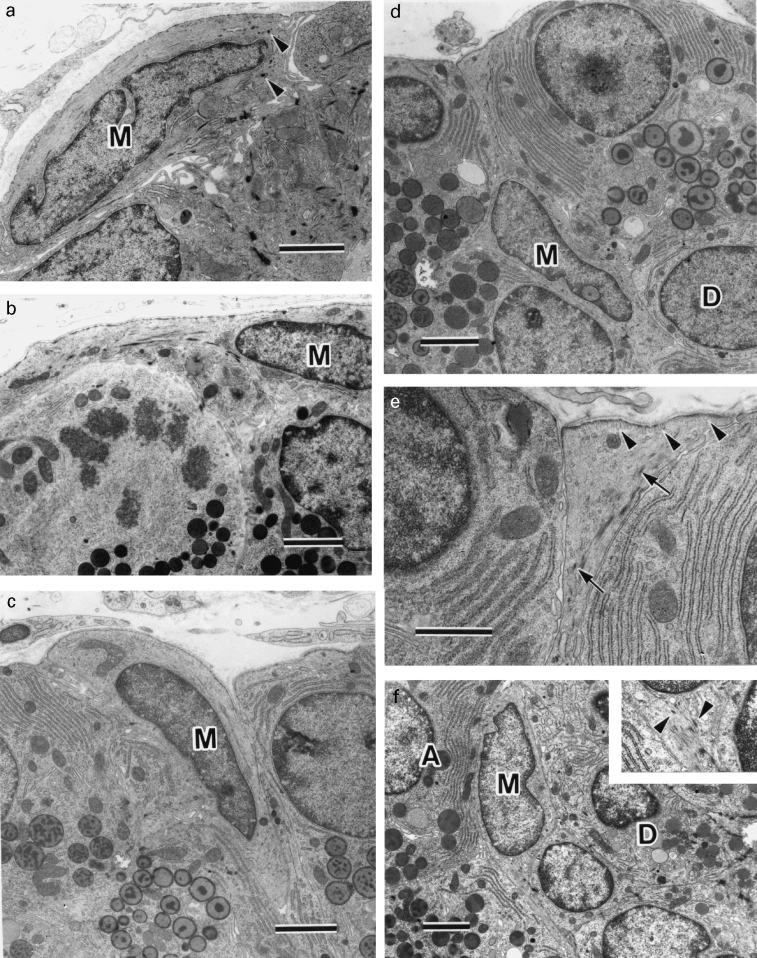
TEM of parotid glands from experimental groups. Throughout M, myoepithelial cells; A, acinar cells; D, duct cells. a, Day 0. Flat myoepithelial cell with dense bodies (arrowheads) is located at periphery of residual duct. b, Day 5. Myoepithelial cell located basal to newly formed acinar cells, one of which is undergoing mitosis. c, Day 10. Myoepithelial cell partially located between acinar cells. d, Day 10. Myoepithelial cell extending thin process to basement membrane, mainly situated among acinar cells and touching duct cell. e, Higher magnification of myoepithelial cell process in (d). Abundant microfilaments sometimes with dense bodies (arrows) and caveolae (arrowheads) along the basal plasma membrane are seen. f, Day 14. Myoepithelial cell is located between acinar cells and duct cells. Inset, Higher magnification of area adjacent nucleus of myoepithelial cell. Microfilaments with dense bodies (arrowheads) are seen. × 7400. Bar = 2 μm (a-d,f); bar = 1 μm (e).
Between day 7 and 14 epithelial cells containing both secretory granules and dense body like materials were seen very rarely (Figure 4). Throughout the experimental period no apoptosis and necrosis of myoepithelial cells was observed in semithin or ultrathin sections.
Figure 4.
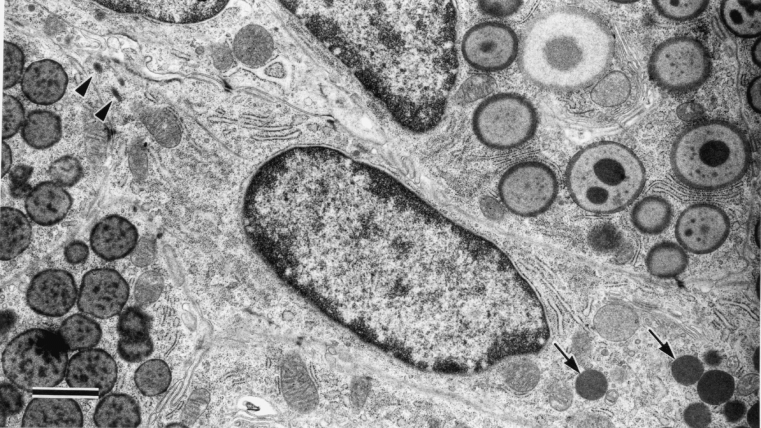
TEM of parotid gland 10 days after removal of clips. The epithelial cell contains dense body like structures (arrowheads) and secretory granules (arrows). Bar = 1 μm.
Discussion
It is difficult to distinguish myoepithelial cells in normal histological preparations, so additional methods are required (Tandler et al. 1970; Redman & Ball 1979; Redman et al. 1980). Myoepithelial cells are very rich in myofilaments confirmed by immunoelectron microscopy to react with anti-muscle actin antibody (Norberg et al. 1992; Takahashi et al. 1997). Therefore muscle actin immunohistochemistry is one of the best markers of myoepithelial cells in glandular tissue and was employed in this study.
Many of residual ducts in parotid gland after one week ligation were surrounded by myoepithelial cells, and many more myoepithelial cells were observed on day 0 than in control parotid glands. Increased myoepithelial cell numbers could be speculated to be caused by two factors, resistance of myoepithelial cells to the effect of obstruction and their proliferation.
It has been shown electron microscopically that myoepithelial cells persist in duct-ligated rat parotid gland (Walker & Gobé 1987), duct-ligated rat submandibular gland (Tamarin 1967, 1971b), human chronic sialadenitis (Matthews & Dardick 1988) and involuting rat mammary gland (Helminen & Ericsson 1968; Walker et al. 1989). However, morphological changes in myoepithelial cells were observed in duct-ligated cat salivary glands (Emmelin et al. 1974) and involuting rat mammary gland (Radnor 1972) and apoptosis of myoepithelial cells in involuting mouse mammary gland (Walker et al. 1989). In this study necrosis and apoptosis of myoepithelial cells were not identified on day 0. This finding suggests that short-term duct obstruction allows persistence of myoepithelial cells.
On the other hand, several investigators have discussed proliferative activity of myoepithelial cells. Batsakis et al. (1989) theorized that myoepithelial cells were end-differentiated cells and amitotic. In developing rat submandibular gland (Cutler & Chaudhry 1973), and cultured and transplanted human mammary gland (Joshi et al. 1986) mature myoepithelial cells are unable to divide, although precursor cells are proliferative. However, mitotic figures in immature myoepithelial cells during rat parotid gland development (Taga & Sesso 1979; Redman et al. 1980) and partly differentiated myoepithelial cells in developing hamster Harderian gland (Lopez et al. 1992) have been identified. In addition, S-phase myoepithelial cells in mouse mammary gland after hormonal stimulation (Sapino et al. 1990) and mitoses of well differentiated myoepithelial cells in maturing rat parotid gland (Redman 1994) have also been reported. Recently Burgess et al. (1996) in similar conditions to this study demonstrated proliferative myoepithelial cells in duct-ligated mature rat parotid gland using anti-proliferating cell nuclear antigen antibody (PCNA). Accordingly, myoepithelial cells were speculated to proliferate, although mitotic figures were not observed.
It was shown immunohistochemically in this study that myoepithelial cell distribution changed during regeneration of parotid gland. Myoepithelial cells embraced acini at an early stage of regeneration but then gradually disappeared from there. TEM observations showed myoepithelial cells shift from the periphery of acinus to the duct-acinar junctional region during this period. These findings suggest that myoepithelial cells migrate from the periphery of acini to duct-acinar junctional regions during regeneration. However, it still remains unclear how myoepithelial cells behave after migration to duct-acinar junctional region. Much higher concentrations of myoepithelial cells are seen at an early stage (day 3 and 5) than later stages (day 21). Although migration of myoepithelial cells serves as a rational explanation of disappearance from the periphery of acini, it is difficult to explain the apparent decrease in myoepithelial cell numbers by migration. Two hypotheses are tenable. One is myoepithelial cell deletion by apoptosis and the other is transformation of myoepithelial cell into other cell types. In the present study, apoptosis of myoepithelial cells was looked for but not identified during regeneration. In the period that myoepithelial cells disappeared from the periphery of acini, epithelial cells with both granules and dense bodies were identified. These epithelial cells may be transitional cells suggesting that myoepithelial cells can transform to duct or acinar cells. However, Batsakis et al. (1989) doubt that highly specialized cell such as myoepithelial cells can transform to another differentiated cell. Further study is needed to clarify this issue.
During development of the rat parotid gland, myoepithelial cells appear at the periphery of not only intercalated ducts but also acini until the age of two weeks, then decrease at the latter site between 18 and 25 days (Redman et al. 1980; Redman 1994). In adult rats only the intercalated ducts are surrounded by myoepithelial cells, which merely extend their processes onto adjacent acini (Dewey 1958; Bogart 1971; Garrett & Emmelin 1979; Redman 1994). It was noticed that the period of myoepithelial cell decrease coincides with the weaning period (Redman 1994). The alteration of myoepithelial cell distribution during parotid gland regeneration in young adult rats shown in the present study is very similar to that during development, suggesting that weaning is not an important factor in the redistribution of myoepithelial cells.
In conclusion, it has been demontrated that myoepithelial cells migrate from the acinar periphery to duct-acinar junctional region during rat parotid regeneration, and that such alterations are closely related to those occurring during rat parotid gland development.
Acknowledgments
The authors gratefully acknowledge the assistance of Dr Neal I. Walker of the Department of Pathology, University of Queensland Medical School, Brisbane, Australia, with the manuscript.
This study is supported by the Grant of Hokkaido Foundation for the Promotion of Scientific and Industrial Technology (Hokscitec), Japan.
References
- Batsakis JG, Kraemer B, Sciubba JJ. The pathology of head and neck tumors: The myoepithelial cell and its participation in salivary gland neoplasia. Head Neck Surg. 1983;5:222–233. doi: 10.1002/hed.2890050307. [DOI] [PubMed] [Google Scholar]
- Batsakis JG, Regezi JA, Luna MA, El-Naggar A. Histogenesis of salivary gland neoplasms: a postulate with prognostic implications. J. Laryngol. Otol. 1989;103:939–944. doi: 10.1017/s0022215100110552. [DOI] [PubMed] [Google Scholar]
- Bhasker SN, Lilly GE, Bhussry B. Regeneration of the salivary glands in the rabbit. J. Dent. Res. 1966;45:37–41. doi: 10.1177/00220345660450012601. [DOI] [PubMed] [Google Scholar]
- Bogart BI. The fine structural localization of acetylcholinesterase activity in the rat parotid and sublingual glands. Am. J. Anat. 1971;132:259–266. doi: 10.1002/aja.1001320210. [DOI] [PubMed] [Google Scholar]
- Burford-Mason AP, Cummins MM, Brown DH, MacKay AJ, Dardick I. Immunohistochemical analysis of the proliferative capacity of duct and acinar cells during induced atrophy and subsequent regeneration of rat parotid gland. J. Oral Pathol. Med. 1993;22:440–446. doi: 10.1111/j.1600-0714.1993.tb00122.x. [DOI] [PubMed] [Google Scholar]
- Burgess KL, Dardick I, Cummins MM, Burford-Mason AP, Bassett R, Brown DH. Myoepithelial cells actively proliferate during atrophy of rat parotid gland. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1996;82:674–680. doi: 10.1016/s1079-2104(96)80443-4. [DOI] [PubMed] [Google Scholar]
- Burgess KL, Dardick I. Cell population changes during atrophy and regeneration of rat parotid gland. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1998;85:699–706. doi: 10.1016/s1079-2104(98)90038-5. [DOI] [PubMed] [Google Scholar]
- Cummins M, Dardick I, Brown D, Burford-Mason AP. Obstructive sialadenitis: a rat model. J. Otolaryngol. 1994;23:50–56. [PubMed] [Google Scholar]
- Cutler LS, Chaudhry AP. Differentiation of the myoepithelial cells of the rat submandibular gland in vivo and in vitro: an ultrastructural study. J. Morphol. 1973;140:343–354. doi: 10.1002/jmor.1051400307. [DOI] [PubMed] [Google Scholar]
- Dardick I, Rippstein P, Skimming L, Boivin M, Parks WR, Dairkee SH. Immunohistochemistry and ultrastructure of myoepithelium and modified myoepithelium of the ducts of human major salivary glands: Histogenetic implications for salivary gland tumors. Oral Surg. Oral Med. Oral Pathol. 1987;64:703–715. doi: 10.1016/0030-4220(87)90173-3. [DOI] [PubMed] [Google Scholar]
- Dewey MM. A histochemical and biochemical study of the parotid gland in normal and hypophysectionized rats. Am. J. Anat. 1958;102:243–271. doi: 10.1002/aja.1001020205. [DOI] [PubMed] [Google Scholar]
- Emmelin N, Garrett JR, Ohlin P. Secretory activity and myoepithelial cells of salivary glands after duct ligation in cats. Arch. Oral Biol. 1974;19:275–283. doi: 10.1016/0003-9969(74)90188-5. [DOI] [PubMed] [Google Scholar]
- Garrett JR, Emmelin N. Activities of salivary myoepithelial cells: a review. Med. Biol. 1979;57:1–28. [PubMed] [Google Scholar]
- Helminen HJ, Ericsson JLE. Studies on mammary gland involution II. Ultrastructural evidence for auto- and heterophagocytosis. J. Ultrastruct. Res. 1968;25:214–227. doi: 10.1016/s0022-5320(68)80070-x. [DOI] [PubMed] [Google Scholar]
- Joshi K, Smith JA, Perusinghe N, Monogham P. Cell proliferation in the human mammary epithelium. Differential contribution by epithelial and myoepithelial cells. Am. J. Pathol. 1986;124:199–206. [PMC free article] [PubMed] [Google Scholar]
- Lopez JM, Tolivia J, Alvares-Uria M. An ultrastructural study of myoepithelium maturation during postnatal development of the hamster Harderian gland. Anat. Embryol. 1992;186:573–582. doi: 10.1007/BF00186980. [DOI] [PubMed] [Google Scholar]
- Matthews TW, Dardick I. Morphological alterations of salivary gland parenchyma in chronic sialadenitis. J. Otolaryngol. 1988;17:385–398. [PubMed] [Google Scholar]
- Morinaga S, Nakajima T, Shimosato Y. Normal and neoplastic myoepithelial cells in salivary glands: an immunohistochemical study. Human Pathol. 1987;18:1218–1226. doi: 10.1016/s0046-8177(87)80404-5. [DOI] [PubMed] [Google Scholar]
- Norberg L, Dardick I, Leung R, Burford-Mason AP, Rippstein P. Immunogold localization of actin and cytokeratin filaments in myoepithelium of human parotid salivary gland. Ultrastruct. Pathol. 1992;16:555–568. doi: 10.3109/01913129209061547. [DOI] [PubMed] [Google Scholar]
- Radnor CJP. Myoepithelium in involuting mammary glands of the rat. J. Anat. 1972;112:355–365. [PMC free article] [PubMed] [Google Scholar]
- Redman RS, Ball WD. Differentiation of myoepithelial cells in the developing rat sublingual gland. Am. J. Anat. 1979;156:543–566. doi: 10.1002/aja.1001560408. [DOI] [PubMed] [Google Scholar]
- Redman RS, Sweney LR, McLaughlin ST. Differentiation of myoepithelial cells in the developing rat parotid gland. Am. J. Anat. 1980;158:299–320. doi: 10.1002/aja.1001580306. [DOI] [PubMed] [Google Scholar]
- Redman RS. Myoepithelium of salivary glands. Microsc. Res. Techn. 1994;27:25–45. doi: 10.1002/jemt.1070270103. [DOI] [PubMed] [Google Scholar]
- Sapino A, MacRi L, Gugliotta P, Bussolati G. Immunocytochemical identification of proliferating cell type in mouse mammary gland. J. Histochem. Cytochem. 1990;38:1541–1547. doi: 10.1177/38.11.2212615. [DOI] [PubMed] [Google Scholar]
- Standish S, Shafer WG. Serial histologic effects of rat submaxillary and sublingual salivary gland. Duct and blood vessel ligation. J. Dent. Res. 1957;36:866–879. doi: 10.1177/00220345570360060801. [DOI] [PubMed] [Google Scholar]
- Taga R, Sesso A. Ultrastructural studies on developing parotid gland of the rat at early postnatal periods. Arch. Histol. Jpn. 1979;42:427–444. doi: 10.1679/aohc1950.42.427. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Domon T, Yamamoto T, Wakita M. Regeneration of myoepithelial cells in rat submandibular glands after yttrium aluminium garnett laser irradiation. Int. J. Exp. Path. 1997;78:91–99. doi: 10.1046/j.1365-2613.1997.d01-244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Schoch E, Walker NI. Origin of acinar cell regeneration after atrophy of the rat parotid induced by duct obstruction. Int. J. Exp. Path. 1998;79:293–301. doi: 10.1046/j.1365-2613.1998.710405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamarin A. Secretory cell alterations associated with submaxillary gland duct ligation. In: Schneyer LH, Schneyer. CA, editors. Secretory Mechanisms of Salivary Glands. New York: Academic Press Inc; 1967. pp. 220–237. [Google Scholar]
- Tamarin A. Submaxillary gland recovery from duct obstruction I. Overall changes and electron microscopic alterations of glandular duct cells. J. Ultrastruct. Res. 1971a;34:276–287. doi: 10.1016/s0022-5320(71)80072-2. [DOI] [PubMed] [Google Scholar]
- Tamarin A. Submaxillary gland recovery from duct obstruction II. Electron microscopic alterations of acinar cells. J. Ultrastruct. Res. 1971b;34:288–302. doi: 10.1016/s0022-5320(71)80073-4. [DOI] [PubMed] [Google Scholar]
- Tandler B, Denning CR, Mandel ID, Kutscher AH. Ultrastructure of human labial salivary gland III. Myoepithelium and ducts. J. Morphol. 1970;130:227–246. doi: 10.1002/jmor.1051300208. [DOI] [PubMed] [Google Scholar]
- Walker NI, Gobé GC. Cell death and cell proliferation during atrophy of the rat parotid gland induced by duct obstruction. J. Pathol. 1987;153:333–344. doi: 10.1002/path.1711530407. [DOI] [PubMed] [Google Scholar]
- Walker NI, Bennett RE, Kerr JFR. Cell death by apoptosis during involution of the lactating breast in mice and rats. Am. J. Anat. 1989;185:19–32. doi: 10.1002/aja.1001850104. [DOI] [PubMed] [Google Scholar]


