Abstract
Cultured murine CD4+ cells from Saccharopolyspora rectivirgula sensitized C3H/HeJ (Th1 bias) donors can adoptively transfer murine experimental hypersensitivity pneumonitis (EHP). We sensitized BALB/c mice (Th2 bias) with S. rectivirgula, obtained spleen and lung associated lymph node (LALN) cells, cultured the cells with specific antigen, and attempted adoptive transfer of EHP. We also treated both C3H/HeJ and BALB/c donor mice with IL4 and anti-IFNγ before exposure to S. rectivirgula and then cultured cells from both spleen and LALN before attempted transfer of EHP. We found that cultured spleen and lung associated lymph node cells can adoptively transfer EHP in both C3H/HeJ and BALB/c mice as demonstrated by infiltration of the recipient lungs with CD4+ lymphocytes. Treatment of both mouse strains with IL4 and anti-IFNγ did not change the ability of cultured cells to adoptively transfer EHP. We conclude that EHP induced by S. rectivirgula can occur in animals with either a Th1 or a Th2 bias and is not altered by treatment with IL4 and anti-IFNγ. This suggests that attributes of the antigen and not genetic background or cytokine environment at the site of initial sensitization determines the results of exposure to S. rectivirgula.
Keywords: hypersensitivity pneumonitis, IL4
Hypersensitivity pneumonitis (HP) is a group of lung diseases that result from repeated pulmonary exposure to various organic, antigenic materials with uncertain immunopathogenetic mechanisms. Despite apparent uniform exposure, most subjects exposed to the agents that cause HP do not develop clinically evident disease in mice (Schuyler 1997).
The relative importance of genetic background, nature of the immune response and antigen attributes in expression of HP are not known. Although early studies suggested an association of specific HLA haplotypes with susceptibility to development of HP, most recent reports do not support this hypothesis. The most consistent association between development of HP and the immune response is the protective effect of current cigarette smoking against expression of HP (Schuyler 1997). Cigarette smoking has many effects on the immune response, in general acting as a mild immunosuppressive agent.
Our model of adoptive transfer of experimental hypersensitivity pneumonitis (EHP, using S. rectivirgula, one agent that causes Farmer's Lung Disease in humans; Schuyler 1997), in mice (Schuyler et al. 1991) is useful for distinguishing events that lead to development of effector cells from events that occur after interaction of effector cells and antigen. Murine adoptive EHP is mediated by appropriately cultured CD4+ cells (Schuyler et al. 1992), and CD4+ cell lines derived from lung associated lymph nodes can be established that either have the characteristics of Th1 cells (high IFNγ and low IL4 secretion) which transfer EHP, or Th2 cells (low IFNγ and high IL4) which do not transfer EHP (Schuyler et al. 1997).
BALB/c mice produce a Th2 type response in various murine models of infectious and autoimmune diseases (Hsieh et al. 1995; Pearlman et al. 1995; Reiner & Locksley 1995; Urban et al. 1996). Since all the previously tested mouse strains in experimental hypersensitivity pneumonitis (C3H/HeJ, SJL/J and C57Bl/6) (Schuyler et al. 1991) exhibit a Th1 bias, we tested the hypothesis that BALB/c mice would respond in a manner different than these strains.
We also attempted to change the nature of the immune response to S. rectivirgula in both C3H/HeJ and the BALB/c mice by administration of IL4 and antibody to IFNγ, methods that have been used to change a Th1 to a Th2 response and alter the response to infectious challenges in other murine models. We examined specific antibody isotype response to S. rectivirgula; and cytokine secretion and total immunoglobulin isotype in cultured cells from both C3H/HeJ and BALB/c mice, as these have been found to reflect the nature of the immune response and to change as a result of experimental manipulation.
We found qualitative, but not quantitative differences between C3H/HeJ and BALB/c mice in ability to adoptively transfer EHP. Treatment of both C3H/HeJ and BALB/c animals with IL4 and anti-IFNγ did not change the ability to adoptively transfer EHP, despite appropriate changes of cytokine secretion patterns in BALB/c animals.
These data indicate that neither genetic background nor manipulation of cytokine environment at the time of initial antigenexposure in vivo substantially changes murine adoptive EHP. This suggests that attributes (including dose and route of administration) of the antigen determines the characteristics of the pulmonary response.
Methods
Animals
Male C3H/HeJCr and BALB/cAnCr mice (NCI, Frederick Cancer Research Facility, Frederick, Md) were housed in laminar flow hoods with HEPA filtered air in the Veterinary Medical Unit at the Albuquerque VA Medical Center which is fully accredited by the American Association for Accreditation of Laboratory Animal Care.
Study design
Our design is to utilize our adoptive transfer model of experimental hypersensitivity pneumonitis in animals to attempt to alter the results of intratracheal (i.t.) administration of the agent, Saccharopolyspora rectivirgula (S. rectivirgula), which causes Farmer's Lung Disease in humans. The dependent variables are the extent of histological abnormalities and bronchoalveolar lavage characteristics. We compared the results of i.t. antigen exposure in mice that exhibit a Th2 bias (BALB/c) to those with a Th1 bias (C3H/HeJ). We also attempted to change the nature of the response by administering IL4 and anti-IFNγ.
Antigen
S. rectivirgula was obtained from V. Kurup, Medical School of Wisconsin, Milwaukee, Wis. and prepared as previously described (Schuyler et al. 1991, 1992, 1993, 1997).
Intratracheal (i.t.) inoculation
Mice were anaesthetized with a mixture of ketamine and xylazine. Lyophilized S. rectivirgula was suspended in sterile pyrogen free normal saline and injected into the trachea per os.
Cell preparation and culture
Lung associated lymph nodes (LALN) were removed from the right hilar and right mediastinal area and dispersed. Spleens were removed and splenic cells dispersed into medium by pressing spleen pieces between the frosted ends of two sterile microscope slides and filtered through a 70-mm cell strainer. Erythrocytes were lysed by exposure to 0.15 m NH4Cl, the cells washed, and cultured in RPMI 1640 with penicillin, streptomycin, 2 mm glutamine, 5 × 105 m 2-mercaptoethanol and 10% in tissue culture dishes at 2 × 106 cells/ml in a 5% CO2 humidified atmosphere for 72 h with a soluble extract of S. rectivirgula (30 μg/ml). Cultures were harvested by centrifugation, supernatants frozen at −70°C for cytokine assays, cells washed, viability determined using trypan blue dye exclusion and 5 × 106 LALN or 20 × 106 spleen cells injected intravenously (i.v.) in 0.3 ml volume into the tail veins of recipients.
In some experiments, mouse lungs were lavaged prior to LALN removal. The trachea was cannulated and 6 × 1 ml washes of normal saline were collected and centrifuged. Bronchoalveolar lavage (BAL) cells were resuspended in RPMI-10, counted, cytocentrifuge preparations made, stained with Diff-Quick (Dade Diagnostics, AguAdA, P.R. USA), and evaluated for cell type.
Antibody isotypes
The amounts of serum specific anti-S. rectivirgula IgG1 and IgG2a and total IgG1 and IgG2a were measured by ELISA. In brief, a 96 well polycarbonate plate (NUNC, Naperville, IL) was coated with 10μg/ml (in PBS) soluble S. rectivirgula, or either goat antimouse IgG1 or IgG2a (Southern Biotechnology Associates, Birmingham, AL, USA). Skim milk 2.5% was used to block nonspecific binding sites and to dilute reagents and sera samples. After washing four times with PBS with Tween 20, sample and/or standard solution were added to each well and incubated at 37°C for 1 h. Horseradish peroxidase labelled goat antimouse IgG1 or IgG2a (Southern Biotechnology Associates) were added to each well, with appropriate washing and incubation steps before addition of an ABTS substrate. Plates were incubated for 30 min at room temperature and absorbance read at 405 nm with a reference wavelength of 550 nm. Unknown serum specific samples were expressed as optical density units (ODU) which is the optical density multiplied by the dilution factor. Unknown total IgG1 and IgG2a samples were compared with standard curves of IgG1 and IgG2a and expressed as ng/ml.
Cytokines
IFNγ and IL10 were measured using an ELISA (Schuyler et al. 1997). IL4 was measured using an IL4 responsive cell line (CT4.S) (Fei et al. 1995). IL5 was measured by ELISA using the Interleukin-5 Minikit (KM-IL5) from ENDOGEN (Cambridge, MA). The lower limit of the assays sensitivity are: IL4: 1.25 pg/ml; IFNγ: 600 pg/ml; IL5: 30 pg/ml; IL10: 62.5 pg/ml.
IL4 and anti-IFNγ
IL4 was obtained from cultures of CHO cells transfected with the IL4 gene (kindly provided by Dr Joan Stein-Streilein, University of Miami School of Medicine) and purified using the Affi-Gel H2 Immunoaffinity kit (BioRad, Richmond, CA). Anti-IFNγ clones R4–6A2 and XMG1.2 were obtained from ATCC, grown in pristane induced ascitic fluid of nude mice and antibody purified by caprylic acid clarification, saturated ammonium sulphate precipitation and ion exchange chromatography. Control animals were treated with 1% BSA (the carrier for IL4) and rat isotype control.
Histological studies
The trachea was cannulated and the lungs inflated with pH 6.9 buffered formalin under 20 cm water pressure for 48 h. After inflation, the lungs were sectioned (transverse for mediastinal lobe and sagittal for the other 4 lobes), embedded in a single paraffin block, and a 5 micron section cut and stained with haematoxylin and eosin. The slides were evaluated without knowledge of treatment. The area covered by an eyepiece grid (0.99 mm × 0.99 mm using × 100 magnification) was judged to be normal or abnormal. An abnormal field is one with increased number of cells in the interstitium or alveoli or both. An average of 300 fields were evaluated from each mouse (50% of the area under the cover slip).
Immunohistochemistry
Some animals lungs were prepared for frozen sections by inflation at 30 cm hydrostatic pressure via the trachea with a 1:3 solution of O.C.T. (Tissue Tek, Miles, Inc., Elkhart, IN) and 20% sucrose in phosphate buffered saline. The lungs were quickly frozen in isopentane followed by liquid nitrogen, and stored at −80°C until sectioned at 8 μm on a cryostat.
To evaluate locations and relative proportions of CD4 and CD8 lymphocytes, serial frozen lung sections were placed on charged glass slides, fixed in 4°C acetone for 10 s, and immunostained as described in detail by Nikula et al. (1997). Briefly, endogenous peroxidase was blocked with H2O2 and serial slides from each mouse were reacted with rat antimouse IgG2a monoclonal antibodies (PharMinigen, Sorrento Valley, CA) to detect mouse CD4+ (CD4; L3T4) and CD8+ (CD8a; Ly-2) lymphocytes. Isotype-specific rat immunoglobulins (IgG2a; PharMinigen) were substituted for primary antibodies on serial immunoglobulin (negative) control slides. Biotinylated rabbit antirat (mouse adsorbed) IgG secondary antibody (Vector Laboratories, Burlingame, CA) followed by an avidin-biotin peroxidase system (ABC Elite Kit; Vector Laboratories) and 3, 3(–diaminobenzidine were used to detect bound CD4 and CD8 antibodies. The slides, which were not counterstained, were evaluated using light microscopy.
Data analysis
Since the percent abnormal (p^) histological fields is obtained from binary outcomes, we first applied the transformation designed to equalize the variances of such(binomial like) responses: y = arcsin (square root (p^) (Feinberg 1980). A concurrent control (RPMI) group was used with each experiment. Analysis of variance using Tukey's hsd procedure was used to compare groups. Multiple anova and Fisher's lsd method of post hoc multiple comparisons was used to compare the effect of cell source and time (Systat 1997).
Results
Determination of pulmonary histological response of BALB/c mice to i.t. S. rectivirgula
Animals were injected intratracheally once with varying amounts of S. rectivirgula and sacrificed 4 days thereafter. The extent of pulmonary inflammation was dependent on the amount of injected S. rectivirgula (Figure 1). The slope of the curve and the y-intercept of the relationship between amount of injected S. rectivirgula in BALB/c mice was similar to that of C57Bl/6, SJL/J and C3H/HeJ mice (Schuyler et al. 1991).
Figure 1.
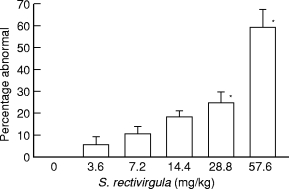
Extent of pulmonary histological abnormalities in animals receiving differing amounts of S. rectivirgula intratracheally. Animals were sacrificed 96 h after receiving a specified amount of S. rectivirgula. Percentage abnormal is percentage of 300 microscopic fields/animal that are abnormal. Six animals per group. Values are expressed as mean and standard error of the mean. *P < 0.05 compared to 3.6 mg/kg S. rectivirgula. 0 mg/kg is different (P < 0.05) from all other values. (Analysis of variance with Tukey's hsd procedure).
Comparison of response of BALB/c to C3H/HeJ to 3 i.t. injections of S. rectivirgula
Serum antibody isotypes
Animals were treated with 3 i.t. injections of 7.2 mg/kg S. rectivirgula every other day. Specific anti-S. rectivirgula IgG1 and IgG2a antibody and serum total IgG1 and IgG2a were measured on day 0, 11, 25 and 35 after the first i.t. exposure to S. rectivirgula (Table 1).
Table 1.
Schedule
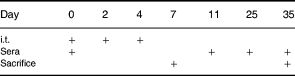
i.t., Intratracheal injection of 7.2 mg/kg S. rectivirgula.
C3H/HeJ mice exhibited more serum IgG1 and IgG2a anti-S. rectivirgula antibody than BALB/c mice (Figure 2) (Multiple anova and Fisher's lsd method of post hoc multiple comparisons). Two-way analysis of variance indicated that there was a significant (P < 0.05) effect of both the strain classification (i.e. BALB/c and C3H/HeJ), and the time from immunization. Total IgG1 was higher inthe BALB/c than the C3H/HeJ mice, but total IgG2a was not different between the strains (Table 2). In addition, total IgG1 increased after immunization (Multiple anova and Fisher's lsd method of post hoc multiple comparisons).
Figure 2.
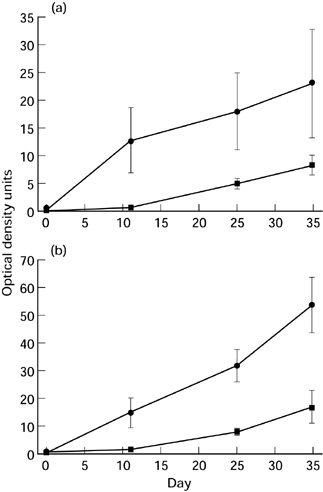
Anti-S. rectivirgula serum antibody in BALB/c (▪) and C3H/HeJ (•) mice after 3 i.t. inoculations with S. rectivirgula. Results are expressed as Optical density units which are the optical density multiplied by the dilution factor. a, Antigen specific IgG1. b, Antigen specific IgG2 Mean, standard error of the mean. Two-way analysis of variance indicated that there was a significant (P < 0.05) effect of both the strain classification (i.e. BALB/c and C3H/HeJ), and the time from immunization.
Table 2.
Total serum immunoglobulin isotypes after immunization with S. rectivirgula
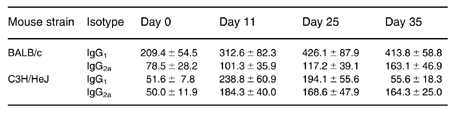
Mean (ng/ml) ± s.e.m. n = 5–7. Days refer to time since the first of 3 i.t. injections of 7.2 mg/kg S. rectivirgula.
Total IgG1 is higher (P < 0.05) in the BALB/c than the C3H/HeJ mice, but total IgG2a is not different between the strains. Total IgG1 increased after immunization in the BALB/c mice (Multiple anova and Fisher's lsd method of post hoc multiple comparisons).
Cytokine and immunoglobulin profile of in vitro cultures
We analysed cytokine profile and immunoglobulin secreted into spleen cell cultures. Spleens were removed and cultured with 30 mg/ml S. rectivirgula for 72 h 7 or 35 days after the first i.t. S. rectivirgula inoculation.
We detected very small amounts of IL4 in cultures from both BALB/c and C3H/HeJ mice, but substantial amounts of IFNγ in supernatants of cultures from C3H/HeJ animals sacrificed 7 days after the first of 3 i.t. injections (Table 3).
Table 3.
Culture supernatant cytokines
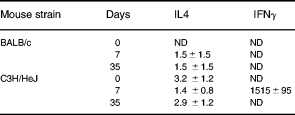
Antigen (30 μg/ml S. rectivirgula) stimulated spleen cell cultures (2 × 106/ml, 72 h). All values are ρg/ml. Days, Days after first of 3 i.t. injections of S. rectivirgula. 0, Cells from naïve animals. ND, None detected. Mean ± s.e.m. n = 3–5.
After 72 h of culture, we detected substantial amounts of total IgG1 and IgG2a in culture supernatants from both C3H/HeJ and BALB/c immunized mice with more IgG1 and IgG2a in supernatants from cultures from C3H/HeJ cells obtained 7 days after the first of 3 i.t. exposures to S. rectivirgula (Table 4).
Table 4.
Culture supernatant immunoglobulin (Total)
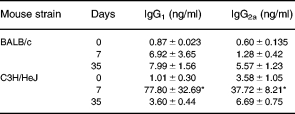
Antigen (30 μg/ml S. rectivirgula) stimulated spleen cell cultures (2 × 106/ml, 72 h). Mean, s.e.m. Days, Days after first of 3 i.t. injections of S. rectivirgula. 0, Cells from naïve animals. n, 3–5.
*P < 0.05 compared to all other values (anova with Tukey's hsd comparison).
Adoptive transfer of EHP
Donor animals were sensitized with 3 i.t. inoculations of 7.2 mg/kg S. rectivirgula (Day 0, 2 and 4) and sacrificed 3 days after the last challenge (Day 7) (Figure 3). LALN and spleen cells were cultured, the cells were harvested and transferred to recipient animals. Eight days thereafter, mice were challenged i.t. with 7.2 mg/kg S. rectivirgula and sacrificed by exsanguination four days later for pulmonary histological examination.
Figure 3.
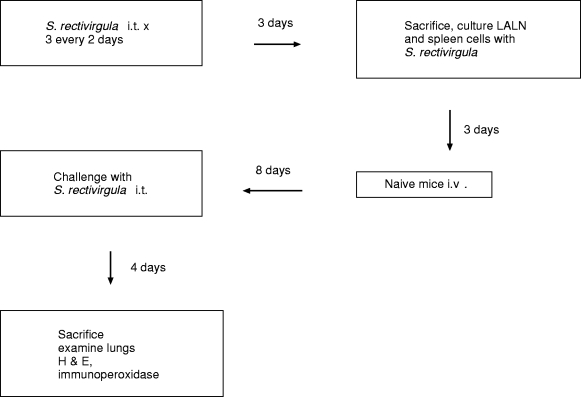
Experimental design of adoptive transfer model. i.t., intratracheal; i.v., intravenous; LALN, Lung associated lymph nodes; H & E, Hematoxylin and eosin.
Cell culture
The yield from culture and the proportion of cells with high cytoplasmic/nuclear ratio (i.e. blast cells) was similar in the two mouse strains (data not shown).
Histology
The extent of histological abnormalities was similar in these 2 strains of mice (Figure 4).
Figure 4.
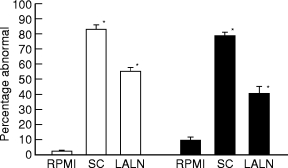
Extent of pulmonary histological abnormalities in BALB/c (□) and C3H/HeJ (▪) recipients of 20 × 106 cultured spleen cells or 5 × 105 lung associated lymph node cells 4 days after intratracheal challenge with S. rectivirgula. Cells were transferred 8 days before intratracheal challenge. Percentage abnormal, percentage of 300 microscopic fields per animal that are abnormal; RPMI, animals treated with RPMI i.v.; LALN, Lung associated lymph nodes. Five to 12 animals per group. *P < 0.05 compared to RPMI group. There are no differences among either the spleen cell or the LALN cell groups (Analysis of variance with Tukey's hsd procedure).
Although the severity of pneumonia was similar in BALB/c and C3H/HeJ mice four days after administration of S. rectivirgula, there were differences in the histological characteristics of the response. BALB/c mice exhibited diffuse interstitial pneumonia with marked perivascular and peribronchiolar cuffing (Figure 5A). The cuffs were densely cellular, and the majority of the cells within the cuffs were lymphocytes (Figure 5C). Mitotic lymphocytes were frequent. Lesser numbers of eosinophils and monocytes were present in the cuffs. There were mild septal alveolar infiltrates of lymphocytes, eosinophils, macrophages, and neutrophils. Few cells were present in the alveolar lumens of BALB/c mice (Figure 5E). When present, lumenal cells were primarily small macrophages.
Figure 5.
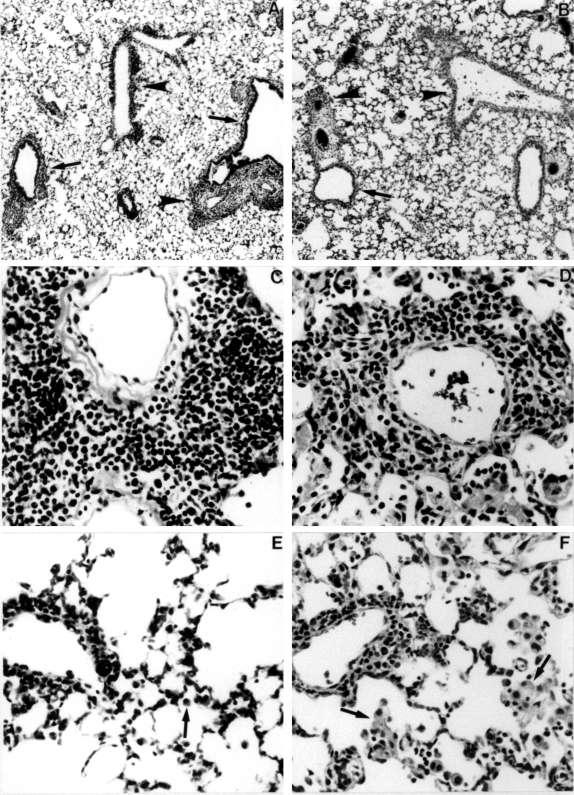
Histopathological characteristics of adoptively transferred EHP. A, Diffuse interstitial pneumonia in a BALB/c mouse. Note the cellular cuffs around bronchioles (arrows) and veins (arrowheads (× 60). B, Interstitial pneumonia in a C3H/HeJ mouse. Cuffs around bronchioles (arrows) and veins (arrowheads) are less prominent compared to the BALB/c mouse. Close inspection shows more cells in alveoli in the C3H/HeJ mouse (× 60). C, Predominantly lymphocytic perivascular cuff in a BALB/c mouse (× 360). D, Pleocellular perivascular cuff in a C3H/HeJ mouse. Numerous macrophages and neutrophils are present in the lumens of adjacent alveoli (× 360). E, Small perivascular lymphocytic cuff and alveolar septal infiltrates in a BALB/c mouse. Few cells are present in alveolar lumens; those present are small macrophages (arrow) (× 300). F, Small perivascular pleocellular cuff and intra-alveolar cellular infiltrate. Alveolar macrophages have abundant cytoplasm (arrows) (× 300).
C3H/HeJ mice exhibited multifocal to diffuse interstitial pneumonia with relatively more intralumenal cells than BALB/c mice (Figure 5B). The perivascular and peribronchiolar cuffs were more pleocellular in C3H/HeJ mice (Figure 5D). Unlike BALB/c mice, which had cuffs around all sizes of veins and venules distributed throughout the lungs, the cuffs in C3H/HeJ mice were less evenly distributed and tended to occur in foci with intralumenal cellular exudates (Figures 5D, 5F). The cells in the cuffs and septal infiltrates were monocytes, eosinophils, lymphocytes, and neutrophils. Alveolar lumenal exudates consisted of macrophages and neutrophils. The macrophages had abundant, frequently vacuolated cytoplasm (Figures 5D, 5F).
Immunohistochemistry
The lymphocytes in perivascular and peribronchiolar cuffs and in septal infiltrates were CD4+ T lymphocytes in both strains of mice (Figures 6A, 6B). CD8 + lymphocytes were infrequent and scattered throughout the lungs (Figures 6A, 6B).
Figure 6.
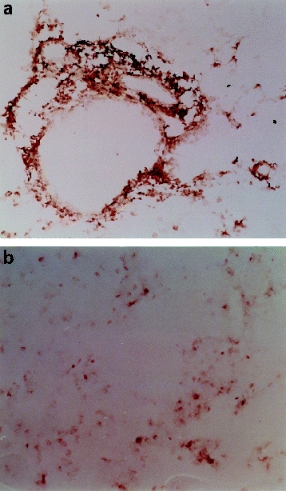
Immunohistochemistry. A, Perivascular cuff in a C3H/HeJ mouse immunoreacted with anti-CD4 primary antibody. CD4 cells in the cuff and adjacent alveolar septa are stained grey to black in this photograph (× 180). B, Serial section immunoreacted with anti-CD8 primary antibody. Only a few cells are stained. Example shown is from a C3H/HeJ mouse; immunophenotyping results were the same in both strains (× 180).
Treatment with IL4 and anti-IFNγ
We attempted to alter the nature of the immune response of both Balb/c and C3H/HeJ mouse strains to S. rectivirgula by treating animals with IL4 and anti-IFNγ during the sensitization period (Figure 7). We determined in preliminary studies that one i.t. exposure to S. rectivirgula is sufficient to produce cells that can adoptively transfer EHP after culture in both mouse strains.
Figure 7.
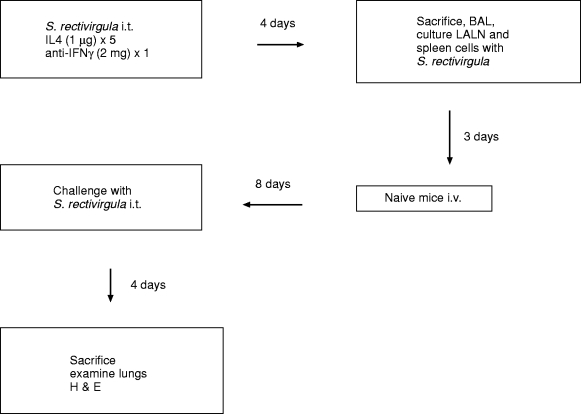
Experimental design of modulation of adoptive transfer model. i.t., intratracheal; i.v., intravenous; LALN, Lung associated lymph nodes; BAL, Bronchoalveolar lavage; H & E, Hematoxylin and eosin.
Three different treatment protocols were used to determine the effects of IL-4 with or without added anti-IFNγ on S. rectivirgula sensitized mice, using doses of IL4 and anti-IFNγ comparable to those which changed the immune response to Leishmania (Scott 1991; Chatelain et al. 1992), Schistosomiasis (Smythies et al. 1992), cerebral malaria (Grau et al. 1989), cardiac transplantation (Piccotti et al. 1998) and models of autoimmune diseases (Racke et al. 1994).
First, BALB/c mice which had received a single i.t. S. rectivirgula, followed by 7 × 1 mg IL-4 intraperitoneal injections (0, 12, 24, 36, 48, 60, 77 h post i.t.), were sacrificed 4 days post i.t. Control animals were treated with 1% BSA (the carrier for IL4). Spleen and BAL cells were collected, and spleen cells were cultured with 30 μg/ml S. rectivirgula for transfer into recipient mice which were subsequently challenged 8 days later with a single i.t. S. rectivirgula, and sacrificed for histological observations 4 days post challenge i.t. We found no effect of treatment of donor animals with IL4 or BSA on the ability of cells to adoptively transfer EHP (data not shown), or in bronchoalveolar cell characteristics (data not shown).
Next we added 2 mg intraperitoneal injections of anti-IFN g (clone R4–6A2) or normal rat IgG (Jackson ImmunoResearch Labs, West Grove, PA), 2 days prior to i.t. S. rectivirgula combined with 5 × 1 mg IL-4 or 1% BSA (−1, 0, 1, 2, 3 days post i.t.). We also examined the ability of cultured LALN cells to adoptively transfer EHP and the cytokine content of supernatants of cultures of both spleen and LALN cells. There were no differences of the numbers of cells in the LALN and spleens harvested from both BALB/c and C3H/HeJ animals treated with IL4 and anti-IFNγ, compared to those treated with BSA and rat isotype control (data not shown). Treatment of BALB/c or C3H/HeJ donor animals with IL4 plus anti-IFNγ had no effect on the ability of either spleen or LALN cells to transfer EHP (Figure 8 and data not shown). There were also no differences of bronchoalveolar lavage parameters (Table 5). However treatment did change the cytokine profile of culture supernatants of BALB/c LALN cell cultures. The amounts of IL4, IL5 and IL10 (Table 6), but not IFNγ (data not shown), were increased in supernatants from cultures of LALN cells from animals treated with IL4 plus anti-IFNγ, compared to untreated animals, or animals treated with control antibody and BSA. Similar treatment of BALB/c mice with IL4 and another anti-IFNγ monoclonal antibody (XMG1.2, 2 mg each at 2 days before, at the time, and 2 days post i.t. S. rectivirgula) produced comparable results in that ability to adoptively transfer was not altered (data not shown).
Figure 8.
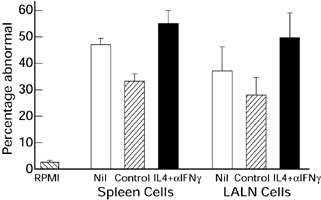
Extent of pulmonary histological abnormalities in BALB/c recipients of 20 × 106 cultured spleen cells or 5 × 105 lung associated lymph node cells 4 days after intratracheal challenge with S. rectivirgula. Cells were transferred 8 days before intratracheal challenge. Percentage abnormal, percentage of 300 microscopic fields per animal that are abnormal; RPMI, Animals treated with RPMI i.v.; Control, Donor animals treated with BSA (carrier for IL4) and isotype control antibody; Nil, Donor animals not treated; IL4+αIFNγ, Donor animals treated with IL4 and anti-IFNγ antibody; LALN, Lung associated lymph nodes. Five to 12 animals per group. The RPMI group is different (P < 0.05) from all spleen cell groups and all LALN groups. There are no differences among either the spleen cell or the LALN cell groups (Analysis of variance with Tukey's hsd procedure).
Table 5.
Bronchoalveolar lavage characteristics C3H/HeJ and BALB/c
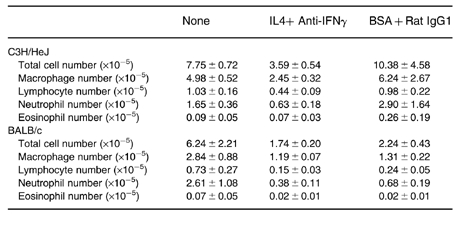
All animals received one i.t. injection with S. rectivirgula. IL4, 5 × 1 μg IL-4 intraperitoneal (i.p.) injections (−1, 0, 1, 2, 3) days post i.t. S. rectivirgula). Anti-IFNγ, Anti-IFNγ (clone R4–6A2), 2 mg i.p. 2 days prior to i.t. S. rectivirgula). BSA, 1% bovine serum albumin (carrier for IL4). Rat IgG1, Rat isotype control. Mean ± s.e.m. 6–7 experiments per group. There are no differences between the groups (anova with Tukey, s hsd comparison).
Table 6.
Culture supernatant cytokines
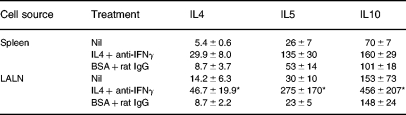
BALB/c mice. Antigen (30 μg/ml S. rectivirgula) stimulated cell cultures (2 × 106/ml, 72 h). Mean, s.e.m. All cytokine concentrations pg/ml. LALN, Lung associated lymph nodes. IL4 + anti-IFNγ, 5 × 1 μg IL-4 (−1, 0, 1, 2, 3 days post i.t. S. rectivirgula); plus 2 mg intraperitoneal anti-IFNγ (clone R4–6A2), 2 days prior to i.t. S. rectivirgula. BSA + rat IgG, 5 injections of 1% BSA using the same schedule as IL4 plus 2 mg of rat IgG using the same schedule as anti-IFNγ. Nil, No treatment. n, 5–12.
*P < 0.05 compared to both Nil; and IL4 + anti-IFNγ treatments (anova with Tukey's hsd comparison).
Discussion
Hypersensitivity pneumonitis is characterized by clinical illness occurring in only a minority of exposed subjects despite evidence of sensitization (i.e. serum antibody and bronchoalveolar lavage lymphocytosis) in most exposed subjects. The reasons for this phenomenon are unknown, but could include characteristics of the host (including the nature of the immune response), or the agent that causes HP. Differences of genetic backgrounds as reflected by HLA haplotypes or TCR usage have not demonstrated differences between exposed but not ill and exposed and ill subjects (Christensen et al. 1975; Rittner et al. 1983; Selman et al. 1987; Terho et al. 1987; Richeldi et al. 1993). However, there are differences in isotypes of antibody directed against agents that cause HP, that suggest that more subtle differences of immune response might determine the result of antigen exposure. Pigeon breeders who remain asymptomatic have a higher level of serum IgG4 antibody directed against pigeon antigens than those who develop HP (Kitt et al. 1986). IgG4 is characteristic of a Th2 response in humans (Ishizaka et al. 1990; Spiegelberg et al. 1991; Schmitt et al. 1994).
Our model of adoptive transfer of EHP allows examination of the role of genetic factors and cytokine environment at the time of initial antigen exposure by separating sensitization, development of effector cells and the results of interaction of effector cells and antigen. Cells from animals sensitized to S. rectivirgula, previously known as Micropolyspora faeni, the organism responsible for Farmer's Lung Disease in humans (Schuyler 1997), are cultured in the presence of S. rectivirgula and injected into recipients. The recipient animals exhibit exaggerated pulmonary histological response to intratracheal (i.t.) challenge with S. rectivirgula. This phenomenon is dependent on sensitization of the donors, culture of the cells with antigen, concentration of antigen and the number of transferred cells (Schuyler et al. 1991). This model (administration of cultured cells from sensitized animals before i.t. challenge with S. rectivirgula), is designated as adoptive EHP (Figure 3).
Current concepts of immunological reactivity derive from seminal observations by Mosmann and Coffman who reported that murine CD4+ cell clones can be driven in vitro to one of several patterns of cytokine secretion (Mosmann et al. 1986). Th1 cells preferentially secrete IFNγ and tumour necrosis factor-β (TNFβ); activate macrophages; are responsible for cell-mediated immunity reactions and CTL (cytotoxic lymphocytes); and provide help for IgG2a production. Th2 CD4+ cells secrete IL-4, IL-5, IL-9, IL-10 and IL-13; provide help for immunoglobulin (particularly IgE and IgG1) secretion; enhance eosinophil production, survival, and activity; and promote mast cell proliferation and maturation. Development of either antigen specific Th1 or Th2 response after in vivo exposure in mice is dependent on many factors, including attributes of the antigen; site of antigen delivery; adjuvant used; age, sex and genetic background of the mouse; nature of the antigen-presenting cell encountered; and involvement of different costimulatory molecules (Gause et al. 1997). Cytokines secreted by one CD4+ subset inhibit the development of the reciprocal subset leading to a predominance of one of the subsets and polarization of the antigen specific immune response (Finkelman 1995; Ria et al. 1998). IL-4 is particularly important in inducing differentiation of Th2 cells, and IL-12 and IFNγ in inducing differentiation of Th1 cells, from Thp (Th precursor) cells. Irreversible commitment to a Th1 or Th2 phenotype is probably mediated by rapid loss of the ability of IL-4, or IL-12 and IFNγ, to initiate intracellular signalling in differentiated Th1 or Th2 cells, respectively (Guler et al. 1997; Nakamura et al. 1997; Szabo et al. 1997). This lack of responsiveness of differentiated Th2 or Th1 cells is caused by either changes in IL4 or IL12 receptors or in cytoplasmic and/or nuclear signal transduction factors, such as phosphorylation of Janus and STAT kinases (Bacon et al. 1995; Jacobson et al. 1995; Pernis et al. 1995; Guler et al. 1997; Szabo et al. 1997).
Recently, it has become evident that the above formulation (Th1 or Th2 cells) may be overly simplistic in that resting memory differentiated Th cells can produce cytokines characteristic of both Th1 and Th2 cells, and that administration of IL12 can increase the antigen specific production of both IFNγ and IL4 by CD4+ cells (Bliss et al. 1996). However, the concept that the cytokine milieu (derived from many possible sources, such as macrophages, dendritic cells, CD4+ cells, CD8 + cells, NK cells, mast cells, eosinophils, γδ T cells) at the time of antigen specific CD4+ cell differentiation, determines the later pattern of cytokine secretion and function of the differentiated cells is valid.
IL12 is necessary for expression of HP in murine models of HP which use repetitive intrapulmonary administration of S. rectivirgula (Gudmundsson et al. 1998). Gudmundsson found that mouse strains differ in susceptibility to HP with C57Bl/6 more sensitive than DBA/2 mice (Gudmundsson et al. 1998). The increased sensitivity of the C57Bl/6 mice correlated with increased expression of IL12. The decreased susceptibility of the DBA/2 mice could be ablated by administration of IL12. These data support the concept that expression of EHP correlates with expression of IL12, the prototypic Th1 cytokine which drives differentiation of the immune response to a Th1 type of response. Our recent demonstration that Th1, but not Th2 cell lines, adoptively transfer EHP (Schuyler et al. 1997) also supports the concept that EHP is a Th1 disease. This is consistent with several animal models of autoimmune disease, such as autoimmune allergic encephalomyelitis, collagen induced arthritis and experimental allergic uveitis (Liblau et al. 1995).
Despite the strong Th2 bias of BALB/c mice, we did not find quantitative differences between BALB/c and C3H/HeJ mice in the pulmonary response to i.t. exposure to S. rectivirgula, or in the ability of cultured sensitized cells to adoptively transfer EHP (Figures 1 and 4). This is consistent with experimental allergic encephalomyelitis in which there was no difference between the response of BALB/c and C57Bl/6 mice to administration of a large amount of a crude antigen mixture (guinea pig spinal cord) (Falcone et al. 1998), but there were differences after administration of a component of spinal cord, proteolipid apoprotein (Tuohy et al. 1988). Since we administered a crude antigen mix, we might have obscured differences that would be evident if we used a single component.
In addition, Shibuya et al. (1998) recently demonstrated that IL1α and TNFα promote the differentiation of Th1 cells in BALB/c mice. S. rectivirgula causes IL1α and TNFα secretion from alveolar macrophages (Denis et al. 1991) and may therefore induce Th1 cells in BALB/c mice.
However, there were qualitative differences between the mouse strains in that the cellular infiltrate tended to include more intralumenal cells in the C3H/HeJ compared to the BALB/c animals. Both strains demonstrated interstitial infiltration with cells, but the cell mixture was more pleomorphic in the C3H/HeJ animals.
The amount of serum total IgG1 was higher in the BALB/c than the C3H/HeJ mice (Table 2) and spleen cells produced less IFNγ from BALB/c than from C3H/HeJ animals (Table 3), consistent with the Th2 bias of BALB/c animals. However, the amount of both IgG1 and IgG2a anti-S. rectivirgula serum antibody was higher in C3H/HeJ than BALB/c mice (Figure 2). This corresponded with in vitro secretion of these antibodies from spleen cells obtained 3 days after the last of 3 i.t. exposures to S. rectivirgula (Table 4). The larger amount of serum IgG2a specific antibody in C3H/HeJ (compared to BALB/c) animals is consistent with their Th1 bias. However, a similar increase of serum IgG1 specific antibody in C3H/HeJ animals is somewhat surprising in view of the general conception that IgG1 is a Th2 cytokine. Results of administration of CpG oligodexynucleotides to BALB/c mice indicate that the amount of specific IgG1 antibody may not invariably reflect Th2 differentiation (Chu et al. 1997).
Treatment with IL4 and anti-IFNγ did not affect BAL cell characteristics (Table 5) or the ability of cultured cells to adoptively transfer EHP (Figure 8), but did change the cytokine profile (increased IL4, IL5 and IL10) in culture supernatants of LALN cells from treated compared to untreated BALB/c animals (Table 6). This indicates that our treatment did have an effect on the nature of the immune response to S. rectivirgula.
Therefore, unlike our ability to change the competence of cultured cells to adoptively transfer EHP by in vitro manipulation of the cytokine environment (Schuyler et al. 1997), we could not change these cells by in vivo manipulation of the cytokine environment present at the time of initial presentation of antigen. Our results are somewhat different from those obtained by Ghadirian & Denis (1992) who demonstrated a decrease in lung inflammatory response in mice administered S. rectivirgula treated with 1 μg IL4 weekly. However there are many differences between the experimental protocols, the most important of which is the mouse strain (C57Bl/6), and the use of multiple (9) injections of S. rectivirgula by Ghadirian & Denis (1992), compared to one injection in our protocol. We used a similar amount of IL4 for each IL4 treatment, but administered more treatments (5 compared to 3) over a shorter duration (4 days compared to 21 days). The lack of effect of treatment with anti-IFN γ on BAL cellularity is consistent with previous results using anti-IFNγ (Denis & Ghadirian 1992) and IFNγ knockout mice (Gudmundsson & Hunninghake 1997), and suggests that IFNγ is not necessary for S. rectivirgula induced lung inflammation as reflected by BAL cells.
Our results may be due to the adjuvant nature of S. rectivirgula, a thermophilic actinomycetes which is related to mycobacteria. Saccharopolyspora rectivirgula is a potent adjuvant (Bice et al. 1974) and mitogen (Smith et al. 1978), promotes the secretion of inflammatory cytokines from macrophages (Denis et al. 1991), and thus tends to produce a Th1 response (Shibuya et al. 1998). Future experiments will explore the ability of S. rectivirgula to induce the secretion of cytokines that cause differentiation of Th1 cells.
In summary, we found that differences in the nature of the murine immune response to intratracheally administered S. rectivirgula, either due to genetic factors or to manipulation of the cytokine environment at the time of initial antigen exposure, did not affect the extent of pulmonary histological changes caused by exposure to this material. In addition, genetic differences and cytokine manipulation in vivo did not affect the ability of cultured cells to adoptively transfer EHP. This implies that attributes of S. rectivirgula, rather than genetic or immunoregulatory differences in the host, determine the outcome of exposure to this agent. Based on these data, manipulation of cytokine environment in vivo would not be expected to change the response of humans to exposure to S. rectivirgula.
Acknowledgments
The authors wish to acknowledge the excellent technical assistance of Ms. Jennifer Berger.
This work was supported by the Veterans Administration Research Service and NHLBI grant HL44253.
References
- Bacon CM, McVicar DW, Ortaldo JR, Rees RC, O'shea JJ, Johnston JA. Interleukin 12 (IL-12) induces tyrosine phosphorylation of JAK2 and TYK2: differential use of Janus family tyrosine kinases by IL-2 and IL- 12. J Exp Med. 1995;181:399–404. doi: 10.1084/jem.181.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bice D, McKarron K, Hoffman E, Salvaggio J. Adjuvant properties of Micropolyspora faeni. Int Arch Allergy Appl Immunol. 1974;55:267–273. doi: 10.1159/000231935. [DOI] [PubMed] [Google Scholar]
- Bliss J, Van Cleave V, Murray K, et al. IL-12, as an adjuvant, promotes a T helper 1 cell, but does not suppress a T helper 2 cell recall response. J Immunol. 1996;156:887–894. [PubMed] [Google Scholar]
- Chatelain R, Varkila K, Coffman RL. IL-4 induces a Th2 response in Leishmania major-infected mice. J Immunol. 1992;148:1182–1187. [PubMed] [Google Scholar]
- Christensen LT, Schmidt CD, Robbins L. Pigeon breeders' disease — a prevalence study and review. Clin Allergy. 1975;5:417–430. doi: 10.1111/j.1365-2222.1975.tb01881.x. [DOI] [PubMed] [Google Scholar]
- Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997;186:1623–1631. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M, Cormier Y, Tardif J, Ghadirian E, Laviolette M. Hypersensitivity pneumonitis: whole Micropolyspora faeni or antigens thereof stimulate the release of proinflammatory cytokines from macrophages. Am J Resp Cell Mol Biol. 1991;5:198–203. doi: 10.1165/ajrcmb/5.2.198. [DOI] [PubMed] [Google Scholar]
- Denis M, Ghadirian E. Murine hypersensitivity pneumonitis: bidirectional role of interferon-gamma. Clin Exp Allergy. 1992;22:783–792. doi: 10.1111/j.1365-2222.1992.tb02819.x. [DOI] [PubMed] [Google Scholar]
- Falcone M, Rajan AJ, Bloom BR, Brosnan CF. A critical role for IL-4 in regulating disease severity in experimental allergic encephalomyelitis as demonstrated in IL-4-deficient C57BL/6 mice and BALB/c mice. J Immunol. 1998;160:4822–4830. [PubMed] [Google Scholar]
- Fei R, Gott K, Edwards B, Schuyler M. Experimental hypersensitivity pneumonitis: in vitro effects of interleukin-2 and interferon-gamma. J Lab Clin Med. 1995;126:485–494. [PubMed] [Google Scholar]
- Feinberg S. Analysis of Cross-Classified Categorical Data. Cambridge, USA: MIT Press; 1980. [Google Scholar]
- Finkelman FD. Relationships among antigen presentation, cytokines, immune deviation, and autoimmune disease. J. Exp. Med. 1995;182:279–282. doi: 10.1084/jem.182.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gause WC, Halvorson MJ, Lu P, et al. The function of costimulatory molecules and the development of IL-4-producing T cells. Immunol Today. 1997;18:115–120. doi: 10.1016/s0167-5699(97)01005-0. [DOI] [PubMed] [Google Scholar]
- Ghadirian E, Denis M. Murine hypersensitivity pneumonitis: interleukin-4 administration partially abrogates the disease process. Microbial Pathogenesis. 1992;12:377–382. doi: 10.1016/0882-4010(92)90100-3. [DOI] [PubMed] [Google Scholar]
- Grau GE, Heremans H, Piguet PF, et al. Monoclonal antibody against interferon gamma can prevent experimental cerebral malaria and its associated overproduction of tumor necrosis factor. Proc Natl Acad Sci USA. 1989;86:5572–5574. doi: 10.1073/pnas.86.14.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudmundsson G, Hunninghake GW. Interferon-gamma is necessary for the expression of hypersensitivity pneumonitis. J Clin Invest. 1997;99:2386–2390. doi: 10.1172/JCI119420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudmundsson G, Monick MM, Hunninghake GW. IL-12 modulates expression of hypersensitivity pneumonitis. J Immunol. 1998;161:991–999. [PubMed] [Google Scholar]
- Guler ML, Jacobson NG, Gubler U, Murphy KM. T cell genetic background determines maintenance of IL-12 signaling: effects on BALB/c and B10.D2 T helper cell type 1 phenotype development. J Immunol. 1997;159:1767–1774. [PubMed] [Google Scholar]
- Hsieh CS, Macatonia SE, O'Garra A, Murphy KM. T cell genetic background determines default T helper phenotype development in vitro. J Exp Med. 1995;181:713–721. doi: 10.1084/jem.181.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka A, Sakiyama Y, Nakanishi M, et al. The inductive effect of interleukin-4 on IgG4 and IgE synthesis in human peripheral blood lymphocytes. Clin Exp Immunol. 1990;79:392–396. doi: 10.1111/j.1365-2249.1990.tb08101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson NG, Szabo SJ, Weber-Nordt RM, et al. Interleukin 12 signaling in T helper type 1 (Th1) cells involves tyrosine phosphorylation of signal transducer and activator of transcription (Stat) 3 and Stat4. J. Exp. Med. 1995;181:1755–1762. doi: 10.1084/jem.181.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitt S, Lee CW, Fink JN, Calvanico NJ. Immunoglobulin G4 in pigeon breeder's disease. J Lab Clin Med. 1986;108:442–447. [PubMed] [Google Scholar]
- Liblau RS, Singer SM, McDevitt HO. Th1 and Th2, CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol Today. 1995;16:34–38. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- Nakamura T, Kamogawa Y, Bottomly K, Flavell RA. Polarization of IL-4- and IFN-gamma-producing CD4+ T cells following activation of naive CD4+ T cells. J Immunol. 1997;158:1085–1094. [PubMed] [Google Scholar]
- Nikula KJ, Swafford DS, Hoover MD, Tohulka MD, Finch GL. Chronic granulomatous pneumonia and lymphocytic responses induced by inhaled beryllium metal in A/J and C3H/HeJ mice. Toxicol Pathol. 1997;25:2–12. doi: 10.1177/019262339702500102. [DOI] [PubMed] [Google Scholar]
- Pearlman E, Lass JH, Bardenstein DS, et al. Interleukin 4 and T helper type 2 cells are required for development of experimental onchocercal keratitis (river blindness) J Exp Med. 1995;182:931–940. doi: 10.1084/jem.182.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernis A, Gupta S, Gollob KJ, et al. Lack of interferon gamma receptor beta chain and the prevention of interferon gamma signaling in TH1 cells. Science. 1995;269:245–247. doi: 10.1126/science.7618088. [DOI] [PubMed] [Google Scholar]
- Piccotti JR, Li K, Chan SY, et al. Alloantigen-reactive Th1 development in IL-12-deficient mice. J Immunol. 1998;160:1132–1138. [PubMed] [Google Scholar]
- Racke MK, Bonomo A, Scott DE, et al. Cytokine-induced immune deviation as a therapy for inflammatory autoimmune disease. J Exp Med. 1994;180:1961–1966. doi: 10.1084/jem.180.5.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Ann Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- Ria F, Penna G, Adorini L. Th1 cells induce and Th2 inhibit antigen-dependent IL-12 secretion by dendritic cells. Eur J Immunol. 1998;28:2003–2016. doi: 10.1002/(SICI)1521-4141(199806)28:06<2003::AID-IMMU2003>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Richeldi L, Sorrentino R, Saltini C. HLA-DP 1 glutamate 69: a genetic marker of beryllium disease. Science. 1993;262:242–244. doi: 10.1126/science.8105536. [DOI] [PubMed] [Google Scholar]
- Rittner C, Sennekamp J, Mollenhauer E, et al. Pigeon breeder's lung: association with HLA-DR 3. Tissue Antigens. 1983;21:374–379. doi: 10.1111/j.1399-0039.1983.tb00186.x. [DOI] [PubMed] [Google Scholar]
- Schmitt E, Hoehn P, Germann T, Rude E. Differential effects of interleukin-12 on the development of naive mouse CD4+ T cells. Eur J Immunol. 1994;24:343–347. doi: 10.1002/eji.1830240211. [DOI] [PubMed] [Google Scholar]
- Schuyler M, Gott K, Haley P. Experimental murine hypersensitivity pneumonitis. Cell Immunol. 1991;136:303–317. doi: 10.1016/0008-8749(91)90354-e. [DOI] [PubMed] [Google Scholar]
- Schuyler M, Gott K, Shopp G, Crooks L. CD3+ and CD4+ cells adoptively transfer experimental hypersensitivity pneumonitis. Am Rev Resp Dis. 1992;146:1582–1588. doi: 10.1164/ajrccm/146.6.1582. [DOI] [PubMed] [Google Scholar]
- Schuyler M, Gott K, Shopp G, Crooks L. CD3+, CD4+, CD8-, Ia- T cells adoptively transfer murine experimental hypersensitivity pneumonitis. Chest. 1993;103:143S–145S. doi: 10.1378/chest.103.2_supplement.143s. [DOI] [PubMed] [Google Scholar]
- Schuyler M. Hypersensitivity pneumonitis. In: Fishman A, editor. Pulmonary Diseases and Disorders. McGraw-Hill: 1997. pp. 1085–1097. [Google Scholar]
- Schuyler M, Gott K, Cherne A, Edwards B. Th1, CD4+ cells adoptively transfer experimental hypersensitivity pneumonitis. Cell Immunol. 1997;177:169–175. doi: 10.1006/cimm.1997.1107. [DOI] [PubMed] [Google Scholar]
- Scott P. IFN-gamma modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J Immunol. 1991;147:3149–3155. [PubMed] [Google Scholar]
- Selman M, Teran L, Mendoza A, et al. Increase of HLA-DR7 in pigeon breeder's lung in a Mexican population. Clin Immunol Immunopath. 1987;44:63–70. doi: 10.1016/0090-1229(87)90052-3. [DOI] [PubMed] [Google Scholar]
- Shibuya K, Robinson D, Zonin F, et al. IL-1 alpha and TNF-alpha are required for IL-12-induced development of Th1 cells producing high levels of IFN-gamma in BALB/c but not C57BL/6 mice. J Immunol. 1998;160:1708–1716. [PubMed] [Google Scholar]
- Smith S, Hill J, Snyder I, Burrell R. Mitogenicity of cell wall fractions of Micropolyspora faeni. Ann Allergy. 1978;40:12–14. [PubMed] [Google Scholar]
- Smythies LE, Coulson PS, Wilson RA. Monoclonal antibody to IFN-gamma modifies pulmonary inflammatory responses and abrogates immunity to Schistosoma mansoni in mice vaccinated with attenuated cercariae. J Immunol. 1992;149:3654–3658. [PubMed] [Google Scholar]
- Spiegelberg HL, Rd OC, Falkoff RJ, Beck L. Interleukin-4 induced IgE and IgG4 secretion by B cells from atopic dermatitis patients. Int Arch Allergy Appl. Immunol. 1991;94:181–183. doi: 10.1159/000235357. [DOI] [PubMed] [Google Scholar]
- SYstat. Systat for Windows v 7.0, Statistics. Evanston, IL: SPSS Inc; 1997. [Google Scholar]
- Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL) -12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terho EO, Husman K, Vohlonen I. Prevalence and incidence of chronic bronchitis and farmer's lung with respect to age, sex, atopy, and smoking. Eur J Resp Dis. 1987;152(Suppl):19–28. [PubMed] [Google Scholar]
- Tuohy VK, Sobel RA, Lees MB. Myelin proteolipid protein-induced experimental allergic encephalomyelitis. Variations of disease expression in different strains of mice. J Immunol. 1988;140:1868–1873. [PubMed] [Google Scholar]
- Urban JF, Fayer R, Chen SJ, Gause WC, Gately MK, Finkelman FD. IL-12 protects immunocompetent and immunodeficient neonatal mice against infection with Cryptosporidium parvum. J Immunol. 1996;156:263–268. [PubMed] [Google Scholar]


