Abstract
The uncontrolled aggregation of amorphous calcium phosphate (ACP) particulate fillers and their uneven distribution within polymer matrices can have adverse effects on the properties of ACP composites. In this paper we assessed the influence of non-ionic and anionic surfactants and poly(ethylene oxide) (PEO) introduced during the preparation of ACP on the particle size distribution and compositional properties of ACP. In addition, the mechanical strength of polymeric composites utilizing such fillers with a photo-activated binary methacrylate resin was evaluated. Zirconia-hybridized ACP (Zr-ACP) filler and its corresponding composite served as controls for this study. Surfactant- and PEO-ACPs had an average water content of 16.8 % by mass. Introduction of the anionic surfactant reduced the median particle diameter about 45 % (4.1 μm vs. 7.4 μm for the Zr-ACP control). In the presence of PEO, however, the dm increased to 14.1 μm. There was no improvement in the biaxial flexure strength (BFS) in any of the dry composite specimens prepared with the surfactant- and/or PEO-ACPs compared to those formulated with Zr-ACP. The BFS of wet composite specimens decreased by 50 % or more after a month-long exposure to saline solutions. Other types of surfactants and/or polymers as well as alternative surface modification protocols need to be explored for their potential to provide better dispersion of ACP into the matrix resin and better mechanical performance ACP composites.
Keywords: amorphous calcium phosphate, particle size distribution, surfactants, poly(ethylene glycol), composite, mechanical strength
Introduction
Various compounds from the calcium phosphate family have been extensively investigated as hard tissue repair materials due to their excellent biocompatibility (1). It has been shown that the resorption rate of poorly crystalline apatites and amorphous calcium phosphate (ACP) coincided closely with the rate of new bone formation (2,3). Additionally, ACP shows better osteoconductivity in vivo than apatite (4) and its biodegradability is higher than that of tricalcium phosphate (5). Although the bioactive, anti-cariogenic ACP composites have a number of desirable properties, they only have moderate mechanical strength due to relatively poor resin-filler interfacial adhesion (6–9). Their clinical applicability may be compromised by the less than optimal filler/matrix interactions and also by the excessive water sorption that occurs in both in the resin and filler phases of these composites. The uncontrolled aggregation of ACP filler particles leads to random clustering of the filler phase within polymerized resin matrices (10) and also appears to have a key role in adversely affecting the strength and durability of these ACP composites. In our continuing pursuit to improve their physical properties while maintaining their adequate remineralizing/anti-cariogenic potential we have focused on: (a) exploring structure/composition/property relationships of resins with ACP fillers, (b) evaluating intra-composite filler/organic matrix interactions, and (c) composite/tooth interactions.
In this study we assessed the possible role of some non-ionic surfactants, an anionic surfactant and the hydrophilic poly(ethylene oxide), PEO, on the particle size distribution, compositional and structural properties of ACP fillers. Additionally, the mechanical stability of the composites made with such ACP fillers after prolonged water immersion was assessed. It was anticipated that surfactants, introduced ab initio during the synthesis of ACP would adsorb on the ACP surface, therefore affecting the degree of spontaneous ACP aggregation. Less aggregated ACPs are expected to have improved dispersion in the resin matrices.
PEO, which has a hydrophilic structural unit found in many surfactants, is widely used in water-compatible polymer systems because of its proven ability to undergo multiple hydrogen bonding interactions and stabilize cations by multiple chelation. If successfully incorporated into ACP during its synthesis, PEO would also be expected to affect ACP’s tendency to form aggregates. Both the PEO and surfactant additives may also affect the water content of the precipitated ACP filler (11). Lower intrinsic water content together with the reduction in the size and number of voids in the composites may also have a beneficial effect on the overall water uptake by the composites, a factor that is important in regulating both ion release kinetics and the mechanical stability of composites.
The goals of this study were twofold: 1) to determine the effects of these additives on morphology, particle size distribution and water content of the modified ACPs and 2) to assess the mechanical strength of composites based on these ACPs. Relevant tasks were to validate the amorphous character of the ACP fillers synthesized in the presence of these additives, to determine their composition and to evaluate the mechanical stability of their polymeric composites before and after aqueous exposure. The experimental results of this study are expected to contribute to a better understanding of the mechanisms that govern resin structure/composition/property relationships in composites with ACP fillers. Also, these results may be helpful in designing improved remineralizing ACP composites for a wider range of dental clinical applications.
Methods
Synthesis and evaluation of ACP fillers
ACP precipitated instantaneously in a closed system at 23 °C upon rapidly mixing equal volumes of a 800 mmol/L Ca(NO3)2 aqueous solution, and 536 mmol/L Na2HPO4 aqueous solution that contained 2 mass % Na4P2O7 as a stabilizer. An appropriate amount of additive (Table 1) was added to either the Ca component (non-ionic surfactant) or the PO4 reactant (anionic surfactant and PEO) prior to mixing. Surfactants were introduced at two different levels 0.05 % and 0.10 % by mass. The chosen concentrations for surfactants correspond to the upper limits of the reported effective range for the fluorinated surfactants (Zonyl) in aqueous adhesive applications (12). PEOs of different molecular masses (8 K, 100 K and 1000 K, respectively) were introduced at a 0.25 % level by mass. The reaction pH varied between 7.9 and 9.0. The suspension was filtered, the solid phase washed subsequently with ice-cold ammoniated water and acetone, and then lyophilized. The control, Zr-ACP was synthesized as previously described (8).
Table 1.
Additives introduced during the ACP synthesis.
| Additive | Name/acronym | Formula | Manufacturer |
|---|---|---|---|
| surfactant | non-ionic: Triton 100 | C14H22O(C2H4O)10H | Res. Prod. International, Elk Grove Village, Il, US |
| Tween 80 Zonyl FSN | C24H43O6(C2H4O)20H
F(C2F4)x(C2H4O)1+yH; x=1–9, y=0–25 |
Fisher, Fair Lawn, NJ, US
DuPont, Wilmington, DE, US |
|
| anionic: Zonyl FSP | F(C2F4)x(C2H4O)yHP(O)(ONH4)z; x=1–7, y+z=3 | DuPont, Wilmington, DE, US | |
|
| |||
| polymer | PEO | H(C2H4O)nH; n=180, 2300 or 22700 | Aldrich, Milwaukee, WI, US |
The amorphous state of the fillers was verified by powder X-ray diffraction (XRD: Rigaku X-ray diffractometer, Rigaku/USA Inc., Danvers, MA, USA) and Fourier-transform spectroscopy (FTIR: Nicolet Magna-IR FTIR System 550 spectrophotometer, Nicolet Instrument Corporation, Madison, WI, USA). Morphological/topological features of the solids, after the specimens were sputter-coated with gold, were examined by scanning electron microscopy (SEM; JEOL 35C instrument, JEOL Inc., Peabody, MA, USA). Particle size distribution (PSD) of the fillers was determined by gravitational and centrifugal sedimentation analysis (SA-CP3 particle size analyzer, Shimadzu Scientific Instruments, Inc., Columbia, MD, USA) following dispersion of the solid in isopropanol and 10 min ultrasonication of the mixture. Water content of the fillers was determined by thermogravimetric analysis (TGA; Perkin Elmer 7 Series Thermal Analysis System, Norwalk, CT, USA). The TGA (3 separate runs) was performed by heating (5 mg to 10 mg) of the filler at the rate of 20 °C/min over a temperature range of (30 °C to 600 °C) in air. The surface or loosely bound water was attributed to the mass loss that occurred from 23 °C to 125 °C. Structural or more tightly bound water was attributed to the mass loss that occurred from 150 °C to 600 °C.
Formulation of the resin
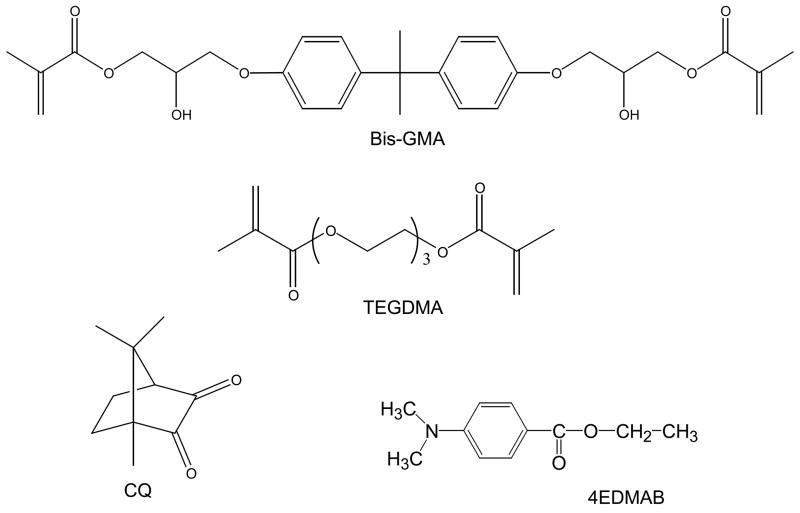
The experimental resin (Fig. 1) was formulated from the commercially available dental monomers: base monomer 2,2-bis[p-(2′-hydroxy-3′-methacryloxypropoxy)phenyl]propane (Bis-GMA) and diluent monomer triethyleneglycol dimethacrylate (TEGDMA) in 1:1 mass ratio (designated as BT resin). It was photo-activated for visible light polymerization by addition of the components of the photo-initiator system: photo-oxidant camphorquinone (CQ) at 0.2 mass % and photo-reductant ethyl-4-N,N-dimethylaminobenzoate (4EDMAB) at 0.8 mass %. The indicated acronyms will be used throughout this manuscript.
Fig. 1.
Chemical structure of the monomers and photo-curing agents utilized in the study.
Preparation of composite specimens
Composite pastes were made from mixing the BT resin (60 mass %) and the appropriate ACP filler (40 mass %) by hand spatulation. The homogenized pastes were kept under a moderate vacuum (2.7 kPa) overnight to eliminate the air entrained during mixing. The pastes were molded into disks (15.8 mm to 19.6 mm in diameter and 1.55 mm to 1.81 mm thick) by filling the circular openings of flat Teflon molds, covering each side of the mold with a Mylar film plus a glass slide, and then clamping the assembly together with spring clips. The disks were photo-polymerized by irradiating sequentially each face of the mold assembly for 120 s with visible light (Triad 2000, Dentsply International, York, PA, USA).
Mechanical strength of composites
Biaxial flexure strength (BFS) values of dry (stored for 24 h in the air at 23 °C) and wet (after 4 weeks of immersion in HEPES-buffered, pH = 7.40, saline solutions at 23 °C) composite disk specimens (three or more specimens per group) were determined by using a computer-controlled Universal Testing Machine (Instron 5500R, Instron Corp., Canton, MA, USA; crosshead speed: 0.5 mm/min) operated by Testworks4 software. BFS values were calculated according to the mathematical expressions given in ASTM F394-78 (13).
Statistical data analysis
One standard deviation (SD) is indicated in this paper for comparative purposes as the estimated standard uncertainty of the measurements. Experimental data were analyzed by ANOVA (α = 0.05). Significant differences between specific groups were determined by all pair-wise multiple comparisons (t-test; unequal variances).
RESULTS
Physicochemical characteristics of ACP fillers
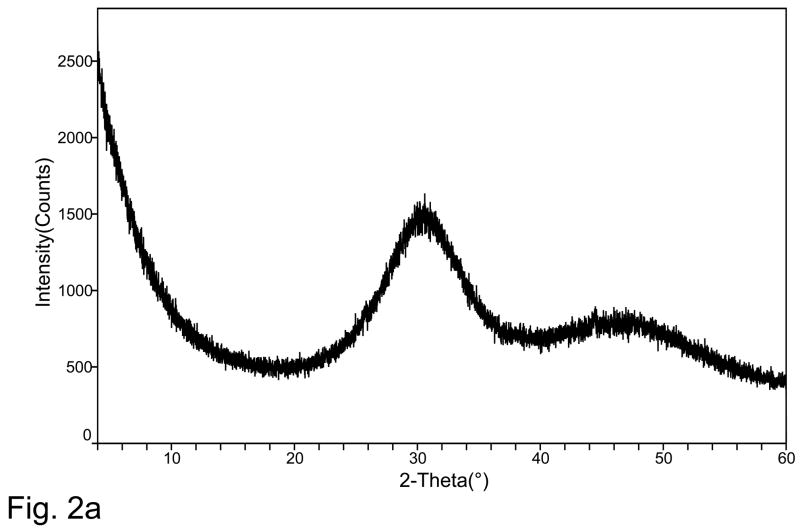
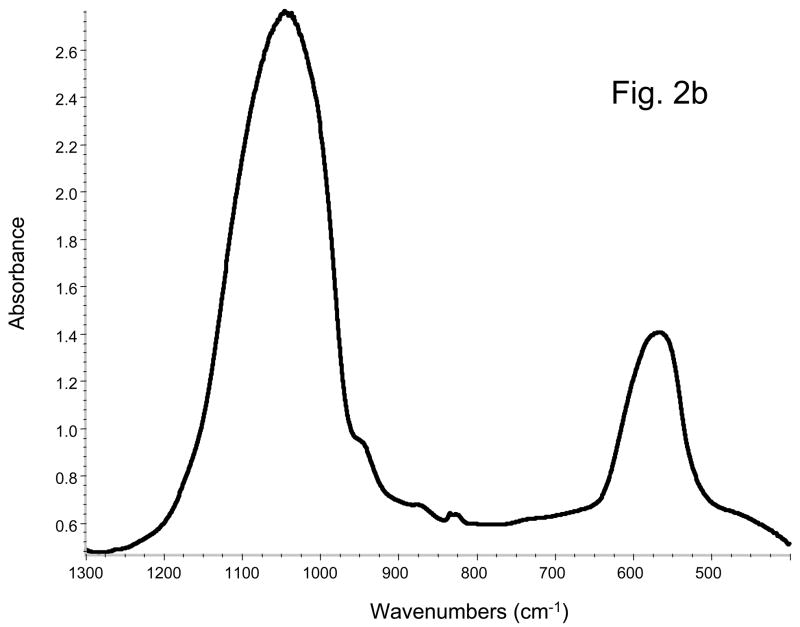
XRD spectrum of a typical ACP prepared in this study showed two diffuse, broad bands resembling the XRD pattern of noncrystalline substances such as glasses and certain polymers (Fig. 2a). A corresponding FTIR spectrum (Fig. 2b) showed two wide bands typical for phosphate stretching and phosphate bending in the region of 1200 cm−1 to 900 cm−1 and 630 cm−1 to 500 cm−1, respectively. The median particle diameter, dm, from the particle size distribution (PSD) analysis and the water content from the thermogravimetric analysis (TGA) are summarized in Table 2. The PSD data revealed heterogeneous distribution with particles ranging from submicron sizes up to 80 μm in diameter. The dm calculated from the PSD data, decreased in the following order: PEO-ACP (irrespective of the PEO’s molecular mass): 14.1 μm) > (Triton-, Tween- FSN- or Zr-ACP; average 7.8 μm) > FSP-ACP (4.1 μm). The heterogeneity of the particle sizes was confirmed by SEM observations (images not shown here). The water content of the precipitated ACPs was not affected by the type of additive used during synthesis. TGA results revealed an average water content of (16.7 ± 1.1) mass %. The ratio of the surface-bound (mobile water) to structurally incorporated water was approximately 2.5 regardless of the type of additive used during ACP’s formation.
Fig. 2.
Typical XRD pattern (a) and FTIR spectrum (b) of modified ACPs utilized in the study.
Table 2.
Median particle size and water content of modified ACPs. Results are shown as mean value with standard deviation indicated in parenthesis. Number of replicate experiments n ≥ 5 in each experimental group.
| ACP type | Median diameter dm (μm) | Water content (mass %) |
|---|---|---|
| Triton 100 | 8.3 (1.4) | 16.3 (1.2) |
| Tween 80 | 8.9 (2.1) | 16.9 (0.9) |
| Zonyl FSN | 6.5 (1.2) | 17.4 (1.1) |
| Zonyl FSP | 4.1 (0.4) | 17.6 (2.1) |
|
| ||
| PEO | 14.1 (4.7) | 15.6 (2.0) |
|
| ||
| Zr (control) | 7.4 (2.1) | 16.1 (2.0) |
Mechanical strength
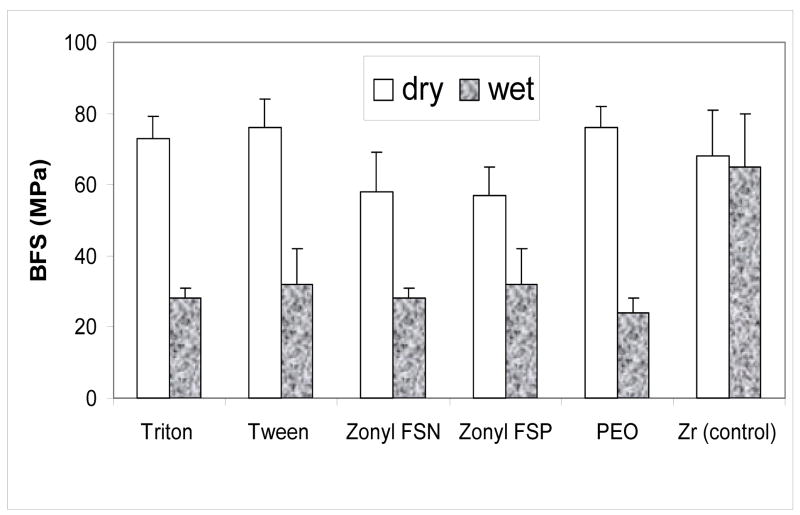
The results of the BFS testing of dry (before immersion) and wet (after 4 weeks of immersion in saline solutions) for the various Bis-GMA/TEGDMA ACP composite specimens are summarized in Fig. 3. There was no significant difference between dry BFS values of Triton-, Tween-, PEO- and Zr-ACP composites (on average 73.3 MPa). The BFS of dry Zonyl-ACPs was, however, lower (average 57.5 MPa). Upon soaking, the BFS of surfactant-ACP and PEO-ACP composites was reduced between 44 % (Zonyl-FSP) and 68 % (PEO) resulting in the average BFS values of wet specimens in the range (23.7 MPa to 32.4 MPa), independent of the additive utilized during ACP filler preparation.
Fig. 3.
Biaxial flexure strength (BFS; mean value + SD (indicated by bars)) of dry and wet composite specimens. The number of runs in each experimental group n ≥ 3.
DISCUSSION
It has been well established that addition of certain compounds to calcium and phosphate supersaturated solutions not only influences the kinetics of crystal growth but also affects the stability of precursor phase(s). The concise literature review on that subject is provided by Amjad (14). The majority of early work focused on the effect of metal cations, primarily magnesium, revealing its stabilizing effect on ACP or inducing the formation of another precursor phase - octacalcium phosphate. It has also been reported that organic additives containing hydroxyl, carboxyl or amino groups markedly affect the precipitation kinetics. Polymeric additives such as poly(acrylic acid), poly(maleic acid) and carboxyl-containing copolymers apparently inhibit the precipitation of calcium phosphates. The effects of polyelectrolytes on the formation and transformation of ACP were studied by Ofir et al. (15). Polystyrene sulfonate, poly-L-lysine and poly-L-glutamic acid at low concentrations induced nucleation, but at high concentrations inhibited nucleation and inhibited growth of crystalline phase in a nonspecific way. Despite the important role crystal aggregation plays in the kinetic interpretation of the spontaneous precipitation from supersaturated solutions (16–18), the agglomeration of HAP has received only very limited attention (19,20), while the ACP’s agglomeration has not been studied at all. It is, however, the spontaneous and uncontrolled agglomeration of ACP particles that occurs during its synthesis and results in a highly clustered filler material, that resists uniform dispersion in matrix resins or polymers. As a direct result of ACP’s heterodispersity, the resulting uneven distribution of ACP filler within the polymeric matrix (10) hinders the interfacial interactions and the ACP composites are mechanically inferior compared to surface-treated, glass-reinforced resin materials. Moreover, the state of aggregation of ACP fillers in composites also affects the ion release profiles of these materials. In this study we have examined the effect that various surfactants and the hydrophilic, non-ionic polymer PEO, introduced ab initio during the synthesis of ACP, have on the structure, composition, morphology and the PSD of the precipitating ACP. The hypothesis was that interactions between the ACP and the surfactants and/or PEO introduced ab initio will reduce the extent of ACP’s aggregation without having a detrimental effect on its stability in aqueous environments, i.e. preserve the morphology and bioactivity of ACP filler as a calcium and phosphate releasing agent.
We found very little experimental evidence to support the hypothesis that additive-ACP surface interaction depends on the chemical nature of the additive itself (non-ionic vs anionic surfactants) or in the case of PEO a molecular mass dependence. Only in the presence of the anionic surfactant was the particle size of ACP moderately reduced. The weak effect of this surfactant may be due to the partial distribution of the headgroup charges to the rest of the molecule, a phenomenon commonly seen in surfactants with linear alkyl tails and sulfonate, sulfate and/or carboxylate headgroups (21). On the other hand, ACP precipitated in the presence of PEO (regardless of PEO’s molecular mass) appeared even more aggregated. A “polymer bridging” mechanism reportedly (20) controls the aggregation of HAP particles in the presence of high-molecular-mass polyacrylate via binding of carboxylic groups of this polymeric additive at positive surface calcium sites.
Several simultaneous events can occur during the precipitation of ACP and each can theoretically influence the resulting particle size distribution of ACP. For example for PEO, the adsorption of polymer molecule on ACP particles, re-arrangement (conformational change) of the adsorbed polymer chains, collision rate between the destabilized particles to form aggregates and/or aggregate break-up due to fluid shear, in fact can control the surface interaction(s) between the PEO molecules and the ACP particulates, but the exact mechanism(s) of interaction has yet to be determined.
In an ideal case, the lower intrinsic water content associated with the reduction in the size and number of voids in the composites (as a result of less agglomerated ACP particulates) may affect the overall water uptake of composites. Water sorption of ACP composites have been reportedly influenced by the type of ACP used as a filler (hybridized vs. unmodified ACPs) in close connection with the type of monomers used in resin formulation (8, 9). ACP formed in the presence of surfactants and PEO did not contain less surface-bound and/or structural water than the control Zr-ACP. This could be explained by the fact that the amounts of the surfactants and/or PEO used were small and did not fully adhere to or cover the ACP. Also, since the precipitated ACPs are thoroughly washed during the workup procedure the level of residual surfactant or PEO probably is extremely small. There was no evidence for the presence of any of the surfactants or PEO from FTIR and TGA analysis.
The average BFS value of wet surfactant- and PEO-ACP Bis-GMA/TEGDMA composites [(28.8 ± 3.3) MPa] is inferior compared to the average BFS [(59.3 ± 10.0) MPa] of wet composite specimens based on unmodified ACP fillers of comparable particle size distribution (dm = 4.5 μm to 7.2 μm) and binary acrylic resins formulated from Bis-GMA, ethoxylated bisphenol A or urethane dimethacrylate and TEGDMA as a diluent co-monomer (8). The reduction in the mechanical strength of ACP composites can, generally, be attributed to: 1) reduction in ACP’s intactness and rigidity at the filler/matrix interface due to spatial changes that may have occurred during the calcium/phosphate ion efflux, internal ACP to apatite conversion, or excessive water absorption. The fact that, after aqueous immersion, the BFS of surfactant- and PEO-ACP composites deteriorated practically independent of filler’s PSD (dm of PEO-ACP was for example 3 times larger than that of Zonyl FSP-ACP and yet their strength was about the same) would suggest that the fillers PSD contributes very little to the composite’s ability to resist plasticization and/or degradation after water exposure. The mechanical strength of PEO-ACP based composites sharply contradicts the claim of Li et al. (22) that poly(ethylene glycol) modifies the surface of ACP and produces a good interface in the polyester/ACP composite.
Water sorption (WS) by dental composites is generally controlled by structure and composition of the matrix monomers, although the poorly understood polymer matrix interface also probably plays an important role as well. However, the relatively hydrophilic ACP filler in the composites may significantly increase the amount of water absorbed; the effect is expected to be proportional to ACP’s mass fraction in the composite. The lack of intimate contact between the surfactant- and/or PEO–modified ACP particles and the Bis-GMA/TEGDMA polymeric network is most likely the critical interphase factor that may have led to excessive water diffusion and enhanced hydration of ACP surfaces. Existence of numerous voids at the ACP filler/BisGMA-based resin interface was experimentally documented by FTIR micro-spectroscopy (10). It has also been shown that these processes were inhibited or reduced in composites with milled ACP filler (23). Studies of Bis-GMA-based resin composites (24–26) emphasized the effects of prolonged exposure to aqueous environments on their fracture toughness, elastic modulus, hardness and flexural strength. To establish which of the above parameters has been adversely affected by introduction of additives would require extensive water sorption/desorption studies and more elaborate mechanical testing. The conclusion derived from the simple BFS screening is that composites formulated with surfactant- and PEO-ACP and subjected to prolonged aqueous exposure are mechanically inferior to Zr-ACP control specimens.
Conclusion
In summary, introducing surfactants during the spontaneous precipitation of ACP from supersaturated calcium and phosphate solutions stabilized the amorphous solid phase against the conversion to apatite. Non-ionic surfactants had no effect and the anionic surfactant moderately reduced the particle size of ACP. Addition of poly(ethylene oxide) of various molecular masses resulted in more pronounced ACP’s agglomeration. ACP’s water content remained unaffected regardless of the type of the additive used. The surfactant- and PEO-ACP composites showed no improvement in their dry biaxial flexure strength compared to the control Zr-ACP composites. However, their strength after prolonged exposure to aqueous milieu was reduced drastically in contrast to composites formulated with Zr-ACP. Alternative ways to reduce the size and the level of ACP’s particle aggregation need to be explored to ensure more intimate ACP dispersion into the polymeric matrix resin. It is expected that those more homogeneous composites would have improved properties that should advance the clinical suitability of ACP composites.
Acknowledgments
This research was supported by the National Institute of Dental and Craniofacial Research (grant R01 DE13169-07), the National Institute of Standards and Technology and the American Dental Association Foundation. We gratefully acknowledge donation of the monomers used in this study from Esstech, Essington, PA.
Footnotes
“Official contribution of the National Institute of Standards and Technology; not subject to copyright in the United States”
Publisher's Disclaimer: Disclaimer: Certain commercial materials and equipment are identified in this work for adequate definition of the experimental procedures. In no instance does such identification imply recommendation or endorsement by the National Institute of Standards and Technology or the American Dental Association Foundation, or that the material and the equipment identified is necessarily the best available for the purpose.
References
- 1.Ambrosio AMA, Sahota JS, Khan Y, Laurencin CT. A novel amorphous calcium phosphate polymer ceramic for bone repair: I. Synthesis and Characterization. J Biomed Mater Res (Appl Biomater) 2001;58:295–301. doi: 10.1002/1097-4636(2001)58:3<295::aid-jbm1020>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 2.Frayssinet P, Trouillet JL, Rouquet N, Azimus E, Autefage A. Osteointegration of macroporous calcium phosphate ceramics having a different chemical composition. Biomaterials. 1993;14:423–429. doi: 10.1016/0142-9612(93)90144-q. [DOI] [PubMed] [Google Scholar]
- 3.Knaack D, Goad MEP, Aiolova M, Rey C, Tofighi A, Chakravarthy P, Lee DD. Resorbable calcium phosphate bone substitute. J Biomed Mater Res (Appl Biomater) 1998;43:399–409. doi: 10.1002/(sici)1097-4636(199824)43:4<399::aid-jbm7>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 4.Nagano M, Nakamura T, Kokubo T, Tanahashi M, Ogawa M. Differences in bone bonding ability and degradation behavior in vivo between amorphous calcium phosphate and highly crystalline apatite. Biomaterials. 1996;17:1771–1777. doi: 10.1016/0142-9612(95)00357-6. [DOI] [PubMed] [Google Scholar]
- 5.Tadic D, Peters F, Epple M. Continuous synthesis of amorphous carbonated apatites. Biomaterials. 2002;23:2553–2559. doi: 10.1016/s0142-9612(01)00390-8. [DOI] [PubMed] [Google Scholar]
- 6.Skrtic D, Antonucci JM, Eanes ED, Eichmiller FC, Schumacher GE. Physicochemical evaluation of bioactive polymeric composites based on hybrid amorphous calcium phosphates. J Biomed Mat Res (Appl Biomater) 2000;53:381–91. doi: 10.1002/1097-4636(2000)53:4<381::aid-jbm12>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 7.Skrtic D, Antonucci JM, Eanes ED. Effect of the monomer and filler system on the remineralizing potential of bioactive dental composites based on amorphous calcium phosphate. Polym Adv Technol. 2001;12:369–79. [Google Scholar]
- 8.Skrtic D, Antonucci JM, Eanes ED. Amorphous calcium phosphate-based bioactive polymeric composites for mineralized tissue regeneration. J Res Natl Inst Stands Technol. 2003;108:167–182. doi: 10.6028/jres.108.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antonucci JM, Skrtic D. Matrix resin effects on selected physicochemical properties of amorphous calcium phosphate composites. J Bioact Compat Polym. 2005;20:29–49. [Google Scholar]
- 10.Skrtic D, Antonucci JM, Eanes ED, Eidelman N. Dental composites based on hybrid and surface-modified amorphous calcium phosphates. Biomaterials. 2004;25:1141–50. doi: 10.1016/j.biomaterials.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Eanes ED. Amorphous calcium phosphate: Thermodynamic and kinetic considerations. In: Amjad Z, editor. Calcium phosphates in biological and industrial systems. Kluwer Academic Publ; Boston: 1998. pp. 21–39. [Google Scholar]
- 12.http://www.dupont.com/zonyl/pdf/FSN.pdf and http://www.dupont.com/zonyl/pdf/FSP.pdf.
- 13.ASTM F394-78 (re-approved 1991): Standard test method for biaxial strength (modulus of rupture) of ceramic substrates.
- 14.Amjad Z. Inhibition of the amorphous calcium phosphate phase transformation reaction by polymeric and non-polymeric inhibitors. Phosphorus Res Bull. 1997;7:45–54. [Google Scholar]
- 15.Ofir PBY, Govrin-Lipman R, Garti N, Furedi-Milhofer H. The influence of polyelectrolytes on the formation and phase transformation of amorphous calcium phosphate. Crystal Growth & Design. 2004;4:177–183. [Google Scholar]
- 16.Ryal RL, Ryal RG, Marshall VR. Interpretation of particle growth and aggregation patterns obtained from the Coulter counter. A simple theoretical model. Inv Urol. 1981;18:396–399. [PubMed] [Google Scholar]
- 17.Skrtic D, Markovic M, Komunjer L, Furedi-Milhofer H. Precipitation of calcium oxalates from high ionic strength solutions. I. Kinetics of spontaneous precipitation of calcium oxalate trihydrate. J Cryst Growth. 1984;66:431–440. [Google Scholar]
- 18.Skrtic D, Markovic M, Furedi-Milhofer H. Precipitation of calcium oxalates from high ionic strength solutions. IV. Testing of kinetic models. J Cryst Growth. 1986;79:791–796. [Google Scholar]
- 19.Hansen NM, Felix R, Bisaz S, Fleish H. Aggregation of hydroxyapatite. Biochim Biphys Acta. 1976;451:549–559. doi: 10.1016/0304-4165(76)90150-1. [DOI] [PubMed] [Google Scholar]
- 20.Nancollas GH, Budz JA. Analysis of particle size distribution of hydroxyapatite crystallites in the presence of synthetic and natural polymers. J Dent Res. 1990;69:1678–1685. doi: 10.1177/00220345900690101001. [DOI] [PubMed] [Google Scholar]
- 21.Huibers PDT. Quantum-chemical calculations of the charge distribution in ionic surfactants. Langmuir. 1999;15:7546–7550. [Google Scholar]
- 22.Li Y, Weng W, Cheng K, Du P, Shen G, Wang J, Han G. Preparation of amorphous calcium phosphate in the presence of poly(ethylene glycol) J Mater Sci Let. 2003;22:1015–1016. [Google Scholar]
- 23.Skrtic D, Lee SY, Antonucci JM, Liu DW. Amorphous calcium phosphate based polymeric composites: Effects of polymer composition and filler’s particle size on composite properties. Key Eng Mater. 2005:284–286. 737–740. [Google Scholar]
- 24.Feilzer AJ, deGee AJ, Davidson CL. Relaxation of polymerization contraction shear stress by hygroscopic expansion. J Dent Res. 1991;69:36–39. doi: 10.1177/00220345900690010501. [DOI] [PubMed] [Google Scholar]
- 25.Sarrett DC, Ray S. The effect of water on polymer matrix and composite wear. Dent Mater. 1994;10:5–10. doi: 10.1016/0109-5641(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 26.Ferracane JL, Berge XH, Condon JR. In vitro aging of dental composites in water. Effect of degree of conversion, filler volume and filler matrix coupling. J Biomed Mater Res. 1998;42:465–472. doi: 10.1002/(sici)1097-4636(19981205)42:3<465::aid-jbm17>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]