Abstract
A series of 3-alkyl-5,5-dimethylhydantoin derivatives were prepared by reacting 5,5-dimethylhydantoin with alkyl bromides with different alkyl chain length (C-2 to C-22). Upon chlorination, the hydration derivatives were transformed into 1-chloro-3-alkyl-5,5-dimethylhydantoins (CADMH). The structures of the samples were fully characterized with FT-IR, 1H NMR, UV, and DSC analyses. The antimicrobial functions of CADMH were challenged with 108-9 CFU/mL of Escherichia coli (gram-negative bacteria) and Staphylococcus aureus (gram-positive bacteria). All the samples provided a total kill of the gram-negative and gram-positive bacteria in less than 30 min. CADMH were used as antimicrobial additives for polymeric materials. It was found that the presence of as low as 1% of CADMH could provide the samples with potent antimicrobial functions. The structure-antimicrobial efficacy relationships of the CADMH were further discussed.
Introduction
Microbial contamination of polymeric materials plays an important role in the transmission of infectious diseases.1-6 Studies have shown that microorganisms have strong abilities to survive on ordinary polymeric materials, and some species can stay alive on various polymers for more than 90 days.3,4 The contaminated polymeric materials could serve as important sources of cross-infections, causing a variety of serious consequences in medical devices, hospital equipment, water purification and delivery systems, bio-protective equipment, etc.3-6 In response to the microbial challenges, the development of antimicrobial polymers, polymers that can effectively inhibit the growth of microorganisms, has attracted considerable research interests. To date, a number of antimicrobial polymeric materials have been developed; some of these studies have achieved very promising results.7-11
Amongst the currently studied antimicrobial agents, organic N-halamines have been demonstrated to be a class of potent biocides with broad inhibitory activities.12-21 An N-halamine is a compound containing one or more nitrogen-halogen covalent bonds that is normally formed by the halogenation of imide, amide, or amine groups.12 When microorganisms come into contact with N-halamines, a halogen exchange reaction occurs, causing the expiration of the cells. Compared with inorganic halogens (e.g., chlorine or bromine), organic N-halamines are more stable, less corrosive, and have much less tendency to generate halogenated hydrocarbons. Thanks to these attractive properties, monomeric N-halamines such as 1,3-dichloro-5,5-dimethyl hydantoin and 3-bromo-1-chloro-5,5-dimethylhydantoin have been widely used as disinfectants.12
Much effort has been devoted to introduce organic N-halamine structures into polymeric materials to provide antimicrobial functions. The most widely used approach is to covalently bind N-halamine precursors (e.g., hydantoins) onto the target polymers. After halogenation, N-halalmine structures are formed in situ, and the resultant polymers provide potent antimicrobial functions against a broad range of microorganisms.14-21 The first N-halamine-based antimicrobial polymer prepared with the covalent binding approach is poly-1,3-dichloro-5-methyl-5-(4′-vinylphenyl) hydantoin, which was synthesized first by a Friedel-Crafts acylation of polystyrene to produce poly(4-vinylacetophenone), followed by a Bucherer-Bergs synthesis of poly[5-methyl-5-(4′-vinylphenyl)hydantoin], and then a chlorination reaction in the presence of chlorine gas.21 Later, 1,3-bis(hydroxylmethyl)-5,5-dimethylhydantoin was used to react with cellulose-containing textile fabrics. After chlorination, the fabrics provided antimicrobial functions against both gram-negative and gram-positive bacteria.16 Most recently, polymerizable hydantoins were synthesized and grafted onto different polymers to achieve antimicrobial functions.17,19
Although the covalent binding approach has been successful in introducing N-halamine structures into a number of polymers,14,16-19,21 it is not suitable for the antimicrobial treatment of many other polymeric materials: in some cases, the polymers are chemically inert, making the covalent binding reactions difficult to perform; in other situations, the covalent binding reaction may deteriorate the physical properties of the polymers and/or complicate the fabrication processes of the final products. A potential solution to these problems is to use N-halamine-based antimicrobial agents as additives for polymers to provide antimicrobial functions.15,20 Apparently, the success of this approach depends heavily on the availability of suitable N-halamine-based antimicrobial additives for different polymers. Given the fact that hydantoin derivatives are effective N-halamine precursors,14,16-19,21 we decided to evaluate the suitability of using hydantoin-based N-halamines as antimicrobial additives. In the current investigation, a series of N-chlorinated hydantoins, 1-chloro-3-alkyl-5,5-dimethylhydantoins (CADMH), were synthesized. The alkyl chains were varied from C-2 to C-22 in order to determine the influences of the alkyl chain length on the antimicrobial activities as well as the solubility and migration rate of the samples in different polymers. The antimicrobial functions of the CADMH against both gram-negative and gram-positive bacteria were tested, and the effectiveness of the CADMH as antimicrobial additives in polymeric materials was preliminarily evaluated.
Experimental Section
Materials
5,5-Dimethylhydantion (DMH), trichloroisocyanuric acid (TCCA), 1-bromoethane (BE) and 1-bromobutane (BB) were purchased from Acros Organics (Morris Plains, NJ). 1-bromohexane (BH), 1-bromododecane (BD) and 1-bromooctadecane (BOD) were provided by Sigma-Aldrich (St. Louis, MO). 1-bromodocosane (BDCS) was obtained from Tokyo Kasei Kogyo Co. LTD. (Portland, OR). Polystyrene (PS, M.W.=100,000) and high density polyethylene (HDPE) provided by Sigma-Aldrich were used as the model polymers to preliminarily evaluate the performances of CADMH as additives. All other regents were analytical grade and used as received.
Instruments
FT-IR spectra were recorded with KBr pellets using a Thermo Nicolet Avatar 370 FT-IR spectrometer (Woburn, MA). 1H NMR analysis was carried out on a Varian Unity-300 spectrometer (Palo Alto, CA) at ambient temperature in deuderated chloroform (CDCl3). UV spectra were taken on a Beckman DU 520 General Purpose UV/Vis Spectrophotometer (Beckman Instruments Inc., Fullerton, CA). Thermal properties of the samples were determined using a Shimadzu DSC-60 instrument at a heating rate of 10 °C/min under N2 atmosphere.
General procedure for the synthesis of 3-alkyl-5,5-dimethylhydantoin
In a typical run, 0.025 mol (3.20 g) of DMH was dissolved in 30 mL methanol in the presence of 0.03 mol (1.68 g) of potassium hydroxide. The mixture was heated and kept at 50 °C for 30 min. After evaporation of the solvent, the potassium salt of DMH was dried in a vacuum oven at 60 °C for three days.18 The anhydrous salt was then dispersed in 100 mL N,N-dimethylformamide (DMF) at 95 °C for 10 min under constant stirring, after which 0.025 mol of the correspondent 1-brominated hydrocarbons was added into the mixtures. The reaction was continued for 2 h at 95 °C. At the end of the reaction, the formed KBr was filtered off.18
Synthesis of 3-ethyl-5,5-dimethylhydantoin (EDMH). After the removal of DMF by distillation under reduced pressure, the residual substance was recrystallized from distilled water. EDMH was obtained as white powders. Yield: 3.65 g (93.5%). Melting point: 96.0 °C.
Synthesis of 3-butyl-5,5-dimethylhydantoin (BDMH). After the removal of DMF by distillation under reduced pressure, 30 mL chloroform were added into the mixtures. The mixtures were filtered, and the chloroform was evaporated under reduced pressure. BDMH was obtained as a clear viscous liquid. Yield: 3.75 g (81.5%).
Synthesis of 3-hexyl-5,5-dimethylhydantoin (HDMH). HDMH was obtained as a clear viscous liquid using the same procedure in the preparation of BDMH. Yield: 3.50 g (66.0%).
Synthesis of 3-dodecyl-5,5-dimethylhydantoin (DDMH). After the removal of KBr by filtration, the filtrate was cooled to 0 °C. The precipitates were collected by filtration and recrystallized from methanol. DDMH was obtained as white powders. Yield: 7.10 g (95.9%). Melting point: 42.0 °C.
Synthesis of 3-octadecyl-5,5-dimethylhydantoin (ODDMH). ODDMH was obtained as white powders using the same procedure in the preparation of DDMH. Yield: 8.34 g (86.2%). Melting point: 68.2 °C.
Synthesis of 3-docosyl-5,5-dimethylhydantoin (DCSDMH). DCSDMH was obtained as white powders using the same procedure in the preparation of DDMH. Yield: 10.20 g (93.4%). Melting point: 76.1 °C.
General procedure for the synthesis of CADMH
To transform the hydantoin derivatives into N-halamines, a known amount of 3-alkyl-5,5-dimethylhydantoin and TCCA (molar ratio: 1:3) were dissolved in acetone. The solution was vigorously stirred for 30 min at room temperature. At the end of the reaction, acetone was evaporated, hexane was added to the mixtures, and the insoluble solids were filtered off. After removing hexane from the filtrate by evaporation, CADMH was obtained as clear liquid or white powders, as described in the sections below.
The active chlorine content of CADMH was determined by iodimetric titration.15 Briefly, around 0.05 g of CADMH was dispersed in 40 mL absolute ethanol containing 1.0 wt% acetic acid. One gram of potassium iodide was added, and the mixture was stirred for 30 min at room temperature under N2 atmosphere. The released iodine was titrated with 0.01 mol/L sodium thiosulfate aqueous solution. The same procedure was applied to the same amount of unchlorinated samples as controls. Percentage of chlorine content was calculated according to the following equation:
| (1) |
where VCl and V0 were the volumes (mL) of sodium thiosulfate solutions consumed in the titration of the chlorinated and unchlorinated samples, respectively; WCl was the weight of the chlorinated sample (g). Each titration was repeated for three times and the average was reported.
Synthesis of 1-chloro-3-ethyl-5,5-dimethylhydantoin (Cl-EDMH). 0.7750 g (0.005 mol) EDMH and 3.50 g (0.015 mol) TCCA were used in the chlorination. After the removal of hexane, 0.4741 g of Cl-EDMH was obtained as while powders. Yield: 50.0%. Melting point: 96.6 °C. Chlorine content determined by titration: 19.11% (theoretical value: 18.63%).
Synthesis of 1-chloro-3-butyl-5,5-dimethylhydantoin (Cl-BDMH). 0.9378 g (0.005 mol) of BDMH and 3.50 g (0.015 mol) of TCCA were used in the chlorination. After the removal of hexane, 0.7890 g of Cl-BDMH was obtained as white powders. Yield: 72.2%. Melting point: 45.4 °C. Chlorine content determined by titration: 16.39% (theoretical value: 16.24%).
Synthesis of 1-chloro-3-hexyl-5,5-dimethylhydantoin (Cl-HDMH). 0.9352 g (0.004 mol) HDMH and 2.79 g (0.012 mol) TCCA were used in the chlorination. After the removal of hexane, 0.7112 g of Cl-HDMH was obtained as a clear viscous liquid. Yield: 65.5%. Chlorine content determined by titration: 13.93% (theoretical value: 14.39%).
Synthesis of 1-chloro-3-dodecyl-5,5-dimethylhydantoin (Cl-DDMH). 0.8477 g (0.003 mol) DDMH and 2.00 g (0.009 mol) TCCA were used in the chlorination. After the removal of hexane, 0.92 g of Cl-DDMH was obtained as a clear viscous liquid. Yield: 97.3%. Chlorine content determined by titration: 10.78% (theoretical value: 10.73%).
Synthesis of 1-chloro-3-octadecyl-5,5-dimethylhydantoin (Cl-ODDMH). 1.0058 g (0.003 mol) ODDMH and 2.00 g (0.009 mol) TCCA wee used in the chlorination. After the removal of hexane, 0.9844 g of Cl-ODDMH was obtained as white powders. Yield: 91.2%. Melting point: 43.0 °C. Chlorine content determined by titration: 8.23% (theoretical value: 8.55%).
Synthesis of 1-chloro-3-docosyl-5,5-dimethylhydantoin (Cl-DCSDMH). 0.8698 g (0.002 mol) DCSDMH and 1.40 g (0.006 mol) TCCA were used in the chlorination. After the removal of hexane, 0.8133 g of Cl-DCSDMH was obtained as white powders. Yield: 86.3%. Melting point: 52.4 °C. Chlorine content determined by titration: 7.68% (theoretical value: 7.54%).
Preparation of CADMH-containing polymeric materials
PS and HDPE were used as the model polymers to preliminarily evaluate the antimicrobial activities of CADMH as additives. In the preparation of CADMH-containing PS films, a certain amount of CADMH was dissolved in 5% PS solutions in THF. The solution was poured onto glass slides. The glass slides were put in a fume hood for 2 days at room temperature to evaporate most of the solvent, and then further dried in a vacuum oven at 30 °C for 3 days to obtain polymer films (100 ± 10 μm of thickness). To prepare CADMH-containing HDPE films, a predetermined amount of CADMH was added into 5% HDPE solutions in hot o-xylene under constant stirring. After evaporation of the solvent, polymer films (thickness: 70 ± 5 μm) were obtained by hot pressing at 140 °C for 15 seconds. The chlorine contents of the CADMH-containing PS and HDPE films were determined by iodimetric titration, as described above. Pure PS or HDPE films without the presence of CADMH were prepared using the same procedures as controls.
Antimicrobial activity of CADMH
Escherichia coli (E. coli, ATCC® 15597™, gram-negative) and Staphylococcus aureus subsp. aureus (S. aureus, ATCC® 6538™, gram-positive) were used as model microorganisms to challenge the antimicrobial functions of the samples. It should be mentioned that the S. aureus species used in the current study are biosafety level-2 microorganisms, which are of moderate hazards to personnel and instruments. Appropriate protective equipment including gloves and gowns were used, and the guidelines provided by the U. S. Department of Health and Human Services were followed in the antimicrobial tests.22
In the microbial studies, the bacteria were grown in broth solutions (LB broth for E. coli; Tryptic Soy broth for S. aureus) overnight at 37 °C. Cells were harvested in a centrifuge, washed twice with phosphate buffered saline (PBS, OmniPur®, NaCl, 8.0 g/L; KCl, 0.20 g/L; Na2HPO4, 1.42 g/L; KH2PO4, 1.36 g/L; pH 7.4), re-suspended in PBS, and then diluted to concentrations of 108-9 CFU/mL. A known amount of CADMH (particle size: 60-80 mesh) was dispersed in 10 mL of sterilized distilled water. The mixture was vortexed and then sonicated for 10 min. Then, 100 μL of the bacteria suspension were added into the CADMH-containing suspension, and the resultant mixture was vortexed for 60 s. After a certain period of contact time under constant shaking, the mixture was poured into 90 mL of 0.03 wt% sterilized sodium thiosulfate aqueous solution to quench the active chlorine and stop the antimicrobial test. Our previous studies have shown that such a treatment does not affect the growth of the bacteria.15 The resulting solutions were vortexed for 2 min and then serially diluted, and each dilution was placed onto LB agar (for E. coli) or Tryptic soy agar (for S. aureus). The same procedure was also applied to the correspondent unchlorinated samples as controls. Bacterial colonies on the plates were counted after incubation at 37 °C for 24 h.
Antimicrobial activity of CADMH-containing polymers
In the antimicrobial tests of CADMH-containing PS or HDPE films, 10 μL of E. coli suspensions (108-109 CFU/mL) were placed onto the surface of a film (4 × 4 cm). The film was then covered with another identical film. A 100 g weight was added onto the whole “sandwich” to ensure sufficient contact. At different contact time, the “sandwich” was transferred into 10 mL of 0.03 wt% sodium thiosulfate aqueous solution to quench the active chlorine of the samples. The mixture was sonicated for 20 min and vortexted for 60 s to remove the adherent bacteria into the solution. The resultant solution was serially diluted, and each dilution was placed onto LB agar plates. The same procedure was also applied to the pure PS or HDPE films as controls. Bacterial colonies were counted after 24 h of incubation at 37°C.
Results and Discussion
Synthesis and characterization of the samples
As illustrated in Scheme 1, the 3-alkyl-5,5-dimethylhydantoins were synthesized by the nucleophilic reaction of alkyl bromide with the potassium salt of DMH, and CADMH was prepared by the chlorine exchange reaction of TCCA with the 3-alkyl-5,5-dimethylhydantoins. All the reactions preceded smoothly with good yields (see the Experimental Section for data).
Scheme 1.
Synthesis of 3-alkyl-5,5-dimethylhydantoin and 1-chloro-3-alkyl-5,5-dimethylhydantoin (CADMH)
FT-IR analysis was used to characterize the reactions. As an example, Figure 1 shows the IR spectra of DMH, BD, DDMH and Cl-DDMH. In the spectrum of DMH, the broad peak centered around 3280 cm-1 is attributable to N-H stretching vibrations, and the 1770, 1732 and 1713 cm-1 bands are caused by the C=O stretching vibrations of the imide and amide groups. The C-H stretching vibrations of BD are presented in the region of 2854 cm-1-2956 cm-1. In the spectrum of DDMH, the C-H peaks of the alkyl chain can be clearly observed. In addition, the carbonyl bands of the imide and amide groups shift to 1782 cm-1 and 1707 cm-1, respectively. Upon treatment with TCCA, the N-H bond in DDMH is transformed into N-Cl bond. Consequently, in the spectrum of Cl-DDMH, the N-H stretching vibration around 3280 cm-1 disappears, and two new bands at 758 and 735 cm-1 can be detected, which are assigned to the N-Cl groups.23-25 Moreover, the transformation of N-H bonds to N-Cl groups is associated with the breakage of N-H---O=C hydrogen bonding in DDMH, and this results in the shifts of the C=O bands from 1782 and 1707 cm-1 in DDMH to 1794 and 1728 cm-1 in Cl-DDMH, respectively.
Figure 1.
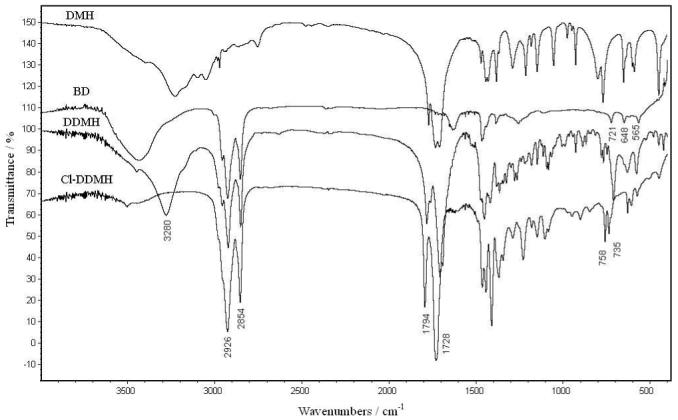
FT-IR spectra of DMH, BD, DDMH and Cl-DDMH
The chemical structures of the samples were also characterized with 1H NMR analysis. As a typical example, Figure 2 shows the proton NMR spectra of BD, DMH, DDMH and Cl-DDMH. The assignments of the signals in BD are: δ = 3.44 (t, H1), 1.80 (m, H2), 1.46 (m, H3), 1.30 (m, H4) and 0.92 ppm (t, H5). In the spectrum of DMH, the methyl protons show signals at 1.64 and 1.47 ppm, the imide proton shows a resonance peak at 8.14 ppm, and the amide proton displays a single peak at 5.79 ppm. After the substitution reaction of DMH with BD, while the alkyl proton signals of BD and the methyl and amide proton resonances of DMH can be clearly observed in the spectrum of DDMH, the imide proton signal (8.14 ppm in DMH) disappears. After chlorination, the amide proton signal can no longer be detected in the spectrum of Cl-DDMH, indicating that the N-H bond in DDMH is transformed into N-Cl group.
Figure 2.
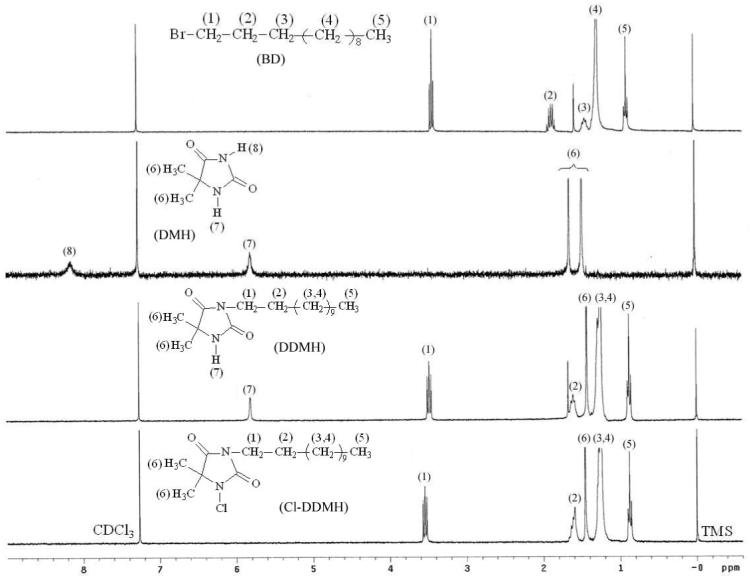
1H NMR spectra of BD, DMH, DDMH and Cl-DDMH
The N-H → N-Cl transformation was further confirmed by UV analysis; typical examples are presented in Figure 3. In the UV spectra of DMH and DDMH (0.05 mol/L in methanol), the UV adsorption centered at 240 nm are caused by the hydantoin ring structures.26-29 After chlorination, DMH is transformed into 1,3-dichloro-5,5-dimethylhydantoin (DCDMH), and DDMH is transformed into Cl-DDMH. At a concentration of 0.05 mol/L, a broad N-halamine peak around 280 is clearly observed in the UV spectra of DCDMH-H and Cl-DDMH, which can be caused by the disruption of the N-Cl bond and/or the transition from a bonding to an antibonding orbital.15 The presence of the 280 nm absorption is further confirmed by the UV spectrum of diluted DCDMH methanol solution (DCDMH-L, 0.01 mol/L). In this spectrum, in addition to the strong hydantoin ring adsorption at 240 nm, a weak shoulder in the range of 270-280 nm can be detected. The UV spectrum of diluted Cl-DDMH was also examined. Unfortunately, because of the relatively low chlorine content of Cl-DDMH (10.78% vs. 36.04% in DCDMH) and the interference of the strong adsorption of the ring structure, the N-Cl adsorption in diluted Cl-DDMH was too weak to be detectable. Similar phenomena were also observed in the UV study of other CADMH.
Figure 3.
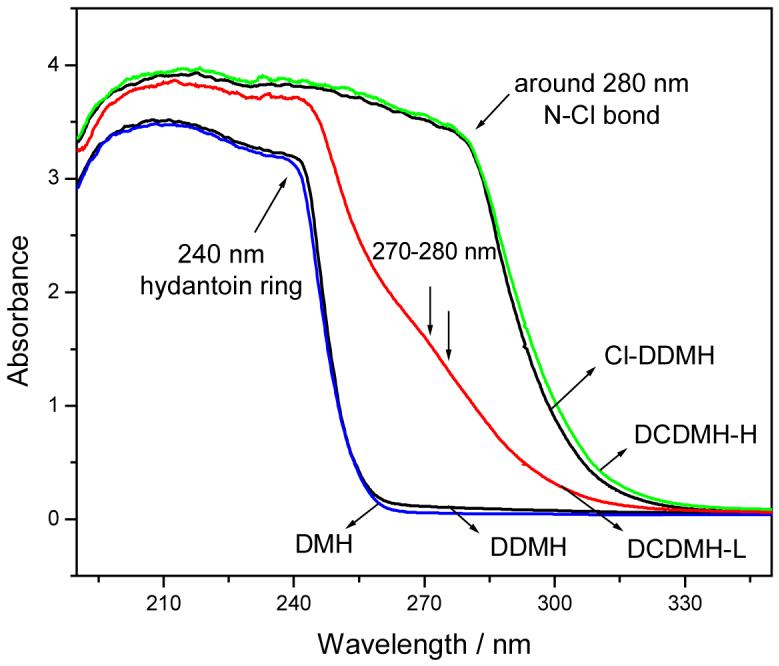
UV spectra of DMH, DDMH, Cl-DDMH, and 1,3-dichloro-5,5-dimethylhydantoin (DCDMH). Solvent: methanol. In the spectra of DMH, DDMH, DCDMH-H or Cl-DDMH, the sample concentration was 0.05 mol/L; in the spectrum of DCDMH-L the sample concentration was 0.01 mol/L.
Thermal properties of the samples were examined with DSC, and the representative DSC curves are shown in Figure 4 using DCSDMH and Cl-DCSDMH as examples. DCSDMH shows a sharp melting peak at 76.1 °C. After chlorination, the N-H bond in DCSDMH is transformed into N-Cl group, and because of the lack of hydrogen bonding, the melting point of Cl-DCSDMH decreases to 52.4°C. Moreover, an intensive exothermic peak at 176.0 °C is observed in the DSC curve of Cl-DCSDMH, which can be caused by the decomposition of the N-Cl bond, in good agreement with the results of our earlier studies.15,20
Figure 4.
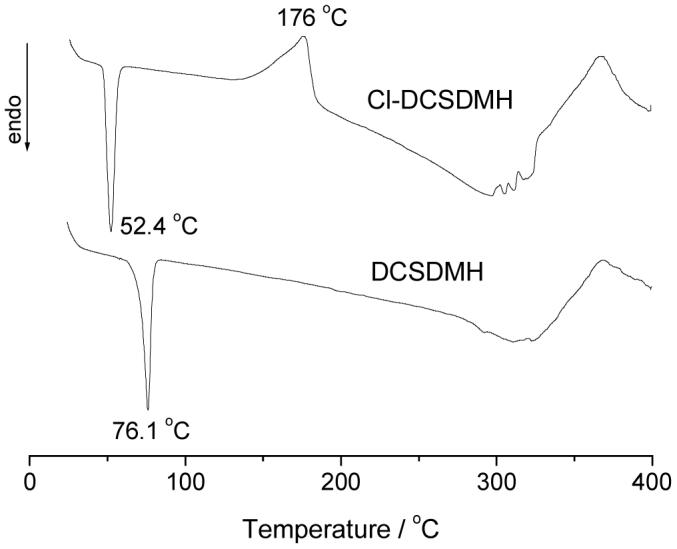
DSC curves of DCSDMH and Cl-DCSDMH
Antimicrobial functions
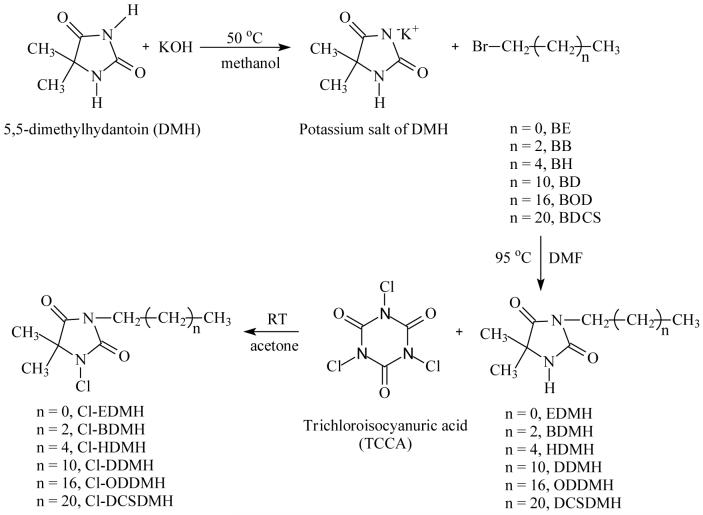
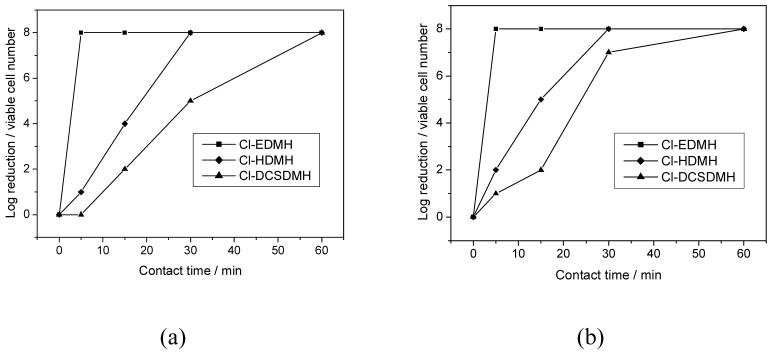
The antimicrobial activities of CADMH were challenged with gram-negative (E. coli) and gram-positive (S. aureus) bacteria. All the samples provided potent antimicrobial functions against both species. As a general observation, shorter alkyl chain length leads to faster antimicrobial actions. For instance, as shown in Figure 5, at 500 ppm of active chlorine content, Cl-EDMH provides a total kill of 108-109 CFU/mL of E. coli and S. aureus in less than 5 min. However, it takes Cl-HDMH 30 min and Cl-DCSDMH 60 min to achieve the same antimicrobial efficacy. When the active chlorine content is increased to 1000 ppm (see Figure 6), the antimicrobial activities of Cl-HDMH and Cl-DCSDMH are significantly improved (20-30 min for total kill), but their bactericidal actions are still slower than that of Cl-EDMH (less than 5 min for total kill).
Figure 5.
Antimicrobial activities of Cl-EDMH, Cl-HDMH and Cl-DCSDMH against (a) E. coli (gram-negative bacteria) and (b) S. aureus (gram-positive bacteria). The active chlorine content in the test suspensions was 500 ppm.
Figure 6.
Antimicrobial activities of Cl-EDMH, Cl-HDMH and Cl-DCSDMH against (a) E. coli (gram-negative bacteria) and (b) S. aureus (gram-positive bacteria). The active chlorine content in the test suspensions was 1000 ppm.
It has been reported that the bactericidal action of N-halamines is a manifestation of a chemical reaction involving the direct transfer of positive chlorines from the N-halamines to the appropriate receptors in the cells.30-32 This chemical reaction can effectively destroy or inhibit enzymatic or metabolic cell processes, resulting in the expiration of the organisms. With the increase of alkyl chain length, the solubility of the CADMH decreases. As a matter of fact, while Cl-EDMH is soluble in water (ca. 3g/L at 23°C), both Cl-HDMH and Cl-DCSDMH are insoluble. The water solubility of Cl-EDMH ensures full contact of the bacteria with Cl-EDMH molecules in the antimicrobial tests, resulting in the most potent antimicrobial activity (total kill in less than 5 min). In the cases of Cl-HDMH and Cl-DCSDMH, however, the bacteria could only contact with the surfaces of the Cl-HDMH and Cl-DCSDMH particles (60-80 mesh) during the microbial tests. Thus, longer contact time is needed for a total kill of the microorganisms.
It is interesting to note that the CADMH provided faster antimicrobial action against gram-positive bacteria than gram-negative bacteria. For example, at 1000 ppm of active chlorine content, after 5 min of contact, Cl-HDMH provides 2 log reduction (99% of kill) of E. coli, but it offers 7 log reduction (99.99999% of kill) of S. aureus (Figure 6). When the contact time is extended to 15 min, Cl-HDMH provides 5 log reduction of E. coli (99.999% of kill), but it inactivates all the S. aureus tested (8 log reduction). This difference can be attributed to the different structures of the bacteria. A major structural difference between gram-negative bacteria and gram-positive bacteria is that the cell wall of the former is overlaid with an outer membrane comprising lipopolysaccharide. This lipopolysaccharide layer offers a supplementary barrier limiting or preventing the penetration of antimicrobial agents into the cell.33-35 Therefore, E. coli (gram-negative) are inactivated in a longer contact period than S. aureus (gram-positive).
The CADMH showed excellent storage stability. After storage for 4 months under ambient conditions (23 ± 2 °C, 70 ± 5% RH), all the samples retained more than 96% of their original chlorine contents. The antimicrobial functions were tested monthly during the storage period, and after 4 months of storage, the minimum contact time of the samples for a total kill of 108-109 CFU/mL of E. coli or S. aureus was unchanged.
The efficacy of CADMH as antimicrobial additives in polymeric materials was preliminarily evaluated. PS and HDPE were used as model polymers. CADMH samples were added into PS through solvent casting, and they were incorporated into HDPE though hot pressing. Iodimetric titration showed that these two approaches did not affect the chlorine contents of the CADMH samples, suggesting the suitability of CADH as potential polymer additives. While neither the original PS nor the HDPE had any antimicrobial effects, the CADMH-containing materials provided potent antimicrobial functions. For instance, when as low as 1% of Cl-DCSDMH (which has the slowest antimicrobial action among all the CADMH synthesized in the current study) was added into PS or HDPE films, the resultant films provided a total kill of 108-109 CFU/mL of E. coli (the more resistant species than S. aureus) after 4 h of contact. After storage for 4 months at 23 ± 2 °C and 70 ± 5% RH, no changes could be observed in the active chlorine contents and the antimicrobial activities of the Cl-DCSDMH-containing PS and HDPE films. Although more studies are needed to further evaluate their performances, these findings point to the great potentials of the CADMH as effective antimicrobial additives for polymeric materials.
Conclusion
A series of hydantoin-based N-halamines were synthesized, and their structures were confirmed with FT-IR, 1H NMH, UV, and DSC analyses. The antimicrobial activities of the samples were challenged with 108-9 CFU/mL of E. coli (gram-negative) and S. aureus (gram-positive). All the samples provided a total kill of the bacteria tested. Shorter alkyl chain length resulted in faster antimicrobial actions. When CADMH were added into PS or HDPE as antimicrobial additives, they offered the polymeric materials with potent and durable antimicrobial functions. More CADMH-containing polymeric materials are being studied in this lab to provide further information concerning the structure-property relationships of the CADMH samples.
Acknowledgements
This work is supported in part by the National Institutes of Health (grant number: DE01640301A1).
Literature Cited
- (1).Binder S, Levitt AM, Sacks JJ, Hughes JM. Emerging Infectious Diseases: Public Health Issues for the 21st Century. Science. 1999;284:1311–1313. doi: 10.1126/science.284.5418.1311. [DOI] [PubMed] [Google Scholar]
- (2).Larson E, Olmsted RN. Research Priorities Project, Year 2000: the Lone Ranger Rides Again. Am. J. Infect. Control. 2001;29:69–72. doi: 10.1067/mic.2001.114194. [DOI] [PubMed] [Google Scholar]
- (3).Neely AN, Maley MP. Survival of Enterococci and Staphylococci on Hospital Fabrics and Plastic. J. Clin. Microbiol. 2000;38:724–726. doi: 10.1128/jcm.38.2.724-726.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Scott E, Bloomfield SF. The Survival and Transfer of Microbial Contamination via Cloths, Hands and Utensils. J. Appl. Bacteriol. 1990;68:271–278. doi: 10.1111/j.1365-2672.1990.tb02574.x. [DOI] [PubMed] [Google Scholar]
- (5).Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial Infections in Medical Intensive Care Units in the United States. Crit. Care. Med. 1999;27:887–892. doi: 10.1097/00003246-199905000-00020. [DOI] [PubMed] [Google Scholar]
- (6).Litsky W. Wanted: Plastics with Antimicrobial Properties. Am. J. Pub. Health. 1990;80:13–15. doi: 10.2105/ajph.80.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Cheng Z, Zhu X, Shi ZL, Neoh KG, Kang ET. Polymer Microspheres with Permanent Antibacterial Surface from Surface-Initiated Atom Transfer Radical Polymerization. Ind. Eng. Chem. Res. 2005;44:7098–7104. [Google Scholar]
- (8).Pernak J, Branicka M. Synthesis and Aqueous Ozonation of Some Pyridinium Salts with Alkoxymethyl and Alkylthiomethyl Hydrophobic Groups. Ind. Eng. Chem. Res. 2004;43:1966–1974. [Google Scholar]
- (9).Tiller JC, Liao C, Lewis K, Klibanov AM. Designing Surfaces that Kill Bacteria on Contact. Proc. Natl. Acad. Sci. USA. 2001;98:5981–5985. doi: 10.1073/pnas.111143098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Jayaraman A, Yarmush ML, Roth CM. Molecular Bioengineering. Ind. Eng. Chem. Res. 2002;41:441–455. [Google Scholar]
- (11).Emerson DW. Slow Release of Active Chlorine and Active Bromine from Styrene-Divinylbenzene Copolymers Bearing N,N- Dichlorosulfonamide, N-Chloro-N-alkylsulfonamide and N-Bromo-N- alkylsufonamide Functional Groups. Polymer Supported Reagents. 6. Ind. Eng. Chem. Res. 1991;30:2426–2430. [Google Scholar]
- (12).Worley SD, Williams DE. Halamine Water Disinfectants. Crit. Rev. Environ. Control. 1988;18:133–175. [Google Scholar]
- (13).Sun Y, Sun G. Novel Refreshable N-Halamine Polymeric Biocides: N-Chlorination of Aromatic Polyamides. Ind. Eng. Chem. Res. 2004;43:5015–5020. [Google Scholar]
- (14).Chen Y, Worley SD, Kim J, Wei CI, Chen TY, Santiago JI, Williams JF, Sun G. Biocidal Poly(styrenehydantoin) Beads for Disinfection of Water. Ind. Eng. Chem. Res. 2003;42:280–284. [Google Scholar]
- (15).Chen Z, Sun Y. N-Chloro-Hindered Amines as Multifunctional Polymer Additives. Macromolecules. 2005;38:8116–8119. [Google Scholar]
- (16).Sun G, Xu X. Durable and Regenerable Antibacterial Finishing of Fabrics: Biocidal Properties. Textile Chem. Colorist. 1998;30:26–30. [Google Scholar]
- (17).Sun Y, Sun G. Durable and Refreshable Polymeric N-halamine Biocides Containing 3-(4′-Vinylbenzyl)-5,5-Dimethylhydantoin. J. Polym. Sci. Part A: Polym. Chem. 2001;39:3348–3355. [Google Scholar]
- (18).Worley SD, Li F, Wu R, Kim J, Wei C-I, Williams JF, Owens JR, Wander JD, Bargmeyer AM, Shirtliff ME. A Novel N-Halamine Monomer for Preparing Biocidal Polyurethane Coatings. Surf. Coat Int. Part B: Coat. Trans. 2003;86:273–277. [Google Scholar]
- (19).Sun Y, Sun G. Synthesis, Characterization, and Antibacterial Activities of Novel N-Halamine Polymer Beads Prepared by Suspension Copolymerization. Macromolecules. 2002;35:8909–8912. [Google Scholar]
- (20).Chen Z, Sun Y. Antimicrobial Functions of N-Chloro-Hindered Amines. Polym. Preprint. 2005;46:835–836. [Google Scholar]
- (21).Worley SD, Sun G. Biocidal Polymers. Trends Polym. Sci. 1996;11:364–370. [Google Scholar]
- (22).Richmond JY, McKinney RW. Biosafaty in Microbiological and Biomedical Laboratories. 4th edition Government Printing Office; Washington, DC: 1999. p. 12. [Google Scholar]
- (23).Moore GE, Badger RM. The Infrared Spectra and Structure of the Chloramines and Nitrogen Trichloride. J. Am. Chem. Soc. 1952;74:6076–6080. [Google Scholar]
- (24).Heasley VL, Kovacic P, Lange RM. N-Haloalkylamines. Analyses and Amination of Toluene. J. Org. Chem. 1966;31:3050–3052. [Google Scholar]
- (25).Petterson RC, Grzeskowiak U, Jules LH. N-Halogen Compounds. II. The N--Cl Stretching Band in Some N-Chloroamides. The Structure of Trichloroisocyanuric Acid. J. Org. Chem. 1960;25:1595–1598. [Google Scholar]
- (26).Cauletti C, Devillanova FA, Isaia F, Verani G, Vondrak T. Electronic Structure of Five-Membered Heterocyclic Compounds Containing Nitrogen, Oxygen, Sulfur and Selenium. Gas-Phase UV Photoelectron Spectra and Auantum-Mechanical Calculations. J. Mol. Struct. 1988;175:447–452. [Google Scholar]
- (27).Santos E, Rosillo I, Del CB, Avendano C. Determination of pKa Values for Hydantoins by Spectrophotometry. J. Chem. Res. Synop. 1982;5:131. [Google Scholar]
- (28).Ajo D, Casarin M, Granozzi G, Poli A, Tondello E. Electronic Structure of Imides by UV Photoelectron Spectroscopy and INDO/S Calculations: Hydantoin and Urazole. J. Mol. Struct. 1982;82:277–282. [Google Scholar]
- (29).Uscumlic GS, Kshad AA, Mijin DZ. Synthesis and Investigation of Solvent Effects on the Ultraviolet Absorption Spectra of 1,3-Bis-Substituted-5,5-Dimethylhydantoins. J. Serbian Chem. Soc. 2003;68:699–706. [Google Scholar]
- (30).Bodor N, Kaminski JJ, Worley SD, Colton RJ, Lee TH, Rabalais JW. Photoelectron Spectra, Hydrolytic Stability, and Antimicrobial Activity of N-Chlorinated Piperidines. J. Pharm. Sci. 1974;63:1387–1391. doi: 10.1002/jps.2600630911. [DOI] [PubMed] [Google Scholar]
- (31).Naquib I, Tsao T, Sarathy PK, Worley SD. Kinetic Versus Thermodynamic Control in Chlorination of Imidazolidin-4-one Derivatives. Ind. Eng. Chem. Res. 1991;30:1669–1671. [Google Scholar]
- (32).Block SS. Disinfection, Sterilization and Preservation. 3rd edition Lea & Febiger; Philadelphia: 1983. pp. 157–182. [Google Scholar]
- (33).Denver SP. Mechanisms of Action of Antibacterial Biocides. Int. Biodet. Biodeg. 1995;36:227–245. [Google Scholar]
- (34).Russell AD. Mechanisms of Bacterial Resistance to Biocides. Int. Biodet. Biodeg. 1995;36:247–265. [Google Scholar]
- (35).Denver SP, Stewart GSAB. Mechanisms of Action of Disinfectants. Int. Biodet. Biodeg. 1998;41:261–268. [Google Scholar]