Abstract
CASE
A 43-year-old female with systemic lupus erythematosus (SLE) was admitted with fever and shortness of breath 1 month after aortic valve replacement. A diagnostic workup including chemistries, complete blood count, blood cultures, chest x-ray, and 2-D echocardiogram was performed to determine the etiology of her symptoms and differentiate between acute bacterial endocarditis and Libman–Sacks endocarditis.
DISCUSSION
By utilizing Duke’s criteria, antiphospholipid antibodies, and serial echocardiography, we were able to make a diagnosis of Libman–Sacks endocarditis. The patient was successfully treated for Libman–Sacks endocarditis and recovered uneventfully.
CONCLUSION
This case highlights the challenges of making the correct diagnosis when 2 disease processes present with similar findings.
KEY WORDS: Libman–Sacks endocarditis, systemic lupus erythematosus, blood cultures, echocardiogram
CASE
A 43-year-old female with a history of systemic lupus erythematosus (SLE) presented to the emergency department with generalized weakness and shortness of breath for 1 week and 1 day of fever to 102°F. She had a nonproductive cough and associated paroxysmal nocturnal dyspnea and orthopnea over the same time period. She had experienced increased dyspnea on exertion with activities of daily living and increasing lower extremity edema. She denied chest pain or palpitations on admission.
Her past medical history was significant for SLE for approximately 16 years complicated by lupus nephritis and end-stage renal disease requiring peritoneal dialysis. One month before this presentation, she had undergone porcine aortic valve replacement for chronic severe aortic regurgitation because of presumed Libman–Sacks endocarditis. She had 3 cesarean sections in the past and a bilateral tubal ligation. Current medications included carvedilol, losartan, phenytoin, erythropoietin, atorvastatin, amlodipine, prednisone 5 mg once a day, dipyridamole, omeprazole, and calcitriol. There was no history of tobacco, alcohol, or drug use. She had 3 living children. There was no family history of connective tissue diseases. Both the patient and her husband were nurses and able to comply with performing peritoneal dialysis at home.
At presentation, her vital signs were remarkable for a temperature of 98.4°F, heart rate of 91 beats per minute, respiratory rate of 24 breaths per minute, and a blood pressure of 166/117 mmHg. Her blood oxygen saturation was 99% while on room air, but she appeared in mild respiratory distress. Her head, eyes, ears, nose, and oropharynx were unremarkable. Neck exam was positive for jugular venous distention to the mandible. Cardiac exam showed a regular rate and rhythm with a new 4 out of 6 harsh holosystolic murmur at the left upper sternal border that was nonradiating. Respiratory examination was remarkable for bilateral crackles in both lung fields halfway up her thorax and presacral pitting edema. Her abdomen was mildly distended with active bowel sounds and a peritoneal dialysis catheter in place that was clean, dry, and intact without evidence of infection. Her extremities had 2+ pitting edema without evidence of splinter hemorrhages, Janeway lesions, or Osler’s nodules. Neurologic exam was without any sensory or motor deficits.
The patient experienced fevers throughout the hospital course from the day of admission and daily thereafter. Given her recent aortic valve replacement, she was empirically started on vancomycin and gentamycin for possible acute bacterial endocarditis after 3 sets of blood cultures were obtained. Three sets of blood cultures drawn on admission and 48 h later did not grow any organisms. Peritoneal fluid cultures were also negative. Q fever antibody IgG titers were less than 1:16. Bartonella henselae antibody IgM and IgG titers were less than 1:16. Complement levels showed C3 of 6 mg/dL (range 88–210 mg/dL) and C4 of 29 mg/dL (range 10–40 mg/dL). A previous C3 level 1 year before admission was within the normal range at 113 mg/dL. A quantitative assay of rheumatoid factor was 10 (range 10–14). The erythrocyte sedimentation rate (ESR) was 106 mm/h (range 0–20 mm/h) and C-reactive protein (CRP) was elevated at 11.4 mg/dL (range <0.9 mg/dL). Her antinuclear antibody (ANA) was positive at a titer of greater than 1:320 in a speckled pattern, and double-stranded DNA antibodies (dsDNA) were also positive at a titer of 1:80. Anticardiolipin antibody IgM and IgG were both moderately positive at 21 IU/mL (range 15 to 80 IU/mL). The anticardiolipin antibody IgG titer from 1 year before admission was previously negative at 9 IU/mL. Her white blood cell count on admission was 8.0 K/mL (range 4.5–11.4 K/mL) and decreased throughout her hospital course to 3.4 K/mL at the time of discharge.
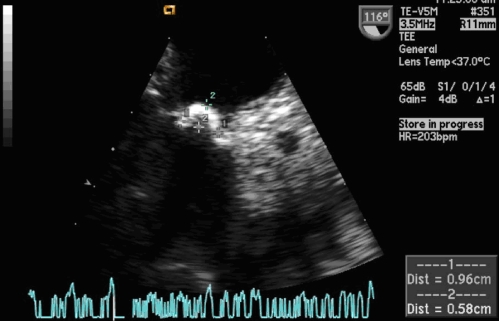
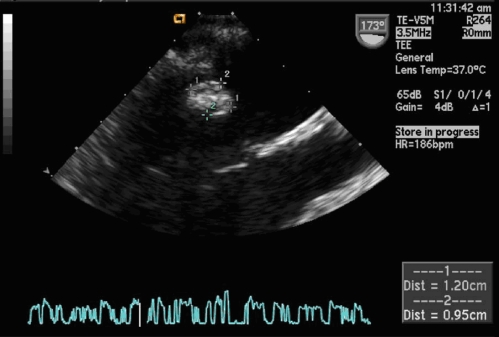
Chest x-ray showed cardiomegaly and bilateral moderate pleural effusions. ECG showed sinus tachycardia with a left anterior fascicular block. A transesophageal echocardiogram (TEE) obtained on admission showed a depressed systolic ejection fraction of 20% and vegetation versus thrombus on the anterior mitral valve leaflet base measuring 7 × 11 mm in the left ventricular outflow track. A TEE done 2 weeks later showed 2 masses, one a 9.6 × 5.8 mm sessile echodense mass at the base of the anterior mitral leaflet on the atrial side and another 12 × 9.5 mm sessile mass on the posterior mitral leaflet on the ventricular side. Figures 1 and 2 demonstrate the mobile echodensity on the mitral valve. Review of the previous intraoperative TEE completed at the time of the aortic valve repair 1 month before this hospital admission showed good aortic valve placement with thickened mitral valve leaflets with normal function. There was no evidence of vegetation on the mitral valve from the intraoperative TEE at the time of the aortic valve replacement surgery. Review of the aortic valve pathology from the prior aortic valve surgery showed fibrosis, myxoid degeneration, neovascularization, and focal minimal chronic valvulitis.
Figure 1.
Mitral valve with mass 9.6 × 5.8 mm in dimension on the anterior/septal leaflet.
Figure 2.
Enlarging mitral valve mass over the 2-week period despite antibiotics.
Despite the initiation of antibiotics on admission, the patient continued to experience high fevers throughout her hospital stay. After the discussion with the patient and consultants, as well as the negative results of blood, peritoneal, and urine cultures over several days, the decision was made to start high-dose prednisone and anticoagulation. The fevers immediately subsided after initiation of high-dose steroids, and antibiotics were discontinued on day 14 of hospitalization. After remaining afebrile on steroids for 36 h, she was discharged home. She was started on hydroxychloroquine as an outpatient and anticoagulation was discontinued.
The patient was continued on her treatment with steroids and hydroxychloroquine as an outpatient. Her follow-up ESR at 6 months postdischarge decreased to 59 mm/h and CRP to 4.8 mg/dL. A follow-up TEE obtained 10 months later showed an improved ejection fraction of 40% and resolution of the vegetative lesions and thickening at the base of the mitral leaflets.
DISCUSSION
In 1924, Libman and Sacks originally described valvular lesions in 4 patients with lupus and added nonrheumatic verrucous endocarditis to the syndrome complex of SLE.1 Libman–Sacks valvular lesions are sterile fibrofibrinous vegetations that favor the left-sided heart valves and usually form on the ventricular surface of the mitral valve.2 The disease progresses from a variable extent of inflammation along with fibrin deposits acutely to end stage or healed forms with a fibrous plaque. The pathogenesis is thought to involve the formation of fibrin–platelet thrombi, which organizes and leads to fibrosis and scarring with subsequent valve dysfunction.3
As clinical therapy for SLE has improved over the decades, patients are living longer with a more chronic form of the disease controlled not just by steroids but a myriad of immunosuppressive medications. Recent data comparing echocardiographic findings of 342 consecutive patients with SLE demonstrated that 11% of these patients had Libman–Sacks vegetations.4 Those patients with Libman–Sacks endocarditis were likely to have had SLE longer with higher disease activity and more frequent episodes of pericarditis, lupus nephritis, and hemolytic anemia. These authors also found that progressive disease with Libman–Sacks lesions was strongly associated with the presence of lupus nephritis.4 Other authors report higher prevalence of Libman–Sacks vegetations, ranging from 53% to 74%.5,6
The development of Libman–Sacks endocarditis adds to the complexity and diagnostic uncertainty in a patient with SLE who presents with a fever and a new cardiac murmur as in this case. Because the occurrence of bacterial endocarditis in patients with persistent Libman–Sacks endocarditis is not uncommon,7 it is imperative to differentiate between the 2 medical conditions, as the management and treatment is quite different.
The modified Duke criteria utilizing pathologic and clinical criteria can be useful in helping differentiate between true infective endocarditis and Libman–Sacks endocarditis. Major Duke criteria include demonstration of typical microorganisms from 2 separate blood cultures or evidence of endocardial involvement by echocardiogram.8 Minor Duke criteria include predisposition from prior heart conditions or intravenous drug use, fever >38.0°C, vascular phenomena, immunologic phenomena, microbiologic evidence with blood cultures not meeting major criteria, or serologic evidence of active infection with organism consistent with infectious endocarditis. Patients may be considered to have definite infectious endocarditis if they have either 2 major criteria or 1 major criterion and 3 minor criteria or 5 minor criteria.8 Our patient met 1 major criterion based on the echocardiographic findings and 2 minor criteria (predisposing heart condition because of the recent aortic valve replacement and fever). This suggested the possibility of infective endocarditis but is not conclusive in establishing the diagnosis.
Some authors have emphasized the importance of certain laboratory tests to help distinguish these 2 disease processes, referring to the condition as pseudoinfective endocarditis.9 Three tests may be useful in making the diagnosis between true lupus endocarditis versus infective endocarditis in this setting. They are:
the white blood cell count,
the CRP level,
the antiphospholipid antibody (aPL) level.2
The white blood cell count in SLE would be expected to be low during a lupus flare. The CRP would be expected to be quite elevated in infection and possibly suppressed in lupus. However, CRP has high sensitivity for any inflammation, and one can argue that SLE as an inflammatory disease process could indeed elevate the CRP. It has been noted by some authors that CRP levels are higher in lupus patients versus controls and poor correlation between established measures of disease activity and biological markers such as anti-dsANA or decrease in C3.10 Finally, it is unlikely that infection would significantly raise the antiphospholipid antibody titers. If levels are moderate to high positive, this is more suggestive of SLE.9 The associated link of antiphospholipids with lupus is well-known11,12 and helps provide an additional mechanism of injury to the cardiac valve as seen in Libman–Sacks endocarditis, possibly one of deposition of immunoglobulin and complement.13 Studies of echocardiographic findings when compared with anticardiolipin and antiphospholipid antibody titers have shown significant correlation for more severe valve dysfunction with increasing titers.8,11
^[var vr_print_hyperlink]Our patient’s white blood cell count was normal on admission and decreased during the course of her hospitalization. Her CRP was elevated. She did have moderately positive anticardiolipin and antiphospholipid antibodies. These laboratory findings coupled with the patient’s multiple negative blood cultures and lack of response to antibiotics led us to the correct diagnosis of Libman–Sacks endocarditis in our patient.
In addition to the laboratory testing that was undertaken, we found that serial echocardiograms allowed good visualization of the mitral valve to assess disease progression and resolution of the vegetations caused by Libman–Sacks endocarditis.
Echocardiography has been shown to be a useful tool in documenting disease progression and valvular dysfunction in patients with SLE. Patients with lupus have also been found to have systolic dysfunction more often whereas those with isolated antiphospholipid antibody syndrome were more likely to have diastolic dysfunction.14 The prevalence of Libman–Sacks vegetations is <10% by transthoracic echocardiogram and up to 30% by TEE. Infective endocarditis lesions are more likely to be located at the leaflet’s line of closure, are homogeneous in echoreflectance, and may show a vibratory or rotary motion.15 In contrast, Libman–Sacks vegetations are usually located at the basal, middle, or tip of leaflets, located on the atrial side of the mitral valve or vessel side of the aortic valve, are of variable sizes and shapes, and heterogeneous in echogenicity.6 The predominant finding in most patients with SLE and valvular abnormalities is valvular thickening. Our patient has masses located on both the atrial and ventricular side of the mitral valve further supporting the diagnosis of Libman–Sacks endocarditis.
CONCLUSION
As medical therapy continues to improve, physicians should expect to see patients with SLE living longer and developing a more chronic disease picture over time. These patients will be more likely to develop cardiac manifestations of lupus, such as valvular regurgitation and possible Libman–Sacks endocarditis. Clinicians treating a lupus patient with fever and a new heart murmur should perform careful evaluation and diagnostic testing to establish the correct diagnosis and begin appropriate treatment. Given the association with antiphospholipid antibodies in this particular condition, further study is warranted as to the optimal treatment regimen once the diagnosis of SLE is firmly established. Optimal treatment, including adequate aggressive anticoagulation therapy, immunosuppressive therapy, and specific treatment for heart failure may play a pivotal role in reducing the severity of valve dysfunction in these patients.16
Acknowledgments
The author would like to thank Philip Sack MD, Cardiology Fellow, Section of General Cardiology, Tulane University Medical School, for his assistance with the echocardiographic images.
Conflict of Interest None disclosed.
References
- 1.Libman E, Sacks B. A hitherto undescribed form of valvular and mural endocarditis. Arch Intern Med. 1924;33:701–707. [Google Scholar]
- 2.Hojnik M, et al. Heart involvement (Libman Sacks endocarditis) in the antiphospholipid syndrome. Circulation. 1996;93:1579–1587. doi: 10.1161/01.cir.93.8.1579. [DOI] [PubMed] [Google Scholar]
- 3.Chartash EK, et al. Aortic insufficiency and mitral regurgitation in patients with systemic lupus erythematosus and the antiphospholipid syndrome. Am J Med. 1989;86:407–412. doi: 10.1016/0002-9343(89)90337-9. [DOI] [PubMed] [Google Scholar]
- 4.Moyssakis I, et al. Libman–Sacks endocarditis in systemic lupus erythematosus: prevalence, associations, and evolution. Am J Med. 2007;120:636–642. doi: 10.1016/j.amjmed.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 5.Roldan CA, Shively BK, Lau CC, et al. Systemic lupus erythematosus valve disease by transesophageal echocardiography and the role of antiphospholipds antibodies. J Am Coll Cardiol. 1992;20:1127–1134. doi: 10.1016/0735-1097(92)90368-w. [DOI] [PubMed] [Google Scholar]
- 6.Roldan CA, Shivley BK, Crawford MH. An echocardiographic study of valvular heart disease associated with systemic lupus erythematosus. N Engl J Med. 1996;335:1424–1430. doi: 10.1056/NEJM199611073351903. [DOI] [PubMed] [Google Scholar]
- 7.Farzaneh-far A, Roman MJ, Lockshin MD, et al. Relationship of antiphospholipid antibodies to cardiovascular manifestations of systemic lupus erythematosus. Arthritis Rheum. 2006;54:3918–3925. doi: 10.1002/art.22265. [DOI] [PubMed] [Google Scholar]
- 8.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 9.Asherson R, Cervera R. Antiphospholipid Antibodies and the Heart. Circulation. 1991;84(2):920–922. doi: 10.1161/01.cir.84.2.920. [DOI] [PubMed] [Google Scholar]
- 10.Barnes EV, Narain S, Naranjo A, et al. High sensitivity C-reactive protein in systemic lupus erythematosus: relation to disease activity, clinical presentation and implications for cardiovascular risk. Lupus. 2005;14:576–582. doi: 10.1191/0961203305lu2157oa. [DOI] [PubMed] [Google Scholar]
- 11.Leszcerynski P, et al. Cardiac valvular disease in patients with systemic lupus erythematosus. Relationship with anticardiolipin antibodies. Clin Rheumatol. 2003;22(6):405–408. doi: 10.1007/s10067-003-0751-0. [DOI] [PubMed] [Google Scholar]
- 12.Amoroso A, Cacciapaglia F, Castro S, et al. The adjunctive role of antiphopholpid antibodies in systemic lupus erythematosus cardiac involvement. Clin Exp Rheumatol. 2006;24(3):286–294. [PubMed] [Google Scholar]
- 13.Perez-Villa F, et al. Severe valvular regurgitation and antiphospholipid antibodies in systemic lupus erythematosus: a prospective, long-term follow up study. Arthritis Rheum. 2005;53(3):460–467. doi: 10.1002/art.21162. [DOI] [PubMed] [Google Scholar]
- 14.Paran D, Caspi D, Levartovsky D, et al. Cardiac dysfunction in patients with systemic lupus erythematosus and antiphospholipid syndrome. Ann Rheum Dis. 2007;66:506–510. doi: 10.1136/ard.2005.044073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roland CA. Valvular disease associated with systemic illness. Cardiol Clin. 1998;16(3):531–550. doi: 10.1016/S0733-8651(05)70030-8. [DOI] [PubMed] [Google Scholar]
- 16.Gonzales-Juanatey C. Libman Sacks endocarditis and primary antiphospholipid syndrome. J Heart Valve Dis. 2005;14:700–702. [PubMed] [Google Scholar]