Abstract
Introduction
Herbal products have gained increasing popularity in the last decade, and are now used by approximately 20% of the population. Herbal products are complex mixtures of organic chemicals that may come from any raw or processed part of a plant, including leaves, stems, flowers, roots, and seeds. Under the current law, herbs are defined as dietary supplements, and manufacturers can therefore produce, sell, and market herbs without first demonstrating safety and efficacy, as is required for pharmaceutical drugs. Although herbs are often perceived as “natural” and therefore safe, many different side effects have been reported owing to active ingredients, contaminants, or interactions with drugs.
Results
Unfortunately, there is limited scientific evidence to establish the safety and efficacy of most herbal products. Of the top 10 herbs, 5 (ginkgo, garlic, St. John’s wort, soy, and kava) have scientific evidence suggesting efficacy, but concerns over safety and a consideration of other medical therapies may temper the decision to use these products.
Conclusions
Herbal products are not likely to become an important alternative to standard medical therapies unless there are changes to the regulation, standardization, and funding for research of these products.
KEY WORDS: herbal medicine, efficacy, safety, regulation
BACKGROUND
Nearly 1 in 5 adults in the United States report taking an herbal product.1 Written records of the use of herbal medicine date back more than 5,000years.2 In fact, for most of history, herbal medicine was the only medicine. Even as recently as 1890, 59% of the listings in the US Pharmacopeia were from herbal products,3 and it has been estimated that as many as one third to one half of currently used drugs were originally derived from plants.4
Although many herbs are primarily of historical interest, thousands of herbal products are available over the counter and commonly used by patients in the United States. Therefore, an understanding of the composition, regulation, safety, and efficacy of herbs may assist clinicians in advising patients about the use of these products.
What’s in an Herb?
An herb can be any form of a plant or plant product, including leaves, stems, flowers, roots, and seeds. These plants can either be sold raw or as extracts, where the plant is macerated with water, alcohol, or other solvents to extract some of the chemicals. The resulting products contain dozens of chemicals, including fatty acids, sterols, alkaloids, flavonoids, glycosides, saponins, and others.5 Because any given herb contains multiple ingredients, some manufacturers attempt to create standardized herbal products by identifying a suspected active ingredient and altering the manufacturing process to obtain a consistent amount of this chemical.
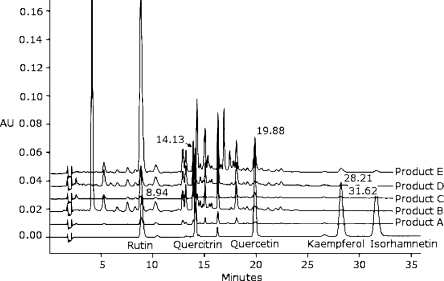
For example, Figure 1 shows 1 high-pressure liquid chromatogram (HPLC) of the herb, Ginkgo biloba.6 This graph allows for the quantification of 5 of the flavonol constituents of ginkgo (rutin, quercitrin, quercetin, kaempferol, and isorhamnetin), and the manufacturer might standardize the extract to a specific level of 1 of these flavonoids. While this type of analysis is a potentially useful method of quantifying the chemical constituents of a given herb, variations in the analytical methods of herbs create uncertainty. For most herbs, the exact chemical, or combination of chemicals, that produce a biological effect is unknown, and it is therefore difficult (if not impossible) to create a precise “chemical fingerprint” of the optimum herbal product. Ginkgo, for example, contains at least 33 different chemical constituents, of which only 5 are shown in Figure 1.6 Similarly, it is not known whether the combination of chemicals in a given plant would produce a superior effect to 1 isolated chemical component of the herb. Finally, the process of performing an HPLC analysis is complicated, and different analytical techniques can lead to different estimations of the constituents of herbs. For Ginkgo, at least 7 different analytical methods have been described.6
Figure 1.
The bottom line (at AU 0.00) shows the reference standard with the labeled flavonol gylcosides: rutin, quercitrin, quercetin, kaempferol, and isorhamnetin. Note that the 5 different ginkgo products (A, B, C, D, E) have substantial variation in the amount of the various glycosides, as reflected by the height of the chromatogram peak. There was more than a 25-fold variation in some constituents between products. (Reproduced with permission from Dubber MJ, Kanfer I. High-performance liquid chromatographic determination of selected flavonols in Ginkgo biloba solid oral dosage forms. J Pharm Pharm Sci. Sep 24 2004;7(3):303–309)
Regulation
The Dietary Supplement Health and Education Act (DSHEA) of 1994 classifies herbs as dietary supplements.7 This law defines supplements quite broadly as “anything that supplements the diet.” Supplements therefore include vitamins, minerals, herbs, amino acids, enzymes, organ tissues, metabolites, extracts, or concentrates. A major difference between a drug and a dietary supplement is that dietary supplements may not claim to “diagnose, cure, mitigate, treat, or prevent illness.”7 It is interesting to note that dietary supplement manufacturers are allowed to make certain “structure/function” claims, which are often vaguely worded claims of health benefits. For example, an Echinacea product (often used to treat or prevent the common cold) might claim to “support the body’s natural defenses.”
Dietary supplements can be produced, sold, and marketed without first demonstrating safety and efficacy, as is required for pharmaceutical drugs. Also, the FDA bears the regulatory burden of proving that a dietary supplement is unsafe before it can be removed from the market—which is in direct contrast to drugs, where a manufacturer must provide the FDA with evidence of safety and efficacy before a product can be sold.7
Not surprisingly, this regulatory structure has led to problems with the consistency and safety of herbal products. Several recent studies have documented dramatically different levels of suspected active ingredients in herbal products. For example, a recent analysis of 25 available ginseng products found a 15- to 200-fold variation in the concentration of 2 ingredients believed to have biological activity: ginsenosides and eleuthrosides.8 Therefore, it may be difficult for patients to ascertain with certainty the precise contents of the products they may be interested in taking.
Efficacy
A recent national survey identified the ten most commonly used herbs in the United States and found that 18.9% of the adult population reported the use of an herb to treat a medical illness within the past year.1 The evidence for efficacy for the most common uses for each herb is shown in Table 1 and discussed below.
Table 1.
The Ten Most Commonly Used Herbal Medicines in the United States
| Herb | Percent use in U.S.* | Common use† | Scientific evidence for efficacy‡ | Safety |
|---|---|---|---|---|
| Echinacea | 7.0 | Upper respiratory tract infection | Inconclusive9 | Side effects similar to placebo9 |
| Ginseng | 4.2 | Physical and cognitive performance | Inconclusive12 | Limited data; hyperactivity and restlessness in case reports12 |
| Ginkgo biloba | 3.7 | Dementia | Likely Effective13 | Side effects similar to placebo;16 case reports of bleeding17 |
| Claudication | Likely effective15 | |||
| Garlic | 3.4 | Hypercholesterolemia | Likely Effective18 | Mild gastrointestinal side effects and garlic odor;18 case reports of bleeding20,21 |
| St. John’s wort | 2.1 | Depression | Likely Effective for mild–moderate depression22,23 | Numerous reports of drug interactions26 |
| Peppermint | 2.1 | Upset stomach / irritable bowel syndrome | Inconclusive27 | Limited data, but side effects appear to be mild27 |
| Ginger | 1.8 | Nausea | Inconclusive28 | No known side effects5 |
| Soy | 1.7 | Menopausal symptoms | Not effective29 | Concerns regarding long-term estrogenic effects29 |
| Hypercholesterolemia | Effective30 | |||
| Chamomile | 1.5 | Insomnia / gastrointestinal problems | No high-quality data | Rare allergic reactions31 |
| Kava kava | 1.2 | Anxiety | Likely Effective32 | Case reports of severe hepatotoxicity33 |
Echinacea is most commonly used for treatment of the common cold. A recent systematic review identified 16 randomized, placebo-controlled trials of Echinacea; 9 were positive and 7 were negative. The authors concluded that although there is some evidence of a possible benefit from Echinacea purpurea for treatment of the common cold, the results are not consistent.9 A subsequent large, high-quality randomized controlled trial found no benefit of Echinacea angustifolia for the treatment of experimentally induced rhinovirus infection.10 However, some authorities believe that a different species (Echinacea purpurea) or a higher dose of the species studied, would have been more likely to find an effect.11 The herb is believed to be safe, with prior studies showing rates of side effects similar in Echinacea and placebo groups.9
Ginseng is primarily marketed in the United States to improve energy and physical or cognitive performance5 and is found in many drinks and tonics. A systematic review identified 16 randomized, placebo-controlled trials of ginseng for physical performance, psychomotor performance, cognitive function, immunomodulation, diabetes mellitus, and herpes simplex type-II infections, and found no compelling evidence for efficacy for any indication.12 Ginseng is believed to be safe, although there are some case reports of excessive arousal and hyperactivity.12
Ginkgo extracts are among the best characterized herbal products, and are generally standardized to 24% flavonoids and 6% terpenoids. Although reviews of prior trials have found inconsistent results, ginkgo is likely effective for dementia, providing a small benefit of approximately 3% in the Alzheimer’s Disease Assessment Scale-Cognitive subtest.13 It is interesting to note that ginkgo was not effective for improving cognitive function in elderly patients without dementia.14 In a systematic review of 8 prior trials, ginkgo was found to improve pain-free walking distance in patients with claudication, a small benefit of unclear clinical significance.15 While side effects from ginkgo and placebo are similar in clinical trials,16 a significant concern regarding the use of ginkgo is the reported association with spontaneous bleeding.17
Garlic is used for many purported medicinal properties, but the most substantial body of research examines the effect on cholesterol. The most recent systematic review concluded that Garlic lowers cholesterol levels by 4–6%,18 which is a modest effect in comparison to the 17–32% reduction achieved with the use of statin drugs.19 The most common side effects are gastrointestinal problems and garlic breath.18 Two case reports suggest a possible increase in the risk of bleeding with garlic use.20,21
Prior trials of St. John’s wort present conflicting and confusing evidence, but the herb is likely effective for the treatment of mild-to-moderate depression.22,23 Two recent studies found it to be ineffective for patients with severe depression.24,25 Enthusiasm for the use of St. John’s wort is tempered by the many well-documented drug interactions.26
Peppermint is a common ingredient in herbal products marketed to treat irritable bowel syndrome. Although a review of 8 prior trials suggests a possible benefit, the quality of the studies was too limited to reach definitive conclusions.27 Side effects appear to be infrequent and mild.27
Ginger is commonly used as a treatment for nausea. Only 3 prior randomized controlled trials have examined the efficacy of ginger for prevention of postoperative nausea, and although 2 suggested a benefit, the combined summary of the 3 studies did not find a statistically significant benefit.28 Other indications for ginger, including seasickness, morning sickness, and chemotherapy-induced nausea, offer preliminary although inconclusive evidence suggesting possible efficacy.28 There are no known side effects.5
Soy, a common source of dietary phytoestrogens that has weak estrogenic activity, is commonly used for the treatment of menopausal symptoms (primarily hot flushes) and for lowering cholesterol. A recent systematic review identified 9 clinical trials examining the effects of increased dietary soy and 9 additional trials examining the efficacy of soy extracts, and concluded that neither was effective for menopausal symptoms.29 A recent review of 11 trials found that soy was effective for lowering total and low-density lipoprotein (LDL) cholesterol by 4–5%,30 a small effect comparable to that observed in studies of garlic.18
Chamomile has been used for thousands of years for numerous ailments and is commonly used in teas (as a mild sedative) or in herbal products used for sleep disorders, anxiety, or gastrointestinal problems. There are no high-quality scientific studies to support efficacy for any of these indications. The herb is generally believed to be safe, but there are case reports of serious allergic reactions.31
Kava is traditionally used in the islands of the south pacific as a sedative and relaxant. Prior clinical studies suggest a small benefit for the treatment of anxiety.32 Use of this herb has been limited by the reported association to several cases of severe hepatotoxicity.33
Five of the top 10 herbs (garlic, ginkgo, garlic, St. John’s wort, soy, and kava) have substantial scientific evidence suggesting efficacy for specific indications. However, even for these commonly used herbs, the scientific evidence often suffers from poor methodology, inconsistent outcome measures, different preparations of the herb, and conflicting results. It has been estimated that there are 20,000 herbal products in this country.34 It is clear that there is limited evidence to support the efficacy of even the top 10 herbs, and there is far less evidence for the remaining 20,000. This lack of evidence does not indicate a lack of benefit, but primarily indicates a lack of conclusive studies, positive or negative, for the efficacy of most herbal products.
Safety, Toxicity, and Side Effects
Because herbs are plants, they are often perceived as “natural” and therefore safe.35 However, many different side effects to herbs have been reported and recently reviewed,35–37 including effects from biologically active constituents from herbs, side effects caused by contaminants, and herb–drug interactions. Case reports of nephropathy caused by the use of certain Chinese herbs are common. A particularly morbid case series describes 105 patients in Belgium who had been taking a Chinese herbal product for weight loss and developed nephropathy caused by the herb Aristolochia fangchi. Forty-three patients developed end-stage renal failure, and 39 had prophylactic kidney removal. Eighteen of these patients were found to have urothelial carcinoma, which was shown to be related to the formation of DNA adducts from the aristolochic acid in this herb.38 Another common toxicity to herbal medicines involves pyrrolizidine alkaloids, which are complex molecules found in certain plants that may be used or inadvertently added to herbal medicines (including comfrey, which is still available in the United States). These alkaloids produce hepatotoxicity through a characteristic veno-occlusive disease that may be rapidly progressive and fatal.39
Contaminants in herbal products may be particularly problematic in medicines imported from Asia. A study examining the contents of 260 Asian patent medicines found that 25% of products contained high levels of heavy metals and another 7% contained undeclared drugs, purposefully and illegally added to produce a desired effect.40
The safety of using most herbs with drugs is not well established. Some herbs are known to interact with pharmaceutical drugs, although most of this information comes from case reports rather than systematic investigations.37 St. John’s wort is the most notoriously interactive herbal product, and has been shown to interfere with numerous drugs metabolized by the cytochrome P-450 liver enzyme system, including protease inhibitors, chemotherapeutic agents, and oral contraceptives.26 Some authorities note that many herbs, including kava, valerian, and St. John’s wort, have the potential to interact with anesthetic agents and other drugs given in the perioperative period.41
Because many herbs contain pharmacologically active compounds, some herbs may cause side effects through excessive biological effects. For example, ephedra, which contains ephedrine, was widely used in traditional Chinese medicine for thousands of years, and then became popular in this country in the 1990s as a component of weight-loss and energy-enhancing products. An analysis of contacts to poison control centers found that, compared with other commonly used herbal products, ephedra was 40 times more likely to lead to a report of a side effect.42 A systematic review found that ephedra led to a 2- to 3-fold increased risk of nausea, vomiting, psychiatric symptoms, and palpitations compared with placebo.43 Because of this and other evidence, ephedra was banned by the FDA on April 12, 2004.44
Shortly after the ephedra ban, the 7 largest manufacturers of ephedra-containing products started marketing “ephedra-free” products, all of which contained the herb citrus aurantium. This herb, also known as bitter orange, contains synephrine, which has many of the same pharmacological properties as ephedrine,45 and therefore the potential to cause many of the same side effects. Use of a combination of an herbal product containing citrus aurantium and caffeine has been shown to cause statistically significant increases in systolic and diastolic blood pressure (approximately 9mmHg) and pulse (16.7 beats per minute) in healthy adults.46 Patients taking such products may have an elevated pulse and blood pressure or report insomnia or feeling “jittery”—all of which may be caused by the caffeine and citrus auranitum-containing supplement.
Unfortunately, the true frequency of side effects for most herbs is not known because most have not been tested in large clinical trials and because surveillance systems are much less extensive than those in place for pharmaceutical products. A review conducted by the Office of the Inspector General concluded that surveillance systems designed to detect adverse reactions to herbs are inadequate and probably detect less than 1% of all events.47
The potential for toxicity from certain herbs is compounded by the frequent use of misleading marketing information. For example, a systematic review of citrus aurantium for weight loss48 identified only 1 methodologically flawed study examining the effect of the herb,49 which incorrectly reported a statistically significant benefit for weight loss (the herb was no more effective than placebo). This misleading article is often cited as “published scientific evidence” of the efficacy of citrus aurantium for weight loss, with no mention of possible side effects.50 Illegal and erroneous marketing claims for herbal products are common. In 1 study of internet marketing, more than half of herbal products illegally claimed to treat, prevent, diagnose, or cure specific diseases.51
CLINICAL USE AND PHYSICIAN ADVICE
As with any decision to use a drug or medical intervention, the decision to use an herbal product should involve a discussion between the patient and clinical provider of the potential risks, benefits, and alternatives. Such a discussion might lead patients to choose a different option, or to proceed with a trial of the product while monitoring for possible side effects. However, a recent survey found that patients believe physicians may be biased against herbs,52 which may explain why most patients rarely discuss these and other complementary and alternative medicine (CAM) therapies with their care providers.53
Unfortunately, because of the current regulatory structure and the limited available data on safety and efficacy, the discussion of risks must highlight the potential for severe side effects from pharmacologically active ingredients, contaminants, or drug interactions. Similarly, the discussion of benefits most often describes a lack of evidence (or inconsistent evidence). Finally, for many indications, there are alternative treatments with established efficacy and safety. For example, in a patient with hypercholesterolemia, the decision to use garlic or soy supplements, which both reduce total cholesterol by approximately 4–6%, does not compare well with the use of statin drugs, which reduce cholesterol by 17–32% and have extensive data to document safety and reductions in cardiovascular disease.19
Despite the generally poor risk–benefit analysis for most herbs, it may be reasonable to use certain herbs for patients who have conditions where there are no known effective treatments, or when standard therapies have not been tolerated or have failed to lead to improvements. Treatment with an herbal product should be closely monitored by a clinical provider, to help detect benefits or side effects and to decide whether treatment should continue.
Future Directions
Several experts have previously suggested a number of important changes to the regulation of herbs that could improve the safety and appropriate use of these products.54, 55 These include: (1) requiring manufacturers to register with the FDA, (2) mandating safety tests similar to those required for over-the-counter drugs, (3) requiring all health claims to be supported by data approved by the FDA, and (4) ensuring that product labels provide an accurate list of all ingredients.
While these changes will clearly help the safety of herbal products, additional changes are needed to improve and promote high-quality research. The most critical element will be to define specific standards for herbal products to ensure consistency between studies. Even recent studies (including the aforementioned study of Echinacea)10 that used high-quality methodology were open to criticism about the formulation of the herbal product used because no clear, well-established standard of the chemical fingerprint exists for most commonly used herbs. The effort to create standards for herbal products is most likely to be successful if supported and coordinated by an appropriately funded branch of the FDA. Research would also proceed more rapidly if some form of patent protection existed, so that manufacturers who invest in expensive studies and document the efficacy of their products could be rewarded financially. Finally, techniques to speed the progress of clinical research would help deal with the “backlog” of 20,000 herbal products that have limited evidence of safety and efficacy. In a recent study conducted entirely over the internet, investigators were able to recruit and enroll 391 patients in just 6 weeks.56 Although this technique is still under investigation, it has the potential to dramatically reduce the duration and cost of clinical trials of herbs.
CONCLUSIONS
Approximately 1 in 5 U.S. adults reports using an herbal product within the past year. Unfortunately, for most of the roughly 20,000 herbal products available in this country, there is little evidence regarding safety or efficacy. However, as one third to one half of all pharmaceutical drugs were originally derived from plants, there is clearly a potential to find effective therapies from the natural environment. The current regulation of herbs does not ensure that available products are safe, and false and illegal marketing claims are common. Several simple changes to the regulation of these products could dramatically improve the appropriate use of herbs. Future research will be best served by the creation of national standards for the constituents of specific herbs, greater incentives for research, and the development of study designs that reduce costs and study duration.
Acknowledgment
The author thanks Erin Hartman, MS, Department of Medicine, University of California, San Francisco, for her editorial assistance on this manuscript. Dr. Bent had full access to all of the data in this presentation and takes responsibility for the integrity of the data and the accuracy of the presentation. This work was supported by Grant Number 1 K08 ATO1338-01 (Dr. Bent) from the National Center for Complementary and Alternative Medicine (NCCAM). The funding organization had no role in the design and conduct of the review; the collection, management, analysis, and interpretation of the data, or the preparation, review, or approval of the manuscript.
Conflict of Interest Statement Dr. Bent previously served as an expert witness for plaintiffs in cases of injury related to the use of ephedra (last case completed in July 2004).
References
- 1.Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002. Adv Data. 2004;343:1–19. [PubMed] [Google Scholar]
- 2.Swerdlow JL. Modern Science Embraces Medicinal Plants. Nature’s Medicine: Plants that Heal. Washington, DC: National Geographic Society; 2000. pp. 110–57. [Google Scholar]
- 3.Swerdlow JL. Medicine Changes: late 19th to early 20th century. Nature’s Medicine: Plants that Heal. Washington, D.C.: National Geographic Society; 2000. pp. 158–91. [Google Scholar]
- 4.Barrett B, Kiefer D, Rabago D. Assessing the risks and benefits of herbal medicine: an overview of scientific evidence. Altern Ther Health Med. 1999;5:40–9. [PubMed] [Google Scholar]
- 5.Rotblatt M, Ziment I. Evidence-based herbal medicine. Philadelphia, PA: Hanley & Belfus; 2002. [Google Scholar]
- 6.Dubber MJ, Kanfer I. High-performance liquid chromatographic determination of selected flavonols in Ginkgo biloba solid oral dosage forms. J Pharm Pharm Sci. 2004;7:303–9. [PubMed] [Google Scholar]
- 7.Dietary Supplements: A Framework for Evaluating Safety. Washington, DC: National Academies Press; 2005. [PubMed] [Google Scholar]
- 8.Harkey MR, Henderson GL, Gershwin ME, Stern JS, Hackman RM. Variability in commercial ginseng products: an analysis of 25 preparations. Am J Clin Nutr. 2001;73:1101–6. doi: 10.1093/ajcn/73.6.1101. [DOI] [PubMed] [Google Scholar]
- 9.Linde K, Barrett B, Wolkart K, Bauer R, Melchart D. Echinacea for preventing and treating the common cold. Cochrane Database Syst Rev. 2006(1):CD000530. [DOI] [PubMed]
- 10.Turner RB, Bauer R, Woelkart K, Hulsey TC, Gangemi JD. An evaluation of Echinacea angustifolia in experimental rhinovirus infections. N Engl J Med. 2005;353:341–8. doi: 10.1056/NEJMoa044441. [DOI] [PubMed] [Google Scholar]
- 11.Blumenthal M, Farnsworth NR. Echinacea angustifolia in rhinovirus infections. N Engl J Med. 2005;353:1971–2. doi: 10.1056/NEJM200511033531818. [DOI] [PubMed] [Google Scholar]
- 12.Vogler BK, Pittler MH, Ernst E. The efficacy of ginseng. A systematic review of randomised clinical trials. Eur J Clin Pharmacol. 1999;55:567–75. doi: 10.1007/s002280050674. [DOI] [PubMed] [Google Scholar]
- 13.Oken BS, Storzbach DM, Kaye JA. The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch Neurol. 1998;55:1409–15. doi: 10.1001/archneur.55.11.1409. [DOI] [PubMed] [Google Scholar]
- 14.Solomon PR, Adams F, Silver A, Zimmer J, DeVeaux R. Ginkgo for memory enhancement: a randomized controlled trial. JAMA. 2002;288:835–40. doi: 10.1001/jama.288.7.835. [DOI] [PubMed] [Google Scholar]
- 15.Pittler MH, Ernst E. Ginkgo biloba extract for the treatment of intermittent claudication: a meta-analysis of randomized trials. Am J Med. 2000;108:276–81. doi: 10.1016/S0002-9343(99)00454-4. [DOI] [PubMed] [Google Scholar]
- 16.Birks J, Grimley EV, Van Dongen M. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst Rev. 2002(4):CD003120. [DOI] [PubMed]
- 17.Bent S, Goldberg H, Padula A, Avins AL. Spontaneous bleeding associated with ginkgo biloba: a case report and systematic review of the literature: a case report and systematic review of the literature. J Gen Intern Med. 2005;20:657–61. doi: 10.1007/s11606-005-0114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevinson C, Pittler MH, Ernst E. Garlic for treating hypercholesterolemia; a meta-analysis of randomized clinical trials. Ann Intern Med. 2000;133:420–9. doi: 10.7326/0003-4819-133-6-200009190-00009. [DOI] [PubMed] [Google Scholar]
- 19.Koren MJ. Statin use in a “real-world” clinical setting: aggressive lipid lowering compared with usual care in the Aggressive Lipid-Lowering Initiation Abates New Cardiac Events (ALLIANCE) trial. Am J Med. 2005;118(Suppl 12A):16–21. doi: 10.1016/j.amjmed.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Burnham BE. Garlic as a possible risk for postoperative bleeding. Plast Reconstr Surg. 1995;95:213. doi: 10.1097/00006534-199501000-00060. [DOI] [PubMed] [Google Scholar]
- 21.Rose KD, Croissant PD, Parliament CF, Levin MB. Spontaneous spinal epidural hematoma with associated platelet dysfunction from excessive garlic ingestion: a case report. Neurosurgery. 1990;26:880–2. doi: 10.1097/00006123-199005000-00026. [DOI] [PubMed] [Google Scholar]
- 22.Linde K, Mulrow CD, Berner M, Egger M. St John’s wort for depression. Cochrane Database Syst Rev. 2005(2):CD000448. [DOI] [PubMed]
- 23.Linde K, Ramirez G, Mulrow CD, Pauls A, Weidenhammer W, Melchart D. St John’s wort for depression—an overview and meta-analysis of randomised clinical trials. BMJ. 1996;313:253–8. doi: 10.1136/bmj.313.7052.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hypericum Depression Trial Study Group Effect of Hypericum perforatum (St John’s wort) in major depressive disorder: a randomized controlled trial. JAMA. 2002;287:1807–14. doi: 10.1001/jama.287.14.1807. [DOI] [PubMed] [Google Scholar]
- 25.Shelton RC, Keller MB, Gelenberg A, et al. Effectiveness of St John’s wort in major depression: a randomized controlled trial. JAMA. 2001;285:1978–86. doi: 10.1001/jama.285.15.1978. [DOI] [PubMed] [Google Scholar]
- 26.Hammerness P, Basch E, Ulbricht C, et al. St John’s wort: a systematic review of adverse effects and drug interactions for the consultation psychiatrist. Psychosomatics. 2003;44:271–82. doi: 10.1176/appi.psy.44.4.271. [DOI] [PubMed] [Google Scholar]
- 27.Pittler MH, Ernst E. Peppermint oil for irritable bowel syndrome: a critical review and metaanalysis. Am J Gastroenterol. 1998;93:1131–5. doi: 10.1111/j.1572-0241.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 28.Ernst E, Pittler MH. Efficacy of ginger for nausea and vomiting: a systematic review of randomized clinical trials. Br J Anaesth. 2000;84:367–71. doi: 10.1093/oxfordjournals.bja.a013442. [DOI] [PubMed] [Google Scholar]
- 29.Lethaby AE, Brown J, Marjoribanks J, Kronenberg F, Roberts H, Eden J. Phytoestrogens for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2007(4):CD001395. [DOI] [PubMed]
- 30.Taku K, Umegaki K, Sato Y, Taki Y, Endoh K, Watanabe S. Soy isoflavones lower serum total and LDL cholesterol in humans: a meta-analysis of 11 randomized controlled trials. Am J Clin Nutr. 2007;85:1148–56. doi: 10.1093/ajcn/85.4.1148. [DOI] [PubMed] [Google Scholar]
- 31.Ulbricht C, Basch E. Natural standard herb and supplement reference: evidence-based clinical reviews. St. Louis, MO: Elsevier Mosby; 2005. [Google Scholar]
- 32.Pittler MH, Ernst E. Kava extract for treating anxiety. Cochrane Database Syst Rev. 2003(1):CD003383. [DOI] [PubMed]
- 33.Centers for Disease Control and Prevention Hepatic toxicity possibly associated with kava-containing products—United States, Germany, and Switzerland, 1999–2002. MMWR Morb Mortal Wkly Rep. 2002;51:1065–7. [PubMed] [Google Scholar]
- 34.Winslow LC, Kroll DJ. Herbs as medicines. Arch Intern Med. 1998;158:2192–9. doi: 10.1001/archinte.158.20.2192. [DOI] [PubMed] [Google Scholar]
- 35.Ernst E. Harmless herbs? A review of the recent literature. Am J Med. 1998;104:170–8. doi: 10.1016/S0002-9343(97)00397-5. [DOI] [PubMed] [Google Scholar]
- 36.Bent S, Ko R. Commonly used herbal medicines in the United States: a review. Am J Med. 2004;116:478–85. doi: 10.1016/j.amjmed.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 37.De Smet PA. Herbal remedies. N Engl J Med. 2002;347:2046–56. doi: 10.1056/NEJMra020398. [DOI] [PubMed] [Google Scholar]
- 38.Nortier JL, Martinez MC, Schmeiser HH, et al. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi) N Engl J Med. 2000;342:1686–92. doi: 10.1056/NEJM200006083422301. [DOI] [PubMed] [Google Scholar]
- 39.Stickel F, Patsenker E, Schuppan D. Herbal hepatotoxicity. J Hepatol. 2005;43:901–10. doi: 10.1016/j.jhep.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Ko RJ. Adulterants in Asian patent medicines. N Engl J Med. 1998;339:847. doi: 10.1056/NEJM199809173391214. [DOI] [PubMed] [Google Scholar]
- 41.Ang-Lee MK, Moss J, Yuan CS. Herbal medicines and perioperative care. JAMA. 2001;286:208–16. doi: 10.1001/jama.286.2.208. [DOI] [PubMed] [Google Scholar]
- 42.Bent S, Tiedt TN, Odden MC, Shlipak MG. The relative safety of ephedra compared with other herbal products. Ann Intern Med. 2003;138:468–71. doi: 10.7326/0003-4819-138-6-200303180-00010. [DOI] [PubMed] [Google Scholar]
- 43.Shekelle PG, Hardy ML, Morton SC, et al. Efficacy and safety of ephedra and ephedrine for weight loss and athletic performance: a meta-analysis. JAMA. 2003;289:1537–45. doi: 10.1001/jama.289.12.1537. [DOI] [PubMed] [Google Scholar]
- 44.DHHS, FDA. Final rule declaring dietary supplements containing ephedrine alkaloids adulterated because they present an unreasonable risk. Final rule. Fed Regist. 2004;69:6787–6854. [PubMed]
- 45.Hoffman BB, Taylor P. Neurotransmission. In: Hardman JG, Limbird LE, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 10. New York, NY: McGraw-Hill; 2001. pp. 115–53. [Google Scholar]
- 46.Haller CA, Benowitz NL, Jacob P., 3rd Hemodynamic effects of ephedra-free weight-loss supplements in humans. Am J Med. 2005;118:998–1003. doi: 10.1016/j.amjmed.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 47.Adverse event reporting for dietary supplements: An inadequate safety valve. US:: Office of the Inspector General, HHS.; 2001. [Google Scholar]
- 48.Bent S, Padula A, Neuhaus J. Safety and efficacy of citrus aurantium for weight loss. Am J Cardiol. 2004;94:1359–61. doi: 10.1016/j.amjcard.2004.07.137. [DOI] [PubMed] [Google Scholar]
- 49.Colker C, Kalman D, Torina G, Perlis T, Street C. Effects of Citrus aurantium extract, caffeine, and St. John’s Wort on body fat loss, lipid levels, and mood states in overweight healthy adults. Curr Ther Res. 1999;60:145–53. doi: 10.1016/S0011-393X(00)88523-9. [DOI] [Google Scholar]
- 50.Miracleburn with Hoodia and Advantra-Z! http://miracleburn.com/productinfo.htm. Accessed March 26, 2008.
- 51.Morris CA, Avorn J. Internet marketing of herbal products. JAMA. 2003;290:1505–9. doi: 10.1001/jama.290.11.1505. [DOI] [PubMed] [Google Scholar]
- 52.Blendon RJ, DesRoches CM, Benson JM, Brodie M, Altman DE. Americans’ views on the use and regulation of dietary supplements. Arch Intern Med. 2001;161:805–10. doi: 10.1001/archinte.161.6.805. [DOI] [PubMed] [Google Scholar]
- 53.Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990–1997: results of a follow-up national survey. JAMA. 1998;280:1569–75. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- 54.Lewis JD, Strom BL. Balancing safety of dietary supplements with the free market. Ann Intern Med. 2002;136:616–8. doi: 10.7326/0003-4819-136-8-200204160-00011. [DOI] [PubMed] [Google Scholar]
- 55.Marcus DM, Grollman AP. Botanical medicines—the need for new regulations. N Engl J Med. 2002;347:2073–6. doi: 10.1056/NEJMsb022858. [DOI] [PubMed] [Google Scholar]
- 56.Jacobs BP, Bent S, Tice JA, Blackwell T, Cummings SR. An internet-based randomized, placebo-controlled trial of kava and valerian for anxiety and insomnia. Medicine (Baltimore) 2005;84:197–207. doi: 10.1097/01.md.0000172299.72364.95. [DOI] [PubMed] [Google Scholar]
- 57.Fugh-Berman A. The 5-Minute Herb & Dietary Supplement Consult. Philadelphia, PA: Lippincott Williams & Wilkins; 2003. [Google Scholar]