Abstract
Background
Hospitalized patients frequently have urinary catheters inserted for inappropriate reasons. This can lead to urinary tract infections and other complications.
Objective
To assess whether stop orders for indwelling urinary catheters reduces the duration of inappropriate urinary catheterization and the incidence of urinary tract infections.
Design
A randomized controlled trial was conducted in three tertiary-care hospitals in Ontario, Canada. Patients with indwelling urinary catheters were randomized to prewritten orders for the removal of urinary catheters if specified criteria were not present or to usual care.
Participants
Six hundred ninety-two hospitalized patients admitted to hospital with indwelling urinary catheters inserted for ≤48 h.
Measurements
The main outcomes included days of inappropriate indwelling catheter use, total days of catheter use, frequency of urinary tract infection, and catheter reinsertions.
Results
There were fewer days of inappropriate and total urinary catheter use in the stop-order group than in the usual care group (difference −1.69 [95% CI −1.23 to −2.15], P < 0.001 and −1.34 days, [95% CI, −0.64 to −2.05 days], P < 0.001, respectively). Urinary tract infections occurred in 19.0% of the stop-order group and 20.2% of the usual care group, relative risk 0.94 (95% CI, 0.66 to 1.33), P = 0.71. Catheter reinsertion occurred in 8.6% of the stop-order group and 7.0% in the usual care group, relative risk 1.23 (95% CI, 0.72 to 2.11), P = 0.45.
Conclusions
Stop orders for urinary catheterization safely reduced duration of inappropriate urinary catheterization in hospitalized patients but did not reduce urinary tract infections.
KEY WORDS: urinary tract infections, urinary catheters, randomized controlled trial, stop order
INTRODUCTION
Approximately one quarter of patients admitted to hospital have indwelling urinary catheters inserted1–3. In 30% to 50% of these patients, a urinary catheter is not medically indicated but have been inserted for either an unclear or inappropriate indication such as urinary incontinence2,4–6. Duration of urinary catheterization is often inappropriately prolonged because physicians forget that their hospitalized patients have catheters in place7. About 80% of hospital-acquired urinary tract infections occur in the presence of an indwelling urethral catheter8. Because bacteriuria develops in up to 50% of patients who have a catheter inserted for 5 days or more9–11, reducing unnecessary use of such catheters may decrease urinary tract infections.
To reduce unnecessary urinary catheterization, we developed stop orders requiring removal of indwelling urinary catheters that did not have a justified indication to continue, based on published recommendations4–6 and feedback from hospital urologists, internists, and nurses. Any one of the following criteria was considered justified to continue catheterization: urinary obstruction, neurogenic bladder and urinary retention, urological surgery, fluid challenge for acute renal failure, open sacral wound care for incontinent individuals, and comfort care for urinary incontinence in terminal illness. We conducted a randomized controlled trial to assess whether this strategy would reduce unnecessary urinary catheter use and lead to a reduction of urinary tract infections in hospitalized patients.
METHODS
Setting and Participants
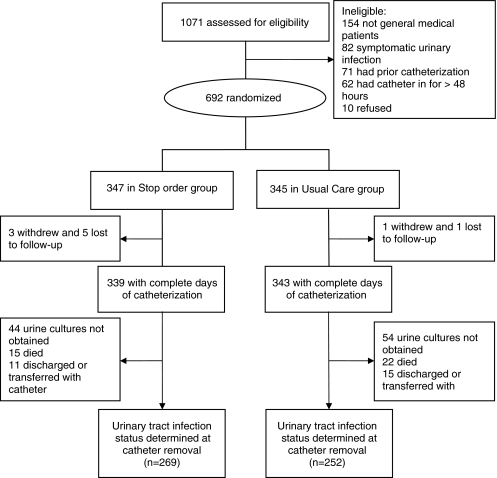
The trial was conducted among patients admitted to one of seven general medical units in three tertiary-care hospitals in Hamilton, Ontario, Canada. Patients with an indwelling catheter (BARDEX Silicon-Elastomere Foley Catheter, Bard, Covington, GA, USA) inserted for ≤48 h were eligible for enrollment. Exclusion criteria included symptomatic urinary tract infection (12) or previously having had an indwelling catheter inserted in the 10 days prior to hospitalization. One thousand seventy-one patients were assessed for eligibility and 692 were randomized (see Fig. 1 for reasons for noneligibility). Enrollment began in January 2004 and ended in June 2006. The study was approved by the Hamilton Health Sciences and McMaster University Research Ethics Board.
Figure 1.
Flow chart of stop-order clinical trial.
Randomization and Interventions
Participants were randomized to either stop orders or to usual care. A computer-generated random numbers sequence was created by a statistician working otherwise independently of the study team. Randomization was stratified by hospital using blocks of four. A remote randomization service using the internet was used to conceal allocation. Although participants in the trial were blinded, because of the nature of the intervention, blinding of the research nurse was not feasible. Assessment of urinary tract infections was done by individuals who were blinded to the study group.
Prior to beginning the trial, information sessions were conducted for nursing staff on participating units to introduce them to the study and explain the stop orders. Attending physicians received a letter notifying them of the stop orders.
Nurses and physicians were notified when patients were enrolled in the study and were informed about how data were going to be collected.
Research nurses identified potential participants during daily rounds of the emergency departments and participating units. They ascertained relevant information from hospital staff and charts and immediately assessed any individuals who might be eligible for the study.
Stop Orders
Prewritten orders were placed in the chart of participants randomized to the stop-order group. Stop orders listed the following six criteria as acceptable for a urinary catheter: urinary obstruction, neurogenic bladder and urinary retention, urological surgery, fluid challenge for acute renal failure, open sacral wound care for incontinent patients, and comfort care for urinary incontinence in terminal illness. Nurses were required to review participants’ medical history and the results of any tests ordered by the attending physician to determine if the required criteria were met and remove catheters in their absence. The research nurse did regular follow-up with nursing staff to ensure that the automatic stop orders were followed.
Usual Care
Research nurses collected data from individuals receiving usual care but no interventions were applied.
Outcomes and Follow-up
The main outcomes in this study were duration of indwelling catheter use and frequency of urinary tract infection. Duration of catheterization was the interval (measured in whole days) between insertion date and date of catheter removal. We assessed days of inappropriate urinary catheter use and total days of catheter use. The planned length of follow-up was indefinite; if the catheter was not removed and the patient was discharged or transferred from the unit, then the difference between the insertion date and the date of discharge or transfer was used. Following catheter removal, we also assessed the number of catheter reinsertions performed by the medical teams caring for study participants.
Urine cultures were obtained at enrollment, upon removal of the indwelling urinary catheter, and at 7 days following catheter removal. Nurses were instructed to collect urine either by aseptic needle puncture from the sampling port after clamping the drainage tubing to avoid contamination or after catheter removal from a clean voided midstream urine sample. The urine was cultured on sheep blood agar plates and incubated under aerobic conditions at 37°C for 24 to 48 h. Each colony type was enumerated and fully identified using standard techniques and procedures12. Urinary tract infection was defined as the presence of >105 colony-forming units per milliliter with one or two bacterial species from specimens sent at the time of urinary catheter removal. If such significant bacteriuria was present upon enrollment, a urinary tract infection was defined as the presence of >105 colony-forming units per milliliter of a bacterium different from those initially present.
A urine culture was obtained if symptomatic urinary tract infection was suspected. Symptomatic urinary tract infection was identified using the Centers for Disease Control and Prevention definition13. This included at least one of the two following criteria: (1) one of the following signs or symptoms with no other recognized cause (temperature >38°C, urgency, frequency, dysuria, or suprapubic tenderness) and >105 colony-forming units per milliliter or urine with no more than two species of microorganisms; (2) at least two of the following signs or symptoms with no other recognized cause (temperature >38°C, urgency, frequency, dysuria, or suprapubic tenderness) and at least one of the following: (a) positive dipstick for leukocyte esterase and/or nitrate; (b) pyuria (urine specimen with ≥10 white blood cells per milliliter urine or ≥3 white blood cells per high-power field of unspun urine); (c) organisms seen on Gram stain of unspun urine; (d) two urine cultures with repeated isolation of the same uropathogen with ≥102 colonies per milliliter in nonvoided specimens; (e) ≥ 105 colonies per milliliter of a single urine pathogen in a patient being treated with an effective antimicrobial agent for a urinary tract infection; (f) physician institutes appropriate therapy for a urinary tract infection.
The treatment of urinary tract infections (symptomatic or asymptomatic), for all study participants, was left to the discretion of the attending physician.
Other outcomes included bacteremia secondary to urinary tract infection (defined by isolation of the same bacterium from blood and urine within 5 days), antimicrobials prescribed to study participants, and isolation of antimicrobial-resistant bacteria from urine (defined as methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococci, Gram-negative bacteria producing extended spectrum beta-lactamase enzymes, and Pseudomonas aeruginosa resistant to three or more antipseudomonal antimicrobials) tested using standard laboratory criteria14.
A trained research nurse assessed outcomes in participants daily from enrollment until removal of their urinary catheter and at 7 days after removal of the catheter. Data were abstracted from hospital charts and laboratory information systems.
Trial registration: ClinicalTrials.gov Identifier NCT00157625
Statistical Analysis
Chi-square tests were used to compare differences in binary variables between study groups and Student’s t-tests for continuous variables. All p values were two-sided with p < 0.05 considered statistically significant. A multivariable logistic analysis, using a prespecified model, was conducted to assess the effect of the intervention adjusting for known covariates. Variables assessed in the model included male sex, diabetes, intervention, and antibiotic exposure. All data were analyzed using SAS version 9.1.3 (SAS Institute, Cary, NC, USA). Outcomes were analyzed on an intention-to-treat basis.
Sample Size
We powered the study to assess whether the intervention would reduce the incidence of urinary tract infection. Based on a 6% equal risk per day of nosocomial catheter-related urinary tract infection15,16 and an average duration of catheterization of 6 days (from our pilot study), we assumed a 30% risk of urinary tract infection in the control group. We assumed that the intervention would reduce catheterization from 6 to 2 days when catheterization was unjustified and because half of urinary catheters evaluated would be unjustified mean urinary catheterization would be reduced from 6 days in the control group to 4 days in the stop-order group. Assuming 80% power and two-sided alpha = 0.05, 626 participants would be needed to detect the resulting reduction of urinary tract infection from 30% in the control group to 20% in the intervention group. We adopted a sample size of 692 to compensate for potential loss to follow-up of some participants.
RESULTS
Participant Characteristics
Six hundred ninety-two participants were enrolled (347 in the stop-order group and 345 in the usual care group; Fig. 1). Characteristics of participants in the study groups were similar (Table 1). There were complete data for duration of urinary catheterization in 682 (98.6%) participants (see Fig. 1) and follow-up data for urinary tract infection at catheter removal in 521 (75%) participants (269 in the stop-order group and 252 in the usual care group). Reasons for lack of follow-up urinary tract infection testing included the following: the research nurse was not notified by staff when the catheter was removed (98 or 14.2%), death prior to catheter removal (37 or 5.3%; all unrelated to urinary tract infection), transfer or discharge prior to catheter removal (26 or 3.8%). In the stop-order group, 203 (58.5%) participants received one or more courses of antimicrobials during catheterization compared to 196 (57.0%) in the usual care group, P = 0.68. Urine cultures at 7 days post catheter removal were obtained for 247 (35.7%) of participants randomized (133 in the stop-order group and 114 in the usual care arm).
Table 1.
Characteristics Upon Enrollment of 692 Study Participants with Indwelling Urinary Catheters
| Characteristic | Stop order (% or standard deviation) N = 347 | Usual care (% or standard deviation) N = 345 |
|---|---|---|
| Mean age (standard deviation), years | 78.6 (12.3), range 24 to 100 | 79.0 (10.7), range 40 to 101 |
| Male, n (%) | 146 (42.1) | 120 (34.8) |
| Co-existing disease, n (%) | ||
| Diabetes | 94 (27.1) | 85 (24.6) |
| Renal disease | 8 (2.3) | 8 (2.3) |
| Dementia | 64 (18.4) | 58 (16.8) |
| Cerebrovascular disease | 75 (21.6) | 78 (22.6) |
| Pulmonary disease | 73 (21.0) | 68 (19.7) |
| Asymptomatic bacteriuria at enrolment* | 61 (17.6) | 70 (20.3) |
| Justified catheter use, n (%) | 247 (71.2) | 219 (63.5) |
| Urinary retention | 35 (10.1) | 29 (8.4) |
| Output monitoring in critically ill | 173 (49.8) | 157 (45.5) |
| Fluid challenge for acute renal failure | 32 (9.2) | 27 (7.8) |
| Open sacral wound care and incontinence | 3 (0.9) | 3 (0.9) |
| Comfort care for urinary incontinence | 4 (1.2) | 3 (0.9) |
| Unjustified catheter insertion, n (%) | 100 (28.8) | 126 (36.5) |
| Unclear indication for catheter use | 83 (23.9) | 109 (31.6) |
| Urinary incontinence | 16 (4.6) | 14 (4.0) |
| Catheter no longer needed for fluid monitoring | 1 (0.3) | 3 (0.9) |
*Asymptomatic bacteriuria = >105 cfu/mL with one or two potential urinary tract infection pathogen
Outcomes
Urine Catheterization
The mean duration of inappropriate urinary catheter days was 2.20 days [SD 1.81] for the stop-order group and 3.89 days [SD 3.92] for the usual care group, mean difference −1.69, 95% CI, −1.23 to −2.15, P < 0.001. The mean duration of total catheterization for participants in the stop-order group was 3.70 days [SD 4.05] compared to 5.04 days [SD 5.28] for those in the usual care group, mean difference −1.34, 95% CI, −0.64 to −2.05, P < 0.001.
Urinary Tract Infection
At urinary catheter removal, 51 participants (19%) in the stop-order group developed urinary tract infection compared with 51 (20%) in the usual care group, relative risk 0.94, (95% CI, 0.66 to 1.33), P = 0.71. At 7 days postcatheterization, 28 of those tested (21.1%) in the stop-order group compared to 19 (16.7%) in the usual care group had urinary tract infections, relative risk 1.26 (95% CI, 0.75 to 2.14), P = 0.38. Seven (2.1%) participants in each study arm developed symptomatic urinary tract infections, P = 0.99.
Other Outcomes
Two (0.6%) participants in the stop-order group had urinary catheter-related bacteremia (where the same organism was isolated from the blood and urine within 5 days), both due to Enterococcus species, compared to no individuals in the usual care group, P = 0.49. Two participants in the usual care group had methicillin-resistant S. aureus detected in their urine and one participant in the stop-order group had multiresistant P. aeruginosa detected in urine. In the usual care group, 196 (57.0%) participants received antibiotics during catheterization, in comparison to 203 (58.5%) in the stop-order group, P = 0.68.
Adverse Events
There were 27 participants (8.6%) who had reinsertion of urinary catheters in the automatic stop-order group compared to 22 participants (7.0%) in the usual care group, P = 0.45.
Multivariable Analysis
In multivariable analysis, the intervention was not significantly associated with urinary tract infection (relative risk 1.04 [95% CI, 0.75 to 1.44], P = 0.80. Being female (relative risk 1.47 [95% CI, 1.01 to 2.03], P = 0.047) was associated with urinary tract infection while receipt of antimicrobials during catheterization (relative risk 0.23 [95% CI, 0.15 to 0.36], P < 0.0001) was protective.
DISCUSSION
The implementation of stop orders for removing urinary catheters among medical patients admitted to acute care hospitals using a prewritten order in the chart along with follow-up by a research nurse reduced duration of inappropriate urinary catheterization. Our data are consistent with recent observational studies, showing that simple interventions can significantly reduce total urinary catheterization in hospitalized patients17–19. However, unlike the observational studies reported by Huang et al.19 and Topal et al.1, we did not observe a significant reduction in incidence rates of urinary tract infections. Even though we do not show a significant difference in infection rates, our results demonstrate that stop orders are effective in reducing inappropriate use of indwelling urinary catheters, a practice that has been characterized as a “one-point restraint” for hospitalized patients20, and may allow patients to achieve earlier mobilization and discharge. In this study, the research nurse played an important role in the implementation of the protocol but we believe that with minimal training and monitoring a similar protocol could be utilized with existing nursing staff.
Although our study did not find a statistically significant difference in urinary tract infection rates with the use of the auto-stop order, as the confidence intervals of our estimates indicate, we cannot rule out the possibility that the intervention reduces infection rates and larger studies with more precise estimates of effect may demonstrate this. Another possible explanation for the lack of effect on urinary tract infection rates is that the overall reduction in duration of catheterization, 1.34 days (95% CI, 0.64 to 2.05), may not have been sufficient to significantly reduce bacteriuria. Because it was not feasible to conduct an entirely blinded study, it is possible that nurses familiar with the protocol may have removed urinary catheters from usual care participants at higher rates than would otherwise have occurred without knowledge of the study. This is suggested by the mean duration of catheterization in our control group being slightly lower to that noted in our pilot study (5 versus 6 days respectively). Consequently, our estimates of reduction in duration of catheterization may be conservative. Our rates of colonization may also be affected by our method and timing of urine collection. Although this would not result in a differential effect in the two groups, future studies may wish to do more frequent sampling using a different method to ensure more accurate identification of bacterial growth and prevent potential contamination from existing catheters. Another possible factor contributing to the lack of difference in urinary tract infections between study groups is that 399 (58%) of study participants were exposed to antimicrobials. The fact that in multivariable analysis antimicrobial exposure showed a protective effect for urinary tract infection confirms the importance of controlling for this variable. Although urinary tract infection status could not be determined in 171 (25%) participants, we believe that bias on this basis is unlikely. The distribution of missing cultures along with the associated reasons (catheter removal prior to the visit by the research nurse, death unrelated to urine infection, or transfer or discharge with no catheter removal) were similar between study groups. A difference in rates of urinary tract infection between participants who could be assessed for urinary tract infection and those that could not would therefore have been unlikely.
We found no significant differences in symptomatic urinary tract infection or bacteremia between the study groups. It has been recently recognized that such events occur at a low rate21, so it is not unexpected that we did not detect differences in these outcomes. We also found similar frequency of catheter reinsertion between study groups suggesting that urinary catheter stop orders do not lead to excessive reinsertion of catheters.
We acknowledge that this study has several limitations. The interpretation of the results is limited by the missing data, as discussed above. Furthermore, we did not assess variables such as mobilization and quality of life which may have demonstrated a potentially important benefit of reducing duration of urinary catheterization in hospital patients20.
In conclusion, stop orders for urinary catheterization safely reduced the duration of urinary catheterization in hospitalized patients. Stop orders for urinary catheters should be considered for hospitalized patients because they can prevent prolonged unnecessary catheterization. Future studies should explore additional outcome variables that may be affected by catheterization as well as interventions to prevent inappropriate urinary catheter placement prior to insertion.
Acknowledgements
This study was funded by the Physician’s Services Incorporated Foundation of Ontario.
Potential Financial Conflicts of interest: None disclosed.
References
- 1.Topal J, Conklin S, Camp K, Morris V, Balcezak T, Herbert P. Prevention of nosocomial catheter-associated urinary tract infections through computerized feedback to physicians and a nurse-directed protocol. Am J Med Qual. 2005;20:121–6. doi: 10.1177/1062860605276074. [DOI] [PubMed] [Google Scholar]
- 2.Gokula RR, Hickner JA, Smith MA. Inappropriate use of urinary catheters in elderly patients at a midwestern community teaching hospital. Am J Infect Control. 2004;32:196–9. doi: 10.1016/j.ajic.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Hazelett SE, Tsai M, Gareri M, Allen K. The association between indwelling urinary catheter use in the elderly and urinary tract infection in acute care. BMC Geriatr. 2006;6:15. doi: 10.1186/1471-2318-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain P, Parada J, Annette D, Smith L. Overuse of the indwelling urinary tract catheter in hospitalized medical patients. Arch Intern Med. 1995;155(13):1425–9. doi: 10.1001/archinte.155.13.1425. [DOI] [PubMed] [Google Scholar]
- 5.Munasinghe RL, Yazdani H, Siddique M, Hafeez W. Appropriateness of use of indwelling urinary catheters in patients admitted to the medical service. Infect Control Hosp Epidemiol. 2001;22:647–9. doi: 10.1086/501837. [DOI] [PubMed] [Google Scholar]
- 6.Gardam MA, Amihod B, Orenstein P, Consolacion N, Miller MA. Overutilization of indwelling urinary catheters and the development of nosocomial urinary tract infections. Clin Perform Qual Health Care. 1998;6:99–102. [PubMed] [Google Scholar]
- 7.Saint S, Wiese J, Amory JK, et al. Are physicians aware of which of their patients have indwelling urinary catheters? Am J Med. 2000;109:476–80. doi: 10.1016/S0002-9343(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 8.Sedor J, Mulholland SG. Hospital-acquired urinary tract infections associated with the indwelling catheter. Urol Clin North Am. 1999;26:821–8. doi: 10.1016/S0094-0143(05)70222-6. [DOI] [PubMed] [Google Scholar]
- 9.Saint S, Lipsky BA. Preventing catheter-related bacteriuria: Should we? Can we? How? Arch Intern Med. 1999;159:800–8. doi: 10.1001/archinte.159.8.800. [DOI] [PubMed] [Google Scholar]
- 10.Stamm WE. Catheter-associated urinary tract infections: epidemiology, pathogenesis, and prevention. Am J Med. 1991;91(suppl 3B):65S–71S. doi: 10.1016/0002-9343(91)90345-X. [DOI] [PubMed] [Google Scholar]
- 11.Warren JW. Catheter-associated urinary tract infections. Infect Dis Clin North Am. 1997;11:609–22. doi: 10.1016/S0891-5520(05)70376-7. [DOI] [PubMed] [Google Scholar]
- 12.BalowsLE, eds. Manual of Clinical Microbiology. 5th ed. Washington DC: American Society for Microbiology; 1991.
- 13.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–40. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 14.Performance standards for antimicrobial susceptibility tests: sixth informational supplement M100-S6. Wayne: NCCLS; 1995. [Google Scholar]
- 15.Haley RW, Hooton TM, Culver DH, et al. Nosocomial infections in US hospitals, 1975–1976: estimated frequency by selected characteristics of patients. Am J Med. 1981;70:947–59. doi: 10.1016/0002-9343(81)90561-1. [DOI] [PubMed] [Google Scholar]
- 16.Garibaldi RA, Burke JP, Dickman ML, Smith CB. Factors predisposing to bacteriuria during indwelling urethral catheterization. N Engl J Med. 1974;291:215–9. doi: 10.1056/NEJM197408012910501. [DOI] [PubMed] [Google Scholar]
- 17.Cornia PB, Amory JK, Fraser S, Saint S, Lipsky BA. Computer-based order entry decreases duration of indwelling urinary catheterization in hospitalized patients. Am J Med. 2003;114:404–7. doi: 10.1016/S0002-9343(02)01568-1. [DOI] [PubMed] [Google Scholar]
- 18.Saint S, Kaufman SR, Thompson M, Rogers MA, Chenoweth CE. A reminder reduces urinary catheterization in hospitalized patients. Jt Comm J Qual Patient Saf. 2005;31:455–62. doi: 10.1016/s1553-7250(05)31059-2. [DOI] [PubMed] [Google Scholar]
- 19.Huang WC, Wann SR, Lin SL, et al. Catheter-associated urinary tract infections in intensive care units can be reduced by prompting physicians to remove unnecessary catheters. Infect Control Hosp Epidemiol. 2004;25:974–8. doi: 10.1086/502329. [DOI] [PubMed] [Google Scholar]
- 20.Saint S, Lipsky BA, Goold SD. Indwelling urinary catheters: a one-point restraint? Ann Intern Med. 2002;137:125–7. doi: 10.7326/0003-4819-137-2-200207160-00012. [DOI] [PubMed] [Google Scholar]
- 21.Tambyah PA, Maki DG. Catheter-associated urinary tract infection is rarely symptomatic: a prospective study of 1,497 catheterized patients. Arch Intern Med. 2000;160:678–82. doi: 10.1001/archinte.160.5.678. [DOI] [PubMed] [Google Scholar]