Abstract
Human kallikrein-related peptidase 6 (KLK6) is a member of the kallikrein family of serine-type proteases, characterized as an arginine-specific digestive-type protease capable of degrading a wide-variety of extracellular matrix proteins. KLK6 has been proposed to be a useful biomarker for breast and ovarian cancer prognosis, is abundantly expressed in the CNS and cerebrospinal fluid, and is intimately associated with regions of active inflammatory demyelination in multiple sclerosis (MS) lesions. Inhibition of KLK6 results in delayed onset and reduced severity of symptoms associated with experimental autoimmune encephalomyelitis, suggesting a key effector role for this protease in CNS inflammatory disease. KLK6 has been shown to autolytically cleave internally, leading to inactivation and suggesting a negative feed-back inhibition control mechanism. Alternatively, the ability of KLK6 to self-activate has also been reported, suggesting a positive feed-back activation loop control mechanism. Activation of pro-KLK6 requires hydrolysis after a Lys residue; however, KLK6 exhibits two orders of magnitude reduced affinity for hydrolysis after Lys versus Arg residues; therefore, the ability to autolytically activate has been called into question. In the present study the catalytic activityof KLK6 towards its pro-sequence and internal autolytic sequence is characterized. The results show that the ability of KLK6 to activate pro-KLK6 is essentially negligible when compared to the rate of the internal autolytic inactivation or to the ability of other proteases to activate pro-KLK6. The results thus show that the primary autolytic regulatory mechanism of KLK6 is negative feed-back inhibition, and activation is likely achieved through the action of a separate protease.
Keywords: kallikrein, kallikrein-related peptidase, protease, KLK6, multiple sclerosis, activation cascade
The human kallikreins are a family of S1 type serine proteases (i.e. containing an identifiable sequence homology to chymotrypsin) that are distributed in a wide variety of tissues and biological fluids. For over 60 years it was believed that the human kallikrein family had only three members (KLK1-3); however, studies within the last decade have revealed that there are a total of 15 members (KLK1-15) clustered at human chromosome loci 19q13.3-q13.4 (1, 2). KLK1-3 are sometimes referred to as the “classical” kallikreins and KLK4-15 as the “neo-” kallikreins to reflect the historical record of their discovery. In the nomenclature recently proposed by Lundwall et al., (3) KLK-1 is tissue kallikrein and the other KLK’s are referred to as “kallikrein-related peptidases.”
The KLK family has gained attention in recent years due to the fact that most members appear to be differentially expressed in normal versus diseased states, and may prove useful as diagnostic or prognostic “biomarkers”. For example, KLK3 (“prostate specific antigen”, or PSA) and KLK2 (sometimes referred to as “glandular kallikrein”) are considered to be the most useful biomarkers known for diagnosis of prostate cancer (4, 5). Other KLK’s have been proposed as diagnostic markers for breast (KLK3, KLK6) and ovarian cancers (KLK6, KLK9, KLK10 and KLK11) (6-10). KLK8 has been implicated in aberrant neuronal function (kindling epileptogenesis) in mice (11, 12), and KLK6 has been implicated in the degradation of β-amyloid, or turnover of amyloid precursor protein (13, 14), inflammatory demyelination (15, 16), and other CNS injuries such as trauma and stroke (17-19). Thus, the physiological functions of the KLK’s and regulation of their activities are of substantial clinical interest.
A review of the reported expression levels of the different KLK’s in human tissues compiled by Diamandis and coworkers lists the majority of the kallikreins (KLK1, 4, 5, 6, 7, 8, 9, 10, 11, 12 and 14) as expressed in the CNS (20). KLK6 is the most abundant kallikrein within the CNS (21) with accumulating evidence pointing to a role in CNS inflammatory demyelinating disease. KLK6 is a broad-specificity digestive-type protease with the ability to rapidly degrade components of the extracellular matrix and myelin-associated proteins (22-24). KLK6 has been shown to be abundantly expressed by oligodendrocytes, and has been postulated to function in normal myelin turnover (25, 26). KLK6 is up-regulated in response to CNS damage and in concert with the demyelination/remyelination processes that take place after such damage (15, 17, 18). KLK6 has been shown to be robustly expressed at sites of active immune mediated demyelination in human MS lesions (16) and in cases of human spinal cord injury (19). Moreover, KLK6 along with several other kallikreins shows regulated expression in T-cells in response to immune cell activation. Importantly, inhibition of KLK6 activity in animal models of MS results in a delay in onset and a reduction in severity of inflammatory demyelination which is paralleled by reduced Th1 cellular responses (27). Together, these data indicate that KLK6 is a pleiotropic enzyme with possible roles not only in normal turnover of myelin, but also in the demyelination thatcharacterizes several CNS inflammatory conditions. The association of KLK6 with inflammatory CNS injury, and the attenuation of disease through selective inhibition, identifies KLK6 as a truly novel therapeutic target of interest for the treatment of MS (alone or in conjunction with existing therapies). To realize this potential, however, details regarding the regulation of KLK6 activity must be elucidated.
The original characterization of rat and human KLK6 identified it as an arginine-specific protease with approximately two orders of magnitude reduced efficiency for hydrolysis after lysine in comparison to arginine (23, 28). Since the proteolytic activation of pro-KLK6 requires hydrolysis after lysine, it was proposed that a separate protease likely regulates KLK6 activation (23, 28, 29). Furthermore, characterization of recombinant mature KLK6 produced from an insect cell host identified an efficient autolytic inactivation mechanism, due to a specific internal hydrolysis after residue Arg76 (28). However, recombinant pro-KLK6 produced from human 293 cells was reported to be activated after concentration of the crude culture medium followed by incubation for 1 week at 4°C (30). Furthermore, recombinant pro-KLK6 produced from Pichia pastoris was reported to undergo a complex sequence of proteolytic steps, involving an initial hydrolysis between a Gln-Asn bond within the pro-peptide, followed by hydrolysis after the pro-peptide P1 Lys residue, resulting in activation (31). In both the above reports of autolytic activation KLK6 hydrolysis of the pro-peptide appeared to proceed with greater efficiency than the autolytic inactivation after Arg76.
The ability of KLK6 to self-activate is an important issue to resolve. If KLK6 can efficiently self-activate, then the regulation of KLK6 function is described as a positive feed-back activation loop. On the other hand, if KLK6 cannot effectively self-activate, but can efficiently autolytically inactivate, then the regulation of function is described as negative feed-back inhibition. In the former case, an initial activating stimulus would be followed by amplification of KLK6 activity; in the latter case an activating stimulus would be followed by rapid degradation of pro-KLK6. KLK6 is one of the major protein components of cerebrospinal fluid and is present as pro-KLK6 (32, 33). If KLK6, were regulated by a feed-back activation loop, the CSF could, in certain disease states, potentially experience an exponential activation of a broad-specificity digestive-type protease.
In the present study the ability of KLK6 to hydrolyze its pro-sequence and internal autolysis site are characterized using several different experimental approaches and with the goal of identifying the relative efficiencies of these two regulatory hydrolyses. The results support the hypothesis that the relevant self-regulating activity of KLK6 is negative feed-back inhibition via autolytic inactivation, and that the activation of pro-KLK6 is achieved through the action of a separate activating protease (of which plasmin or KLK5 are identified as potential physiologically-relevant candidates).
Materials and Methods
Production of recombinant KLK6 and pro-KLK6
Production of recombinant KLK6 from an insect cell/baculovirus host has previously been described in detail (23, 28). Pro-KLK6 was designed to be expressed as a fusion construct with a C-terminal Strep-tag and 8x His-tag, respectively. The expression host for pro-KLK6 was a human embryonic kidney epithelium cell line (HEK293, ATCC number CRL-1573). The cDNA encoding human pre-pro-KLK6 was cloned into the pSecTag2/HygroB expression vector (Invitrogen, Carlsbad, CA). In this construct, the intrinsic KLK6 signal sequence was utilized for protein secretion into the media. HEK293 cells at log phase of growth were transfected with the vector DNA mixed with Liopfectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA) according to the manufacture’s instructions. The secretion of pro-KLK6 into the media was monitored by Western blot using rabbit anti-KLK6 polyclonal antibody (produced in-house using recombinant KLK6 as antigen). The presence of the C-terminal His-tag was confirmed by Western blot using Anti-His HRP conjugate kit (Qiagen, Valencia, CA). The HEK293 culture media was harvested 2 days after transfection for purification of secreted pro-KLK6. The recombinant pro-KLK6 protein was purified by sequential affinity chromatography utilizing Ni-NTA (Qiagen, Valencia, CA) and Strep-Tactin Superflow (Qiagen, Valencia, CA) media, respectively, taking advantage of the C-terminal His-tag and Strep-tag fusion peptides.
Purity and homogeneity of recombinant KLK6 and pro-KLK6 were evaluated by 16.5% Tricine SDS-PAGE and N-terminal sequencing. The concentration of the purified proteins was determined using molar extinction coefficients determined by the method of Gill and von Hippel (34) (33,381 M-1 cm-1 for KLK6, and 38,764 M-1 cm-1 for pro-KLK6, respectively). In order to confirm the absence of contaminating host protease activity, purified pro-KLK6 was concentrated to 40μM and incubated at 37°C for 6 hours at pH8.0, and the integrity of the protein quantified by 16.5% Tricine SDS-PAGE analysis.
Characterization of pro-peptide versus autolysis loop hydrolyses using internally-quenched fluorogenic peptides
Throughout this study we have utilized a 100:1 ratio of substrate:enzyme with the intent of evaluating potential catalytic as opposed to stoichiometric concentrations (e.g. 10:1 or even 1:1) of activating protease. Internally-quenched fluorogenic peptide substrates were utilized to compare the relative rate of KLK6 pro-peptide hydrolysis (resulting in activation of pro-KLK6) versus autolysis loop hydrolysis (resulting in autolytic inactivation). These substrates contain ortho-aminobenzoic acid (Abz) at the amino-terminus as the fluorophore, and ethylenediamine 2,4-dinitrophenyl (EDDnp) at the carboxyl-terminus as the quenching agent (35). The pro-peptide substrate (Abz-EEQNKLVH-EDDnp; “PRO”) utilizes the entire pentameric pro-peptide as well as the first three residues of the KLK6 sequence (with activation of pro-KLK6 occurring via hydrolysis between the K-L bond). The autolysis loop substrate (Abz-NLRQRESS-EDDnp; “AL”) spans residue positions 72-79 of the KLK6 autolysis loop and includes Arg76, the site of inactivating autolytic attack in KLK6 (28). Internally-quenched fluorogenic peptides were purchased from Peptides International (Louisville, KY) and stock solutions of 100mM were prepared in dimethyl sulfoxide (DMSO). The total releasable fluorescence of each peptide was determined by 24 hr hydrolysis with 20nM sequencing grade trypsin (Promega, Madison, WI) in 50mM Tris-HCl, 0.1 mM EDTA, and pH 8.5 at 37°C. Fluorescence detection was performed with an excitation wavelength of 320nm and emission wavelength of 420nm using a Varian Cary Eclipse fluorescence spectrophotometer. A standard curve of total releasable fluorescence versus peptide concentration, from 0 to 100μM, was prepared for each peptide to permit normalization of experimentally-generated fluorescence signal to molar concentration, and to identify potential intermolecular quenching at high peptide concentrations.
Hydrolysis of the PRO and AL substrates by recombinant KLK6 utilized enzyme concentrations of either 1.0μM (for the PRO substrate) or 20nM (for the AL substrate), respectively. Assays were performed in 300μl of assay buffer at 37°C with substrate concentrations spanning 0-100μM. Generation of fluorescence signal as a function of time was normalized to an enzyme-free control, and all data were collected in triplicate. The conversion from fluorescence intensity to molar concentration was performed using the experimentally-derived standard curve of total-releasable fluorescence after trypsin hydrolysis. Hydrolyses were performed at both pH 8.0 (100mM Tris, 0.1mM EDTA) and pH 6.0 (100mM sodium phosphate, 0.1mM EDTA).
Proteolytic activity of KLK6 against pro-KLK6
Recombinant pro-KLK6 (26μM) was incubated with recombinant KLK6 (0.26μM; a 100:1 molar ratio) in 100mM Tris-HCl pH 8.0, or 100mM sodium phosphate pH 6.0, 100mM NaCl, at 37°C. At various time intervals between 0-6 hrs, 2.2μl aliquots (corresponding to 2μg protein) of the reaction mixture were removed and snap-frozen in dry-ice. The time course aliquots were subsequently resolved on 16.5% Tricine SDS-PAGE, under both reducing and non-reducing conditions, and visualized by Coomassie Brilliant Blue stain. The resolved polypeptide bands were electroblotted onto polyvinylidene difluoride (PVDF) membrane and subjected to amino-terminal peptide sequencing using an Applied Biosystems Procise protein sequencer (Applied Biosystems, Foster City, CA).
Gelatin zymography of pro-KLK6 activation
15μM of recombinant pro-KLK6 was incubated with 0.15μM (100:1 molar ratio) of either KLK6, enterokinase (EK; Roche Applied Sciences, Indianapolis, IN), or no added protease, in 100mM Tris HCl, 0.1mM EDTA, pH8.0, or in 100mM sodium phosphate, 0.1mM EDTA, pH 6.0. Samples were incubated at 37°C, and volumes representing 50ng of pro-KLK6 in each case were withdrawn at 1, 2 and 6 hrs, added to SDS sample buffer and incubated for 5 min at 95°C (0 time point controls contained no added KLK6). The samples were subsequently resolved on 15% SDS-PAGE. Gelatin zymography of the gels was performed essentially as described by Liotta and Stetler-Stevenson (36) but with 100mM Tris, 0.1 mM EDTA, pH 8.0 as the gelatin digestion buffer. The gelatin digest was performed by 4 hr incubation at 37°C prior to Coomassie Brilliant Blue staining. Stained gels were scanned and converted to the negative image for visualization.
Comparative activation of pro-KLK6 by KLK,6enterokinase , plasmin and KLK5
The ability of KLK6, enterokinase, plasmin and KLK5 to activate pro-KLK6 was evaluated using a coupled assay procedure with the KLK6-sensitive internally-quenched fluorogenic peptide Abz-AFRFSQ-EDDnp (29). In the first step of the pro-KLK6 activation assay, 400 nM of pro-KLK6 in 100mM Tris, 0.1 mM EDTA, pH 8.0 was mixed with 4 nM (i.e. 100:1 molar ratio) of KLK6, EK, plasmin (Roche Applied Sciences, Indianapolis, IN) or KLK5 (R&D Systems, Inc., Minneapolis, MN). The samples were incubated for 1 hr at 37°C (comprising the pro-KLK6 “activation step”). Following this activation step, the KLK6-sensitive fluorogenic substrate Abz-AFRFSQ-EDDnp was added to a concentration of 2μM, and the generation of released fluorescence due to hydrolysis was monitored at 37°C for 5 min using a Cary Eclipse fluorescence spectrophotometer, with excitation wavelength of 320nm and emission wavelength of 420nm (“detection step”). Controls were subtracted to account for any background hydrolysis. 0, 2.5, 5, 10, 20 and 40 nM of KLK6 were added to 2μM of Abz-AFRFSQ-EDDnp substrate to permit normalization of arbitrary fluorescence units to molar concentration of KLK6 produced in the sample. The above incubation conditions were chosen such that less than 10% of the pro -KLK6 substrate was activated in the initial activation step, and less than 10% of the fluorogenic peptide substrate was subsequently hydrolyzed in the detection step. In this way, the reaction rates for both steps were determined under conditions of essentially constant substrate concentration.
Results
Production of recombinant KLK6 and pro-KLK6
Approximately 8-10mg of purified pro-KLK6 was isolated per liter of HEK293 culture media. N-terminal sequence analysis showed that KLK6 started with the sequence LVHG, and pro-KLK6 started with EEQN, the expected sequences for intact protein in both cases, with no detectable minor sequences. Under non-reducing conditions both the KLK6 and pro-KLK6 samples resolved as essentially homogenous bands on SDS-PAGE (Fig. 1). KLK6 contains an N-linked glycosylation site at position Asn132. The extent of glycosylation at this position is variable, giving rise to heterogeneity of mass and a characteristic smearing of the resolved SDS-PAGE bands (28). The pro-KLK6 produced in HEK293 cells does not exhibit glycosylation heterogeneity to the same extent (Fig. 1). KLK6 contains 223 amino acids with a mass of 25,866 Da. The KLK6 pro-peptide provides an additional 5 amino acids; however, the presence of the C-terminal purification tags adds additional 16 residues; thus, the pro-KLK6 protein resolves from the non-tagged KLK6 on SDS-PAGE. Under reducing conditions both KLK6 and pro-KLK6 exhibit the presence of a minor component (<5% by scanning densitometry) of a lower mass band consistent with a characteristic internal cleavage after Arg76 (28). This cleavage, likely due to the activity of a trypsin-like host protease, inactivates KLK6; thus, approximately 95% of the purified pro-KLK6 is activatable. Self-incubation of the purified pro-KLK6 at 37°C, pH 8.0, showed no detectable proteolytic degradation on SDS-PAGE after 6 hrs (data not shown) and all activation studies were performed within this time frame.
Figure 1.
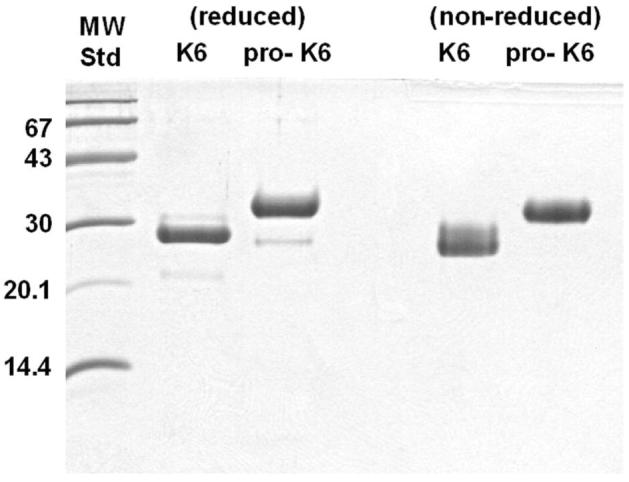
16.5% tricine SDS-PAGE of purified recombinant KLK6 and pro-KLK6 proteins, under both reducing and non-reducing conditions.
Characterization of KLK-6 pro-peptide versus autolysis loop hydrolyses using internally-quenched fluorogenic peptides
The standard curve of total-releasable fluorescence exhibited a fit to a linear function over the range of 1 - 100 μM, for both PRO and AL substrates, with a deviation from linearity (suggesting intermolecular quenching) detectable at concentrations above 100 μM (data not shown); therefore, 1 - 100 μM concentration range was utilized for all kinetic studies using these substrates. Michaelis-Menten constants were determined for the hydrolysis of the AL substrate by KLK6 (table 1). The rate of hydrolysis of the PRO substrate by 20nM KLK6 was essentially undetectable (at either pH 8.0 or pH 6.0), and for this reason the enzyme concentration was increased to 1.0μM (i.e. the maximum concentration permitting a 1:100 molar ratio of enzyme: substrate for the highest substrate concentration of 100μM) and the incubation period was extended to 30 min. Even with these adjustments to the protocol, it was not possible to determine Michaelis-Menten constants for the hydrolysis of the PRO substrate by KLK6 due to generally-low rates of hydrolysis. Therefore, the rate of hydrolysis of the 100mM PRO substrate data point was quantified, normalized to enzyme concentration, and used to determine the comparative rate of hydrolysis of the two substrates by KLK6, at both pH 8.0 and 6.0. At pH 6.0 the rate of hydrolysis of the AL peptide decreased two-fold, whereas, the rate of hydrolysis of the PRO peptide increased approximately five-fold, in comparison to pH 8.0. However, despite these changes, the AL peptide was still hydrolyzed with 2-3 orders of magnitude greater efficiency in comparison to the PRO peptide for the pH range 6.0 - 8.0 (table 1).
Table 1.
Kinetic constants associated with KLK6 hydrolysis of internally-quenched fluorogenic peptide substrates, representing the KLK6 pro-peptide and autolysis loop sequences, at pH 8.0 and 6.0
| Abz-EEQNKLVH-EDDnp (KLK6 pro-peptide sequence) | Abz-NLRQRESS-EDDnp (KLK6 autolysis loop sequence) | |||
|---|---|---|---|---|
| pH6.0 | pH8.0 | pH6.0 | pH8.0 | |
| Km(μM) | - | - | 95.3±10.8 | 85.6±5.9 |
| kcat(s-1) | - | - | 0.069±0.007 | 0.141±0.012 |
| kcat/Km (M-1s-1) | - | - | 0.72±0.05 × 103 | 1.67±0.16 × 103 |
| 100μM substrate hydrolysis rate (s-1) | 1.24±0.33 × 10-4 | 2.49±2.06 × 10-5 | 3.44±0.14 × 10-2 | 7.63±0.27 × 10-2 |
Proteolytic activity of KLK6 against pro-KLK6
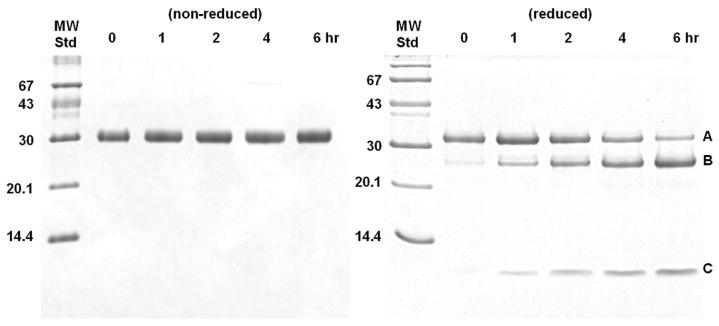
A time-course incubation of pro-KLK6 with KLK6 (100:1 molar ratio) resulted in loss of the intact 32 kDa protein band, and the simultaneous production of 25 kDa and 7 kDa bands under reducing conditions (Fig. 2). The non-reducing SDS-PAGE shows a 32 kDa band throughout. Amino acid sequence analysis of the resolved bands from the reducing SDS-PAGE analysis for digestion at pH 8.0 yielded peptide sequences associated with the pro-KLK6 amino-terminus; no detectable sequence corresponding to the mature amino-terminus was observed for any of the peptides (table 2). A similar amino acid sequence analysis for digestion at pH 6.0 yielded similar results, but with a detectable minor sequence corresponding to the mature N-terminus of KLK6 within band C (table 2, Fig. 3).
Figure 2.
16.5% tricine SDS-PAGE analysis of a time course digest of pro-KLK6 by KLK6 (100:1 ratio). 2 μg samples were run under either non-reducing (left panel) or reducing (right panel) conditions. Bands A, B and C in the reducing gel yielded N-terminal sequences indicated in table 2.
Table 2.
Amino-terminal sequence analysis of the SDS-PAGE bands from the hydrolysis of pro-KLK6 by KLK6 (see Fig. 2)
| Band | Molecular Mass (kDa) | Sequence | Location in KLK6a |
|---|---|---|---|
| pH 8.0 | |||
| A | 32 | EEQNKLVHG | E11E12Q13N14K15L16V17H18G19 |
| B | 25 | ESSQEQSSV | E77S78S79Q80E81Q82S83S84V85 |
| C | 7 | EEQNKLVHG | E11E12Q13N14K15L16V17H18G19 |
| pH 6.0 | |||
| A | 32 | EEQNKLVHG | E11E12Q13N14K15L16V17H18G19 |
| B | 25 | ESSQEQSSV | E77S78S79Q80E81Q82S83S84V85 |
| C | 7 | EEQNKLVHG | E11E12Q13N14K15L16V17H18G19L16V17H18G19G20 (minor seq)b |
Numbering scheme of chymotrypsin; with residue 11 being the pro-sequence N-terminus, and residue 16 being the sequence N-terminus.
See Fig. 3.
Figure 3.
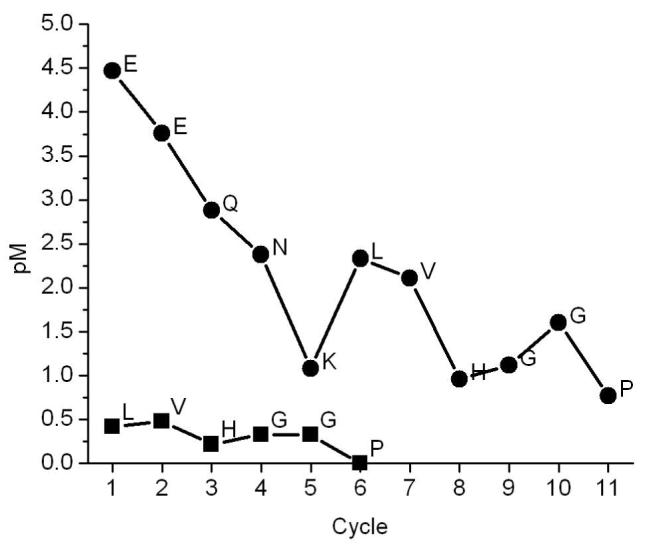
Sequencing information for band C (Fig. 2) of 100:1 pro-KLK6:KLK6 6hr digest at pH 6.0.
Gelatin zymography of pro-KLK6 activation
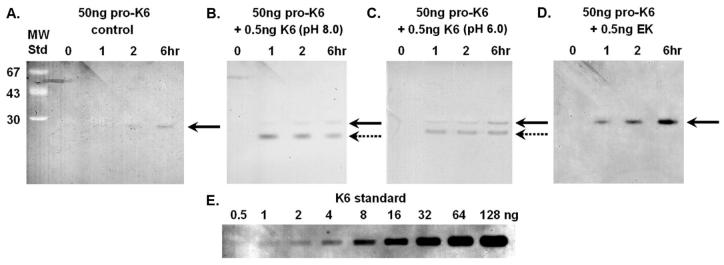
KLK6 has previously been shown to be an arginine-specific degradative-type protease (28) and gelatin zymography permits detection of enzymatic activity to approximately 0.5ng (Fig. 4). The control sample of 50ng purified pro-KLK6 exhibited no detectable enzymatic activity on the gelatin zymography (Fig. 4). There is a faint suggestion of enzymatic activity with the longest time point evaluated (6 hrs; panel A, Fig. 4), essentially at the detection limit of the zymogram. Incubation of 50ng of pro-KLK6 with 0.5ng KLK6 at pH 8.0 indicates the presence of the 0.5ng of active KLK6 added to the sample (cleanly resolved from the C-tagged pro-KLK6) but only a faint suggestion of activated pro-KLK6 even after the longest incubation period (6 hrs, panel B, Fig. 4), and again, at the detection limit of the zymogram. Incubation of 50ng of pro-KLK6 with 0.5ng KLK6 at pH 6.0 shows a similar result as the pH 8.0 digestion, although with marginally increased activity; however, the amount of pro-KLK6 activated is essentially at the limit of detection (panel C, Fig. 4). Incubation of pro-KLK6 with the lysine-specific protease enterokinase (100:1 molar ratio) demonstrates a time-dependent activation of pro-KLK6, yielding approximately 5-10ng of KLK6 after 6 hr incubation (panel D, Fig. 4).
Figure 4.
Gelatin zymography (reverse image) of pro-KLK6 activation. Panel A: time course incubation of 50 ng of pro-KLK6 control. Panel B: time course incubation with 0.5 ng of added KLK6 (pH 8.0). Panel C: time course incubation with 0.5 ng of added KLK6 (pH 6.0). Panel D: time course incubation with 0.5 ng of added enterokinase (EK). The solid arrow indicates the migration of KLK6 enzymatic activity derived from the C-terminal tagged form of pro-KLK6 and the dashed arrow indicates the migration KLK6 enzymatic activity derived from the non-C-terminal tagged mature KLK6. Panel E: zymography standards of 0.5-128 ng of KLK6.
Comparative activation of pro-KLK6 by KLK,6enterokinase , plasmin and KLK5
The relative ability of enterokinase, plasmin, KLK5 and KLK6 to activate pro-KLK6 in a coupled fluorogenic assay is shown in Fig. 5. Based upon a standard curve of KLK6 with this substrate (data not shown), the normalized reaction rates for activation of pro-KLK6 by the different proteases tested is listed in table 3. Enterokinase and plasmin exhibit approximately equivalent efficiencies in activating pro-KLK6 under the conditions evaluated (100mM Tris, 0.1mM EDTA, pH 8.0), with K5 exhibiting approximately 50% the efficiency of these two proteases. There was essentially no detectable activity of pro-KLK6 by KLK6 under these conditions. To evaluate whether the lack of detectable activation of pro-KLK6 by KLK6 might be due to initial activation followed by subsequent autolytic inactivation, a further 1 hr incubation was performed in the presence of 4nM EK. In this case, significant activity resulted, indicating the presence of activatable pro-KLK6 remaining in the sample (data not shown).
Figure 5.
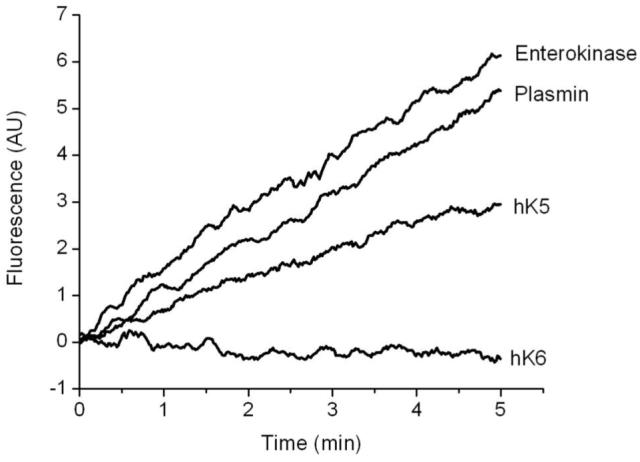
Relative activation rates of pro-KLK6 by enterokinase, plasmin, KLK5 or KLK6. Activation was quantified using the KLK6-sensitive internally-quenched fluorogenic substrate Abz-AFRFSQ-EDDnp (29). Normalized reaction rates are given in table 3.
Table 3.
Activation rates for 400nM pro-KLK6 by enterokinase, plasmin, KLK5 and KLK6 (100mM Tris, 0.1mM EDTA, pH 8.0, at 37°C)
| Protease | Activation rate (min-1) |
|---|---|
| Enterokinase | 3.21 × 10-2 |
| Plasmin | 2.88 × 10-2 |
| KLK5 | 1.50 × 10-2 |
| KLK6 | (Not detected) |
Discussion
Production of recombinant KLK6 and pro-KLK6
An important consideration in the production of recombinant proteases for enzymatic studies is the presence of contaminating host proteases. A reported expression of recombinant pro-KLK5 from Pichia pastoris resulted in the isolation of mature KLK5, suggesting KLK5 could auto-activate (37). However, this KLK5 was subsequently shown to be incapable of activating pro-KLK5 produced from Chinese hamster ovary cells, thus indicating that yeast host proteases were responsible for activating the pro-KLK5 (37). The original report of auto-activation of recombinant pro-KLK6 involved a week-long incubation of concentrated crude cell culture supernatant (30); thus, activation due to a contaminating host protease is a possibility. The reported sequential autolytic activation of pro-KLK6, first by hydrolysis between a Gln-Asn bond, then by hydrolysis between a Lys-Leu bond (31), is either indicative of an efficient dual-substrate specificity for KLK6, or the activity of a contaminating host protease. Since a clearly-defined dual-specificity for KLK6 has yet to be confirmed, the possibility that the reported auto-activation pathway of KLK6 was the result of a contaminating protease cannot be excluded. In the present case, we observed that controlled 6 hr incubations of purified recombinant pro-KLK6 did not result inany significant hydrolysis , indicating thatthe pro -KLK6 sample is substantially free of any contaminating host proteases that might contribute to a false positive activation.
In addition to providing affinity purification, the inclusion of a C-terminal His-tag and Strep-tag on pro-KLK6 permits resolution of pro-KLK6 from added catalytic amounts of KLK6 on SDS-PAGE (Fig’s 1 and 4). The individual enzymatic contributions derived from KLK6 and any activated pro-KLK6 within a mixture of these proteins can therefore be resolved and unambiguously identified in zymography (Fig. 4). The C-terminus of KLK6 is almost exactly anti-polar to the active site and N-terminus regions (28, 38) and is thus not structurally positioned to interfere with either pro-KLK6 activation or KLK6 active site function (Fig. 6).
Figure 6.
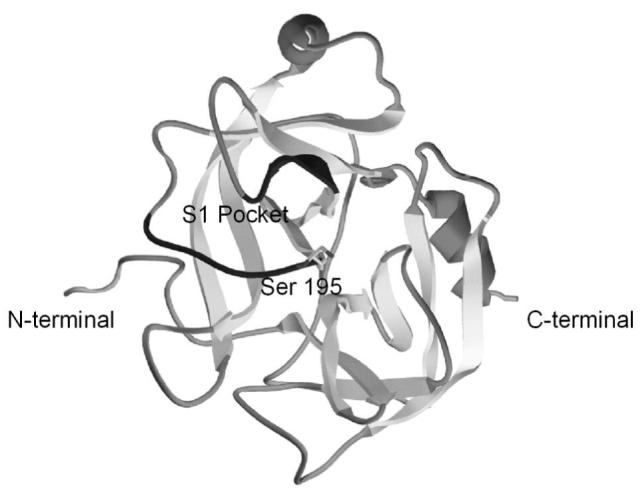
Ribbon diagram of pro-KLK6 x-ray structure (38) showing the location of the N- and C-termini, as well as the active site serine 195 and the region forming the S1 pocket (dark grey). His-tag and Strep-tag peptide sequences were attached to the C-terminal, which is distal to the pro-sequence and active site region.
Characterization of pro-peptide versus autolysis loop hydrolyses using internally-quenched fluorogenic peptides
The internally-quenched fluorogenic PRO peptide (representing the KLK6 pro-peptide sequence) was poorly hydrolyzed by KLK6; in contrast, the AL peptide representing the internal autolytic cleavage site at position Arg76 yielded the kinetic constants listed in table 1. Trypsin incubation to determine the magnitude of the total releasable fluorescence for each substrate yielded equivalent values; thus, the lack of hydrolysis of the PRO substrate by KLK6 is an intrinsic property of KLK6 activity towards this substrate. The relative rates of hydrolysis, at the highest concentration of both substrates (100mM), indicates that KLK6 hydrolyses the AL substrate at a rate 2-3 orders of magnitude faster than the PRO substrate (table 1). The rates of hydrolysis of these peptides is modulated somewhat by pH, with detectable increase in reaction rate for the hydrolysis of the PRO peptide by KLK6 at pH 6.0; however, even under acidic pH conditions the AL peptide is hydrolyzed by KLK6 with 2 orders of magnitude greater rate than the PRO peptide.
The results of these peptide studies therefore suggest that the internal KLK6 autolysis loop sequence represents a far more efficient substrate target for KLK6 than the KLK6 pro-sequence. Pro-KLK6 contains both the PRO and internal AL sequences; thus, the results indicate that if KLK6 was incubated with pro-KLK6, and engaged in a single proteolytic event, there is a 2-3-order of magnitude greater probability of this occurring at the internal autolytic site in comparison to the activation pro-sequence. Cleavage of the AL sequence of KLK6 inactivates the enzyme (23, 28), thus, cleavage of pro-KLK6 at the AL sequence would result in an internally-clipped pro-form that wouldbe incapable of subsequent activation.
Proteolytic activity of KLK6 against pro-KLK6
The incubation of pro-KLK6 with KLK6 resulted in the generation of two fragments of 25 and 7 kDa connected by a reducible disulfide bond (Fig. 2). The sequence analysis of these fragments shows that this internal hydrolysis occurs between residues Arg76 and Glu77 (the site of autolysis in KLK6 leading to inactivation (28)). With digestion at pH 8.0 both the 7 kDa and 32 kDa bands exhibit the N-terminal sequence of intact pro-KLK6; there was no sequence detected for the mature KLK6 N-terminus (i.e. IVHG). An identical fragment pattern on SDS PAGE was observed with digestion at pH 6.0 (data not shown). However, sequence analysis of the fragments indicates the presence of a minor sequence corresponding to the mature N-terminus in fragment C (Fig. 3). These results show that the in vitro activity of KLK6 against pro-KLK6 results in overwhelming internal cleavage at position Arg76 in relationship to hydrolysis of the pro-sequence. Arg76 is located within a surface turn, and is the most solvent-exposed Arg side chain in the x-ray structure (28). The crystal structure of pro-KLK6 shows that the N-terminus exhibits even greater solvent accessibility (38). Thus, the lack of hydrolysis of the KLK6 pro-peptide appears to be a direct consequence of the intrinsic specificity of KLK6 for Arg versus Lys residues, and not an issue of accessibility. This result agrees with the previously reported two-orders of magnitude greater preference of KLK6 for hydrolysis of arginine containing substrates as compared to lysine-containing substrates (28) and agrees with the 2-3 orders of magnitude greater hydrolysis rate for the internally-quenched AL versus PRO peptides.
Gelatin zymography of pro-KLK6 activation
The gelatin zymography of pro-KLK6 demonstrates that purified pro-KLK6 is essentially catalytically inactive. The use of the C-tagged form of pro-KLK6 permits discrimination of zymographic activity due to added (non-C-tagged) KLK6; in this case, there is a detectable zymography band for the added KLK6 (lanes 7-9, Fig. 4). The added KLK6 (0.5ng) represents 1% of the total pro-KLK6 present in these samples (50ng). The extent of activation of the C-tagged KLK6 by KLK6 after the 6 hr incubation time point, at either pH 8.0 or 6.0, is essentially at the detection limit of the assay, and therefore indicates that less than 1% of the pro-KLK6 has been activated. A comparison of the pH 8.0 to pH 6.0 incubations suggests a slight enhancement of pro-KLK6 activation under acidic conditions (Fig. 4). This agrees with the sequence analysis (Fig. 3) for the pH 6.0 digest of pro-KLK6 by KLK6, and the observed increase in hydrolysis rate of the PRO internally-quenched peptide at pH 6.0 (table 1).
Given the comparatively low rate of activation of pro-KLK6 by KLK6 (in relationship to the efficient rate of internal autolysis) an important question is whether the lack of activation is due to some intrinsic problem with the pro-KLK6 protein, i.e. is the pro-peptide accessible and can it actually be activated? The KLK6 pro-peptide (EEQNK) is similar to that of pro-trypsin (DDDDK), which is hydrolyzed by enterokinase; therefore, we evaluated the ability of enterokinase to activate pro-KLK6. The zymography results show that pro-KLK6 can be activated by enterokinase (Fig. 4, lanes 11-13), and also demonstrates that enterokinase is far more effective than KLK6 at activating pro-KLK6. These results show that the recombinant pro-KLK6 can indeed be activated, but that KLK6 is not efficiently performing this hydrolysis.
Comparative activation of pro-KLK6 by KLK,6enterokinase , plasmin and KLK5
The gelatin zymography result shows that the Lys-specific processing protease enterokinase is capable of activating pro-KLK6 with substantially greater efficiency than KLK6 (Fig. 4), therefore, the ability of KLK6, enterokinase and two other proteases (plasmin, a member of the fibrinolytic system, and KLK5, another human kallikrein-related peptidase expressed in the CNS) to active pro-KLK6 was evaluated using a coupled fluorogenic peptide assay. KLK5 exhibits a preference for hydrolysis after basic residues (both Arg and Lys)(37, 39, 40). KLK5 is second only to KLK6 in terms of abundance in normal human brain samples (21) and this along with Lys specificity, suggests that KLK5 might be a kallikrein of physiological relevancewith regard to potential activation of pro-KLK6 in the CNS.
A wealth of data implicates proteases of the plasminogen activator system in inflammatory disorders of the CNS. Levels of tissue plasminogen activator (tPA) are increased in the cerebrospinal fluid of MS patients; furthermore, myelin proteins and ECM proteins are prone to proteolysis by plasmin (41, 42). Importantly, the ability of plasmin to activate the MMP procollagenase was a breakthrough result that demonstrated a link between these two major proteolytic systems (43, 44). The specificity of plasmin includes hydrolysis after Lys residues, and the presence of plasmin in the CNS identified it as another protease of potential physiological relevance with regard to pro-KLK6 activation.
The results of the pro-KLK6 activation assay by plasmin and KLK5 shows that each of these proteases is far more efficient than KLK6 in activating pro-KLK6 (Fig. 5, table 3); this narrow list of screened proteases has therefore already identified more likely physiological candidates for activating pro-KLK6 than KLK6. Previous studies of the autolytic inactivation of KLK6, in conjunction with the present report, indicates that the consequence of an encounter of KLK6 with pro-KLK6 would almost certainly result in internal hydrolysis at position Arg76 (resulting in destruction of the potential catalytic function) than activation due to hydrolysis of the pro-peptide. Juliano and coworkers have previously reported a study of the activity of KLK6 against a series of peptide substrates (including the pro-KLK6 sequence) under various conditions of pH, cosmotropic salts, and glycosaminoglycans (29). Their results showed that while the activity of KLK6 could be modulated by these agents, in no case was the pro-peptide sequence efficiently hydrolyzed, whereas, various arginine-containing peptides were hydrolyzed with comparatively two-orders of magnitude greater efficiency. Thus, while the present study has not evaluated the effects of various added solutes, the results are consistent with peptide studies performed under a wide variety of conditions.
In total, the results of this report supportthe postulate that t he primary autolytic function of KLK6 is that of a negative feed-back loop that serves to inactivate KLK6 function and is unlikely to effectively serve as a positive feed-back activation loop to increase KLK6 activity. These data support the view that pro-KLK6 is activated by a separate activating protease (possibly plasmin or KLK5 in the CNS) (Fig. 7), the further characterization of which may point to novel targets for the treatment of CNS disorders such as MS. The results are therefore consistent with KLK6 being a tightly-regulated digestive-type protease within the CNS.
Figure 7.
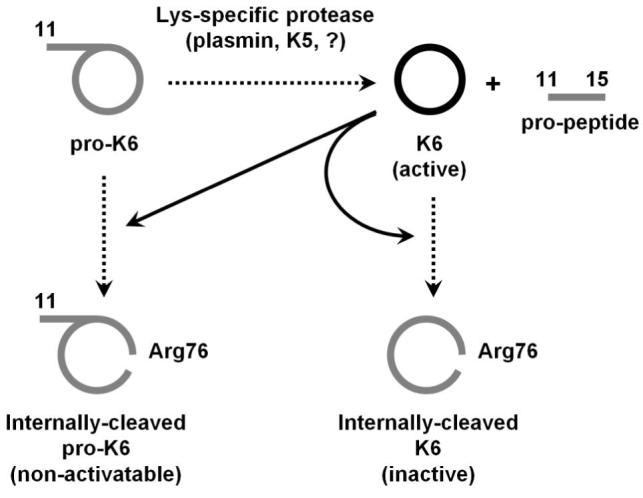
The proteolytic activity of KLK6 serves as a feed-back inhibitory mechanism via internal hydrolysis after Arg76. By comparison, the ability of KLK6 to function as a feed-back activator of pro-KLK6 appears substantially less significant, and distinct activating proteases such as plasmin or KLK5 are much more effective activators of pro-KLK6.
Acknowledgement
We thank Ms. Rani Dhanrajan and Ms. Margaret Seavy in the Molecular Cloning Laboratory and Analytical Laboratory, respectively, of the FSU Biological Sciences Department for assistance with cloning and amino terminal sequence data.
This work was supported by research grants RG 3406-A-2 (M.B.) and RG 3367-B4 (I.A.S.) from the National Multiple Sclerosis Society, 1 R15 NS057771-01 from the NIH (M.B.), the Neilsen Foundation (I.A.S.) and Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP) (M.A.J.).
Abbreviations used
- PBS
phosphate buffered saline
- DMSO
dimethyl sulfoxide
- PCR
polymerase chain reaction
- Ni- NTA
nickel-nitriloacetic acid
- EK
enterokinase
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- Abz
ortho-aminobenzoic acid
- EDDnp
ethylenediamine 2,4-dinitrophenyl
- PRO
Abz-EEQNKLVH-EDDnp internally-quenched fluorogenic peptide
- AL
Abz-NLRQRESS-EDDnp internally-quenched fluorogenic peptide
- MS
multiple sclerosis
References
- (1).Qin H, Kemp J, Yip M, Lam-Po-Tang PRL, Morris BJ. Localization of human glandular kallikrein-1 gene to chromosome 19q13.3-13.4 by in-situ hybridization. Human Heredity. 1991;41:222–226. doi: 10.1159/000154005. [DOI] [PubMed] [Google Scholar]
- (2).Riegman PH, Vlietstra RJ, Suurmeijer L, Cleutjens CB, Trapman J. Characterization of the human kallikrein locus. Genomics. 1992;14:6–11. doi: 10.1016/s0888-7543(05)80275-7. [DOI] [PubMed] [Google Scholar]
- (3).Lundwall A, Blaber M, Clements JA, Courty Y, Diamandis EP, Fritz H, Lilja H, Malm J, Maltais LJ, Olsson AY, Petraki C, Scorilas A, Sotiropoulou G, Stenman U-H, Stephan C, Talieri M, Yousef G. A comprehensive nomenclature for serine proteases with homology to tissue kallikreins. Biological Chemistry. 2006;387:637–641. doi: 10.1515/BC.2006.082. V., B. [DOI] [PubMed] [Google Scholar]
- (4).Oesterling JE. Prostate-specific antigen: a critical assessment of the most useful tumor marker for adenocarcinoma of the prostate. Journal of Urology. 1991;145:907–923. doi: 10.1016/s0022-5347(17)38491-4. [DOI] [PubMed] [Google Scholar]
- (5).Catalona WJ, Smith DS, Ratliff TL, Dodds KM, Coplen DE, Yuan JJ, Petros JA, Andriole GL. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. New England Journal of Medicine. 1991;324:1156–1161. doi: 10.1056/NEJM199104253241702. [DOI] [PubMed] [Google Scholar]
- (6).Diamandis EP, Okui A, Mitsui S, Luo LY, Soosaipillai A, Grass L, Nakamura T, Howarth DJ, Yamaguchi N. Human kallikrein 11: a new biomarker of prostate and ovarian carcinoma. Cancer Res. 2002;62:295–300. [PubMed] [Google Scholar]
- (7).Yousef GM, Kyriakopoulou LG, Scorilas A, Fracchioli S, Ghiringhello B, Zarghooni M, Chang A, Diamandis M, Giardina G, Hartwick WJ, Richiardi G, Massobrio M, Diamandis EP, Katsaros D. Quantitative expression of the human kallikrein gene 9 (KLK9) in ovarian cancer: a new independent and favorable prognostic marker. Cancer Res. 2001;61:7811–8. [PubMed] [Google Scholar]
- (8).Diamandis EP, Yousef GM, Soosaipillai AR, Bunting P. Human kallikrein 6 (Zyme/Protease M/Neurosin): a new serum biomarker of ovarian carcinoma. Clin. Biochem. 2000;33:579–583. doi: 10.1016/s0009-9120(00)00182-x. [DOI] [PubMed] [Google Scholar]
- (9).Diamandis EP, Yousef GM, Luo L-Y, Magklara A, Obiezu CV. The new human kallikrein gene family: Implications in carcinogenesis. Trends Endocrinol. Metab. 2000;11:54–60. doi: 10.1016/s1043-2760(99)00225-8. [DOI] [PubMed] [Google Scholar]
- (10).Luo LY, Katsaros D, Scorilas A, Fracchioli S, Piccinno R, Rigault de la Longrais IA, Howarth DJ, Diamandis EP. Prognostic value of human kallikrein 10 expression in epithelial ovarian carcinoma. Clin Cancer Res. 2001;7:2372–9. [PubMed] [Google Scholar]
- (11).Kishi T, Kato M, Shimizu T, Kato K, Matsumoto K, Yoshida S, Shiosaka S, Hakoshima T. Crystallization and preliminary X-ray analysis of neuropsin, a serine protease expressed in the limbic system of mouse brain. J Struct Biol. 1997;118:248–51. doi: 10.1006/jsbi.1997.3862. [DOI] [PubMed] [Google Scholar]
- (12).Kishi T, Kato M, Shimizu T, Kato K, Matsumoto K, Yoshida S, Shiosaka S, Hakoshima T. Crystal structure of neuropsin, a hippocampal protease involved in kindling epileptogenesis. J Biol Chem. 1999;274:4220–4. doi: 10.1074/jbc.274.7.4220. [DOI] [PubMed] [Google Scholar]
- (13).Ogawa K, Yamada T, Tsujioka Y, Taguchi J, Takahashi M, Tsuboi Y, Fujino Y, Nakajima M, Yamagoto T, Akatsu H, Mitsui S, Yamaguchi N. Localization of a novel type trypsin-like serine protease, neurosin, in brain tissues of Alzheimer’s disease and Parkinson’s disease. Psychiatry and Clinical Neurosciences. 2000;54:419–426. doi: 10.1046/j.1440-1819.2000.00731.x. [DOI] [PubMed] [Google Scholar]
- (14).Diamandis EP, Yousef GM, Petraki C, Soosaipillai AR. Human kallikrein 6 as a biomarker of alzheimer’s disease. Clin Biochem. 2000;33:663–7. doi: 10.1016/s0009-9120(00)00185-5. [DOI] [PubMed] [Google Scholar]
- (15).Scarisbrick IA, Towner MD, Isackson PJ. Nervous system specific expression of a novel serine protease: regulation in the adult rat spinal cord by excitotoxic injury. J. Neurosci. 1997;17:8156–8168. doi: 10.1523/JNEUROSCI.17-21-08156.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Scarisbrick IA, Blaber SI, Lucchinetti CF, Genain CP, Blaber M, Rodriguez M. Activity of a newly identified serine protease in CNS demyelination. Brain. 2002;125:1283–1296. doi: 10.1093/brain/awf142. [DOI] [PubMed] [Google Scholar]
- (17).He X-P, Shiosaka S, Yoshida S. Expression of neuropsin in oligodendrocytes after injury to the CNS. Neuroscience Research. 2001;39:455–462. doi: 10.1016/s0168-0102(01)00200-0. [DOI] [PubMed] [Google Scholar]
- (18).Terayama R, Bando Y, Takahashi T, Yoshida S. Differential expression of Neuropsin and Protease M/Neurosin in oligodendrocytes after injury to the spinal cord. Glia. 2004;48:91–101. doi: 10.1002/glia.20058. [DOI] [PubMed] [Google Scholar]
- (19).Scarisbrick IA, Sabharwal P, Cruz H, Larsen N, Vandell A, Blaber SI, Ameenuddin S, Papke LM, Fehlings MG, Reeves RK, Blaber M, Windebank AJ, Rodriguez M. Dynamic role of kallikrein 6 in traumatic spinal cord injury. Eur. J. Neurosci. 2006;24:1457–1469. doi: 10.1111/j.1460-9568.2006.05021.x. [DOI] [PubMed] [Google Scholar]
- (20).Yousef GM, Diamandis EP. The new human tissue kallikrein gene family: structure, function, and association to disease. Endocr. Rev. 2001;22:184–204. doi: 10.1210/edrv.22.2.0424. [DOI] [PubMed] [Google Scholar]
- (21).Scarisbrick IA, Blaber SI, Tingling JT, Rodriguez M, Christophi GP. Potential scope of action of tissue kallikreins in CNS immune-mediated disease. Journal of Neuroimmunology. 2006;178:167–176. doi: 10.1016/j.jneuroim.2006.05.022. M., B. [DOI] [PubMed] [Google Scholar]
- (22).Matsui H, Kimura A, Yamashiki N, Moriyama A, Kaya M, Yoshida I, Takagi N, Takahashi T. Molecular and biochemical characterization of a serine proteinase predominantly expressed in the medulla oblongata and cerebellar white matter of mouse brain. J. Biol. Chem. 2000;275:11050–11057. doi: 10.1074/jbc.275.15.11050. [DOI] [PubMed] [Google Scholar]
- (23).Blaber SI, Scarisbrick IA, Bernett MJ, Dhanarajan P, Seavy MA, Jin Y, Schwartz MA, Rodriguez M, Blaber M. Enzymatic properties of rat myelencephalon specific protease. Biochemistry. 2002;41:1165–1173. doi: 10.1021/bi015781a. [DOI] [PubMed] [Google Scholar]
- (24).Ghosh MC, Grass L, Soosaipilla A, Sotiropoulou G, Diamandis EP. Human kallikrein 6 degrades extracellular matrix proteins and may enhance the metastatic potential of tumour cells. Tumour Biol. 2004;25:193–199. doi: 10.1159/000081102. [DOI] [PubMed] [Google Scholar]
- (25).Yamanaka H, He X, Matsumoto K, Shiosaka S, Yoshida S. Protease M/neurosin mRNA is expressed in mature oligodendrocytes. Molecular Brain Research. 1999;71:217–224. doi: 10.1016/s0169-328x(99)00187-4. [DOI] [PubMed] [Google Scholar]
- (26).Scarisbrick IA, Asakura K, Blaber S, Blaber M, Isackson PJ, Beito T, Rodriguez M, Windebank AJ. Preferential expression of myelencephalon specific protease by oligodendrocytes of the adult rat spinal cord white matter. Glia. 2000;30:219–230. doi: 10.1002/(sici)1098-1136(200005)30:3<219::aid-glia2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- (27).Blaber SI, Ciric B, Christophi GP, Bernett M, Blaber M, Rodriguez M, Scarisbrick IA. Targeting kallikrein 6 proteolysis attenuates CNS inflammatory disease. FASEB J. 2004;18:920–922. doi: 10.1096/fj.03-1212fje. [DOI] [PubMed] [Google Scholar]
- (28).Bernett MJ, Blaber SI, Scarisbrick IA, Dhanarajan P, Thompson SM, Blaber M. Crystal structure and biochemical characterization of human kallikrein 6 reveals a trypsin-like kallikrein is expressed in the central nervous system. J. Biol. Chem. 2002;277:24562–24570. doi: 10.1074/jbc.M202392200. [DOI] [PubMed] [Google Scholar]
- (29).Angelo PF, Lima AR, Alves FM, Blaber SI, Juliano L, Juliano MA. Substrate specificity of human kallikrein 6: salt and glycosaminoglycan effects. J Biol Chem. 2006;281:3116–3126. doi: 10.1074/jbc.M510096200. I.A., S., M., B. [DOI] [PubMed] [Google Scholar]
- (30).Little SP, Dixon EP, Norris F, Buckley W, Becker GW, Johnson M, Dobbins JR, Wyrick T, Miller JR, MacKellar W, Hepburn D, Corvalan J, McClure D, Liu X, Stephenson D, Clemens J, Johnstone EM. Zyme, a novel and potentially amyloidogenic enzyme cDNA isolated from Alzheimer’s disease brain. J. Biol. Chem. 1997;272:25135–25142. doi: 10.1074/jbc.272.40.25135. [DOI] [PubMed] [Google Scholar]
- (31).Bayes A, Tsetsenis T, Ventura S, Vendrell J, Aviles FX, Sotiropoulou G. Human kallikrein 6 activity is regulated via an autoproteolytic mechanism of activation/inactivation. Biological Chemistry. 2004;385:517–524. doi: 10.1515/BC.2004.061. [DOI] [PubMed] [Google Scholar]
- (32).Okui A, Kominami K, Uemura H, Mitsui S, Yamaguchi N. Characterization of a brain-related serine protease, neurosin (human kaillikrein 6), in human cerebrospinal fluid. Neuroreport. 2001;12:1345–50. doi: 10.1097/00001756-200105250-00011. [DOI] [PubMed] [Google Scholar]
- (33).Yuan X, Russell T, Wood G, Desiderio DM. Analysis of the human lumbar cerebrospinal fluid proteome. Electrophoresis. 2002;23:1184–1196. doi: 10.1002/1522-2683(200204)23:7/8<1185::AID-ELPS1185>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- (34).Gill SC, von Hippel PH. Calculation of protein extinction coefficients from amino acid sequence data. Analytical Biochemistry. 1989;182:319–326. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
- (35).Chagas JR, Juliano L, Prado ES. Intramolecularly quenced fluorogenic tetrapeptide substrates for tissue and plasma kallikreins. Analytical Biochemistry. 1991;192:419–425. doi: 10.1016/0003-2697(91)90558-b. [DOI] [PubMed] [Google Scholar]
- (36).Liotta LA, Stetler-Stevenson WG. Metalloproteinases and cancer invasion. Seminars inCancer Biology. 1990;1:99–106. [PubMed] [Google Scholar]
- (37).Michael IP, Sotiropoulou G, Pampalakis G, Magklara A, Ghosh M, Wasney GA, Diamandis EP. Biochemical and enzymatic characterization of human kallikrein 5 (hK5), a novel serine protease potentially involved in cancer progression. Journal of Biological Chemistry. 2005;280:14628–14635. doi: 10.1074/jbc.M408132200. [DOI] [PubMed] [Google Scholar]
- (38).Gomis-Ruth FX, Bayes A, Sotiropoulou G, Pampalakis G, Tsetsenis T, Villegas V, Aviles FX, Coll M. The structure of human prokallikrein 6 reveals a novel activation mechanism for the kallikrein family. J Biol Chem. 2002;277:27273–81. doi: 10.1074/jbc.M201534200. [DOI] [PubMed] [Google Scholar]
- (39).Brattsand M, Stefansson K, Lundh C, Haasum Y, Egelrud T. A proteolytic cascade of kallikreins in the stratum corneum. J Invest Dermatol. 2005;124:198–203. doi: 10.1111/j.0022-202X.2004.23547.x. [DOI] [PubMed] [Google Scholar]
- (40).Yousef GM, Kapadia C, Polymeris ME, Borgono C, Hutchinson S, Wasney GA, Soosaipillai A, Diamandis EP. The human kallikrein protein 5 (hK5) is enzymatically active, glycosylated and forms complexes with two protease inhibitors in ovarian cancer fluids. Biochim Biophys Acta. 2003;1628:88–96. doi: 10.1016/s0167-4781(03)00116-7. [DOI] [PubMed] [Google Scholar]
- (41).Cuzner ML, Gveric D, Strand C, Loughlin AJ, Paemen L, Opdenakker G, Newcombe J. The expression of tissue-type plasminogen activator, matrix metalloproteases and endogenous inhibitors in the central nervous system in multiple sclerosis: comparison of stages in lesion evolution. J. Neuropath. Exp. Neurol. 1996;55:1194–1204. doi: 10.1097/00005072-199612000-00002. [DOI] [PubMed] [Google Scholar]
- (42).Cuzner ML, Opdenakker G. Plasminogen activators and matrix metalloproteases, mediators of extracellular proteolysis in inflammatory demyelination of the central nervous system. J Neuroimmunol. 1999;94:1–14. doi: 10.1016/s0165-5728(98)00241-0. [DOI] [PubMed] [Google Scholar]
- (43).Eeckhout Y, Vaes G. Further studies on the activation of procollagenase, the latent precursor of bone collagenase. Effects of lysosomal cathepsin, plasmin and kallikrein, and spontaneous activation. Biochem. J. 1977;166:21–31. doi: 10.1042/bj1660021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Werb Z, Mainardi CL, Vater CA, Harris EDJ. Endogenouse activation of latent collagenase by rheumatoid synovial cells. Evidence for a role of plasminogen activator. N Engl J Med. 1977;296:1017–1023. doi: 10.1056/NEJM197705052961801. [DOI] [PubMed] [Google Scholar]