Abstract
Background
Muscle effects are the most common reported adverse effects of 3-hydroxy-3-methylglutaryl coenzyme A inhibitors (statins). However, in placebo-controlled trials the incidence of muscle pain is most often similar for placebo and active control groups.
Objective
We sought to evaluate whether statin use was associated with a higher prevalence of musculoskeletal pain in a nationally representative sample.
Methods
Cross-sectional analysis using data from the National Health and Nutrition Examination Survey (NHANES) 1999–2002. Participants were 3,580 adults ≥40 years without arthritis who were interviewed at home and examined in a mobile examination center. Participants were asked about sociodemographic characteristics, health conditions, medication use, and musculoskeletal pain. Height, weight, blood pressure, ankle brachial index, and cholesterol were measured.
Measurements and Main Results
Prevalence and adjusted odds ratios (OR) of any musculoskeletal pain and musculoskeletal pain in 4 different anatomical regions (neck/upper back, upper extremities, lower back, and lower extremities) by statin use during the last 30 days. Among statin users (n = 402), 22.0% (95%CI 18.0–26.7%) reported musculoskeletal pain in at least 1 anatomical region during the last 30 days, compared with 16.7% (95%CI 15.1–18.4%) of those who did not use a statin. Compared to persons who did not use statins, those who used statins had multivariable-adjusted odds ratios (95%CI; p value) of 1.50 (1.07–2.11; p = .01) for any musculoskeletal pain, 1.59 (1.04–2.44, p = .03) for lower back pain, and1.50 (1.02–2.22, p = .03) for lower extremity pain.
Conclusion
Musculoskeletal pain is common in adults ≥40 years without arthritis. In this nationally representative sample, statin users were significantly more likely to report musculoskeletal pain.
KEY WORDS: statin myopathy, hydroxymethylglutaryl-coa reductase inhibitors, musculoskeletal pain
BACKGROUND
About 25 million individuals use 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) inhibitors (statins) worldwide, including about 13 million in the United States.1 Statins significantly lower cholesterol levels and have been shown to significantly reduce morbidity and mortality associated with heart disease.2,3 Generally, statins are well tolerated with few serious side effects.4 However, adherence can be limited by patients’ concern about serious muscle toxicity5 and by more frequent but less severe side effects of muscle aches, pain, weakness, and cramps.6 Because statins must be taken long-term and are potentially life-saving, their tolerability and adherence is a major concern.5
Overall, muscle side effects (including symptoms or creatine kinase elevations or both) are recognized as the most common adverse effect associated with statin use, but their extent is not well-defined.7 The incidence of myalgia (generally defined as muscle symptoms without significant creatine kinase elevation) approximates 1–5% of statin-users in randomized clinical trials, with rates similar to placebo groups.8,9 However, the frequency of muscle complaints reported in usual care settings appears to be higher than in clinical trials with a frequency of 9% to 20% in outpatient settings.10–12 Because clinical trials have mainly focused on serious muscle toxicity, with limited data reported on less severe musculoskeletal effects that occur without substantial increases in creatine kinase, we aimed to evaluate the association between musculoskeletal pain and statin use in a nationally representative sample.
METHODS
The National Health and Nutrition Examination Survey (NHANES) is a stratified multistage probability survey of the noninstitutionalized U.S. population administered by the National Center for Health Statistics (NCHS). NHANES includes an in-home interview for demographic and basic health information and a health examination in a mobile examination center. Informed consent was obtained from all participants, and the protocol was approved by the institutional review board of the NCHS.13 Public use data files were obtained from the NHANES Website (http://www.cdc.gov/nchs/nhanes.htm), and these analyses were approved by the Beth Israel Deaconess Medical Center Committee on Clinical Investigation.
Study Population
This study consisted of 3,580 adults aged ≥40 years participating in survey years 1999–2002, who had both interview and examination data available. Individuals reporting a doctor’s diagnosis of arthritis were not included because of the high prevalence of musculoskeletal pain associated with this condition.
Study Variables
Respondents to NHANES were asked, “During the past month have you had a problem with pain that lasted more than 24 hours?” Those who answered “yes” were asked to specify the location of their pain. For this study we assessed pain according to location of pain at 4 different musculoskeletal regions including neck or upper back, upper extremities (shoulder, arm, wrist or hand), lower back, and lower extremities (buttock, leg, or foot). Any musculoskeletal pain was defined as pain in any one of these regions.
To ascertain medication use, survey participants were asked if they had taken a medication in the past month for which they needed a prescription. Those who answered “yes” were asked to show the interviewer their medication containers so that the product’s complete name could be obtained. If no container was available (∼10%), the participant was asked the name of the medication.
Age, sex, race/ethnicity, education level, smoking status, physical activity, and health status were obtained by self-report. Race/ethnicity was classified as non-Hispanic black, non-Hispanic white, Mexican American, and all others. Exercise-related physical activity was based on the last 30 days and was classified as vigorous if participants reported “vigorous activity for at least 10 minutes that caused heavy sweating, or large increases in breathing or heart rate” or moderate if participants reported “moderate activities for at least 10 minutes that caused only light sweating or a slight to moderate increase in breathing or heart rate,” or sedentary if neither vigorous nor moderate activity was reported. Alcohol intake was based on current intake over the last year and was categorized as <1 drink per day, 1 to 2 drinks per day, or more than 2 drinks per day. Having a usual place for health care was defined as having at least 1 place to go when sick or in need of advice about health. Coronary heart disease was defined by the respondent’s self-report of a doctor’s diagnosis of coronary heart disease, angina, or a previous heart attack. Diabetes was defined by self-report of a doctor’s diagnosis of diabetes, a measured glucose level of >200 mg/dl, or use of an antidiabetic medication. Cancer was defined by self-report of a doctor’s diagnosis, excluding non-melanoma skin cancer. Body mass index was calculated as measured weight in kilograms divided by the square of measured height in meters. The means of 2 blood pressure measurements in the right arm and in each ankle were used to calculate ankle brachial index (ABI) for the right and left sides. We used the lower of the 2 ABI values measured to categorize ABI and defined peripheral artery disease as an ABI ≤.97.
Statistical Methods
We carried out statistical analyses using SAS (version 9.1; SAS Institute, Cary, NC) and SUDAAN (version 9.0; Research Triangle Institute, Research Triangle Park, NC). Prevalence estimates of demographics, health characteristics, statin use, and musculoskeletal pain were weighted to represent the U.S. population, to account for oversampling in specific demographic subgroups, and to account for nonresponses.
We compared the characteristics of participants according to statin use with t tests for continuous measurements, and chi-square tests for categorical variables. We calculated the prevalence of musculoskeletal pain in any region and in the following 4 regions: neck/upper back, upper extremities, lower back, and lower extremities.
We performed multivariable logistic regression to assess the association of statin use with musculoskeletal pain. From an a priori list of potential confounders, we included the following in the final multivariable model: age, sex, race/ethnicity, coronary heart disease, diabetes, cancer, systolic blood pressure, ABI, BMI, total cholesterol (quintiles), smoking (never, past, current), and health status (excellent/very good, good, fair/poor). We included quadratic terms to examine nonlinear effects of continuous variables (age, BMI, and systolic blood pressure). We classified ABI as ≤.90, 91–.99, 1.00–1.29, ≥1.29, and missing and explored whether using different ABI cutoffs to define peripheral artery disease (ABI ≤.97, yes/no) or severe peripheral artery disease (ABI ≤.69, yes/no) altered the results of our final models.
Other factors examined, but not included in our final models because they were not associated with musculoskeletal pain and did not appear to confound the relation of statin use with pain, included educational level, physical activity, alcohol intake, and having a usual place for health care. As a post hoc analysis, we used interaction terms to examine whether the association between statin use and any pain, lower back pain, or lower extremity musculoskeletal pain differed by hypothesized effect modifiers, including age, sex, ABI, BMI, general health status, coronary heart disease status, or alcohol use. Results of multivariable models are presented as odds ratios (OR) with 95% confidence intervals.
RESULTS
Of 3,580 persons aged 40 years and older (representing 74.6 million U.S. individuals) examined, 402 (8.2 million, 11%) had used a statin in the last 30 days. Fifty-one percent of the participants were male, 75% were non-Hispanic white, and 56% had received education beyond a high school diploma. Table 1 shows the prevalence of demographic and health characteristics of the study sample according to statin use. Compared to statin nonusers, statin users were older, more likely to be male, non-Hispanic white, and former smokers, and to report poor/fair health status, coronary heart disease, diabetes, and cancer.
Table 1.
Characteristics of the Sample Population (N = 3580)
| Statin User | Statin Nonuser | p value* | |
|---|---|---|---|
| (n = 402) | (n = 3178) | ||
| Age, % | <.001 | ||
| 40–49 | 16.8 | 48.6 | |
| 50–59 | 31.3 | 27.6 | |
| 60–69 | 24.5 | 12.7 | |
| 70+ | 25.6 | 11.2 | |
| female, % | 39.9 | 50.1 | .01 |
| Race/ethnicity, % | <.001 | ||
| Non-Hispanic White | 82.8 | 74.7 | |
| Non-Hispanic Black | 5.7 | 9.8 | |
| Mexican-American | 2.1 | 5.7 | |
| All others | 9.3 | 9.8 | |
| Education, % | .08 | ||
| Less than high school | 14.8 | 19.7 | |
| High school diploma | 26.1 | 24.0 | |
| More than high school | 59.2 | 56.3 | |
| Physical activity, % | .46 | ||
| Sedentary | 36.2 | 37.7 | |
| Moderate | 34.4 | 30.1 | |
| Vigorous | 29.4 | 32.2 | |
| Smoking status, % | <.001 | ||
| Never | 43.1 | 49.6 | |
| Former | 42.7 | 29.8 | |
| Current | 14.3 | 20.6 | |
| Alcohol consumption (drinks/day), % | 0.76 | ||
| <1 | 85.5 | 85.3 | |
| 1–2 | 8.6 | 9.5 | |
| >2 | 5.9 | 5.2 | |
| Self-reported health, % | .007 | ||
| Excellent/very good | 49.9 | 57.9 | |
| Good | 29.2 | 28.7 | |
| Poor/fair | 20.9 | 13.3 | |
| Has usual place for health care, % | 99.5 | 87.1 | <.001 |
| Co-morbidity, % | |||
| Coronary heart disease | 28.6 | 3.7 | <.001 |
| Diabetes | 19.6 | 6.9 | <.001 |
| Peripheral artery disease | 7.2 | 13.9 | .003 |
| Cancer | 10.6 | 6.0 | .006 |
| Systolic blood pressure, mean (mm Hg) | 130.2 | 125.9 | .004 |
| Body mass index, mean (Kg/m2) | 28.5 | 27.8 | .03 |
| Total cholesterol, mean (mg/dl)† | 195.7 | 213.4 | <.001 |
Population characteristics by statin use among US adults ≥40 years who do not have arthritis. Results from the National Health and Nutrition Examination Survey 1999–2002
*Based on t tests for continuous variables and chi-square tests for categorical variables.
†SI conversion factor: to convert cholesterol to millimoles per liter, multiply by 0.0259.
Figure 1 shows the estimated prevalence of musculoskeletal pain in different anatomical regions during the last 30 days according to statin use for US adults aged 40 years and older without arthritis. Overall, 22.0% (95% CI, 18.0–26.7%) of those who used a statin reported musculoskeletal pain, compared with 16.7% (95% CI, 15.1–18.4%) of those who did not use a statin. The prevalence of lower extremity pain was particularly high among statin users compared with statin nonusers (10.7%; 95% CI, 7.7–14.7% vs 6.4%; 95% CI, 5.3–7.7%).
Figure 1.
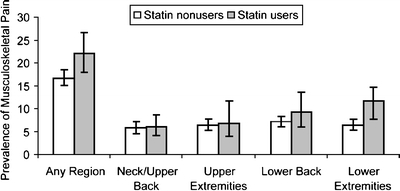
Unadjusted weighted prevalence estimates (percent) and 95% confidence intervals of any musculoskeletal pain and musculoskeletal pain at 4 different anatomical regions according to statin use (p = .01, p = .86, p = .80, p = .26, p = .03, respectively).
Table 2 shows the unadjusted and adjusted odds ratios of musculoskeletal pain in different anatomical regions for statin users compared to statin nonusers. When all potential confounders were included simultaneously in a multivariable model, the odds ratio for lower extremity pain was slightly attenuated but remained significant, whereas the odds ratios increased for both lower back pain and any musculoskeletal pain (Table 2). Age was the most important confounder included as it was associated with statin use and was inversely associated with greater report of pain in any region and of the lower back.
Table 2.
Odds Ratios of Musculoskeletal Pain at Different Anatomical Locations for Statin Users Compared to Nonusers
| Region | Unadjusted OR (95%CI) | p value | Adjusted* OR (95%CI) | p value |
|---|---|---|---|---|
| Any Region | 1.39 (1.08–1.80) | .01 | 1.50 (1.07–2.11) | .01 |
| Neck/Upper Back | 1.04 (0.70–1.54) | .85 | 1.14 (0.71–1.82) | .54 |
| Upper Extremities | 1.10 (0.63–1.90) | .74 | 1.00 (0.51–1.98) | .99 |
| Lower Back | 1.33 (0.86–2.06) | .18 | 1.59 (1.04–2.44) | .03 |
| Lower Extremities | 1.72 (1.16–2.54) | .01 | 1.50 (1.02–2.22) | .03 |
*Adjusted for age, sex, race (non-Hispanic white, non-Hispanic black, Mexican-American, others), smoking status (never, former, current), self reported health (excellent/very good, good, poor/fair), coronary heart disease, diabetes, cancer, systolic blood pressure (SBP and SBP2), body mass index (BMI and BMI2), total cholesterol (quintiles), and ankle brachial index (≤0.90, 0.91–0.98, 0.99–1.08, 1.09–1.29, ≥1.30, and missing)
Independent of statin use, a number of factors were found to be associated with pain. The presence of coronary heart disease was also independently associated with lower extremity pain (OR, 2.03; 95% CI, 1.10–3.75, p = .02) but not with musculoskeletal pain in other regions. Self-reported fair/poor health status compared to excellent/very good health was associated with greater likelihood of musculoskeletal pain in any region (OR, 2.85, 95% CI, 2.08–3.90), neck (OR, 3.66; 95% CI, 2.31–5.80), upper extremity (OR, 3.15, 95% CI, 1.94–5.12), lower back (OR, 2.79; 95% CI, 1.89–4.12), and lower extremity (OR 3.24; 95% CI, 1.82–5.41). Compared to males, females had a higher odds of having lower extremity pain (OR, 1.43; 95% CI, 1.02–2.0), and tended to have a lower odds of having low back pain (OR 0.65; 95% CI, 0.42–1.01). Compared to those who had never smoked, current smokers reported more lower extremity pain, lower back pain and overall pain (OR 1.69; 95% CI, 1.01–3.84; OR, 1.59; 95% CI, 1.02–2.48; OR, 1.48; 95% CI, 1.08–2.03, respectively). Neither physical activity nor alcohol intake was associated with musculoskeletal pain in any region on bivariable or multivariable models. Neither BMI nor ABI (nor peripheral artery disease based on an ABI ≤.97 nor severe peripheral artery disease based on an ABI <.69), was associated with musculoskeletal pain in multivariable models.
No statistically significant interactions were observed for any, lower back, or lower extremity musculoskeletal pain when we included interaction terms for statin use by age, ABI, BMI, general health status, or alcohol intake. However, the association of statin use with greater reports of lower extremity pain tended to be stronger among those with coronary heart disease (OR, 4.59; 95% CI, 1.22–17.26) compared to those without coronary heart disease (OR, 1.04; 95% CI 0.58–1.86; p value interaction = 0.06) and among females (OR, 2.06; 95% CI, 1.07–3.97) compared to males (OR, 1.12; 95% CI, 0.64–1.96; p value interaction = 0.06).
Comment
This study is the first to our knowledge to examine musculoskeletal pain and statin use in a nationally representative U.S. sample. In this study, statin use was associated with significantly increased odds of musculoskeletal pain. Compared with adults who did not use statins, those who used statins had 50% greater odds of having any musculoskeletal pain, and 50–60% greater odds of musculoskeletal pain in the lower back and lower extremities.
It is important to note the prevalence of pain was high among both statin users and nonusers. Therefore, pain occurring during statin use may be unrelated to statin use and suggests that another trial of statin use may be appropriate in patients with musculoskeletal pain who require statins and do not have contraindications to their continued use (e.g., substantially elevated muscle enzymes).
The results of this study suggest that the burden of musculoskeletal pain that is associated with statin use may be substantial. Using the NHANES-supplied sample weights, we estimate that 8.2 million individuals ≥ 40 years of age use statins in the United States. Of these, 22% (representing 1.8 million people) report musculoskeletal pain in at least 1 anatomical region and 11% (representing 0.9 million people) report lower extremity pain in the last month. Based on the observed differences in the prevalence of pain according to statin use (22% among statin users compared to 16.7% among non-statin users), we estimate that nearly 25% of cases of musculoskeletal pain at any region among statin users (corresponding to 450,000 individuals) appear to be associated with statin use. Likewise, we estimate that statins could account for approximately 45% of cases of lower extremity pain among statin users.
Our finding of a significant association between musculoskeletal pain and statin use are compatible with prior observational10–12,14 and post-marketing surveillance studies of statins, but conflict with randomized clinical trials of statin therapy that show no significant association of statins with myalgia.14 These differences may be caused by characteristics of participants involved in clinical trials, who may differ from patients using statins in the general population. With a lower prevalence of musculoskeletal pain in this group, clinical trials may have had limited power to detect differences of this magnitude. In addition, randomized clinical trials have varied greatly in reporting data on muscle symptoms, and differences in the prevalence of pain may be related to the more comprehensive assessment used in NHANES. Although symptomatic muscle effects associated to statin use range from nonspecific muscle aches without elevated serum creatine kinase concentration to severe myositis, most trials have reported few details about muscle symptoms that occur without substantial increases in levels of creatine kinase. In some trials, only myalgia that resulted in withdrawal from the study or myalgia thought to be attributed to the intervention (criteria not defined) have been reported.15–18 Finally, studies based on study designs that included active treatment phases before randomization19,20 necessarily underestimate the incidence of myalgia, given that individuals who demonstrate intolerance during active pre-randomization phases are actively excluded.
The mechanism of statin-associated muscle effects remains unknown. Common hypotheses for the cause of muscle symptoms involves a deficiency of 1 of the many synthetic products of the HMG-CoA reductase pathway, including impaired cholesterol synthesis leading to abnormal cell membrane stability; impaired ubiquinone (coenzyme Q10 or CoQ10) synthesis, leading to decreased mitochondrial enzyme activity; or prenylated protein synthesis abnormalities causing altered intracellular messaging.21,22 Preliminary studies have suggested that some patients who demonstrate muscle symptoms with statins may have underlying genetic or metabolic muscle abnormalities, which predispose them to statin intolerance.23,24 However, additional research is needed to clarify these possible links.
Several limitations should be considered when interpreting these results. First, because this is a cross-sectional analysis, it is not possible to determine the causal nature or direction of the association between musculoskeletal pain and statin use. Second, comorbidity was based on self-report of a doctor’s diagnosis, and thus may be subject to misclassification bias. Also, self-reported exercise activity was coarsely defined, and therefore, the lack of association between greater exercise activity and musculoskeletal pain should be interpreted with caution, given such associations have been noted in prior observational studies.25 In addition, the fact that an association between pain (particularly lower extremity pain) and peripheral artery disease was not observed suggests that episodic pain, such as exertional musculoskeletal pain, was not captured in this study. Third, the timeframe of the musculoskeletal pain and statin use referred only to the month before the interview. Thus, we did not know the duration of statin use or whether nonusers had recently discontinued a statin. Furthermore, we did not have sufficient numbers of statin users to assess outcomes by statin type, and also lacking dose information, we could not examine possible associations with higher statin dose, which has been shown to be a factor in statin muscle-related side effects.6 In the future, additional information on medication history, including dose, which could be easily obtained from prescription bottles, as well as frequency and length of use and prior discontinuance would be valuable data to collect. In this study, we did not have information on creatine kinase levels, so we were unable to examine the relation between muscle symptoms and creatine kinase. Such data as well as longitudinal follow-up will be useful in clarifying musculoskeletal adverse effects of statins. Fourth, exclusion of participants with arthritis, while necessary for internal validity, limits the generalizability of this study. Finally, NHANES does not include institutionalized persons, whose poorer health and comorbidity may render them more susceptible to adverse effects of medications.
Our post hoc analysis revealed probable interactions between statin use and coronary heart disease and sex in the odds of reporting pain, suggesting that pain associated with statin use may be more common among women and those with coronary heart disease. However, because these analyses were based on a relatively small number of participants and involved several tests of possible modifiers, the results should be interpreted with caution.
Recent recommendations made by an expert panel called for more careful monitoring of minor statin-related muscle problems, even when they occur in the absence of creatine kinase elevations.6 Our study suggests the risk of musculoskeletal pain with statin use may be substantially greater than previously reported and statins may be responsible for nearly a quarter of the musculoskeletal pain experienced by individuals who use them. It remains crucial to investigate side effects of statins in a broad population of patients, including those who have greater comorbidity, and include an adequate number of women.
CONCLUSION
Musculoskeletal pain is common among persons without arthritis. Statin use is significantly associated with greater prevalence of musculoskeletal pain overall, lower extremity pain, and lower back pain.
Acknowledgments and Conflict of Interest
Dr Mittleman has received research funding from Pfizer; has served as a scientific consultant to Pfizer, Bayer, Lily ICOS, CV Therapeutics, AstraZeneca, and Reliant; and has coauthored peer-reviewed publications with individuals who have been employed by industry either currently or in the past. Drs. Buettner, Davis, Leveille, and Mukamal have no conflicts of interest, financial interests nor relationships or affiliations relevant to the subject matter or materials discussed in this manuscript.
No internal or external funding supported this research project.
The authors’ affiliated organizations did not participate in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
References
- 1.Mitka M. Expanding statin use to help more at-risk patients is causing financial heartburn. JAMA. 2003;290(17):2243–5. [DOI] [PubMed]
- 2.Cannon CP, Steinberg BA, Murphy SA, Mega JL, Braunwald E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol. 2006;48(3):438–45. [DOI] [PubMed]
- 3.Fonarow GC, Watson KE. Effective strategies for long-term statin use. Am J Cardiol. 2003;92(1A):27i–34i. [DOI] [PubMed]
- 4.Black DM. A general assessment of the safety of HMG CoA reductase inhibitors (statins). Curr Atheroscler Rep. 2002;4(1):34–41. [DOI] [PubMed]
- 5.Sacks FM. Adherence to statin therapy: why aren’t we doing better? Am J Med. 2002;113(8):685–6. [DOI] [PubMed]
- 6.Thompson PD, Clarkson PM, Rosenson RS. An assessment of statin safety by muscle experts. Am J Cardiol. 2006;97(8A):69C–76C. [DOI] [PubMed]
- 7.McKenney JM, Davidson MH, Jacobson TA, Guyton JR. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97(8A):89C–94C. [DOI] [PubMed]
- 8.Pasternak RC, Smith SC Jr., Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol. 2002;40(3):567–72. [DOI] [PubMed]
- 9.Armitage J. The safety of statins in clinical practice. Lancet. 2007;370(9601):1781–90. [DOI] [PubMed]
- 10.de Sauvage Nolting PR, Buirma RJ, Hutten BA, Kastelein JJ. Two-year efficacy and safety of simvastatin 80 mg in familial hypercholesterolemia (the Examination of Probands and Relatives in Statin Studies with Familial Hypercholesterolemia [ExPRESS FH]). Am J Cardiol. 2002;90(2):181–4. [DOI] [PubMed]
- 11.Bruckert E, Hayem G, Dejager S, Yau C, Begaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther. 2005;19(6):403–14. [DOI] [PubMed]
- 12.Franc S, Dejager S, Bruckert E, Chauvenet M, Giral P, Turpin G. A comprehensive description of muscle symptoms associated with lipid-lowering drugs. Cardiovasc Drugs Ther. 2003;17(5–6):459–65. [DOI] [PubMed]
- 13.National Center for Health Statistics, National Health and Nutrition Examination Survey Institutional Review Board Approval. (Accessed April 18, 2007, at http://www.cdc.gov/nchs/about/major/nhanes/intro_mec.htm).
- 14.Kashani A, Phillips CO, Foody JM, et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation. 2006;114(25):2788–97. [DOI] [PubMed]
- 15.Jukema JW, Bruschke AV, van Boven AJ, et al. Effects of lipid lowering by pravastatin on progression and regression of coronary artery disease in symptomatic men with normal to moderately elevated serum cholesterol levels. The Regression Growth Evaluation Statin Study (REGRESS). Circulation. 1995;91(10):2528–40. [DOI] [PubMed]
- 16.Hunninghake DB, Stein EA, Dujovne CA, et al. The efficacy of intensive dietary therapy alone or combined with lovastatin in outpatients with hypercholesterolemia. N Engl J Med. 1993;328(17):1213–9. [DOI] [PubMed]
- 17.Davidson M, Ma P, Stein EA, et al. Comparison of effects on low-density lipoprotein cholesterol and high-density lipoprotein cholesterol with rosuvastatin versus atorvastatin in patients with type IIa or IIb hypercholesterolemia. Am J Cardiol. 2002;89(3):268–75. [DOI] [PubMed]
- 18.Kerzner B, Corbelli J, Sharp S, et al. Efficacy and safety of ezetimibe coadministered with lovastatin in primary hypercholesterolemia. Am J Cardiol. 2003;91(4):418–24. [DOI] [PubMed]
- 19.MRC/BHF. Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7–22. [DOI] [PubMed]
- 20.Blankenhorn DH, Azen SP, Kramsch DM, et al. Coronary angiographic changes with lovastatin therapy. The Monitored Atherosclerosis Regression Study (MARS). Ann Intern Med. 1993;119(10):969–76. [DOI] [PubMed]
- 21.Antons KA, Williams CD, Baker SK, Phillips PS. Clinical perspectives of statin-induced rhabdomyolysis. Am J Med. 2006;119(5):400–9. [DOI] [PubMed]
- 22.Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289(13):1681–90. [DOI] [PubMed]
- 23.Oh J, Ban MR, Miskie BA, Pollex RL, Hegele RA. Genetic determinants of statin intolerance. Lipids in Health and Disease. 2007;6:7. [DOI] [PMC free article] [PubMed]
- 24.Vladutiu GD, Simmons Z, Isackson PJ, et al. Genetic risk factors associated with lipid-lowering drug-induced myopathies. Muscle Nerve. 2006;34(2):153–62. [DOI] [PubMed]
- 25.Sinzinger H, O’Grady J. Professional athletes suffering from familial hypercholesterolaemia rarely tolerate statin treatment because of muscular problems. Br J Clin Pharmacol. 2004;57(4):525–8. [DOI] [PMC free article] [PubMed]


