Abstract
Particulate matter (PM) elicits inflammatory and toxic responses in the lung specific to its constituents, which can vary by region, time, and particle size. To identify the mechanism of toxicity in PM collected in a rural area in the San Joaquin Valley of Central California, we studied coarse particles of 2.5 – 10 μm diameter (PM2.5-PM10). Potential pro-inflammatory and toxic effects of PM2.5-PM10 in the lung were investigated using intratracheally instilled mice. We determined total and differential cell profiles and inflammatory chemokines in lung lavage fluid, and biomarkers of toxicity resulting from coarse PM exposure. Responses of the mice were readily observed with total doses of 25–50 ug of PM per mouse. Changes in pro-inflammatory cellular profiles and chemokines showed both dose and time response; peak responses were observed 24 hours after PM instillation, with recovery as early as 48 hours. Furthermore, macrophage inflammatory protein (MIP-2) profiles following PM exposures were correlated to levels of measured macrophages and neutrophils recovered from lung lavage fluid of PM treated animals. Our data suggest that pro-inflammatory effects observed from coarse PM collected during the summer months from California’s hot and dry Central Valley are driven largely by the insoluble components of the PM mixture, and are not caused by endotoxin.
Keywords: PM10, Intratracheal Instillation, Lung inflammation, Endotoxin, Bronchoalveolar Lavage, Air pollution
Introduction
Particulate Matter (PM) is a mixture of small particles regulated as one of the National Ambient Air Quality Standard (NAAQS) pollutants by the Environmental Protection Agency (EPA) under the Clean Air Act. Currently, PM standards are based on total mass and size, which can range from a few nanometers to tens of micrometers in aerodynamic diameter. Coarse particles of 2.5 μm – 10 μm diameter (PM2.5-PM10) are of particular health interest due to the capacity to penetrate into the lung and potentially elicit health effects. Experiments evaluating lung response to ultrafine, fine, and coarse PM found that it was coarse size (2.5 – 10 μm) that showed the most potency at equivalent masses (Monn et al., 1999; Soukup et al., 2001; Hetland et al., 2005). Additionally, epidemiological studies have shown growing and convincing evidence of the association between increase in morbidity and respiratory diseases with increased levels of coarse ambient particulate matter, especially among susceptible populations (Dockery et al., 1993; Schwartz, 1994; Pope et al., 1995). Although these standards set forth by the EPA in an attempt to limit total PM concentration, they do not take into account the specific PM constituents that may be responsible for the adverse effects seen in response to exposure.
However, beyond aerodynamic diameter and surface area, it remains unclear which constituent(s) in the particulate mixture are responsible for respiratory effects (Dreher, 2000; Schwarze et al., 2006). A proposed explanation for reported adverse effects elicited by PM is its mixture of primary emissions and secondary air pollutants, but it has yet to be determined which specific component or combinations of coarse PM components are responsible for the observed inflammatory and toxic effects in the respiratory system. The literature suggests that possible toxic and pro-inflammatory effects of coarse PM may be attributed to biological agents (Becker et al., 1996; Dong et al., 1996), metals (Pope et al., 1989; Costa et al., 1998; Ghio et al., 1999), or ultrafine particles adhered to coarse particles (Oberdorster et al., 1994). Two common approaches used to investigate complex mixtures are to either 1) study individual components that make up the whole mixture, or 2) fractionate the whole mixture and investigate sets of constituents. The goal of either of these two approaches is to identify key constituent(s) which may be responsible for the activity of the mixture. Both of these approaches have been utilized in investigating possible key constituents in particulate matter that may be responsible for inflammatory and toxic health effects.
Complex particulate mixtures can be analyzed by fractionation techniques based on the physical chemical properties of the constituents making up the whole mixture. There have been a handful of studies that have used this approach in both in vitro and in vivo PM experiments; fractionating the mixture into soluble and insoluble fractions, (Ghio et al., 1999; Imrich et al., 2000; Soukup et al., 2001; Becker et al., 2003; Adamson et al., 2004). Both fractions in these experiments have shown different biological activities based on the constituents in each of the two fractions. Although many studies have attributed inflammatory and toxic responses observed in the lung to classes of constituents in either fraction, the biological effects of specific constituents of PM remain elusive and require further evaluation.
Numerous studies have focused on the role of biological constituents in PM such as β-glucans, mold spores, and endotoxin (Schwarze et al, 2006). Endotoxin, a common constituent of particulate matter, also known as lipopolysaccharide (LPS) is a component of the outer membrane of gram negative bacteria. Endotoxin can elicit inflammatory responses in the lung and is found more often in the coarse PM2.5–10 fraction as compared to the fine PM2.5 fraction (Monn et al., 1999; Soukup et al., 2001; Schins et al., 2004). Endotoxin exposure, via inhalation, has been associated with an increase in neutrophil influx (Schwarze et al., 2006). One study reported that PM containing endotoxin collected from Mexico City played an important role in pro-inflammatory reactions (Osornio-Vargas et al., 2003), suggesting that the mechanisms of endotoxin-mediated effects are not pro-cytotoxic but rather pro-inflammatory (Osornio-Vargas et al., 2003; Vernath et al., 2004).
In vivo studies evaluating respiratory tract inflammation and toxicity from particulate matter use various exposure methods (e.g., inhalation, intratracheal or intranasal instillation, and others). While inhalation mimics the most natural route of exposure, there are some experimental limitations (Driscoll et al., 2000; Costa et al., 2006). One important limitation is estimation of the actual dose delivered to the lungs, which is one of the most powerful advantages of intratracheal instillation. It allows delivery of an actual dose; this can be very beneficial in characterizing dose response to a particulate matter sample. Although there are limitations to using intratracheal instillation as a method of exposure, this method serves as an effective technique for the identification and evaluation of the individual constituents of complex mixtures that make up PM, and to further elucidate the specific components that drive inflammatory and toxicological responses. We set out to test the hypothesis that the insoluble fraction of coarse particulate matter collected from the Central Valley of California is responsible for toxicological and pro-inflammatory responses in the mouse lung utilizing intratracheal instillation.
Materials and Methods
Animals
Pathogen-free male BALB/c mice 8–10 weeks old (25–30g) were purchased from Charles River Breeding Laboratories (Wilmington, MA). Animals were housed, 4 animals per cage, in filtered Bio-Clean facilities in the Animal Resources Center (UC Davis, CA). Mice received water and standard feed (Purina Rat Chow) ad lib and were allowed to acclimate for a week prior to any experimental procedures. The animals were kept on a 12-h light/dark cycle at room temperature (20–25°C) and 30–70% relative humidity.
Source of PM
The coarse PM used in this study was collected with a high-volume sampler in the summer months from a small town in California’s San Joaquin Valley. Previous studies have also been done with samples collected from this area (Smith et al., 2003; Chow et al., 1992; 2006).
Particulate Matter Elution
Pre-weighed Teflon filters stored in −20°C were acclimated to room temperature prior to elution of PM. Filters were cut and placed into labeled 15ml conical vials. Phosphate-buffered saline (PBS), pH 7.6 (Cellgro, Mediatech, Inc, Herndon, VA) was added to the first conical vial (1ml/kg). Serial elution was performed by vortex mixing the first vial for 60 seconds, removing the contents and placing them into the second vial, and vortex mixing for an additional 60 seconds; this was repeated 3 times per vial in a serial fashion. All eluted contents were pooled into one vial, stored as aliquots in separate vials and stored at −80°C until 30 minutes prior to an experiment.
Soluble and Insoluble Particulate Matter Fractions
Eluted PM suspensions in PBS were fractionated into soluble and insoluble fractions (Ghio et al., 1999). PM preparations were mixed and centrifuged at 12,850 × g for 30 minutes. The supernatant (soluble fraction) was transferred to a vial, while the pellet (insoluble fraction) was either suspended in a volume of PBS equal to the volume of supernatant removed or was further treated to inactivate biological material by heating the contents at 120°C for 20 hours (modified from Alexis et al., 2006).
Endotoxin Spiking of PM
Aliquots of the PM suspension were spiked with a known concentration (1.25 ug/mouse) of endotoxin (Escherichia coli; 055:B5, Lonza). Concentrations of endotoxin were determined by preliminary dose-response pilot experiments (data not shown) utilizing pulmonary cellular influx as a measure of inflammatory potential. Whole PM spiked with endotoxin was fractionated into soluble and insoluble fractions as described above. Insoluble and soluble spiked PM was administered separately to mice via IT instillation. An aliquot of insoluble spiked PM was exposed to 120°C for 24 hours to inactivate the biological material (modified from Alexis et al., 2006) and then administered via IT.
Experimental Design
Mice (5–6 animals for each exposure group studied) were instilled with either 50 ul or 25 ul, at a concentration of 1 mg/ml of PM. PM exposures include: 1) whole particulate fraction, 2) soluble fraction, 3) insoluble fraction, 4) heated insoluble particulate fraction, 5) whole LPS-spiked particulate fraction, 6) soluble LPS-spiked particulate fraction, 7) insoluble LPS-spiked particulate fraction, 8) heated LPS-spiked whole particulate fraction, or 9) heated insoluble LPS-spiked particulate fraction. Bronchoalveolar lavage (BAL) and tissue were collected 6 hours after PM instillation. BAL and tissues were also collected in a time course experiment and processed at 3, 6, 18, 24, 48, or 72 hours after IT instillation. PBS-instilled controls (50 ul/per animal) were included in each experiment and at each time point in the time course experiment.
Intratracheal Instillations
Prior to instillation, mice were anesthetized with Attane (isoflurane) via inhalation in an enclosed box chamber. Mice were positioned in a supine position and the jaw and tongue were drawn away from the esophageal region using forceps while inserting a 22-gauge glass syringe (Model #1705, Hamilton, Reno, NV) into the trachea and instilling 50 ul of the PM suspension. Prior to instillation, PM preparations were vortex mixed for 30 seconds to suspend the material. Tracheal insertion was confirmed by palpating the tracheal rings with the tip of the syringe. After instillation, the mouse was allowed to recover under visual control before placement back into the cage for a predetermined period after exposure.
Bronchoalveolar Lavage
Mice were euthanized with an overdose of pentobarbital/dilantin via intraperitoneal injection, usually 6 hours after PM instillation. Mice were exsanguinated by cardiac puncture and blood collected and stored at −80° C for future biochemical analyses. The tracheas were cannulated and the lungs were lavaged two times each with 1ml instillations (2 ml total) of phosphate-buffered saline (PBS), pH 7.6 (Mediatech, Inc, Herndon, VA). More than 80% of the initial instilled PBS volume was recovered in the lavage fluid. The total lavage fluid from each mouse lung was combined and centrifuged at 1800–2000 rpm for 10 minutes using a bench top unit (Centrifuge #5415C, Eppendorf, New York, NY). The supernatant was removed and the resulting pellet was suspended in ACK Lysis Buffer (0.15M NH4Cl, 1.0 M KHCO3, 0.1 mM Na2EDTA, H2O) and centrifuged for another 10 minutes. The supernatant was discarded and the pellet was suspended in 1ml of PBS. The trypan blue exclusion method (Mishell and Shiigi. 1980) was used to count the total number of viable cells in each BAL sample using a hemocytometer. Additional cell cytospin preparations were performed with 100 ul of BAL fluid using a cytocentrifuge, (StatSpin Cytofuge 2, Iris, Westwood, MA). Prepared cytospin slides were stained with DiffQuick® (International Reagent Corp, Kobe, Japan). Cell differentials were counted using methods previously performed in our laboratory (Kenyon et al. 2006). Briefly, cells were counted and classified by counting 10 fields (400x) from each stained cytospin slide using a light microscope. Cell counts were expressed as percentages of total cell counts.
Lung Isolation and Fixation
Lavaged lungs were either fixed at 30 cm pressure with 1% paraformaldehyde in PBS for 24 hours for histopathological assessment or separated into right and left lobes and stored at −80°C for additional toxicological analyses. Fixed lungs were separated into right and left lobes, where the left lobe was processed for analysis via light microscopy in paraffin embedded histological sections (5μm) and the right lobes were stored in 70% ethanol for future use.
Immunochemical and Biochemical Analyses
Macrophage Inflammatory Protein (MIP-2) was analyzed from bronchoalveolar lavage fluid (BALF) supernatant. MIP-2 was analyzed using an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
Statistical Analysis of Data
Prism 4.0 (GraphPad Software, San Diego, CA) was used for the analysis of data. All values are expressed as mean values ± SE (standard error) unless otherwise noted. Parametric analysis of data was conducted using Student’s t-test. Welch’s correction was applied if variances were assumed to be unequal. For experiments using more than two groups, data were analyzed by ANOVA followed by post-testing with Tukey’s honestly significant difference test. Linear regression was performed using a 95% confidence interval. Differences were considered significant if the p value was <0.05.
Results
Particulate Matter Elution and IT Instillation
Recovery of particulates from the Teflon filters was at least 80%. Nominal doses presented in the Figures were not adjusted for actual particulate recoveries, but are based upon the assumption of 100% recovery of PM by mass from the filters. Previous evaluation of homogeneity of distribution using this method of IT instillation in our laboratory (Greenberg et al., 1978) suggests that instillation of material upon injection follows a homogenous pattern throughout the lung.
Effects of PM Exposure on Dose-Response
The first question we addressed was whether the coarse PM preparation we tested had a pro-inflammatory effect on the lung. We examined three groups of mice instilled with: (1) PBS, (2) 25 ug of PM/mouse, or (3) 50 ug of PM/mouse. Mice were sacrificed 6 hours after instillation and their lungs were lavaged with PBS. Total viable cells recovered were counted. We found about 1.0×105 cells per ml in normal mice instilled with PBS (Fig. 1). Increased numbers of viable cells were found in the mice instilled with PM, with an apparent dose-response relationship between amounts of PM instilled and total cells recovered in the lung lavage fluid (Fig. 1). Differential cell counts showed that essentially all of the cells recovered from the mice instilled with PBS were macrophages (Fig. 2). Increased numbers of macrophages were found in both groups of mice instilled with PM. However, the mice instilled with 50 ug of PM also showed a significant increase in the number of neutrophils recovered in their lung lavage as compared with either of the other two groups of mice (Fig. 3). The relative percentage of neutrophils in the group instilled with 50 ug of PM was about 30% of the total cells recovered, as compared with neutrophil contents of less than 5% of the total cells in lung lavage from the mice instilled with either PBS or 25 ug of PM.
Figure 1.
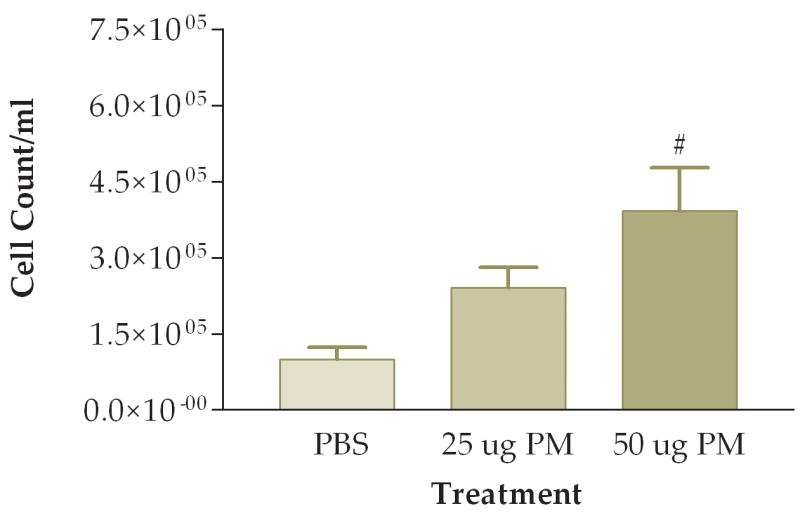
Average Cell Count of recovered cells in lung lavage fluid 6 hours after instillation of PM (#, P<0.01 compared to PBS control, ANOVA). The bars represent ± SE in this and all subsequent figures. N, the number of mice per experimental group, was 5–6 for all of the experiments in this and the following figures.
Figure 2.
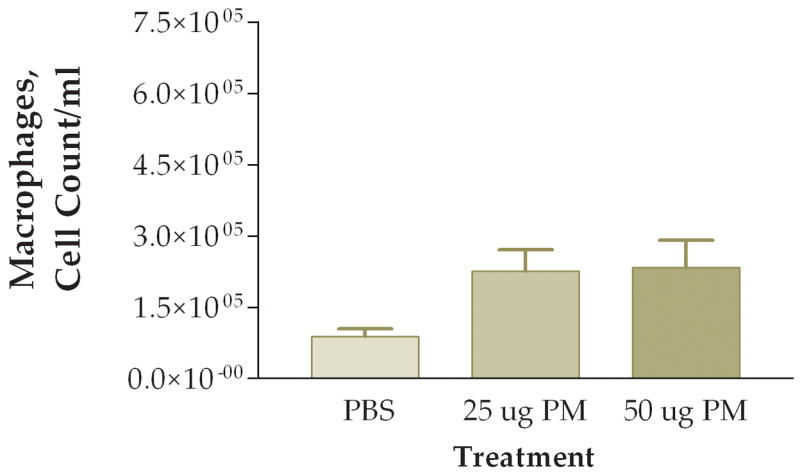
Number of macrophages found in lung lavage fluid 6 hours after instillation of 50 ug of PM.
Figure 3.
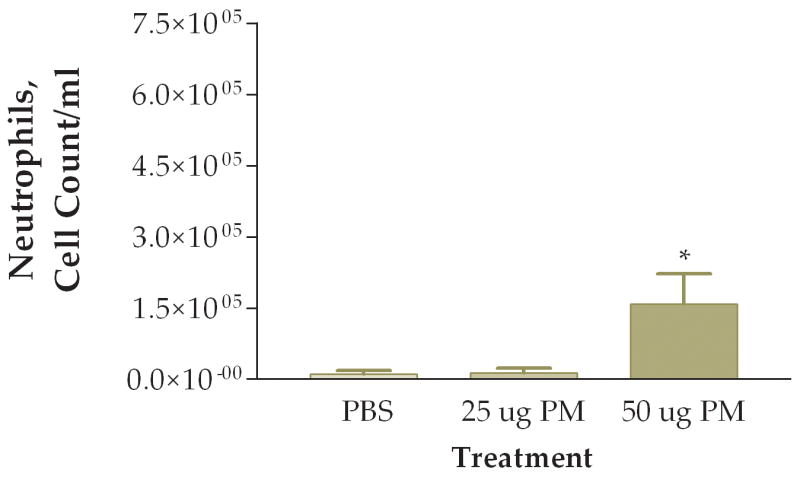
Number of neutrophils found in lung lavage fluid 6 hours after instillation of 50 ug of PM (*, P<0.05 compared to PBS control, ANOVA).
Time Dependent Effects of PM Exposure
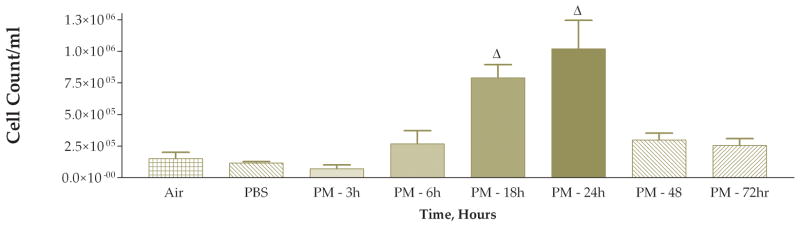
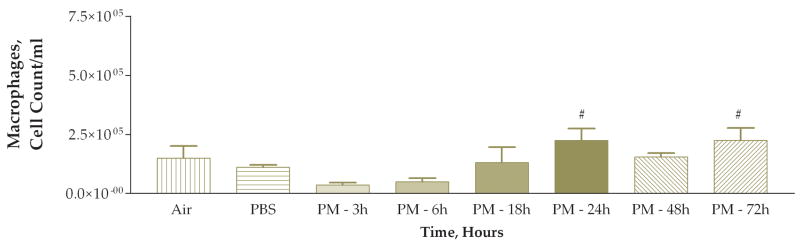
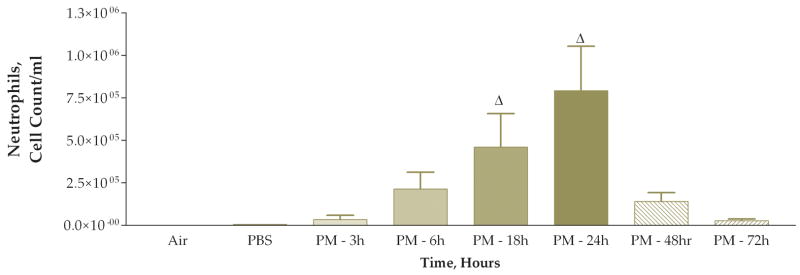
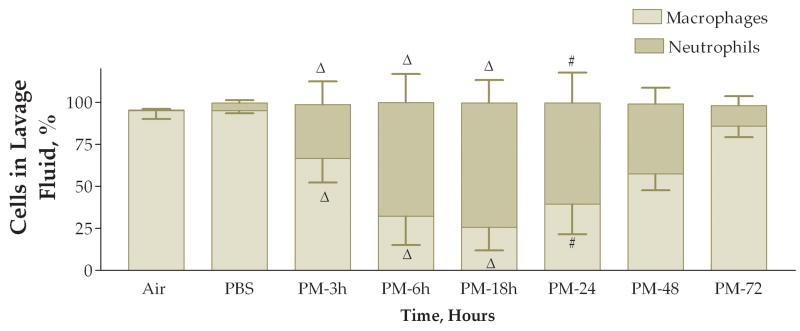
The next experiment examined the time course of the response to coarse PM exposure. Mice were divided into 6 treatment groups each receiving 50 ug of PM with different time intervals after instillation before necropsy: 3, 6, 18, 24, 48, or 72 hours. We also included matched control groups that received only PBS instilled at each time point, 3, 6, 18, 24, 48, or 72 hours, after instillation. The PBS-instilled mice were processed identically to their matched PM-instilled counterparts. We found a stepwise increase in the total viable cells recovered from lung lavage fluid (Fig. 4). This apparent time-response relationship was significant when the 6, 18, 24, 48, and 72-hour groups were compared to either the 3-hour group or the pooled data from the PBS control groups. The cell differentials also exhibited an apparent time-response relationship (Figs. 5 and 6). Both the macrophage and neutrophil numbers recovered from the lung lavage fluid show steady increases with longer times after instillation, with a peak at 24 hours. We found that the neutrophils recovered from lung lavage fluid were increased at all time points (32–74%); however, the relative percentage of neutrophils peaked at 18 hours after instillation and was slightly decreased (to 60%) at the 24 hr time point with continued decreases at 48 and 72 hours (41–12%) (Fig. 7). After instillation, there was a reciprocal relationship between the relative percentage of macrophages in the lung lavage fluid and the relative percentage of neutrophils; however, it should be noted that the absolute number of macrophages (and the total cell count) was increased at the later time points examined after 6 hours.
Figure 4.
Average cell count of recovered cells in lung lavage fluid 3, 6, 18, 24, 48, and 72 hours after instillation of 50 ug of PM (Δ, P<0.001 compared to PBS control, ANOVA; PBS control group data for all of the time points were pooled).
Figure 5.
Number of macrophages found in lung lavage fluid 3, 6, 18, 24, 48, and 72 hours after instillation of 50 ug of PM (#, P<0.01 compared to PBS control, ANOVA; PBS control group data for all of the time points were pooled).
Figure 6.
Number of neutrophils found in lung lavage fluid 3, 6, 18, 24, 48, and 72 hours after instillation of 50 ug of PM (Δ, P<0.001 compared to PBS control, ANOVA; PBS control group data for all of the time points were pooled).
Figure 7.
Percentage of macrophages (light bars) and neutrophils (dark bars) in lung lavage fluid 3, 6, 18, 24, 48, and 72 hours after instillation of 50 ug of PM (#, P<0.01; ΔP<0.001 compared to PBS control, ANOVA). Top bars, symbols are for the macrophages; bottom bars, symbols are for the neutrophils.
Lung Responses to Soluble and Insoluble PM Constituents
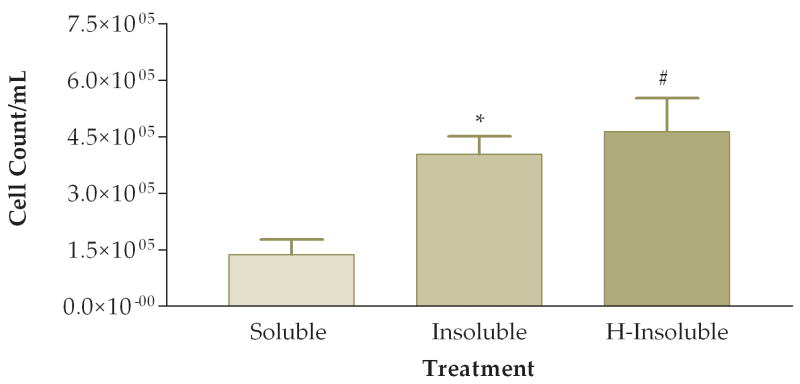
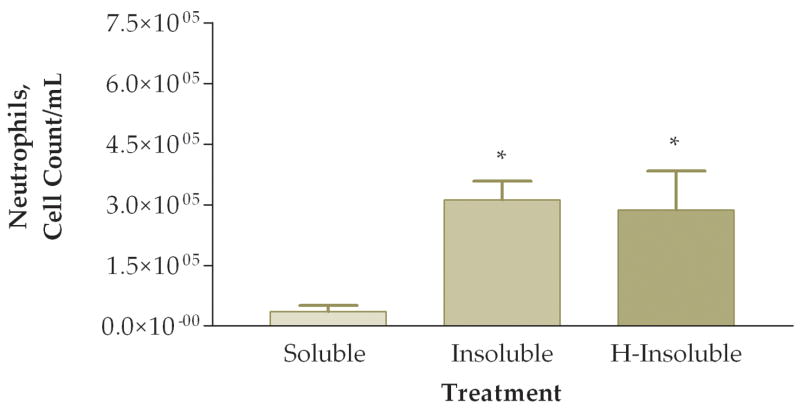
To answer the question of whether the pro-inflammatory effects were attributed to the soluble or insoluble fractions of the PM sample, we fractionated PM samples and exposed animals to 50 ug of soluble or insoluble PM. Note that in these experiments we did not actually instill 50 ug of either soluble or insoluble PM into the mice. Rather, we instilled the volume equivalent of 50 ug of unfractionated PM from each preparation. Preliminary experiments indicated that essentially all of the pro-inflammatory activity was in the insoluble fraction. The next experiment addressed whether heat treatment of the insoluble fraction destroyed any biological constituents that would result in a decreased pro-inflammatory response on the lung. In this experiment mice were instilled with either: (1) soluble fraction (S), (2) insoluble fraction (I), or (3) heat-treated insoluble fraction (HI). We found a significant difference between the three groups when comparing total viable cells recovered from lung lavage fluid (Fig. 8). Mice exposed to either the I or HI fraction exhibited a higher pro-inflammatory response as compared to the S fraction. Differential cell counts also showed a significant increase of neutrophils and a trend towards decreased macrophages in the I fraction as compared to the S and HI groups (Fig. 9). The relative percentages of macrophages were higher in the S (83%) and HI (43%) groups and the percentage of neutrophils was higher in the I (78%) group (Fig. 10). The HI group showed no significant difference in the relative percentages of neutrophils and macrophages as compared to the I group.
Figure 8.
Average cell count of recovered cells in lung lavage fluid 6 hours after instillation of soluble, insoluble, or heated (H)-insoluble PM fractions (*, P<0.05; #, P<0.01 compared to the soluble PM fraction, ANOVA).
Figure 9.
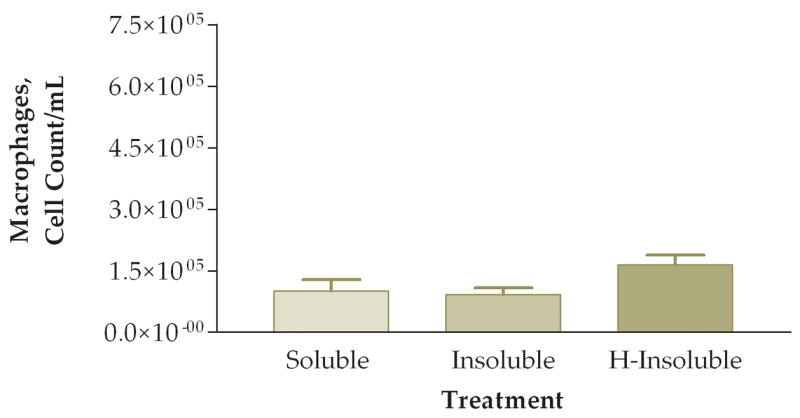
Number of macrophages (A) and neutrophils (B) in lung lavage fluid 6 hours after instillation of soluble, insoluble, or heated (H)-insoluble PM fractions (*, P<0.05, ANOVA).
Figure 10.
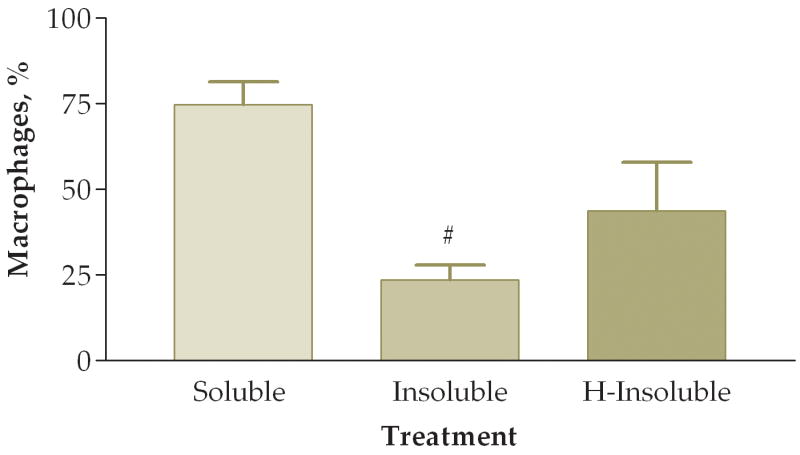
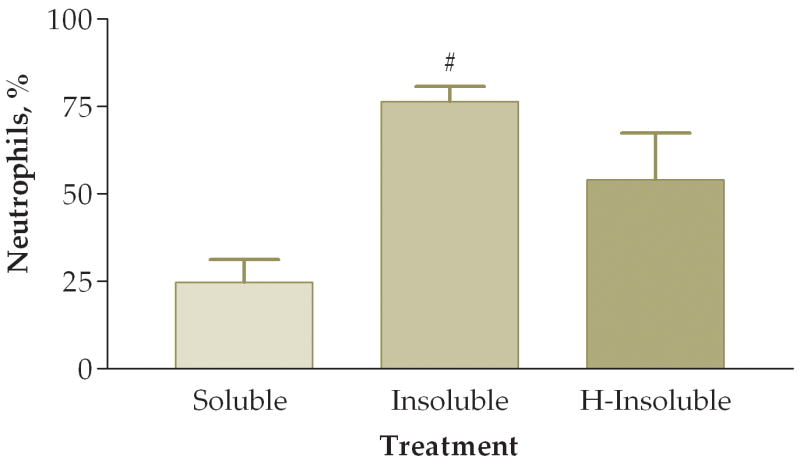
Percentage of macrophages (A) and neutrophils (B) in lung lavage fluid 6 hours after instillation of soluble, insoluble, or heated (H)-insoluble PM fractions (#, P<0.01 compared to the soluble PM fraction, ANOVA). No significant differences were observed between insoluble and H-insoluble cellular responses.
To determine whether the heat treatment actually destroyed any endotoxin present, we added known concentrations of soluble LPS to whole PM samples. Experiments were also performed to determine whether LPS would be found in the soluble fraction, the insoluble fraction, or both when we extracted our coarse PM sample with PBS. In this experiment mice were divided into 5 treatment groups: 1) spiked whole PM, 2) heat-treated spiked whole PM, 3) soluble fraction of spiked whole PM, 4) insoluble fraction of spiked whole PM, 5) heat-treated insoluble fraction of spiked whole PM. Preliminary experiments showed that 1.25 ug of endotoxin/mouse elicited an increase in cellular response of 4.8×105 cells per ml of lavage. Instillation of whole PM spiked with 1.0 ug/mouse showed a total cell count consistent with an additive response of the PM alone plus the endotoxin alone. When we treated the spiked whole PM with heat, we observed a response consistent with that observed with whole PM to which no endotoxin had been added. Thus, we conclude that the heat treatment used successfully inactivated endotoxin in the PM preparation (see Fig. 11). We also observed trends (that would have been significant with a one-tailed t-test) towards decreased total cells and decreased numbers (and percentages) of neutrophils in the soluble fraction (but not the heat-treated insoluble fraction) prepared from spiked whole PM, demonstrating that the endotoxin partitioned into the soluble fraction of the PM when we treated it with PBS.
Figure 11.

Average cell count of recovered cells in lung lavage fluid 6 hours after instillation of LPS-spiked PM or heat-treated LPS-spiked PM (*, P<0.05, one-tailed t-test with Welch’s correction).
Chemokine Content of Lung Lavage Fluid
MIP-2- Dose Response
We next addressed whether the presence of neutrophils in the each of the treated groups was positively correlated with localized concentrations of macrophage inflammatory protein-2 (MIP-2). To measure the concentrations of MIP-2 we used lung lavage fluid supernatant from each of the treated groups: (1) PBS, (2) 25 ug of PM/mouse, (3) 50 ug of PM/mouse. Animals instilled with either PBS or 25 ug of PM exhibited comparable concentrations of MIP-2 measured in the lung lavage fluid supernatant. However, the group instilled with the higher dose (50 ug of PM) showed an apparent (not statistically significant) increase in MIP-2 concentrations as compared to both the PBS- and 25 ug of PM-instilled groups. We found a positive correlation between measured MIP-2 concentrations and increased neutrophil counts for mice instilled with 25 or 50 ug of PM. We found no correlation between levels of MIP-2 and number of macrophages in lung lavage fluid across all groups.
MIP-2 – Time Dependent Response
To investigate whether there is an apparent time-response relationship between MIP-2 and neutrophil response, we measured MIP-2 concentrations from the lung lavage fluid supernatant at each of the post instillation periods. We found a significantly increased, by about 143%, peak MIP-2 concentration at the earliest time point examined (3 hours), with a return to values that were not significantly higher than the air controls at each time point thereafter (6–72 hours, see Figure 12). We correlated neutrophil numbers from lung lavage fluid to MIP-2 concentrations from lung lavage fluid and found a significant positive correlation at 3 hours after exposure. This positive correlation of neutrophils to MIP-2 concentrations apparently steadily decreased (and was not significant) at later time points. We measured MIP-2 concentrations in matched PBS-instilled mice from each time point and in air exposed mice. Air exposed, non-treated, animals had measurable levels of MIP-2, 176 ± 12 pg/ml. MIP-2 levels are transiently elevated in PBS-instilled mice to 248±40 pg/ml at the 3 hour time point, with a return to the air control value (174 ± 15 pg/ml) at the 6 hour time point and subsequently.
Figure 12.
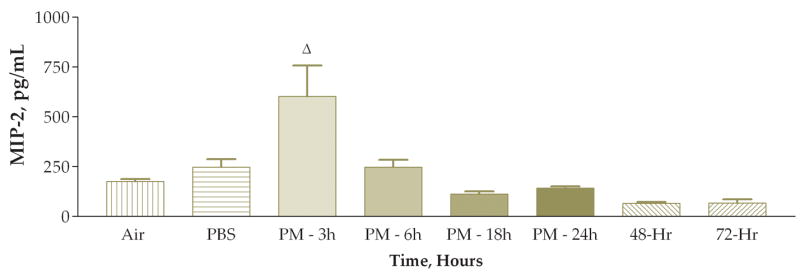
Macrophage Inflammatory Protein-2 (MIP-2) content (pg/ml) of lung lavage fluid supernatant from mice sacrificed at various times after instillation of 50 ug of PM (Δ, P<0.001 compared to the PBS control group, ANOVA).
Discussion and Conclusions
The initial intent of this study was to determine if a single IT dose of coarse PM collected from the California’s Central Valley would elicit pro-inflammatory and cytotoxic effects in a mouse model. California’s Central Valley, which has a hot and dry climate except in the winter months (rainy season), has the highest ambient levels of coarse PM reported in the United States (2002 data, CARB). The experiments reported in this paper were all performed with a single batch of coarse PM collected with a high-volume sampler from an agricultural community in the San Joaquin Valley of California, near Fresno, during the summer months. Detailed chemical analyses of coarse PM collected from this location at different times were previously reported by Chow et al. (1992; 2006). We have, however, replicated these findings with another source of coarse PM, also collected in the summer months, from a different location in the Central Valley of California. Smith and colleagues (2003) previously reported that total PM mass from Fresno, CA (collected as concentrated ambient particles) was comprised mainly of ammonium nitrate and organic carbon. Pinkerton and colleagues have also used detection of birefringent particles in lung sections by polarized light to identify silicates in inhaled PM from this region (2000). Although our sample was taken at a different location and time and collected in a different way than these well characterized PM samples from nearby Fresno, we can speculate that our samples contained ammonium nitrate, organic carbon, and aluminum silicates as major components by mass. We would expect that organic carbon and aluminum silicates would fractionate into the insoluble fraction, while ammonium nitrate would fractionate into the soluble fraction after PBS extraction.
We identified birefringent particles in the coarse PM preparation we studied, which prompted us to use these birefringent particles as a biomarker of PM deposition in cells obtained by lung lavage from PM-treated animals. Fireman and colleagues (1999) used this technique (MacDonald et al., 1995) to identify mineral particles in lung lavage fluid in exposed workers and reported rapid and reliable results compared to scanning electron microscopy in detecting the same particles. We found significantly higher amounts of birefringent particles in the macrophages and neutrophils of mice instilled with 6 hours earlier with 50 ug of PM. We also found greater amounts of polarized particles in the insoluble fraction (particulate phase) as compared to the soluble fraction (aqueous phase). Although we cannot mechanistically link the pro-inflammatory effects with the presence of polarized particles, we can conclude that the IT-instilled PM are phagocytosed by cells recovered in the lung lavage fluid, especially pulmonary macrophages and neutrophils.
Our demonstration of pro-inflammatory effects of coarse PM in mice is consistent with earlier observations by other groups (Monn et al., 1999; Soukup et al., 2001; Schins et al., 2004; Hetland et al., 2005). We used intratracheal instillation as a means of acute administration as opposed to inhalation or other exposure methods due to the small amount of PM available and to develop a bioassay method that would facilitate comparative studies between different batches of PM collected by high-volume sampling. Costa and colleagues (2006) have demonstrated that intratracheal instillation used as a means of PM exposure can provide appropriate and relevant information on the potential toxicity and lung injury caused by inhalable particulates as long as conditions of dust overloading are avoided and concentrations are reasonable with respect to actual ambient concentrations that might be encountered. We can calculate that an intratracheal dose of 50 ug/mouse is approximately equivalent to the amount of coarse PM that would be inhaled during a 1–2 week exposure to PM at the average coarse PM concentration reported for an 8-hour exposure period at a site in the region, 44 mg/m3 (2006 data, CARB). In addition, intratracheal instillation offers many advantages over inhalation including control of the total dose administered and reproducibility.
There was good correlation between average cell counts recovered in the lung lavage fluid and PM dose as evaluated 6 hours after IT instillation. There have been many other studies that have also shown such a dose response upon exposure of animals or cultured cells to coarse PM (Gerlofs-Nijland et al., 2005; Happo et al., 2007). The total number of, and the relative percentage of, neutrophils recovered in the lung lavage fluid (Henderson et al., 1985) were better indicators of pro-inflammatory effects with increasing doses of PM than the measurement of total cells recovered by lung lavage, which showed a great deal of variability from animal to animal. When we examined the time course of this response, we found a significant pro-inflammatory neutrophilic influx, gradually increasing over time, with a peak neutrophilic influx 24 hours after instillation of the PM. There was a significant decrease in neutrophilic inflammation at 48 and 72 hours, with an increased relative percentage of macrophages. Other studies have reported a peak neutrophilic responses at 12 hours (Happo et al., 2007) and, similar to our results, 24 hours (Adamson et al., 2004; Gerlofs-Nijland et al., 2005) after exposure to PM.
The identification of specific constituents that are responsible for pro-inflammatory and cytotoxic effects observed in the lung after coarse PM exposure remains elusive. Two common approaches used to identify potentially “active” constituents in PM use either additive or a subtractive approaches. An additive approach commonly includes screening individual PM components and their simple mixtures, and examines pro-inflammatory and cytotoxic effects of each individual component. The subtractive approach takes the PM sample as a whole and gradually fractionates the mixture into parts (Ghio et al., 1999; Imrich et al., 2000; Soukup et al., 2001; Becker et al., 2003; Adamson et al., 2004). The advantage of using a subtractive approach with fractionation includes identifying potential groups of constituents, based on their physical and chemical properties, which might be responsible for eliciting deleterious effects in the lung. In this study we divided the whole PM fraction into two fractions, soluble and insoluble in an aqueous buffer (Ghio et al., 1999; Imrich et al., 2000; Soukup et al., 2001; Becker et al., 2003; Adamson et al., 2004), to minimize any alterations of the native state of the particulate matter sample. Our results indicate that the constituents from this single sample that drive a pro-inflammatory response fractionate into the insoluble (particulate) fraction. We have also found that the insoluble fraction of particulates collected from another location in the Central Valley of California, also collected in the summer months, is responsible for significant pro-inflammatory effects after IT instillation using the same mouse model (unpublished results). Imrich and colleagues (2000) have previously shown that alveolar macrophages (in vitro) exposed to particle suspensions responded to the insoluble components of the particles. Adamson et al., (2004) looked at TNF-α production and release after instillation of EHC-93 particles and also found that the insoluble fraction was responsible for these effects. However, other studies have reported contradictory data suggesting that the soluble fraction, particularly metals, drive pro-inflammatory effects in the lung (Costa et al., 1997; Kodavanti et al., 1998; Ghio et al., 1999). In an in vitro study, Soukup and Becker (2001) reported strong induction of inflammatory mediators driven by the insoluble fraction of PM10 (collected from Chapel Hill, North Carolina) by alveolar macrophages. Stoeger et al, (2006) reported significant correlations between neutrophil influx, MIP-2, IL-1β, and KC concentrations measured in the lung lavage fluid after IT instillation of 5, 20, or 50ug of carbonaceous ultrafine PM into BALB/c mice. There are obvious difference between mice and cultured cells, and probably important differences between different preparations of PM studied (seasonal, spatial, dose, and size differences) and different types of cells studied in culture that might account for the apparently contradictory findings of the different studies.
Another important variable that might account for these apparently contradictory findings from different laboratories is the occurrence of endotoxin in some coarse PM samples. Often when there is a marked neutrophilic influx after exposure of animals or cells to PM, endotoxin is reported as the major initiator (Becker et al., 1996; Dong et al., 1996; Ning et al., 1999; Schins et al., 2004; Schwarze et al., 2006). Although soluble in its most common purified form, LPS, endotoxin is often associated with the particulate phase of coarse PM, especially when it is present as Gram-negative bacterial cell walls or Gram-negative bacteria themselves (Monn et al., 1999; Soukup et al., 2001; Schins et al., 2004). To determine if the pro-inflammatory responses observed in the mice we studied could be attributed to endotoxin; we inactivated any putative endotoxin present in the coarse PM samples by heat-treating the whole and insoluble PM fractions. There was no significant decrease in pro-inflammatory activity of PM after we heat-treated the whole PM or the insoluble fraction (the fraction containing all of the pro-inflammatory activity in our experiments). To validate that the heat treatment regimen we used was an effective means of inactivating endotoxin as has been reported by others (Moesby et al., 2005; Alexis et al., 2006); we instilled either LPS-spiked whole PM or heat-treated LPS-spiked PM into our mouse model. As expected, we observed a significant decrease in inflammatory activity in the heat-treated PM sample after inactivation of endotoxin. This has also been observed in a human cohort study conducted by Alexis and colleagues (2006) where they report no significant effects on neutrophil influx after inhalation of heated PM material using a similar protocol. These results indicate the whole PM sample we studied contained little or no endotoxin. We would speculate that endotoxin contamination of coarse PM samples collected from ambient air might be a problem with samples collected under conditions of high relative humidity that would favor bacterial contamination and bacterial growth, but may not be a significant factor with PM samples collected in ambient air from the arid climates of the Western United States.
The most striking response of the mouse lung to instillation of coarse PM in the present study was a rapid and extensive inflammatory neutrophil influx. There are several known macrophage and epithelial cell-derived chemoattractants that might be directly or indirectly responsible for recruitment of neutrophils to the lung, for example MIP-2 (Wolpe et al., 1989; Driscoll, 1994; Ramos et al., 2006), Keratinocyte-derived cytokine (KC) and Monocyte chemoattractant protein-1 (MCP-1) (Johnston et. al., 1999Beck-Schimmer et al., 2005;). We initially focused on measurement of the well-known neutrophil chemoattractant, MIP-2 because of its relative ease and reproducibility of measurement in the lung lavage fluid, and because previous studies have suggested that it plays a major role in PM-induced neutrophilia (Hetland et al., 2002; Sandstrom et al., 2005; Stoeger et al., 2006). We found a strong correlation between MIP-2 concentrations in lung lavage fluid and both percentage and number of recovered neutrophils from lavage fluid at the 3-hour time point after instillation, with a gradually decreasing correlation at the later time points studied. MIP-2 levels were below the limit of detection in the plasma of PM-treated animals, as expected since MIP-2 is released from macrophages and epithelial cells in the localized areas of inflammatory response. Gupta and colleagues (1996) report from in vivo rat experiments that MIP-2 released in specific alveoli recruit neutrophils selectively to the alveoli affected. Their conclusion was drawn from observations of selective neutrophil influx 2 hours after recombinant MIP-2 instillation. We have not yet examined earlier time points than 3 hours after instillation of PM, but we would predict that MIP-2 concentrations in the lavage fluid would be at their highest levels between 1 and 3 hours after PM exposure, and that there would be other neutrophil chemoattractants present in the lavage fluid at later time points. We also found higher concentrations of MIP-2 in lavage fluid of mice instilled with the insoluble PM fraction than in mice instilled with the soluble fraction, with no apparent decrease in MIP-2 concentrations elicited after heat treatment of the insoluble fraction. Elevated MIP-2 levels have been reported after LPS exposure (Xing et al., 1994), however, absence of decreased MIP-2 concentration following H-I instillation suggests other PM constituents are responsible for MIP-2 expression.
In summary, this study suggests that pro-inflammatory lung response to instillation of coarse PM from the Central Valley of California is largely driven by the insoluble components of the PM sample, not driven by endotoxin, and is readily quantified by a simple mouse bioassay. These bioassay methods should have broad applications for studying the toxicity of specific components of the PM mixture, and for defining the detailed mechanisms responsible for lung inflammation in mice exposed to coarse PM.
Acknowledgments
We would like to thank Drs. Norman Kado, Steven Cliff and Kent Pinkerton for providing the particulate matter samples used in this experiment, and for many helpful discussions. We also thank Lisa M. Franzi for her skilled technical assistance and generous support. This work was funded in part through a grant from the NIEHS (ES-05707). Teresa C. Wegesser was supported by an NIH training grant (ES-07059-30) during this study.
Footnotes
CONFLICT OF INTEREST STATEMENT: Neither of the authors has any conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamson IYR, Prieditis H, Renaud V. Soluble and Insoluble Air Particle Fractions Induce Differential Production of Tumor Necrosis Factor-α in Rat Lung. Exp Lung Res. 2004;30(5):355–368. doi: 10.1080/01902140490438933. [DOI] [PubMed] [Google Scholar]
- Alexis NE, Lay JC, Zeman K, Bennett WE, Peden DB, Soukup JM, Devlin RB, Becker S. Biological material on inhaled coarse fraction particulate matter activates airway phagocytes in vivo in healthy volunteers. J Clin Immunol. 2006;117(6):1396–1403. doi: 10.1016/j.jaci.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Becker S, Soukup JM, Gilmour IM, Devlin RB. Stimulation of human and rat alveolar macrophages by urban air particulates: Effects of oxidant radical generation and cytokine production. Toxicol Appl Pharmacol. 1996;141:637–648. doi: 10.1006/taap.1996.0330. [DOI] [PubMed] [Google Scholar]
- California Air Resources Board. California Air Resources Board; Sacramento, CA: 2002. Central California Air Quality Studies Homepage. http://www.arb.ca.gov/airways/ccaqs.htm. [Google Scholar]
- Chow JC, Watson JG, Lowenthal DH, Chen Antony LW, Magliano KL. Particulate carbon measurements in California’s San Joaquin Valley. Chemosphere. 2006;62:337–348. doi: 10.1016/j.chemosphere.2005.04.094. [DOI] [PubMed] [Google Scholar]
- Chow JC, Watson JG, Lowenthal DH, Solomon PA, Magliano KL, Ziman SD, Richards LW. PM10 source apportionment in California’s San Joaquin Valley. Atmos Environ. 1992;26A(18):3335–3354. [Google Scholar]
- Costa DL, Dreher KL. Bioavailable transition metals in particulate matter mediate cardiopulmonary injury in healthy and compromised animal models. Environ Health Perspect. 1997;105(suppl 5):1053–1060. doi: 10.1289/ehp.97105s51053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa DL, Lehmann JR, Winsett D, McGee J, Ghio A. Pulmonary toxicity of Utah Valley PM: Are empirical indices of adverse health effects coherent with the epidemiology. Am J Respir Cri Care Med. 1998;157(3):A880. [Google Scholar]
- Costa DL, Lehmann JR, Winsett D, Richards J, Ledbetter AD, Dreher KL. Comparative pulmonary toxicological assessment of oil combustion particles following inhalation or instillation exposure. Toxicol Sci. 2006;91(1):237–46. doi: 10.1093/toxsci/kfj123. [DOI] [PubMed] [Google Scholar]
- Dockery DW, Pope CA, III, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG, Speizer FE. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;29:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- Donaldson KT, Mill S, MacNee W, Robinson S, Newby D. Role of inflammation in cardiopulmonary health effects of PM. Toxicol Appl Pharmacol. 2005;207(2 suppl 1):483–488. doi: 10.1016/j.taap.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Dong W, Lewtas J, Luster MI. Role of endotoxin in tumor necrosis factor α expression from alveolar macrophages treated with urban air particles. Exp Lung Res. 1996;22:577–592. doi: 10.3109/01902149609046043. [DOI] [PubMed] [Google Scholar]
- Dreher KL. Particulate Matter Physiochemistry and Toxicology: In Search of Causality – A Critical Perspective. Inhal Toxicol. 2000;12(Supplement):45–57. doi: 10.1080/08958378.2000.11463230. [DOI] [PubMed] [Google Scholar]
- Driscoll KE. Macrophage Inflammatory Proteins - Biology and Role in Pulmonary Inflammation. Exp Lung Res. 1994;20(6):473–490. doi: 10.3109/01902149409031733. [DOI] [PubMed] [Google Scholar]
- Driscoll KE, Costa DL, Hatch G, Henderson R, Oberdoster G, Salem H, Schlesinger RB. Forum: Intratracheal Instillation as an Exposure Technique for the Evaluation of Respiratory Tract Toxicity: Uses and Limitations. Toxicol Sci. 2000;55:24–35. doi: 10.1093/toxsci/55.1.24. [DOI] [PubMed] [Google Scholar]
- Fireman E, Greif J, Schwarz Y, Man A, Ganor E, Ribak Y, Lerman Y. Assessment of Hazardous Dust Exposure by BAL and Induced Sputum. Chest. 1999;115:1720–1728. doi: 10.1378/chest.115.6.1720. [DOI] [PubMed] [Google Scholar]
- Ghio AJ, Stonehuerner J, Dailey LA, Carter JD. Metals associated with both the water soluble and insoluble fractions of an ambient air pollution particle catalyze an oxidative stress. Inhal Toxicol. 1999;11:37–49. doi: 10.1080/089583799197258. [DOI] [PubMed] [Google Scholar]
- Gilmour PS, Schladweiler MC, Richards JH, Ledbetter AD, Kodavanti UP. Hypertensive Rats are Susceptible to TLR4-Mediated Signaling Following Exposure to Combustion Source Particulate Matter. Inhal Toxicol. 2004;6(suppl 1):5–18. doi: 10.1080/08958370490442827. [DOI] [PubMed] [Google Scholar]
- Greenberg DB, Lyons SA, Last JA. Paraquat-Induced Changes in the Rate of Collagen Biosynthesis by Rat Lungs Explants. J Lab Cli Med. 1978;92(6):1033–1042. [PubMed] [Google Scholar]
- Henderson RF, Benson JM, Hahn FF, Hobbs CH, Jones RK, Mauderly JL, McClellan RO, Pickrell JA. New Approaches for the Evaluation of Pulmonary Toxicity: Bronchoalveolar Lavage Fluid Analysis. Fundam App Toxicol. 1985;5:451–458. doi: 10.1016/0272-0590(85)90092-2. [DOI] [PubMed] [Google Scholar]
- Hetland RB, Cassee FR, Lag M, Refsnes M, Dybing E, Schwarze PE. Cytokine release from alveolar macrophages exposed to ambient particulate matter: heterogeneity in relation to size, city and season. Part Fibre Toxicol. 2005;2:4. doi: 10.1186/1743-8977-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imrich A, Ning Y, Kobzik L. Insoluble components of concentrated air particles mediate alveolar macrophage responses in vitro. Toxicol Appl Pharmacol. 2000;167:140–150. doi: 10.1006/taap.2000.9002. [DOI] [PubMed] [Google Scholar]
- Kenyon NJ, Last MS, Eiserich JP, Morrissey BM, Temple LM, Last JA. Differentiation of the roles of NO from airway epithelium and inflammatory cells in ozone-induced lung inflammation. Toxicol Appl Pharmacol. 2006;215:250–259. doi: 10.1016/j.taap.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodavanti UP, Schladweiler MC, Ledbetter AD, McGee JK, Walsh L, Gilmour PS, Highfill JW, Davies D, Pinkerton KE, Richards JH, Crissman K, Andrews D, Costa DL. Consistent Pulmonary and Systemic Responses from Inhalation of Fine Concentrated Ambient Particles: Roles of Rat Strains Used and Physicochemical Properties. Environ Health Perspect. 2005;113(11):1561–1568. doi: 10.1289/ehp.7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Last JA, Kishorchandra G, Mathrani VC, Kenyon NJ. Systemic responses to inhaled ozone in mice: Cachexia and down-regulation of liver xenobiotic metabolizing genes. Toxicol Appl Pharmacol. 2005;208:117–126. doi: 10.1016/j.taap.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Last JA, Ward R, Temple LM, Kenyon NJ. Ovalbumin-Induced Airway Inflammation and Fibrosis in Mice also Exposed to Ozone. Inhal Toxicol. 2004;16:33–43. doi: 10.1080/08958370490258237. [DOI] [PubMed] [Google Scholar]
- Last JA, Ward R, Temple LM, Pinkerton KE, Kenyon NJ. Ovalbumin-Induced Airway Inflammation and Fibrosis in Mice Also Exposed to Ultrafine Particles. Inhal Toxicol. 2004;16:93–102. doi: 10.1080/08958370490265077. [DOI] [PubMed] [Google Scholar]
- MacDonald WJ, Roggli VL. Detection of silica particles in lung tissue by polarizing light microscopy. Arch Pathol Lab Med. 1995;119:242–246. [PubMed] [Google Scholar]
- Mishell BB, Shiigi SM. Selected Methods in Cellular Immunology. Freeman and Co; San Francisco: 1980. pp. 16–19. [Google Scholar]
- Moesby L, Hansen EW, Christense JD, Hoyer CH, Juhl GL, Olsen HB. Dry and moist sterilization cannot inactivate pyrogenicity of Gram positive microorganisms. Eur J Pharm Sci. 2005;26:318–323. doi: 10.1016/j.ejps.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Monn C, Becker S. Cytotoxicty and induction of proinflammatory cytokines from human monocytes exposed to fine (PM2.5) and coarse particles (PM10-2.5) in outdoor and indoor air. Toxicol Appl Pharmacol. 1999;155:245–252. doi: 10.1006/taap.1998.8591. [DOI] [PubMed] [Google Scholar]
- Oberdorster G, Ferin J, Lehnert BE. Correlation between particle size, in vivo particle persistence, and lung injury. Environ Health Perspect. 1994;102(suppl 5):173–179. doi: 10.1289/ehp.102-1567252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oslund KL, Miller LA, Usachenko JL, Tyler NK, Wu R, Hyde DM. Oxidant-Injured Airway Epithelial Cells Upregulate Thioredoxin but Do Not Produce Interleukin-8. Am J Respir Cell Mol Biol. 2004;30:597–604. doi: 10.1165/rcmb.2002-0273OC. [DOI] [PubMed] [Google Scholar]
- Osornio-Vargas AR, Bonner JC, Alfaro-Moreno E, Martinez L, Garcia-Cuellar G, Ponce-de-Leon RS, Miranda J, Rosas I. Proinflammatory and cytotoxic effects of Mexico City air pollution particulate matter in vitro are dependent on particle size and composition. Environ Health Perspect. 2003;65:1261–1272. doi: 10.1289/ehp.5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA., III Respiratory disease associated with community air pollution and a steel mill, Utah Valley. Am J Public Health. 1989;79:623–628. doi: 10.2105/ajph.79.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA, III, Thun MJ, Namboodiri MM, Dochery DW, Evans JS, Speizer FE, Clark CW., Jr Particulate air pollution as a predictor of mortality in a prospective study of U. S. adults. Am J Respir Cri Care Med. 1995;151:669–674. doi: 10.1164/ajrccm/151.3_Pt_1.669. [DOI] [PubMed] [Google Scholar]
- Ramos CDL, Fernandes KSS, Canetti C, Teixeria MM, Silva JS, Cunha FQ. Neutrophil recruitment in immunized mice depends on MIP-2 inducing the sequential release of MIP-1α, TNF-α and LTB4. Eur J Immunol. 2006;36:2025–2034. doi: 10.1002/eji.200636057. [DOI] [PubMed] [Google Scholar]
- Schwartz J. Air pollution and daily mortality. A review and meta analysis. Environ Res. 1994;64:36–52. doi: 10.1006/enrs.1994.1005. [DOI] [PubMed] [Google Scholar]
- Schwarze PE, Ovrevik J, Lag M, Refsnes M, Nafstad P, Hetland RB, Dybing E. Particulate matter properties and health effects: consistency of epidemiological and toxicological studies. Hum Exp Toxicol. 2006;25:559–579. doi: 10.1177/096032706072520. [DOI] [PubMed] [Google Scholar]
- Schins RPF, Lightbody JH, Borm PJA, Shi T, Donaldson K, Stone V. Inflammatory effects of coarse and fine particulate matter in relation to chemical and biological constituents. Toxicol Appl Pharmacol. 2004;195:1–11. doi: 10.1016/j.taap.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Smith KR, Kim S, Recendez JJ, Teague SV, Menache MG, Grubbs DE, Sioutas C, Pinkerton KE. Airborne Particles of the California Central Valley Alter the Lungs of Health Adult Rats. Environ Health Perspect. 2003;111(7):902–908. doi: 10.1289/ehp.5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soukup JM, Becker S. Human alveolar macrophage responses to air pollution particulates are associated with insoluble components of coarse material, including particulate endotoxin. Toxicol Appl Pharmacol. 2001;171:20–26. doi: 10.1006/taap.2000.9096. [DOI] [PubMed] [Google Scholar]
- Veranth JM, Reilly GA, Vernath MM, Moss TA, Langelier CR, Lanza DL, Yost GS. Inflammatory cytokines and cell death in BEAS-2B lung cells treated with soil dust, lipopolysaccharide, and surface-modified particles. Toxicol Appl Pharmacol. 2004;82:88–96. doi: 10.1093/toxsci/kfh248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpe SD, Sherrry B, Juers D, Davatelis G, Yurt RW, Cerami A. Identification and characterization of macrophage inflammatory protein 2. Immunology. 1989;86:612–616. doi: 10.1073/pnas.86.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Z, Jordana M, Kirpalani H, Driscoll KE, Schall TJ, Gauldie J. Cytokine expression by neutrophils and macrophages in vivo: endotoxin induces TNF-alpha, MIP-2, IL-1beta, and IL-6 but not RANTES or TGF beta1 mRNA expression in acute lung inflammation. Am J Respir Cell Mol Biol. 1994;10:148–153. doi: 10.1165/ajrcmb.10.2.8110470. [DOI] [PubMed] [Google Scholar]