SUMMARY
Information on the status of long chain polyunsaturated fatty acids (LC-PUFA) in pregnancy and breast milk in very high fish eating populations is limited. The aim of this study was to examine dietary intake and changes in fatty acid status in a population of pregnant women in the Republic of Seychelles. Serum docosahexaenoic acid (DHA) decreased significantly between 28 weeks gestation and delivery (n=196). DHA status did not correlate significantly with length of gestation and was not associated with self reported fish intake which was high at 527 g/wk. In breast milk, the ratio of DHA to arachidonic acid (AA) was consistent with those observed in other high fish eating populations. Overall the data suggest that high exposure to LCPUFAs from habitual fish consumption does not prevent the documented decrease in LCPUFA status in pregnancy that occurs as a result of fetal accretion in the third trimester of pregnancy.
INTRODUCTION
The long chain polyunsaturated fatty acids (LCPUFAs) docosahexaenoic acid (DHA; 22: 6n−3) and arachidonic acid (AA; 20: 4n−6) are important structural fatty acids in neural tissue, especially brain and retina [1–2] In pregnancy there is an increased demand for DHA and AA as a result of increased accretion of placental and fetal tissue. [3] This is of particular significance in the third trimester when fetal brain growth is at its greatest. [4–5] The increased fetal demands for LCPUFAs are indicated by a concomitant decrease in concentrations of DHA and AA in the maternal circulation as pregnancy progresses. [6–8]
Definitive fetal requirements for LCPUFAs for optimal development are not known. It has been estimated that in the last trimester, when fetal uptake of DHA is at its peak, the average fetal accretion is 50 – 60 mg of n−3 LCPUFA a day, most of this is DHA. [5] However, this is likely an underestimation as a total figure of 6–7 g has been estimated as the net fetal accretion of DHA in the last trimester. [9] Although total maternal requirements for LCPUFA during pregnancy are not clear, it has been estimated that pregnant women may need to consume as much as 300mg/d to provide for the additional requirement owing to increased fetal accretion. [10]
Intervention studies have shown that fish oil supplementation or increased intakes of oily fish increase DHA status of both the mother and fetus [10–13]. This increase in LCPUFA status is reported to produce improvements in physiological outcomes, such as reducing the risk of preeclampsia, [14–15], and enhancing cognitive development and visual acuity in infants [16] and reducing the incidence of cardiovascular disease in adults. [17–18] Other reported benefits of higher LCPUFA intake during pregnancy include a positive association with gestation length [19] and neonatal birth weight. [20–21]
Recommendations for LCPUFA intake during pregnancy are available [22–23], and usually given as either a % of total fat intake or a recommendation for number of fish portions to be consumed per week. However, recommendations for total fat intake and subsequent LCPUFA intake are not standardised. Combined with variability in what constitutes an average portion of fish, the differing amounts of LCPUFAs in different fish species and a lack of knowledge concerning precise amounts of LCPUFA required for maternal health and optimal fetal development, means that guidelines are at best vague, and at the worst confusing.
The aim of the current study was to assess the alterations of serum fatty acids during pregnancy, and to examine fatty acid content of breast milk in women living in the Republic of Seychelles, an archipelago in the Indian Ocean, as part of the Seychelles Child Development Nutrition Study (SCDNS). Although the Seychellois have been reported to habitually consume a diet high in fish, dietary intake and nutritional status in this population have not been assessed. Associations between fatty acid status, gestation length and birth weight were also examined.
MATERIALS AND METHODS
Subjects
A total of 300 pregnant women were recruited between August and December, 2001 from nine antenatal clinics on Mahé, the main island of the Republic of Seychelles. Attendance at antenatal clinics in Seychelles is typical for all pregnant women and at their first antenatal visit, women were invited to participate in the study and those who consented were enrolled. With an average annual birth rate of 1464 between the years of 2001 to 2006, the sample size recruited represented approximately one fifth of all pregnancies that year in Seychelles. Participants completed a number of questionnaires requesting information on socioeconomic, demographic and health and lifestyle factors. Inclusion criteria were: aged over 16y, resident on Mahé, and native born Seychellois. Subjects were excluded if they were vegetarian, or if they had a serious medical illness such as insulin dependent diabetes, toxaemia with seizures, or a haematological disorder such as thalassemia or sickle cell anaemia. The study was reviewed and approved by the Research Subjects Review Board in Seychelles and the appropriate Research Subjects Review Boards of the other collaborating partners.
Blood and breast milk sample collection
Non-fasting blood samples (30 ml) were taken from mothers at 28 weeks gestation, and again one day after delivery. Samples were collected by anticubital venepuncture into evacuated serum tubes, placed on ice and allowed to sit for 30 minutes prior to being centrifuged at 2500 rpm for 15 minutes. Samples were then aliquoted and stored at −80°C until analysis. Breast milk samples were collected at one month postpartum into 50 ml containers and frozen at −80°C until analysis. Samples were accompanied and transported to the University of Ulster on dry ice and analysed at the University of Ulster.
Anthropometry
All measuring equipment in each of the participating antenatal clinics and maternity wards were calibrated prior to initiation of the study, and regularly throughout the project, by the Seychelles Bureau of Standards. Maternal height and weight were measured by trained nurses at enrolment into the study and used to calculate maternal BMI.
Fatty acid analyses
Total lipids were extracted from serum and breast milk samples using a modified method of Folch et al. [24] Two internal standards (Sigma Aldrich Co Ltd, Poole, Dorset, UK), heptadecaenoic acid (C17:0) and heneicosanaenoic (C21:0) were added to the samples prior to extraction. Lipid extracts were esterified with boron trifluoride in methanol (Sigma Aldrich Co Ltd). Samples were flushed with nitrogen at all stages of extraction and esterification. Fatty acid methyl esters were analysed and quantified using a ThermoFinnegan TRACE MS with Xcalibur software (Thermofinneigan, Hemel Hempstead, UK) with 30m FAMEWAX (Restek Ireland, Belfast, UK) capillary column with an internal diameter of 0.25mm and a 0.25-μm film thickness. Run conditions involved a starting oven temperature of 120°C which was ramped at 7°C a minute up to a final temperature of 220°C where it was held for 20 minutes. Samples were run using split injection techniques with an injector temperature of 250°C, a source temperature of 250°C and a detector temperature of 220°C. Optimisation of the method included determination of the linearity of the detector response to the various fatty acids. Quantitative precision and identification of the individual fatty acids was confirmed using commercially available standards (Sigma Aldrich Co Ltd).
Fish analyses
Samples of fifteen of the most commonly consumed fish in Seychelles were collected by the Seychelles Bureau of Standards. Samples were stored at −80°C and transported in dry ice to the University of Ulster. The main macronutrient content of fifteen species of fish and n−3 and n−6 composition of six of the fifteen species of fish were analysed by CCFRA Technology Ltd, Chipping Campden, UK. The data from these analyses were incorporated into the dietary database WISP version 2.0 (Tinuviel Software, Warrington, UK).
Dietary assessment
At 28 weeks gestation, subjects completed a Food Use Questionnaire (FUQ) devised specifically for this study to ascertain retrospectively intakes of fish and fish products in the two weeks prior to their visit to the antenatal clinic. Detailed dietary information was also collected from each subject by means of a 4d semi-quantitative diet diary (two consecutive week days and two weekend days). Following conversion to gramme weights, food intake data were analysed using the nutrient database package WISP version 2.0 (Tinuviel Software, Warrington, UK), which was supplemented with food composition and recipe data for foods consumed in Seychelles.[25–26] In addition, the basic nutrient composition of the fifteen most commonly consumed fish were analysed and entered into the database.
Statistics
All data were analysed using the SPSS 12.0 for Windows statistical package (SPSS Inc., Chicago, IL). Data for all variables were tested for normality and adjusted where necessary. All fatty acid data were log transformed prior to statistical analysis. Change in fatty acid status from 28 weeks gestation to delivery was analysed using mixed model analysis adjusting for maternal age, maternal BMI, length of gestation and parity. Associations among DHA and AA status and neonatal anthropometric outcomes were analysed using linear regression, adjusting for maternal age, maternal BMI, parity, sex of infant and length of gestation. Bivariate correlations were used to examine for relationships among DHA concentrations in maternal serum at 28 weeks gestation, DHA concentrations in breast milk at one month postpartum and dietary fish intake. A significance level of p < 0.05 was used to evaluate all statistical outcomes.
RESULTS
Of the original 300 subjects recruited, 24 did not complete the study because of miscarriage, neonatal death or delivery overseas. Among the remaining 276 subjects complete data sets were available for 196 subjects to determine alterations of fatty acid status in pregnancy (28 weeks gestation and delivery). There was no significant difference with respect to maternal height, weight, BMI and dietary intake between the 276 mothers who delivered babies and those included in the final analysis (n = 196). This was also true for anthropometric outcomes of the neonates. For breast milk analyses 166 mothers were included. Dietary data was available for 195 of the 196 subjects.
Anthropometry
Mean (SEM) age, height and BMI of subjects are presented in Table 1 along with information on neonatal anthropometry. Maternal BMI was positively associated with parity (r = 0.179; p = 0.012). Mean (SD) neonatal weight (3241.5g ± 504.1) was significantly associated with length of gestation (r = 0.320; p = 0.000) and age of the mother at enrolment (r = 0.170; p = 0.017). Neonatal length (50.3 cm ± 3.2) was positively associated with length of gestation (r = 0.292; p = 0.000).
Table 1.
Maternal (enrolment) and infant characteristics
| Subject Characteristics(n = 196) | Mean | SD |
|---|---|---|
| Maternal characteristics at enrolment | ||
| Age (y) | 26.98§ | 6.38 |
| Weight (kg) | 67.00 | 17.20 |
| Height (m) | 1.60 | 0.07 |
| BMI (kg/m2) | 26.07* | 6.64 |
| Weeks gestation (wk) | 13.20 | 4.08 |
| Parity (n) | 2.33* | 1.54 |
| Infant characteristics at delivery | ||
| Gestational age (wk) | 38.74†‡ | 1.36 |
| Weight (g) | 3241.50†§ | 504.13 |
| Length (cm) | 50.81‡ | 3.19 |
| Head circumference (cm) | 33.48 | 1.43 |
Mean values with like symbols are significantly and positively associated
Dietary data
Mean (SD) daily energy intake (n = 195) at 28 weeks gestation was 8865.1 (179.5) kj or 2109 kcal (586) Results from the FUQ indicated a high fish intake with volunteers consuming on average nine fish containing meals a week. The most commonly consumed fish was karang (Carangoides fulvoguttatus) in both the diet diaries (32% of all fish consumed) and FUQ. Mean (SD) weekly intake of fish was 526 (327) g/wk. Average fish composition (mean ± SD) in terms of total fat was 2.58 (1.36) g/100g fish. Contribution (%) of AA, EPA and DHA to total fat were 3.32 (1.56), 3.22 (1.44) and 16.21 (5.43) g/100g fat respectively based on chemical analysis of six commonly consumed fish in Seychelles.
Fatty acid results
Fatty acid status was assessed as total serum fatty acids because in the maternofetal unit, LCPUFAs which are transferred across the placenta are reported to originate from maternal triglycerides and free fatty acids [27–28], not from phospholipids. Participants with complete serum fatty acid compositional data at 28 weeks gestation and delivery were included in the analyses (n=196). A subset (n = 166) of the 196 subjects donated breast milk (at one month post partum) for fatty acid analyses. Fatty acid composition of serum and breast milk are presented as a % of weight by total (Table 2). There was a significant decrease (11.9 %) in DHA concentration between 28 weeks gestation and delivery (p = 0.003). AA status (%) also decreased between 28 weeks and delivery, whereas status of the saturated fatty acids capric acid (10 : 0), and lauric acid (14 : 0), increased. After adjusting for parity, however, these differences were not significant.
Table 2.
Fatty acid (%) in serum and breast milk from Seychellois women
| Fatty acids(% by weight of total) | 28 weeks serum Mean (SD) | Delivery serum Mean (SD) | One month breast milk Mean (SD) |
|---|---|---|---|
| 10:0 (capric) | 0.23 (0.05) | 0.29 (0.13) | 1.71 (1.00) |
| 12:0 (lauric) | 0.21 (0.16) | 0.23 (0.24) | 5.68 (1.34) |
| 14:0 (myristic) | 2.13 (0.75) | 2.11 (1.61) | 8.68 (2.28) |
| 16:0 (palmitic) | 34.80 (4.18) | 35.31 (4.09) | 21.57 (2.67) |
| 16:1 (palmitoleic) | 2.68 (1.23) | 2.83 (0.75) | 3.38 (1.15) |
| 18:0 (stearic) | 8.19 (2.03) | 8.21 (1.80) | 10.14 (1.26) |
| 18:1 (oleic) | 19.08 (2.86) | 20.66 (3.03) | 32.30 (3.17) |
| 18:2n−6 (linoleic) | 29.24 (3.82) | 27.12 (3.62) | 15.77 (3.19) |
| 18:3n−3 (αlinolenic) | 0.04 (0.01) | 0.04 (0.02) | 0.10 (0.06) |
| 20:4n−6 (arachidonic) | 2.58 (0.52) | 2.47 (0.56) | 0.29 (0.13) |
| 20:5n−3 (eicosapentaenoic) | 0.08 (0.06) | 0.07 (0.06) | 0.02 (0.02) |
| 22:6n−3 (docosahexaenoic) | 0.76 (0.23) | 0.65 (0.20)* | 0.31 (0.20) |
significantly different from 28 weeks gestation (p < 0.05) after adjusting for maternal age, parity, and BMI.
In breast milk, mean DHA (% of weight by total) were 0.31 % and a 1:1 ratio between DHA:AA concentrations was observed. The concentrations of shorter chain fatty acids such as lauric and myristic were higher in milk samples than serum, while the concentration of the LCPUFAs, AA and DHA were higher in serum.
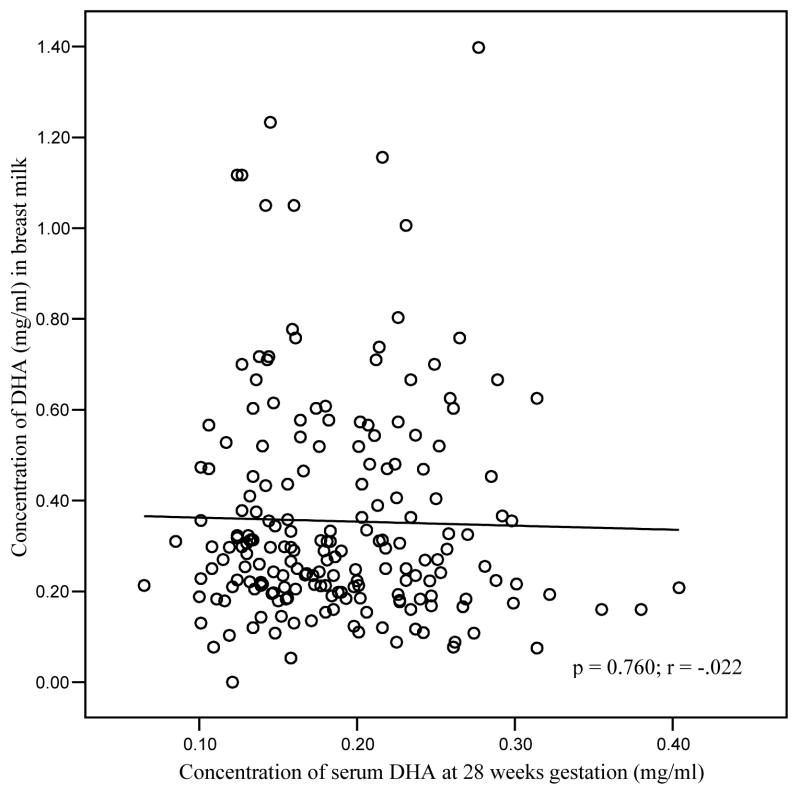
A significant positive correlation was observed between DHA status at delivery and parity, after adjusting for maternal age, length of gestation and maternal BMI. Higher DHA concentrations were observed in mothers with more than one child (p = 0.04; data not shown). No relationship was observed, however, between DHA status at delivery and gestational length (p = 0.833), nor with neonatal birth weight and length (p = 0.878, p = 0.296 respectively) after controlling for maternal age, maternal BMI and length of gestation. Fish intake (g/wk) was not associated with maternal serum DHA status (mg/ml) at 28 weeks or with breast milk DHA concentration (mg/ml) at one month postpartum (Figs. 1 and 2). No association was observed between DHA status in serum samples at 28 weeks gestation and breast milk at one month postpartum (Fig. 3).
Fig 1.
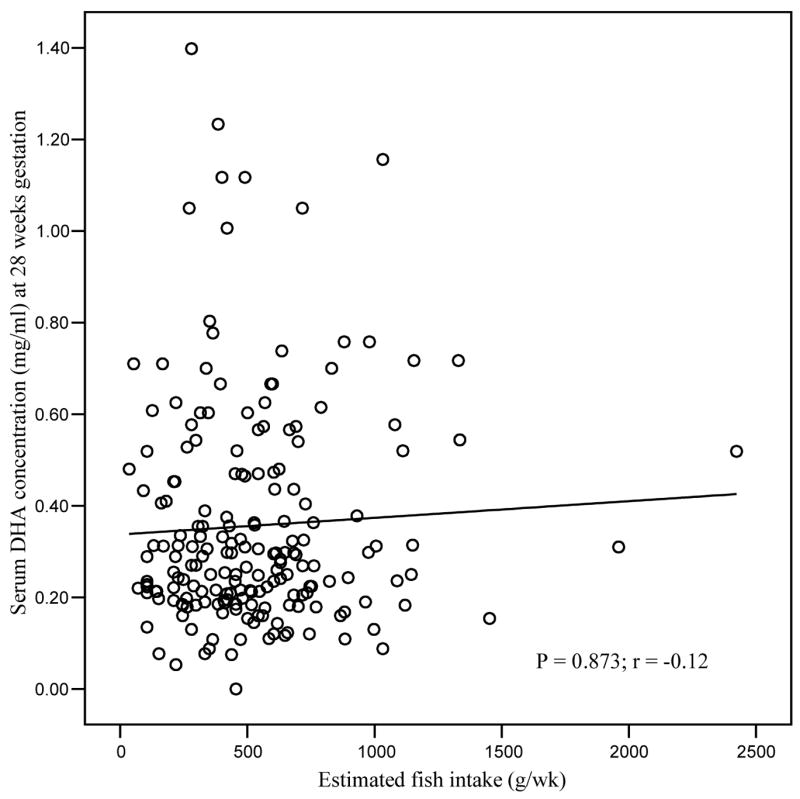
Correlation of estimated fish intake (g/wk) with serum DHA status (mg/ml) at 28 weeks gestation
Fig 2.
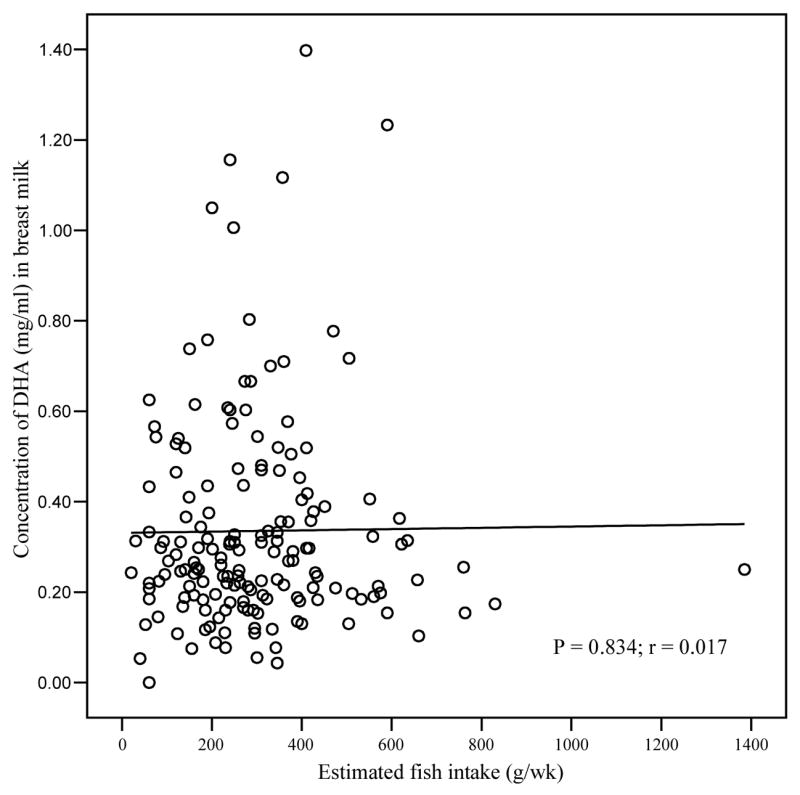
Correlation of estimated fish intake (g/wk) with DHA status (mg/ml) in breast milk at one month post partum
Fig 3.
Correlation of DHA concentration in serum at 28 weeks gestation with DHA concentration in breast milk at one month postpartum (n=166)
DISCUSSION AND CONCLUSIONS
This paper reports dietary intakes during pregnancy and maternal serum fatty acid concentrations and breast milk in a sample of pregnant women, in the Republic of Seychelles where habitual fish consumption is high.
We found that the fatty acid composition of serum from pregnant women in the Seychelles who consume fish daily to be similar to that observed for other populations. [29] The concentration of both maternal DHA and AA in serum fell between 28 weeks gestation and delivery; a decrease that remained significant for DHA after adjusting for confounding factors. This fall has been observed in other studies [7–8] and is believed indicative of increased accretion of LCPUFAs for the development of neural tissue by the fetus. DHA status in maternal serum phospholipids has been observed to be lower in multiparous than in primiparous women. [30] This inverse association has been attributed to the delay (up to six months post partum) for LCPUFA status to return to pre-pregnancy levels. [31] In contrast, we observed a positive association between parity and serum DHA. This finding is partly supported by Dunstan et al, [12] who observed no association between parity and LCPUFA status of erythrocyte phospholipids in women supplemented with fish oil and concluded that, if adequate LCPUFAs are available from the diet, DHA depletion associated with pregnancy can be avoided. Postnatal rapid repletion of maternal DHA status may have benefits for children born from subsequent pregnancies.
We did not find any association between maternal DHA status and the length of gestation, a finding which has previously been reported in both observational and intervention studies [32–33]. Furthermore, we found no evidence of the previously reported association between maternal DHA status and neonatal birth weight. [19] However, our findings are in agreement with other observational [34] and n-3 PUFA intervention studies [14, 35–36] and supported the conclusions of Desci & Koletzko [37] who after reviewing the literature concluded that no unequivocal effect of LCPUFA status was evident. We speculate that associations among DHA status and neonatal outcomes may only be evident in populations where DHA status is limiting and may not be apparent in populations such as the current one where fish (and thereby DHA) intake are high.
Fish consumption in the Seychelles is high compared to other populations. In the UK, average fish consumption is 140 g/week.[22] In the Seychelles the mean weekly intake of fish was 527 g. Recommendations for fish intake in pregnancy are generally based on obtaining adequate intakes of n−3 PUFAs but concerns of adverse effects of prenatal methylmercury exposure have lead to stringent guidelines being set. The UK Scientific Advisory Committee on Nutrition [22] base their recommendations on consuming not more than 2 ×140 g portions of oily fish per week (rich in n-3 PUFAs) whereas the US FDA and US EPA jointly recommend not more than 2 × 170 g portions of fish (low in mercury) a week for pregnant women.[23] A recent publication by Hibbeln at al [38] found no evidence to support the US advisory on fish intake in pregnancy. In a large cohort of pregnant women (n = 11875) low fish intake (< 340 g/week) did not protect against adverse outcomes. Conversely, fish consumption exceeding 340g/wk was associated with beneficial effects on child development. The population in Seychelles currently exceeds both thee UK and US recommendations, albeit fish consumption is declining. Changes in the traditional diet have been observed in Seychelles with a two fold increase in the per capita consumption of eggs and chicken and a three fold increase in processed meats between 1983 and 1992 leading to a 36 % decrease in fish intake per capita.[39] In keeping with this trend we did observe a significant correlation between age of mother and dietary fish intake (data not shown) with younger mothers consuming less fish.
Dietary fish intake, as estimated from the food diaries, did not correlate with LCPUFA status in either maternal serum or breast milk. These findings are supported by recently published work demonstrating no relation between concentrations of n−3 fatty acids in serum and total dietary fish intake [40] in non-pregnant volunteers and are in agreement with other studies in high fish consuming populations.[41] It is highly likely that fatty acid status in pregnant women is influenced by a number of physiological pregnancy related factors, not least fetal requirements, so it is perhaps not surprising that such associations were not evident. Measurement error from self-reporting of habitual diet is also a potential confounding factor although the reporting error, if any, associated with estimating portion sizes would likely have been consistent between subjects as only one trained nutritionist at the was responsible for the interpretation and entry of dietary data.
The concentrations of the various fatty acids observed in breast milk at one month postpartum were similar to those reported in other populations. A comparative study of breast milk samples from diverse populations reported concentrations of the saturated and monosaturated fats similar to those observed in the Seychelles. In this comparative study the DHA concentration of breast milk varied between 0.17 and 0.99 % with the highest DHA status evident in high fish eating populations from Asia. [42] A recent metaanalysis incorporating 106 studies reported a mean DHA content of breast milk of 0.32 %, remarkably similar to the average of 0.31% observed in this study. [43] The 1:1 ratio of AA to DHA observed in breast milk from Seychellois women is in keeping with a previous study undertaken in Tanzania.[44] The authors of that study attributed their finding to the high fish consumption of the population. In contrast, studies in populations with lower fish intake report that the ratio of AA : DHA approaches 3:1 in breast milk.[45–46] Whilst there is no gold standard defining optimal fatty acid status in formula milk, typical recommendations for term infants are AA ≥ 0.35 % and DHA ≥ 0.2%.[47] Mean concentrations of AA and DHA in the breast milk of Seychellois women are 0.29 % and 0.31 % respectively. Thus, breastfed infants born to Seychellois women, who habitually consume a high fish diet, are receiving a DHA : AA ratio similar to that recommended for optimal development.
In conclusion, we report that even with high fish consumption, fetal accretion is so great that maternal status of DHA declines during the later stages of pregnancy. However, our results indicate that the n-3 : n-6 ratio in breast milk is high, which may exert potentially beneficial effects on cognitive development of breast fed children in Seychelles. Furthermore, our findings contribute to the ongoing debate on the influence of fatty acid status on gestational length and neonatal outcomes by the observation that in our high fish eating cohort fatty acid status is not a significant determinant of gestational length and neonatal anthropometric outcomes.
Acknowledgments
This paper was in part supported by the following grants from the National Institute of Environmental Health Sciences: RO1 ES10219, P30 ES01247 (Centre Grant), and T32 ES007271 (Training Grant). Additional support was provided by the Ministry of Health, Republic of Seychelles and the University of Ulster, Coleraine, Northern Ireland.
We thank Octavie Choisy, Maygane Jean and Anne-Marie Bibi and the maternity nurses for all their work with the subjects in the Seychelles. We also acknowledge Seychelles Bureau of Standards and CCFRA Technology Ltd, Chipping Campden, UK for their laboratory analyses of fish samples. We thank Dr Ian Bradbury for statistical advice.
Support National Institute of Environmental Health Sciences: Grant no: RO1 ES10219
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sastry PS. Lipids of nervous tissue: composition and metabolism. Prog Lipid Res. 1985;24:169–76. doi: 10.1016/0163-7827(85)90011-6. [DOI] [PubMed] [Google Scholar]
- 2.Svennerholm L. Distribution and fatty acid composition of phosphoglycerides in normal human brain. J Lipid Res. 1986;9:570–579. [PubMed] [Google Scholar]
- 3.De Vriese SR, Matthys C, De Henauw S, De Backer G, Dhont M, Christophe AB. Maternal and umbilical fatty acid status in relation to maternal diet. Prostaglandins Leukot Essent Fatty Acids. 2002;67:389–396. doi: 10.1054/plef.2002.0446. [DOI] [PubMed] [Google Scholar]
- 4.Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW. Intrauterine fatty acid accretion rates in human brain: implications for fatty acid requirements. Early Hum Dev. 1980;4:121–129. doi: 10.1016/0378-3782(80)90015-8. [DOI] [PubMed] [Google Scholar]
- 5.Clandinin MT, Chappell JE, Heim T, Swyer GW PR. Fatty acid accretion in fetal and neonatal liver: implications for fatty acid requirements. Early Hum Dev. 1981;5:7–14. doi: 10.1016/0378-3782(81)90066-9. [DOI] [PubMed] [Google Scholar]
- 6.Montgomery C, Speake BK, Cameron A, Sattar N, Weaver LT. Maternal docosahexaenoic acid supplementation and fetal accretion. Br J Nutr. 2003;90:135–145. doi: 10.1079/bjn1079/2003888. [DOI] [PubMed] [Google Scholar]
- 7.Al MD, van Houwelingen AC, Kester AD, Hasaart TH, Jong AE, Hornstra G, et al. Maternal essential fatty acid patterns during normal pregnancy and their relationship to the neonatal essential fatty acid status. Br J Nutr. 1995;74:55–68. doi: 10.1079/bjn19950106. [DOI] [PubMed] [Google Scholar]
- 8.Al MD, van Houwelingen AC, Hornstra G. Relation between birth order and the maternal and neonatal docosahexaenoic acid status. Eur J Clin Nutr. 1997;51:548–553. doi: 10.1038/sj.ejcn.1600444. [DOI] [PubMed] [Google Scholar]
- 9.Cashel K, English R, BennettS S, et al. National dietary survey of adults: No. 1 foods consumed. Canberra: Australian Government Publishing Service; 1986. p. 1124. [Google Scholar]
- 10.Connor WE, Lowensohn R, Hatcher L. Increased docosahexaenoic acid levels in human newborn infants by administration of sardines and fish oil during pregnancy. Lipids. 1996;31:s183–s187. doi: 10.1007/BF02637073. [DOI] [PubMed] [Google Scholar]
- 11.van Houwelingen AC, Sorensen JD, Hornstra G, Simonis MM, Boris J, Olsen SF, Secher NJ. Essential fatty acid status in neonates after fish-oil supplementation during late pregnancy. Br J Nutr. 1995;74:723–731. doi: 10.1079/bjn19950175. [DOI] [PubMed] [Google Scholar]
- 12.Dunstan JA, Mori TA, Barden ALJ, et al. Effects of n-3 polyunsaturated fatty acid supplementation in pregnancy on maternal and fetal fatty acid composition. Eur J Clin Nutr. 2004;58:429–437. doi: 10.1038/sj.ejcn.1601825. [DOI] [PubMed] [Google Scholar]
- 13.Sanjurjo P, Matorras R, Perteagudo L. Influence of fatty acid fish intake during pregnancy in the polyunsaturated fatty acids of erythrocyte phospholipids in the mother at labor and newborn infant. Acta Obstet Gynecol Scand. 1995;74:594–598. doi: 10.3109/00016349509013468. [DOI] [PubMed] [Google Scholar]
- 14.Olsen SF, Secher NJ. A possible preventive effect of low-dose fish oil on early delivery and preeclampsia: indications from a 50-year old controlled trial. Br J Nutr. 1990;64:599–609. doi: 10.1079/bjn19900063. [DOI] [PubMed] [Google Scholar]
- 15.Williams MA, Zingheim RW, King IB, Zebelman AM. Omega-3 fatty acids in maternal erythrocytes and risk of pre-eclamsia. Epidemiology. 1995;6:232–237. doi: 10.1097/00001648-199505000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Cheatham CL, Colombo J, Carlson SE. n-3 fatty acids and cognitive and visual acuity development: methodologic and conceptual considerations. Am J Clin Nutr. 2006;83:1456s–1466s. doi: 10.1093/ajcn/83.6.1458S. [DOI] [PubMed] [Google Scholar]
- 17.Breslow JL. N-3 fatty acids and cardiovascular disease. Am J Clin Nutr. 2006;83:1477s–1482s. doi: 10.1093/ajcn/83.6.1477S. [DOI] [PubMed] [Google Scholar]
- 18.He K, Song Y, Daviglus ML, et al. Accumulated evidence on fish consumption and coronary heart disease mortality: a metaanalysis of cohort studies. Circulation. 2004;109:2705–2711. doi: 10.1161/01.CIR.0000132503.19410.6B. [DOI] [PubMed] [Google Scholar]
- 19.Olsen SF, Hansen HS, Sorensen TI, et al. Intake of marine fat, rich in (n3) PUFA, may increase birth weight by prolonging gestation. Lancet. 1986;2:367–369. doi: 10.1016/s0140-6736(86)90055-3. [DOI] [PubMed] [Google Scholar]
- 20.Leaf AA, Leighfield MJ, Costeloe KL, Crawford MA. Long chain polyunsaturated fatty acids and fetal growth. Early Hum Dev. 1992;30:183–191. doi: 10.1016/0378-3782(92)90068-r. [DOI] [PubMed] [Google Scholar]
- 21.Felton CV, Chang TC, Crook D, Marsh M, Robson SC, Spencer JA. Umbilical vessel wall fatty acids after normal and retarded fetal growth. Arch Dis Child. 1994;70:F36–39. doi: 10.1136/fn.70.1.f36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.UK Scientific Advisory Committee on Nutrition. Advice on fish consumption: benefits and risk. Joint report of the Scientific Advisory Committee on Nutrition (SACN) and Committee on Toxicity, UK Food Standards Agency. [last accessed 20 November, 2006];2004 www.sacn.gov.uk/pdfs/fics_sacn_advice_fish.pdf.
- 23.Institue of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, D. C: National Academies Press; National Academies Press; 2002. 2002. [Google Scholar]
- 24.Folch J, Lees M, Sloane-Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 25.Sayed N, Frans Y, Schönfeldt H H. Composition of South African Foods: Milk and Milk Products; Eggs; Meat and Meat Products. South Africa: Medical Research Council; 1999. [Google Scholar]
- 26.Ather N, McLaughlin J, Taylor G. The Concise New Zealand Food Composition Tables. New Zealand Institute for Crop and Food Research Limited – A Crown Research Institute. 6. Palmerston North; New Zealand: 2003. [Google Scholar]
- 27.Herrera E. Implications of dietary fatty acids during pregnancy on placental, fetal and postnatal developmenta review. Placenta. 2002;23:S9–S19. doi: 10.1053/plac.2002.0771. [DOI] [PubMed] [Google Scholar]
- 28.Dutta-Roy AK. Cellular uptake of longchain fatty acids: role of membrane-associated fattyacid binding/transport proteins. Cell Mol Life Sci. 2000;57:1360–1372. doi: 10.1007/PL00000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stark KD, Beblo S, Murthy M, et al. Comparison of bloodstream fatty acid composition from AfricanAmerican women at gestation, delivery, and postpartum. J Lipid Res. 2005;46:516525. doi: 10.1194/jlr.M400394-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Al MD, van Houwelingen AC, Hornstra G. Longchain polyunsaturated fatty acids, pregnancy, and pregnancy outcome. Am J Clin Nutr. 2000;71:285s–291s. doi: 10.1093/ajcn/71.1.285s. [DOI] [PubMed] [Google Scholar]
- 31.Otto SJ, van Houwelingen AC, BadartSnook A, Hornstra G. Comparison of the peripartum and postpartum phospholipid polyunsaturated fatty acid profiles of lactating and non-lactating women. Am J Clin Nutr. 2001;73:1074–1079. doi: 10.1093/ajcn/73.6.1074. [DOI] [PubMed] [Google Scholar]
- 32.Olsen SF, Secher NJ. Low consumption of seafood in early pregnancy as a risk factor for preterm delivery: prospective cohort study. BMJ. 2002;324:15. doi: 10.1136/bmj.324.7335.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smuts CM, Huang M, Mundy D, Lasse TP, Major S, Carlson SE. A randomized trial of docosahexaenoic acid supplementation during the third trimester of pregnancy. Obstet Gynecol. 2003;101:469–479. doi: 10.1016/s0029-7844(02)02585-1. [DOI] [PubMed] [Google Scholar]
- 34.Oken E, Kleinman KP, Olsen SF, Rich-Edwards JW, Gillman MWSF. Associations of seafood and elongated n-3 fatty acid intake with fetal growth and length of gestation: Results from a US pregnancy cohort. Am J Epidemiol. 2004;160:774–783. doi: 10.1093/aje/kwh282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malcolm CA, Hamilton R, McCulloch DL, Montgomery C, Weaver LT. Scotopic electroretinogram in term infants born of mothers supplemented with docosahexaenoic acid during pregnancy. Invest Ophthalmol Vis Sci. 2003;44:3685–3691. doi: 10.1167/iovs.02-0767. [DOI] [PubMed] [Google Scholar]
- 36.Krauss-Etschmann S, Shadid R, Campoy C, et al. Effects of fishoil and folate supplementation of pregnant women on maternal and fetal plasma concentrations of docosahexaenoic acid and eicosapentaenoic acid: a European randomized multicenter trial. Am J Clin Nutr. 2007;85:1392–1400. doi: 10.1093/ajcn/85.5.1392. [DOI] [PubMed] [Google Scholar]
- 37.Desci T, Koletzko B. N-3 fatty acids and pregnancy outcomes. Curr Opin Clin Nutr Metab Care. 2005;8:161–166. doi: 10.1097/00075197-200503000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Hibbeln JR, Davis JM, Steer C, Emmett P, Rogers I, Williams C, Golding J. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet. 369:578–585. doi: 10.1016/S0140-6736(07)60277-3. [DOI] [PubMed] [Google Scholar]
- 39.Shamlaye CF, Shamlaye H, Brewer R. Health in Seychelles – an overview. Seychelles Medical and Dental Journal. 2004;7:13–20. www.smdj.sc.
- 40.Philibert A, Vanier C, Abdelouahab N, Chan HM, Mergler D. Fish intake and serum fatty acid profiles from freshwater fish. Am J Clin Nutr. 2006;84:1299–1307. doi: 10.1093/ajcn/84.6.1299. [DOI] [PubMed] [Google Scholar]
- 41.Hjartaker A, Lund E, Bjerve KS. Serum phospholipid fatty acid composition and habitual intake of marine foods registered by a semi-quantitative food frequency questionnaire. Eur J Clin Nutr. 1997;51:736–742. doi: 10.1038/sj.ejcn.1600475. [DOI] [PubMed] [Google Scholar]
- 42.Yuhas R, Pramuk K, Lien EL. Human milk fatty acid composition from nine countries varies most in DHA. Lipids. 2006;41:851–858. doi: 10.1007/s11745-006-5040-7. [DOI] [PubMed] [Google Scholar]
- 43.Brenna JT, Varamini B, Jensen RG, Diersen-Schade DA, Boettcher JA, Arterburn LM. Docosahexaenoic and arachadonic acid concentrations in human breast milk worldwide. Am J Clin Nutr. 2007;85:14571464. doi: 10.1093/ajcn/85.6.1457. [DOI] [PubMed] [Google Scholar]
- 44.Kuipers RS, Fokkema MR, Smit EN, van der Meulen J, Boersma ER, Muskiet FA. High contents of both docosahexaenoic and arachidonic acids in milk of women consuming fish from lake Kitangiri (Tanzania). Targets for infant formulae close to our ancient diet? Prostaglandins Leukot Essent Fatty Acids. 2005;72:279288. doi: 10.1016/j.plefa.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 45.Jensen CL, Maude M, Anderson RE, Heird WC. Effect of docosahexaenoic acid supplementation of lactating women on the fatty acid composition of breast milk lipids and maternal and infant serum phospholipids. Am J Clin Nutr. 2000;71:292s–299s. doi: 10.1093/ajcn/71.1.292s. [DOI] [PubMed] [Google Scholar]
- 46.Da Cunha J, da Costa THM, Ito MK. Influences of maternal dietary intake and suckling on breast milk and fatty acid composition in low-income women from Brasilia, Brazil. Early Hum Dev. 2005;81:303–311. doi: 10.1016/j.earlhumdev.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 47.Koletzko B, Agostoni C, Carlson SE, et al. Long chain polyunsaturated fatty acids (LCPUFA) and perinatal development. Acta Pediatr. 2001;90:460–464. [PubMed] [Google Scholar]