Abstract
Background
There are limited data describing how pre-existing heart failure affects mortality following pneumonia.
Objective
To examine the association between history and severity of heart failure and mortality among patients hospitalized for pneumonia.
Design
Population-based cohort study in Western Denmark between 1994 and 2003.
Patients
33,736 adults with a first-time hospitalization for pneumonia. Heart failure was identified and categorized based on data linked from population-based health care databases.
Measurements
We compared 30-day mortality between patients with pre-existing heart failure and other pneumonia patients, while adjusting for age, gender, comorbidity, and medication use.
Results
The 30-day mortality was 24.4% among heart-failure patients and 14.4% among other patients, with an adjusted 30-day mortality rate ratio (MRR) of 1.40 (95% CI: 1.29–1.51). Adjusted MRRs increased according to severity of pre-existing heart failure, as indicated by medication regimen: thiazide-based, MRR = 1.09 (95% CI: 0.79–1.50); loop-diuretics, MRR = 1.25 (95% CI: 1.10–1.43); loop-diuretics and digoxin, MRR = 1.35 (95% CI: 1.18–1.55); loop-diuretics and spironolactone, MRR = 1.72 (95% CI: 1.49–2.00). Pre-existing heart valve disease and atrial fibrillation substantially increased mortality.
Conclusion
History and severity of heart failure are associated with a poor outcome for patients hospitalized with pneumonia.
KEY WORDS: heart failure, pneumonia, outcome, clinical epidemiology
INTRODUCTION
Heart failure, affecting approximately 5 million Americans and 10 million Europeans, is a huge public-health burden.1,2 Patients with heart failure carry a high risk of recurring hospital admissions for decompensated heart failure and cardiovascular events.3–6 Acute respiratory tract infection is the main precipitating event for 3–16% of patients hospitalized with decompensated heart failure;7–9 conversely, heart failure is a risk factor for pneumonia.10
Pneumonia-related mortality rates in general patient populations remain at 10%-15%, increasing with advanced age,11 and it is plausible that pre-existing heart failure may worsen outcome. Only a few recent studies have examined heart failure among a variety of predictors for pneumonia outcome. These studies have suggested mortality increases of up to 50% associated with heart failure.11,12 To date, no population-based cohort study has focused on heart failure patients with pneumonia or examined the impact of heart failure severity or such frequent coexisting conditions as atrial fibrillation or diabetes on pneumonia outcome.
Hospitalizations for heart failure and pneumonia are increasingly common among the elderly.13 Data on associated mortality are needed to understand the clinical course of these diseases and potentially prevent post-pneumonia deaths. We conducted a large population-based cohort study of more than 30,000 patients hospitalized with pneumonia in a well-defined European population, examining the association between a preadmission history of heart failure and 30-day mortality.
SUBJECTS AND METHODS
Setting
The study cohort consisted of patients with pneumonia ascertained from population-based health care databases in the counties of North Jutland, Aarhus, and Viborg, a mixed rural and urban region in northwestern Denmark, with 1,400,000 Caucasian inhabitants. The Danish National Health Service provides tax-funded healthcare for all residents, guaranteeing free access to hospitals and primary medical care and reimbursing costs of most physician-prescribed medications.
For the study purposes, three different data collection periods were defined, based on the availability of prescription data records: starting on January 1, 1994, in North Jutland County; on January 1, 1996, in Aarhus County; and on January 1, 1998, in Viborg County. In all counties, the end date for data retrieval was December 31, 2003. Civil registration numbers, assigned to every Danish citizen since 1968 and encoding gender and date of birth, permitted accurate record linkage among multiple health databases.
Data on Hospitalizations for Pneumonia
We identified patients aged at least 15 years of age, with a first-time hospitalization for pneumonia during the three data collection periods, using counties’ hospital discharge registries merged into a research database, as previously described 13. The registries contain key information on all patient discharges from non-psychiatric hospitals in the counties since 1977 (from 1995, all outpatient visits at hospitals are also recorded). Data include patients’ civil registration numbers, admission and discharge dates, and up to 20 discharge diagnoses coded exclusively by physicians according to the International Classification of Diseases [8th revision (ICD-8) until the end of 1993; 10th revision (ICD-10) thereafter]. The ICD-10 codes used for pneumonia hospitalizations were J12-J18, A481, and A709.
Data on Heart Failure
Data on markers for heart failure among study patients were obtained from the counties’ hospital discharge registries. Heart failure was defined as a previous hospital discharge diagnosis or outpatient diagnosis of congestive heart failure; pulmonary edema; left ventricular failure; unspecified heart failure; cardiomyopathy; or hypertensive heart disease with congestive heart failure (with or without hypertensive renal disease or renal failure). We considered diagnoses recorded within five years preceding the date of hospitalization for pneumonia (see Appendix for specific ICD codes). We further disaggregated patients with heart failure into five categories of heart failure-related conditions: 1) cardiomyopathy (with or without any of the following diagnoses); 2) heart valve disease (with or without any of the other diagnoses except cardiomyopathy); 3) myocardial infarction (with or without atrial fibrillation), 4) atrial fibrillation only; 5) none of the above diagnoses.
Databases containing information on prescriptions for all reimbursed drugs dispensed from pharmacies in the counties 14 permitted us to classify the heart failure patients according to preadmission medication use, which we used as a surrogate measure of heart failure severity. Based on European and North American guidelines 1,2 and observed prevalence of preadmission medication use, we classified treatment as follows: 1) thiazide-based regimens without loop-diuretics; 2) loop-diuretic-based regimens excluding digoxin and spironolactone; 3) loop-diuretic-based regimens including digoxin but not spironolactone; 4) loop-diuretic-based regimens including spironolactone; 5) other heart failure medications not including any diuretics (at least one prescription for slow-acting nitrates, ACE inhibitors, angiotensin reuptake blockers, or oral anticoagulants); and 6) none of the above heart failure medications [see Appendix for specific ATC codes].
Data on Potential Confounding Factors
We obtained data on comorbidity and other covariates from the hospital and prescription databases. For each study subject, we collected data on different major disease categories (except heart failure) as included in the Charlson comorbidity index [Charlson et al. 15], based on the complete hospital discharge history before the pneumonia hospitalization and, for diabetes, also based on data on prescriptions for insulin/antidiabetic drugs.16 We defined three comorbidity levels as low (Charlson score of 0), medium (1–2), and high (3+). We also collected hospital discharge data on previous alcoholism-related disorders (yes/no) not included in the Charlson index. From the prescription databases, we furthermore retrieved data on prescriptions for immunosuppressive drugs, including corticosteroids prescribed within one year, and systemic antibiotics prescribed within 6 months prior to the hospitalization with pneumonia.
The prevalence of diabetes is very high among heart failure patients 17, while hyperglycemia and diabetes have been associated with increased mortality from pneumonia.18 We therefore conducted a separate interaction analysis by examining pneumonia mortality among patients with heart failure but not diabetes, patients with diabetes but not heart failure, and patients with joint exposure of heart failure and diabetes.
Mortality Data
The study outcome was death within 30 days following the pneumonia admission date. We ascertained mortality from the Danish Civil Registry System, which is updated daily.19
Statistical Analysis
Duration of follow-up was computed from the date of hospital admission with pneumonia until death, or up to 30 days post-admission. We constructed Kaplan-Meier curves and life-table estimates according to the following variables: heart failure; gender; age group (15–64 years, 65–79 years, and ≥80 years); study period (1994–1998 and 1999–2003); individual disease categories (according to Charlson index categories); alcoholism-related conditions; preadmission use of immunosuppressive drugs and/or systemic antibiotics; and cumulative mortality 30 days post-hospitalization. For each variable, the category with the lowest risk of death was used as the reference. Cox’s regression was used to estimate 30-day mortality rate ratios (MRRs) for pneumonia patients with a history of heart failure compared with other pneumonia patients, while controlling for all confounding factors. We then repeated the mortality analyses among heart failure patient subgroups according to preadmission heart failure medications and heart failure-related conditions, compared with other pneumonia patients. Finally, cumulative mortality from heart failure and adjusted MRRs were stratified by the diabetes status (present/absent), using pneumonia patients with neither heart failure nor diabetes as the reference cohort. The assumption of proportional hazards in the Cox’s model was assessed graphically and found appropriate. We used SAS software (version 9.1.3, SAS Institute Inc., Cary, NC) for the analyses. The study was approved by the Danish Data Protection Agency and by the Aarhus University Hospital Registry Board.
RESULTS
We identified 33,736 patients with a first-time hospitalization for pneumonia, of whom 3,210 (9.5%) had a previous diagnosis of heart failure (Table 1). The median age was 73 years (interquartile range 61 to 81), and 47% of patients were women. Pneumonia patients with heart failure were, on average, older and more likely to have a history of hospital-diagnosed comorbidity as defined by the Charlson index, compared with other pneumonia patients (83% vs. 52%) (Table 1).
Table 1.
Characteristics of 33,736 Patients With a First Hospitalization for Pneumonia, According to Heart Failure Status
| Characteristic | Heart Failure Patients ( = 3210) | Other Patients ( = 30,526) | Total ( = 33,736) |
|---|---|---|---|
| Sex | |||
| Females | 1374 (42.8) | 14,330 (46.9) | 15,704 (46.6) |
| Males | 1836 (57.2) | 16,196 (53.1) | 18,032 (53.4 |
| Age, years | |||
| 15–39 | 11 (0.3) | 2624 (8.6) | 2635 (7.8) |
| 40–64 | 328 (10.2) | 7393 (24.2) | 7721 (22.9) |
| 65–79 | 1475 (46.0) | 11707 (38.4) | 13,182 (39.1) |
| ≥80 | 1396 (43.5) | 8,802 (28.8) | 10,198 (30.2) |
| Comorbidity level | |||
| Score* low (0) | 560 (17.4) | 14,509 (47.5) | 15,069 (44.7) |
| Score medium (1–2) | 1674 (52.2) | 11,889 (39.0) | 13,563 (40.2) |
| Score high (>2) | 976 (30.4) | 4128 (13.5) | 5104 (15.1) |
| Alcoholism-related disorders | 96 (3.0) | 1311 (4.3) | 1407 (4.2) |
| Preadmission medications | |||
| Immunosuppressive drugs ≤ 1 year before admission | 803 (25.0) | 5650 (18.5) | 6453 (19.1) |
| Systemic antibiotic therapy ≤ 6 months before admission | 1651 (51.4) | 14,931 (48.9) | 16,582 (49.2) |
| Period of study | |||
| 1994–1998 | 941 (29.3) | 9189 (30.1) | 10,130 (30.0) |
| 1999–2003 | 2269 (70.7) | 21,337 (69.9) | 23,606 (70.0) |
Data are given as (%) of individuals
* Level of Charlson index score
Thirty-day mortality from pneumonia was 24.4% among heart failure patients vs. 14.4% among other pneumonia patients. With the exception of the small and relatively young group of cardiomyopathy patients, mortality in all subgroups of heart failure patients was over 20%. Poor outcomes were observed for patients with a history of heart valve disease and for those with atrial fibrillation alone, as well as for those with no recorded heart failure-related conditions (Figure 1, Panel A). Patients who received thiazides only, loop-diuretics without addition of digoxin or spironolactone or those receiving no diuretics before admission had a lower risk of death than those who received loop-diuretic-based regimens with addition of digoxin or spironolactone. Patients who, despite previous hospital-diagnosed heart failure, used no heart failure medication before the admission for pneumonia had particularly high mortality during the first weeks following this event (Figure 1, Panel B).
Figure 1.
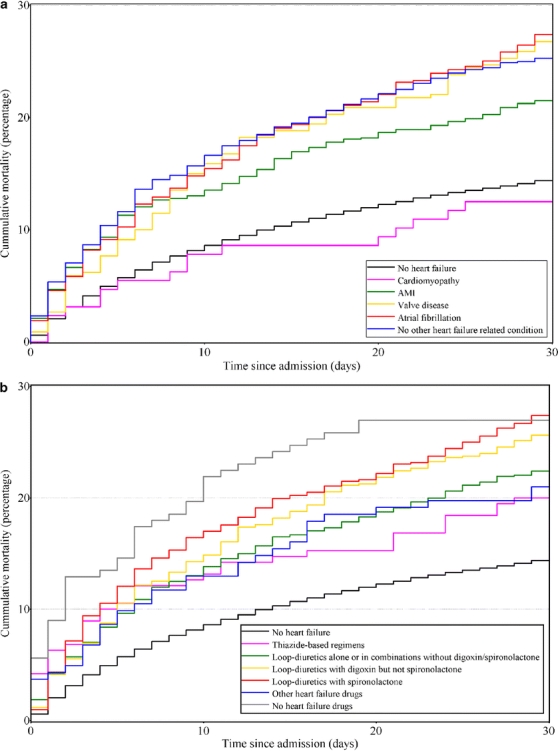
Unadjusted mortality curves for hospitalized pneumonia patients without heart failure and for pneumonia patients with heart failure, according to presence of heart failure-related conditions (panel A) and preadmission heart failure medications (panel B).
Compared with other patients hospitalized with pneumonia, the crude 30-day MRR for heart failure patients was 1.80 (95% CI: 1.67–1.95). After controlling for potential confounding factors, the 30-day MRR decreased, considerably, to 1.40 (95% CI: 1.29–1.51) (Table 2). The adjusted increases in mortality associated with heart failure were present in all age groups, but MRRs decreased with increasing age: MRR = 1.98 (95% CI: 1.45–2.72) among patients aged 40–64 years; MRR = 1.56 (95% CI: 1.37–1.77) among those aged 65–79 years; and MRR = 1.25 (95% CI: 1.12–1.40) among patients 80 years or older (no deaths occurred among the 11 heart failure patients younger than 40 years). By contrast, the absolute differences in mortality were similar across the age groups: 40–64 years, 7.2%; 65–79 years; 7.7%; ≥ 80 years, 5.2%.
Table 2.
Overall and Stratified Crude and Adjusted 30-day Mortality Rate Ratios (MRRs) for Patients With a First-time Hospitalization for Pneumonia, According to Presence or Absence of Heart Failure
| Variable, Stratification Type | (%) | 30-day Mortality (%) | Crude 30-day MRR (95% CI) | Adjusted* 30-day MRR (95% CI) | value Adjusted MRR |
|---|---|---|---|---|---|
| No heart failure | 30526 (90.5) | 14.4 | 1.0 (reference) | 1.0 (reference) | |
| Heart failure present | |||||
| Overall | 3210 (9.5) | 24.4 | 1.80 (1.67–1.95) | 1.40 (1.29–1.51) | <0.01 |
| Classified by presence of heart failure related condition† (% of heart failure) | |||||
| Cardiomyopathy | 128 (4.2) | 12.5 | 0.86 (0.53–1.41) | 1.03 (0.63–1.68) | 0.92 |
| Heart valve disease | 340 (10.6) | 26.8 | 1.97 (1.60–2.43) | 1.52 (1.23–1.88) | <0.01 |
| Myocardial infarction | 814 (25.4) | 21.5 | 1.57 (1.35–1.83) | 1.21 (1.01–1.45) | 0.04 |
| Atrial fibrillation only | 635 (19.8) | 27.4 | 2.04 (1.75–2.37) | 1.42 (1.22–1.65) | <0.01 |
| None of these recorded | 1293 (40.3) | 25.3 | 1.89 (1.69–2.12) | 1.47 (1.31–1.65) | <0.01 |
| Classified by preadmission heart failure medications (% of heart failure) | |||||
| Thiazide-based regimens | 190 (5.9) | 20.0 | 1.46 (1.06–2.01) | 1.09 (0.79–1.50) | 0.59 |
| Loop-diuretic-based regimens not including digoxin or spironolactone | 1,121 (34.9) | 22.4 | 1.63 (1.44–1.86) | 1.25 (1.10–1.43) | <0.01 |
| Loop-diuretic-based regimens including digoxin, but not spironolactone | 847 (26.4) | 25.6 | 1.89 (1.65–2.17) | 1.35 (1.18–1.55) | <0.01 |
| Loop-diuretic-based regimens including spironolactone | 712 (22.2) | 27.4 | 2.05 (1.78–2.37) | 1.72 (1.49–2.00) | <0.01 |
| Other heart failure drugs only‡ | 162 (5.0) | 21.0 | 1.53 (1.09–2.14) | 1.34 (0.96–1.89) | 0.09 |
| No heart failure drugs | 178 (5.5) | 27.0 | 2.11 (1.59–2.81) | 1.81 (1.36–2.41) | <0.01 |
*Adjusted for sex, age, individual disease categories in the Charlson index (except heart failure), alcoholism-related disorders, antibiotic and immunosuppressive drug use, and period of study
†See ‘Subjects and methods’ for categorization of heart failure related conditions
‡”Other heart failure drugs only” refers to patients with at least one prescription for slow-acting nitrates, ACE inhibitors, Angiotensin reuptake blockers, or oral anticoagulants
After adjustment for confounding, the magnitude of relative increase in mortality followed the gradient of heart failure severity as indicated by medication regimens: thiazide-based, MRR = 1.09 (95% CI: 0.79–1.50); loop-diuretics, MRR = 1.25 (95% CI: 1.10–1.43); loop-diuretics and digoxin, MRR = 1.35 (95% CI: 1.18–1.55); and loop-diuretics and spironolactone, MRR = 1.72 (95% CI: 1.49–2.00 [p-value for trend = 0.004] (Table 2). This trend was observed during both 1994–1998 and 1999–2003. Among digoxin users, mortality was increased among patients without atrial fibrillation (MRR = 1.52 (95% CI: 1.24–1.86)), but not among those with atrial fibrillation (MRR = 0.81 (95% CI: 0.62–1.06)). Non-use of any heart failure medication prior to hospital admission remained a strong prognostic indicator for mortality following pneumonia (MRR = 1.81 (95% CI: 1.36–2.41)).
Heart failure in combination with previous heart valve disease (adjusted MRR = 1.52 (95% CI: 1.23–1.88)) or atrial fibrillation only (adjusted MRR = 1.42 (95% CI: 1.22–1.65)) predicted increased mortality. This was less pronounced for heart failure in combination with previous myocardial infarction (adjusted MRR = 1.21 (95% CI 1.01–1.45)). Supplementary analyses showed that heart valve disease was associated with substantially increased mortality regardless of the presence or absence of other heart failure-related conditions (MRR = 1.54 (95% CI: 1.19–2.00) and 1.49 (95% CI: 1.05–2.11), respectively), while heart failure in combination with previous myocardial infarction was a weaker prognostic indicator, both with and without concomitant atrial fibrillation (MRR = 1.06 (95% CI: 0.79–1.41) and 1.29 (95% CI: 1.06–1.59), respectively).
Among heart failure patients with pneumonia, 19.2% had known diabetes before admission. Stratified analyses showed similarly increased 30-day mortality and adjusted MRRs from heart failure, regardless of presence of diabetes. Nor did presence of diabetes appear to strengthen the association between heart failure and mortality from pneumonia on the absolute scale (Table 3).
Table 3.
Cumulative 30-day Mortality and Adjusted 30-day Mortality Rate Ratios (MRRs) for Patients With a First-time Hospitalization for Pneumonia, According to Presence or Absence of Diabetes Mellitus
| Stratified by Diabetes: | Patients with First-time Hospitalization for Pneumonia | ||||
|---|---|---|---|---|---|
| 30-day Mortality | Adjusted* 30-day MRR(95% CI) | value Adjusted MRR | |||
| No diabetes | No heart failure | 27,794 | 14.0% | 1.0 (reference) | |
| Heart failure present | 2,595 | 24.2% | 1.41 (1.29–1.54) | <0.01 | |
| Diabetes present | No heart failure | 2,732 | 18.0% | 1.17 (1.06–1.28) | <0.01 |
| Heart failure present | 615 | 25.2% | 1.55 (1.32–1.82) | <0.01 | |
*Adjusted for sex, age, individual disease categories in the Charlson index (except heart failure and diabetes), alcoholism-related disorders, antibiotic and immunosuppressive drug use, and period of study
Reference group = patients with neither heart failure nor diabetes
DISCUSSION
In this large population-based cohort study of more than 33,000 patients hospitalized for pneumonia, including 3,210 persons with preexisting heart failure, we found that heart failure was an important prognostic indicator of increased 30-day mortality. Our data indicated that pneumonia mortality increased with preadmission heart failure severity. Thus, patients receiving heart failure drugs used for heart failure NYHA class III-IV were at a particularly high risk for death associated with pneumonia, while heart failure patients using a thiazide as the sole diuretic experienced a mortality rate similar to that of other pneumonia patients. Non-use of any heart failure medication prior to admission was strongly associated with poor outcome, particularly during the first few weeks following hospitalization, when death is expected to be pneumonia-related.20 Sufficient diuretic therapy during this period may be critical to clearing acute infection from the alveoli. Additionally, patients with heart failure whose physicians do not prescribe appropriate medications—or patients who choose not to fill their prescriptions—may be less educated, have poorer general health, or experience delayed hospital admission for acute infection. In heart failure patients using digoxin or spironolactone, mortality rates continued to rise after the first few post-admission weeks, which may, to a large extent, reflect the generally very poor prognosis among patients with severe chronic heart failure. Detailed clinical data collected in future studies may shed further light on these hypotheses.
Heart valve disease combined with heart failure predicted a poor outcome, suggesting that affected patients may be especially vulnerable to pneumonia-induced exacerbation of heart failure. Also, 30-day mortality was substantially elevated among patients with preexisting atrial fibrillation, but less among those with prior myocardial infarction. This finding corroborates results of a recent Canadian cohort study of 30-day mortality among patients hospitalized for clinical heart failure.21 Close to 20% of heart failure patients in our study had diabetes, compared with 32–34% of heart failure patients with diabetes reported in Canada and the United States.17 Recent population-based studies have challenged the hypothesis that diabetes increases mortality from pneumonia.11,16,22 We found that diabetes per se was associated with a slightly increased mortality but did not worsen outcomes among heart failure patients with pneumonia.
Denmark’s national health care system allowed for a population-based design, with inclusion of all incident pneumonia hospitalizations and complete follow-up for mortality, thereby preventing selection bias. Our study population was sufficiently large to permit comparisons of different subgroups of heart failure patients. Moreover, we ascertained heart failure status based on extensive prospectively recorded hospital discharge and prescription data collected prior to the date of admission for pneumonia, thus ensuring independence of heart failure diagnosis from the pneumonia episode.
The estimated positive predictive value of a registry pneumonia diagnosis in the cohort is 90% (95% CI, 82%-95%).13 We could not distinguish between community-acquired and nosocomial pneumonia, but our earlier study showed that only 13% of pneumonia discharge diagnoses in our region represented nosocomial episodes.13 Closer surveillance of patients with heart failure might have led both to earlier diagnosis of pneumonia and to a higher proportion of patients being hospitalized. However, this would probably lead to underestimation of mortality in our study. Heart failure patients were similarly likely as other pneumonia patients to receive preadmission antibiotics in our study, which argues against closer surveillance.
Ascertainment of disease status from hospital databases could be hampered by inaccurate coding, yet previous studies have demonstrated high positive predictive value of ICD-coded heart failure diagnoses in our data sources.23,24 While our study included a complete prescription history, we lacked information on patient compliance. Nondifferential patient non-compliance would be statistically expected to bias our MRR estimates for different treatment categories towards unity.
We were able to adjust for a wide range of prognostic factors assumed to be important for pneumonia, including malignancies, cardiovascular disease, chronic lung disease, liver cirrhosis, and renal disease.11,12 Misclassification of data on confounders might have left some residual confounding. However, registration of previous diagnoses should be at least as complete for heart failure patients as for other individuals, leading to conservative mortality estimates. Similarly, non-ascertainment of heart failure patients who had never been previously hospitalized would have lowered our mortality estimates.
In our study, the overall 30-day mortality from pneumonia was higher than that reported in previous selected patient cohorts or hospitals 11,22,25, most likely because we had access to comprehensive population-wide health information, covering nursing-home residents and all types of hospitals 13. Our estimated 1.8-fold overall increase in mortality among heart failure patients was slightly lower than the odds-ratio-based estimate of 2.4-fold mortality increase associated with congestive heart failure, calculated in meta-analysis of pneumonia prognosis by Fine et al. 26, based on six pneumonia outcome studies (conducted between 1966 and 1995) with data on heart failure. Our crude MRR was similar to that published in a recent Canadian cohort study of pneumonia patients [crude relative risk for in-hospital mortality with heart failure = 1.65 (95% CI 1.20–2.29); adjusted estimate not given] 22. However, our findings showed that much of the mortality increase associated with heart failure disappeared after controlling for age and comorbidity. Our adjusted MRR of 1.40 was comparable to estimates from the few recent studies reporting multivariable-adjusted pneumonia mortality specifically for heart failure, i.e., a study of U.S. Medicare recipients (adjusted in-hospital mortality relative risk = 1.53 (95% CI 1.47–1.59)) 11 and in a multinational study of community-acquired pneumococcal bacteremia (adjusted mortality OR = 1., -value > 0.5; CI not given) 27.
Our findings are consistent with current knowledge of pulmonary physiology.28 In patients with heart failure, varying degrees of alveoli flooding may 29 interfere with normal physiological mechanisms operating in the alveolar lining fluid at the interphase between air and the lung tissue (including effective opsonins and macrophages), both increasing the risk of infection and hampering clearance once infection is established. Conversely, an episode of pneumonia may induce or worsen cardiogenic pulmonary edema as cardiac output fails to meet the needs during severe infection. Pneumonia and severe sepsis may further induce noncardiogenic pulmonary edema or respiratory distress syndrome, whereas aggressive fluid resuscitation, recommended for severe sepsis, may lead to risk of volume overload and further pulmonary edema.30
In summary, our data showed that among adults with a first-time hospitalization for pneumonia, medical history and severity of hospital-diagnosed heart failure is associated with a poor outcome. The increased mortality observed throughout 30 post-admission days indicates that current hospitalization strategies for patients with chronic heart failure and pneumonia and their surveillance during and after hospitalization can be improved. Heart failure patients with NYHA class III-IV seem to be a particular high-risk group for death associated with pneumonia; and thus a group of pneumonia patients that clinicians should maintain high vigilance in caring for. The particularly poor pneumonia outcome among heart failure patients not receiving heart failure medications before admission is worrying and warrants further investigation.
Acknowledgements
This study received financial support from the Western Danish Research Forum for Health Sciences, and from the “Klinisk Epidemiologisk Forskningsfond”, Denmark.
Conflict of interest None disclosed.
Appendix
World Health Organization International Classification of Diseases ICD-8 and ICD-10 codes and Anatomical Therapeutical Chemical classification system (ATC) codes used in this study.
Pneumonia
ICD-10: J12-J18, A481, and A709.
Heart failure
ICD-8: 427.09, 427.10, 427.11, 427.19, 428.99, 782.49, 425.99; ICD-10: I50, I11.0, I13.0, I13.2, I42.0, I42.6, I42.7, I42.8, I42.9, I25.5
Diabetes
ICD-8: 249, 250; ICD-10: E10, E11; ATC codes A10A, A10B
Heart failure-related conditions:
Myocardial infarction
ICD-8: 410; ICD-10: I21, I22, I23
Atrial fibrillation
ICD-8: 427.93, 427.94; ICD-10: I48
Heart valve disease
ICD-8: 394, 395, 396, 397, 398, 424; ICD-10: I34, I35, I05, I06, I07, I08, I091, I098, I099, I36, I37, I38, I39
Cardiomyopathy
ICD-8: 425; ICD-10: I42
Alcoholism-related disorders
ICD-8: 291, 303, 979, 980, 577.10; ICD-10: F10, K86.0, Z72.1, R78.0, T51
Heart failure drugs
ATC codes: B01AA, C01AA, C01CA08-C01CA14, C03A, C03C, C07AB, C02E, C02L, C09A, C09B, C08DA51, C09C, C03DA01.
Systemic antibiotics
ATC code: J01.
Immunosuppressive drugs
ATC codes: L01, L04, and H02AB.
References
- 1.Remme WJ, Swedberg K. Task force for the diagnosis and treatment of chronic heart failure, European Society of Cardiology. Guidelines for the diagnosis and treatment of chronic heart failure. Eur Heart J. 2001;22:1527–60. [DOI] [PubMed]
- 2.Hunt SA. Guideline update for the diagnosis and management of chronic heart failure in the adult: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). ACC/AHA 2005 J Am Coll Cardiol. 2005;46:e1–82. [DOI] [PubMed]
- 3.Krumholz HM, Parent EM, Tu N, et al. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med. 1997;157:99–104. [DOI] [PubMed]
- 4.McDermott MM, Feinglass J, Lee P, et al. Heart failure between 1986 and 1994: temporal trends in drug-prescribing practices, hospital readmissions, and survival at an academic medical center. Am Heart J. 1997;134:901–9. [DOI] [PubMed]
- 5.Khand AU, Gemmell I, Rankin AC, Cleland JGF. Clinical events leading to the progression of heart failure: insights from a national database of hospital discharges. Eur Heart J. 2001;22:153–64. [DOI] [PubMed]
- 6.Gonseth J, Guallar-Castillon P, Banegas JR, Rodriguez-Artalejo F. The effectiveness of disease management programmes in reducing hospital re-admission in older patients with heart failure: a systematic review and meta-analysis of published reports. Eur Heart J. 2004;25:1570–95. [DOI] [PubMed]
- 7.Ghali JK, Kadakia S, Cooper R, Ferlinz J. Precipitating factors leading to decompensation of heart failure. Traits among urban blacks. Arch Intern Med. 1988;148:2013–6. [DOI] [PubMed]
- 8.Chin MH, Goldman L. actors contributing to the hospitalization of patients with congestive heart failure. Am J Public Health. 1997;87:643–8. [DOI] [PMC free article] [PubMed]
- 9.Michalsen A, Konig G, Thimme W. Preventable causative factors leading to hospital admission with decompensated heart failure. Heart. 1998;80:437–41. [DOI] [PMC free article] [PubMed]
- 10.Jackson ML, Neuzil KM, Thompson WW, et al. The burden of community-acquired pneumonia in seniors: results of a population-based study. Clin Infect Dis. 2004;39:1642–50. [DOI] [PMC free article] [PubMed]
- 11.Kaplan V, Angus DC, Griffin MF, Clermont G, Scott WR, Linde-Zwirble WT. Hospitalized community-acquired pneumonia in the elderly: age- and sex-related patterns of care and outcome in the United States. Am J Respir Crit Care Med. 2002;165:766–72. [DOI] [PubMed]
- 12.O’Meara ES, White M, Siscovick DS, Lyles MF, Kuller LH. Hospitalization for pneumonia in the Cardiovascular Health Study: incidence, mortality, and influence on longer-term survival. J Am Geriatr Soc. 2005;530:1108–16. [DOI] [PubMed]
- 13.Thomsen RW, Riis A, Nørgaard M, et al. Rising incidence and persistently high mortality of hospitalized pneumonia: a 10-year population-based study in Denmark. J Intern Med. 2006;259:410–7. [DOI] [PubMed]
- 14.Gaist D, Sørensen HT, Hallas J. The Danish prescription registries. Dan Med Bull. 1997;44:445–8. [PubMed]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. [DOI] [PubMed]
- 16.Thomsen RW, Hundborg HH, Lervang HH, Johnsen SP, Sørensen HT, Schønheyder HC. Diabetes and outcome of community-acquired pneumococcal bacteremia: a 10-year population-based cohort study. Diabetes Care. 2004;27:70–6. [DOI] [PubMed]
- 17.Ko DT, Tu JV, Masoudi FA, et al. Quality of care and outcomes of older patients with heart failure hospitalized in the United States and Canada. Arch Intern Med. 2005;165:2486–92. [DOI] [PubMed]
- 18.Falguera M, Pifarre R, Martin A, Sheikh A, Moreno A. Etiology and outcome of community-acquired pneumonia in patients with diabetes mellitus. Chest. 2005;128:3233–9. [DOI] [PubMed]
- 19.Frank L. Epidemiology. When an entire country is a cohort. Science. 2000;287:2398–9. [DOI] [PubMed]
- 20.Mortensen EM, Coley CM, Singer DE, et al. Causes of death for patients with community-acquired pneumonia: results from the pneumonia patient outcomes research team cohort study. Arch Intern Med. 2002;162:1059–64. [DOI] [PubMed]
- 21.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003;290:2581–7. [DOI] [PubMed]
- 22.McAlister FA, Majumdar SR, Blitz S, Rowe BH, Romney J, Marrie TJ. The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community-acquired pneumonia. Diabetes Care. 2005;28:810–5. [DOI] [PubMed]
- 23.Goff DC Jr, Pandey DK, Chan FA, Ortiz C, Nichaman MZ. Congestive heart failure in the United States: is there more than meets the I(CD code)? The Corpus Christi Heart Project. Arch Intern Med. 2000;160:197–202. [DOI] [PubMed]
- 24.Ingelsson E, Sundstrom J, Arnlov J, Zethelius B, Lind L. Insulin resistance and risk of congestive heart failure. JAMA. 2005;294:334–41. [DOI] [PubMed]
- 25.Mortensen EM, Kapoor WN, Chang CC, Fine MJ. Assessment of mortality after long-term follow-up of patients with community-acquired pneumonia. Clin Infect Dis. 2003;37:1617–24. [DOI] [PubMed]
- 26.Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA. 1996;275:134–41. 8531309 [DOI]
- 27.Kalin M, Örtqvist Å, Almela M, et al. Prospective study of prognostic factors in community-acquired bacteremic pneumococcal disease in 5 countries. J Infect Dis. 2000;182:840–7. [DOI] [PubMed]
- 28.Donowitz GR, Mandell GL. Acute pneumonia. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett’s priciples and practice of infectious diseases. Philadelphia: Elsevier; 2005:819–45.
- 29.Ware LB, Matthay MA. Acute pulmonary edema. N Engl J Med. 2005;353:2788–96. [DOI] [PubMed]
- 30.Dellinger RP, Carlet JM, Masur H, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858–73. [DOI] [PubMed]


