Abstract
Background
Telephone counseling in chronic disease self-management is increasing, but has not been tested in studies that control for quality of medical care.
Objective
To test the effectiveness of a six-session outpatient telephone-based counseling intervention to improve secondary prevention (behaviors, medication) in patients with acute coronary syndrome (ACS) following discharge from hospital, and impact on physical functioning and quality of life at 8 months post-discharge.
Design
Patient-level randomized trial of hospital quality improvement (QI-only) versus quality improvement plus brief telephone coaching in three months post-hospitalization (QI-plus). Data: medical record, state vital records, patient surveys (baseline, three and eight months post-hospitalization). Analysis: pooled-time series generalized estimating equations to analyze repeated measures; intention-to-treat analysis.
Participants
Seven hundred and nineteen patients admitted to one of five hospitals in two contiguous mid-Michigan communities enrolled; 525 completed baseline surveys.
Measurements
We measured secondary prevention behaviors, physical functioning, and quality of life.
Results
QI-plus patients showed higher self-reported physical activity (OR = 1.53; = .01) during the first three months, with decline after active intervention was withdrawn. Smoking cessation and medication use were not different at 3 or 8 months; functional status and quality of life were not different at 8 months.
Conclusions
Telephone coaching post-hospitalization for ACS was modestly effective in accomplishing short-term, but not long-term life-style behavior change. Previous positive results shown in primary care did not transfer to free-standing telephone counseling as an adjunct to care following hospitalization.
KEY WORDS: clinical trials, disease management, guidelines, chronic disease, quality improvement, patient-centered care, acute coronary syndrome, telephone counseling, decision support techniques
The efficacy of surgical and medical therapies for acute coronary syndrome (ACS) is well established, as is the efficacy of medical and behavioral secondary prevention. Accomplishing secondary prevention, however, requires substantial effort and resources from patients and from clinicians.1 To realize the benefits of secondary prevention, it is important to adopt the most effective and efficient set of interventions that is feasible in routine care. Quality improvement (QI) efforts have been shown to improve guideline implementation in hospitals.2,3 Behavior change interventions have been shown to be effective in outpatient settings, especially brief patient advice utilizing a smoking cessation model.4–8. However, the latter studies frequently fail to establish the independent contribution of outpatient behavioral interventions, which are often confounded with the quality of care the patient has received leading up to the behavior change. To date, few researchers have addressed the problem of gauging the independent and cumulative effects on intermediate and long-term outcomes of interventions that link hospital and outpatient care. Thus, we hypothesized that post-hospitalization health status and behavior of ACS patients would be influenced by the intervention, and by patient level variables, such as illness severity, depressive symptoms and functioning, socio-economic characteristics and prior smoking behaviors as well as hospital treatments. We report results of a trial of a six-session telephone intervention conducted in the first three months after hospitalization for acute coronary syndrome (ACS), one year after successful implementation of a hospital quality improvement (QI) program. All patients received care enhanced by the QI program. The hospitals and the quality improvement program (guidelines applied to practice [GAP]) have been described previously.9 To determine the independent effects of the outpatient coaching intervention, we randomized patients to two groups: 1) a six-session telephone coaching intervention in addition to in-hospital QI (QI-plus) and 2) hospital QI alone (QI-only).
METHODS
Participants
A sample of 719 subjects consented and were enrolled by trained nurse recruiters from January 14, 2002 through April 13, 2003 at five community hospitals in two geographically contiguous Michigan communities, with comparable patient demographics, employment patterns, insurance coverage, proportions of minority, unemployed and low-income residents.10–13 All five were community teaching hospitals. Based on peer grouping criteria, three were large hospitals and two were moderately sized hospitals (none were small volume hospitals). Four out of five had facilities for coronary artery bypass graft surgery. Four out of five had greater than 10% minority patients with ACS discharged per year.
Patient inclusion criteria were a working diagnosis of ACS in the medical record, a documented serum troponin I level greater than the upper limits of normal, and age greater than 21 years. Patients were excluded if unable to speak English or complete study interviews, or discharged to a non-home setting.
Randomization
Patients who agreed to participate were randomized within each hospital to QI-only or QI-plus. Randomization was originally begun at recruitment for the first 98 patients (19% of baseline interviewed) patients, but was changed to post-baseline interview for the remaining 427 (81%) to correct an imbalance in study group sizes. The imbalance in study group sizes developed not as a result of the initial (blocked) randomization, but from unknown factors related to the logistics of interviewer contacts, which happened to be more successful among subjects assigned to the intervention than the control groups. Based on comparison of demographic variables, we cannot find any introduced bias. The study was approved by the University Committee on Research Involving Human Subjects of Michigan State University and by each hospital IRB before study data were collected.
Intervention
Participants were recruited from hospitals that participated in the American College of Cardiology Guidelines Applied to Practice (GAP) QI program one year prior to the present trial.9 Both intervention and control groups received the QI program by virtue of having been admitted to GAP hospitals. The added counseling intervention was provided to the QI-plus intervention group only. The two programs are described in Table 1 according to the American Heart Association Taxonomy for Disease management.14
Table 1.
Comparison of QI only* and QI Plus Coaching†
| Amer. Heart Assoc. Taxonomic Domain‡ | Hospital QI (GAP) | Coaching added to QI (HARP) |
|---|---|---|
| Intervention recipient | Providers | Patients |
| Patient population | Recruited patients with ACS admitted to 5 hospitals between 14 Jan 2002 and 13 April 2003 | Random sample of recruited ACS patients from five hospitals between 14 Jan. 2002 and 13 April 2003 |
| Intervention content | Hospital kick off event/Grand Rounds site visit; Guideline oriented tools to improve adherence to key quality indicators; Rapid cycle quality improvement; | Initial patient contact approximately 2 weeks post-hospital discharge; 6 weekly sessions, 15- to 30-minutes each (behavioral staging, goal setting, relapse prevention, and social support), 25-page booklet describing achievable risk reduction, goal sheets |
| Identification and assignment of physician and nurse opinion leader; pre- and post- measurement of quality indicators | ||
| Delivery personnel | Oversight committee (clinician leaders, community health coalition, peer review organization) | One health educator (coach) trained in behavior change and motivational counseling |
| Implementation teams in each hospital | ||
| Method of communication | Physician and nurse leaders in each hospital through meetings periodically over one year | Telephone counseling |
| Intensity and complexity | Added clinician time per patient was minimal | Time per patient was 1.5–3.0 hours total for six sessions |
| Environment | Hospital-based, in-patient | Home-based, outpatient |
| Clinical outcomes | Patient behavior, functional status, quality of life | Patient behavior, functional status, quality of life |
GAP QI-Only GAP is a translational program shown to improve physician adherence to guidelines, but ends at discharge.3,9 GAP patients received a written discharge contract listing recommended outpatient medications, cardiac rehabilitation recommendations, and health behavior changes (smoking cessation, diet modification, and exercise), as well as numerical values for ejection fraction and cholesterol. Both discharge planner and patient signed the contract and the patient received a copy.
HARP QI-Plus Telephone Coaching The Heart After-Hospital Recovery Planner (HARP) intervention was adapted from a successful smoking cessation program,8 and was designed for multiple risk behaviors. Patients in the QI-plus arm received a six-session health behavior change telephone counseling program delivered by a trained health educator (the coach) during the first three months after discharge. Primary behavior goals included: reduction or elimination of smoking, increasing physical activity, and eating a healthier diet. Coaching telephone sessions averaged 15 to 30 minutes and occurred weekly for six weeks. Behavior change strategies included behavioral staging, motivational interviewing, goal setting, relapse prevention, and obtaining social support.15–18 Patients were encouraged to identify at least one current behavior they intended to improve and set weekly goal(s). Patients who selected smoking cessation were encouraged to use pharmacotherapy for assistance with cessation. Each patient and his/her family received an information booklet and goal worksheets, described elsewhere.19
The content of the program was standardized through a semi-structured counseling program, the adoption of individual goal sheets, and the use of the same counselor for all participants. The program was thus tailored to each patient’s goals. The content of patient goals is described in detail elsewhere.19 Of the 175 patients entering the program, all completed more than four sessions, with a mean number of sessions of 5.9 (SD = 0.34). Those who did not enter the program after randomization ( = 93) were not different from those that did complete the program, with the exception of the non-completers were less likely to have a BMI over 30 (24.5% versus 9.9%; = .010). Reasons for non-participation in the program included: telephone change/disconnected (33%), changed mind and declined participation (18%), unable to contact due to privacy manager/caller ID (14%), unspecified reasons (19%), too ill (13%), and patient moved (5%).
Measures
Primary outcomes are secondary prevention behaviors; secondary outcomes are physical functioning and quality of life. Predictor variables to control for study baseline health status include severity of illness, socio-economic status, and hospital treatment. Race and ethnicity were assessed using the US Census self-report classification.
Secondary prevention behaviors Physical activity, weight loss, and smoking were assessed on a stages-of-change scale.19–21 Self-report physical activity asked whether the patient was engaging in physical activity for a total of at least 30 minutes a day at least five days a week. Responses on the five-item scale were re-coded into two categories: 1 = Engaging in the behavior (action or maintenance) or 0 = Not Engaging in the behavior (pre-contemplation, contemplation or preparation). Smoking status at the time of hospitalization was established by: 1) medical record (history of current and past smoking), and 2) the baseline interview. In the three-month and eight-month interviews, smoking status was divided into three categories: current smoker, former smoker/quitter, and non-smoker (never smoked). In-hospital and discharge medications were obtained from the medical record.
Physical functioning was measured by the Duke Activity Status Index (DASI), a weighted composite score computed from answers to questions about 12 activities of daily living of progressive intensity.22 DASI scores range from 0 to 58.2, with a higher score indicating greater functional capacity.
The quality of life EQ-5D measure evaluates: 1) mobility, 2) self-care, 3) usual activity, 4) pain/discomfort, and 5) anxiety/depression.23 It produces a score in which full health has a value of 1 and death has a value of 0.
Health status ACS case-mix was evaluated by abstracting from the medical record 1) ejection fraction (EF) and 2) comorbidity count. Ejection fractions were dichotomized at EF above and below 35%.22 Charlson comorbidity index (CCI) definitions were used, with the index ACS episode not counted as a comorbidity.24 The distribution of comorbid conditions is lopsided (Table 2). Since comorbidity effects are often non-linear, the discrete categorical variable (0–1, 2–3, ≥4) was used because it captures these effects better than a continuous variable.
Table 2.
Sample Characteristics at Baseline
| Intervention = 268 | Control = 257 | -value | |
|---|---|---|---|
| Sex: | |||
| Female | 173 (65%) | 161 (63%) | |
| Male | 95 (35%) | 96 (38%) | 0.65 |
| Education, years (mean [SD]) | 12.5 [2.3] | 12.6 [2.3] | 0.58 |
| Marital status: | |||
| Married | 183 (68%) | 167 (65%) | |
| Not married | 85 (32%) | 89 (35%) | 0.46 |
| Race: | |||
| Non-Hispanic white | 228 (85%) | 215 (84%) | 0.50 |
| African American | 32(11.9%) | 28(10.9%) | |
| Hispanic white | 5(1.9%) | 7(2.7%) | |
| American Indian/Aleuts | 3(1.1%) | 7(2.7%) | |
| Patient age (mean [SD]) | 59.0 [12.0] | 60.5 [11.9] | 0.93 |
| Drinks alcohol | 75% | 78% | 0.47 |
| Smoking status | |||
| Never smoked | 61 (23%) | 80 (31%) | |
| Quit before entering index hospital | 125 (47%) | 107 (42%) | 0.10 |
| Smoked when entering index hospital | 82 (31%) | 70 (27%) | |
| # of Co-morbid conditions | |||
| 0–1 | 145 (54%) | 140 (55%) | |
| 2–3 | 106 (40%) | 100 (39%) | 0.99 |
| 4+ | 17 (6%) | 16 (6%) | |
| Ejection fraction | |||
| 15–34 | 23 (9%) | 27 (11%) | |
| 35–44 | 44 (16%) | 39 (15%) | |
| 45+ | 160 (60%) | 160 (60%) | |
| Missing info | 41 (15%) | 31 (12%) | 0.62 |
| Most invasive hospital procedure | |||
| None | 21 (8%) | 22 (9%) | |
| Catheterization | 51 (19%) | 56 (22%) | |
| PTCA | 127 (48%) | 126 (50%) | |
| CABG | 65 (25%) | 50 (20%) | 0.57 |
| Quality of life (EuroQol)* | 0.75 | 0.74 | 0.55 |
| Functional status (DASI)† | 29.12 | 30.00 | 0.55 |
| Depression (CESD)‡ | 14.00 | 13.09 | 0.32 |
Depression was measured by the Centers for Epidemiologic Studies of Depression (CES-D), a 20-item symptom measure with a range from 0 (no depressive symptoms) to 60 (highest level of symptoms). A CES-D score of 16 or greater is highly correlated with a diagnosis of depression.25
Data Collection
Study data were collected from 1) medical records, 2) post-discharge patient telephone surveys, and 3) State of Michigan Vital Records (mortality). Nurse medical record reviewers in each hospital were supervised by the project field manager. The nurses developed the code book together, practiced on pilot charts, and refined the project code book in an iterative process. The project field manager continued to sample each reviewer’s charts to maintain quality control. Reliability ≥98% was maintained throughout.
Telephone surveys lasting approximately 30 to 40 minutes were conducted by survey researchers from the Michigan State University (MSU) Institute for Public Policy and Social Research. The baseline interview was conducted within four weeks of discharge (mean = 14.1 days, SD = 9.6). Telephone surveys assessed medical care utilization, medication use, hospital readmissions, emergency department visits, cardiac rehabilitation program participation, and secondary prevention behaviors, at each interview. Medication use was assessed in the survey by asking patients to collect the bottles of each of their currently used prescription medications and read the names and dosages to the interviewer. Data collectors were blinded to group membership (QI-only or QI-plus).
Outcome assessments were obtained from the three-month and eight-month interviews and from State of Michigan Vital Records (mortality).
Statistical Analysis
We employed pooled-time series generalized estimating equations to analyze the survey data. This approach allows for: 1) regression models with multiple link functions to accommodate either categorical or continuous outcome variables (such as the health status and behavioral variables), 2) the incorporation of correlated errors to accommodate within-subject variation between the baseline and follow-up interviews, and 3) regression models with all available observations as well as intention-to-treat analysis, applying the principle of last observation carried forward.26,27 Overall, we employed five behavioral outcome variables to test for post-intervention differences between the intervention and control groups. To reduce the probability of obtaining significant results due to multiple tests, we applied a Bonferroni adjustment to the probability of a Type I error, taking into account that the outcomes variables were moderately correlated (average correlation 0.24). Consequently, the alpha-level for ‘statistical significance’ was set at 0.02. Similarly, for the ‘ultimate’ outcomes of the DASI and EQ5D, which correlated highly at = 0.6, the Bonferroni adjustment led to the adoption of an alpha value of 0.04.
For all main results, we have adequate power to detect a difference between groups. For two primary behavioral outcome measures, weight loss and physical activity, we have power of approximately 0.89 (Type I error of 0.05, a one-sided test of a 25% relative improvement). For the secondary outcomes, the DASI and EQ-5D, standardized effects sizes translate to 2 point (small ES) and 5 point (moderate ES) differences on the EQ-5D.28 For the DASI, this translates to mean differences of 3.44 and 8.6, respectively.
RESULTS
Baseline Characteristics
Of 719 ACS patients who met eligibility criteria and consented to participate, 525 (73%) could be re-contacted by phone and participated in the baseline interview, conducted, on average, two weeks after discharge. Attrition is shown in Figure 1. Patients who consented, but did not participate in the baseline interview differed from the interviewees in that they were older (63 years vs. 60 years, < .01) more likely to have received anti-anxiety medications (OR: 2.58, < 0.01), and were more likely to be minorities (OR: 2.02, < 0.01). Sample characteristics of the intervention ( = 268) and control groups ( = 257) participating in the baseline interview are shown in Table 2. There was no difference in attrition by group assignment. Fifteen post-discharge deaths occurred between baseline and the eight-month follow-up (intervention = 8, control = 7, = 0.84)
Figure 1.
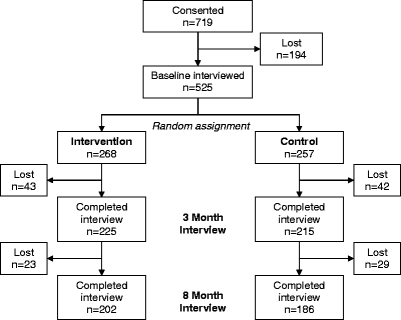
Patient attrition.
Secondary Prevention
There were no statistically significant differences in medication use between the intervention and control groups for beta blockers, aspirin, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, and lipid lowering medication at the three time points.
The intervention increased self-reported physical activity at 3 and 8 months (OR = 1.53, < 0.02) (Table 3). Differences in the odds of smoking cessation and weight loss participation were not statistically significant, and there were no difference in functional status or quality of life by intention-to-treat.
Table 3.
Behavioral Outcomes at 3 and 8 Months (GEE Logistic Regression Models: Multivariate Odds-ratios and [95% Confidence Intervals])
| Variables | Weight loss attempted† = 434║ | Quit smoking‡ = 136¶ | Phys. activity 150 min/week§ = 432† |
|---|---|---|---|
| Group assignment | |||
| Intervention vs. control | 1.08 [0.45–2.29] | 2.34 [0.44–12.5] | 1.53* [1.09–2.15] |
| Demographics | |||
| Age (in years) | 0.95* [0.89–0.99] | 1.01 [0.92–1.11] | 1.00 [0.99–1.02] |
| Female gender | 2.06* [1.02–6.09] | 1.61 [0.28–9.26] | 0.88 [0.67–1.16] |
| Minority race | 0.68 [0.16–2.38] | 1.19 [0.82–1.72] | 0.69 [0.39–1.22] |
| Married | 4.18* [1.96–15.8] | 1.07 [0.45–2.54] | 1.69* [1.11–2.57] |
| Years of education | 0.94 [0.78–1.21] | 0.66* [0.48–0.91] | 1.02 [0.95–1.10] |
| Clinical and health status characteristics | |||
| Ejection fraction: <35% # | |||
| 35–45% | 1.57 [0.24–12.6] | 4.54 [0.79–26.1] | 1.72 [0.85–3.48] |
| ≥45% | 4.05* [1.11–32.2] | 4.88 [0.28–85.1] | 2.03* [1.10–3.75] |
| Missing | 6.54* [1.45–98.8] | 0.74 [0.12–4.56] | 1.58 [0.77–3.24] |
| Charlson comorbidity index: | |||
| 0–1 # | |||
| 2–3 | 1.05 [0.36–2.78] | 0.37 [0.08–4.40] | 0.77 [0.52–1.14] |
| ≥4 | 1.01 [0.15–6.81] | 12.76 [0.34–479.9] | 0.79 [0.31–2.01] |
| Baseline funct. Status (DASI) | 0.99 [0.97–1.01] | 1.06* [1.01–1.11] | 0.99 [0.97–1.02] |
| Baseline depression (CESD) | 1.03 [0.99–1.07] | .97 [0.89–1.06] | 0.99 [0.97–1.01] |
| Smoking Status | |||
| Current smoker # | |||
| Former smoker | 6.86* [1.74–27.0] | 1.70 [0.89–3.25] | |
| Non-smoker | 7.82* [1.72–35.6] | 1.89 [0.92–3.88] | |
| Hospital procedures | |||
| No invasive procedure # | |||
| CATH | 6.36* [1.32–30.6] | 15.75 [0.29–855.4] | 1.57 [0.68–3.62] |
| PTCA | 7.50* [1.84–30.6] | 6.60 [0.38–114.6] | 1.70 [0.78–3.71] |
| CABG | 2.79 [0.42–18.5] | 98.71* [1.32–7382] | 2.23 [0.92–5.41] |
*statistically significant ( ≤ .05)
†Respondents who engaged (action or maintenance) in a weight loss regimen
‡Respondents who quit (action or maintenance) smoking at time of data collection
§Respondents who engaged (action or maintenance) in physical activity for 30 minutes 5 days a week
║Out of N = 440 who participated in the 3-month interview and N = 388 who participated in the 8-month interview, due to missing information on predictor variables,
¶Subset of smokers at the time of hospitalization who participated in panel survey
#reference category
CONCLUSIONS
The GAP QI intervention was previously shown to be successful in increasing physician adherence to guidelines in-hospital, and to reduce mortality.3,9,29 We evaluated an added telephone counseling intervention post-discharge. Over a period of 8 months after hospitalization, we found the telephone counseling intervention to add minimally to self-reported behavior change during the intervention, with no significant changes in health status or quality of life. While we found a small difference in physical activity in the intervention group, our results are largely negative. We conclude that a telephone counseling intervention added to hospital QI during the 3-month period post-hospitalization failed to produce a meaningful advantage in terms of health status and quality of life.
What is the explanation for our negative results? One explanation could be that the intervention approach itself was not a good match for these patients. While our follow-up intervals exceeded the usual three-month interval found in most studies, it is possible that the increased physical activity reported, if maintained for an even longer time period, could result in health status and quality-of-life benefits. However, our previous success in smoking cessation in primary care did not carry over to multiple risk factor intervention post-hospitalization for ACS.30–32 This approach did not produce gains in physical activity, weight, functional status or quality of life beyond what patients accomplished spontaneously with medical management.
We believe the main explanation for the failure of the telephone intervention to show additional benefits is that it came on top of an ongoing QI program in which patients consistently received standard in-hospital counseling. This suggests that for the majority of patients, instruction in hospital appears to have been important and effective, and that additional counseling outside the context of follow-up office care added only a little benefit. It may well be that, at least following ACS, patients largely followed the discharge advice, including relatively high medication adherence. The QI protocol required in-hospital counseling and a discharge patient contract that provided the patient with numerical values for ejection fraction and cholesterol, and made medication and behavior change recommendations.
Do we conclude that telephone-delivered health behavior change is not effective in accomplishing secondary prevention? In part, the answer is yes. We show that in our study, telephone counseling for patients added little when the clinicians were participating in rigorous quality improvement. In other settings that do not have active QI protocols, telephone counseling may act as a “reminder” to a patient to raise guideline-specific issues with a clinician, though we are not aware of research testing this approach. Putting our results in a broader context, they are consistent with a recently published Cochrane review of telephone follow-up following discharge from hospital. The review included 33 randomized trials or quasi-randomized trials that followed a total of 5,110 patients.33 The review authors found the studies to be of generally low methodological quality that varied widely in terms of type of health professional involved. However, they concluded that overall, clinically equivalent results were found in the telephone follow-up and control groups. Our study is more rigorous, followed patients for eight months rather than three, and followed a known standardized quality improvement protocol in the five hospitals. Our findings corroborate the more tentative ones of Mistiaen and Poot in the Cochrane review33. This suggests that where resource allocation choices are being made, higher payoff for secondary prevention post-hospital may be found in consistent delivery of guideline-consistent care.
What is the impact of other patient-oriented interventions? Several reviews of related standardized, written patient information show clear improvement in patient knowledge.34,35 Reviews of patient-centered care, and coaching show improvements in patient satisfaction and question asking.36,37 Free-standing patient-oriented behavior change interventions, however, show limited impact on behavior and health outcomes, including layperson led chronic disease self-management courses.38,39 At the same time, some disease-specific interventions that provide information to both clinician and patient at the point of care do show impact on health outcomes in at least some patient-oriented chronic disease interventions.40,41
We conclude that patient-oriented interventions, to be both effective and efficient must be integrated into on-going medical care. Whether or not brief behavior change interventions can contribute substantially to clinical care beyond the clear success of smoking cessation requires further testing. Future research should investigate both brief and intensive behavior change interventions that are well-integrated into care delivery systems.
Acknowledgments
Special thanks to Chrystal Price, MS, for data entry, Camille Proden, RN, BSN, community project manager, for supervision of field staff, study recruitment and medical record auditing, and to Cynthia Alderson (deceased) for project management. The sponsor had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation review or approval of the manuscript. Presented in part at: the Society for General Internal Medicine Annual Meeting, 2006.
Funding/Support Supported in part by an AHRQ R01 grant (HS10531), “Translating Research: Patient Decision Support/Coaching” (Dr. Margaret Holmes-Rovner, Principal Investigator). None of the authors receives compensation from any of the hospitals studied. No consultancies, honoraria, stock ownership, expert testimony, grants received, grants pending, patients pending, patients received or royalties or other relationships represent a conflict of interest for any of the authors.
References
- 1.Smith SC Jr., Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Circulation. 2006;113:2363–72. [DOI] [PubMed]
- 2.Peterson ED, Roe MT, Mulgund J, et al. Association between hospital process performance and outcomes among patients with acute coronary syndromes. JAMA. 2006;295:1912–20. [DOI] [PubMed]
- 3.Mehta RH, Montoye CK, Gallogly MJ, et al. Improving quality of care for acute myocardial infarction: The Guidelines Applied in Practice (GAP) Initiative. JAMA. 2002;287:1269–76. [DOI] [PubMed]
- 4.Koertge J, Weidner G, Elliott-Eller M, et al. Improvement in medical risk factors and quality of life in women and men with coronary artery disease in the Multicenter Lifestyle Demonstration Project. Am J Cardiol. 2003;91:1316–22. [DOI] [PubMed]
- 5.Steptoe A, Kerry S, Rink E, Hilton S. The impact of behavioral counseling on stage of change in fat intake, physical activity, and cigarette smoking in adults at increased risk of coronary heart disease. Am J Public Health. 2001;91:265–9. [DOI] [PMC free article] [PubMed]
- 6.Stead LF, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database of Systematic Reviews. 2006 Issue 3.Art. No. CD002850 doi:10.1002/14651858. CD002850.pub2. [DOI] [PubMed]
- 7.Vale MJ, Jelinek MV, Best JD, et al. Coaching patients On Achieving Cardiovascular Health (COACH): a multicenter randomized trial in patients with coronary heart disease. Arch Intern Med. 2003;163:2775–83. [DOI] [PubMed]
- 8.Wadland WC, Soffelmayr B, Ives K. Enhancing smoking cessation of low-income smokers in managed care. J Fam Pract. 2001;50:138–44. [PubMed]
- 9.Mehta RH, Montoye CK, Faul J, et al. Enhancing quality of care for acute myocardial infarction: shifting the focus of improvement from key indicators to process of care and tool use: the American College of Cardiology Acute Myocardial Infarction Guidelines Applied in Practice Project in Michigan: Flint and Saginaw Expansion. J Am Coll Cardiol. 2004;43:2166–73. [DOI] [PubMed]
- 10.Watson RE, Stein AD, Dwamena FC, et al. Do race and gender influence the use of invasive procedures? J Gen Intern Med. 2001;16:227–34. [DOI] [PMC free article] [PubMed]
- 11.Barber K, Stommel M, Kroll J, Holmes-Rovner M, McIntosh B. Cardiac rehabilitation for community-based patients with myocardial infarction: factors predicting discharge recommendation and participation. J Clin Epidemiol. 2001;54:1025–30. [DOI] [PubMed]
- 12.Dwamena FC, El Tamimi H, Watson RE, et al. The use of angiotensin-converting enzyme inhibitors in patients with acute myocardial infarction in community hospitals. Michigan State University Inter-Institutional Collaborative Heart (MICH) Study Group. Clin Cardiol. 2000;23:341–6. [DOI] [PMC free article] [PubMed]
- 13.Census Bureau. County Population Datasets. State of Michigan. 2006. Lansing Michigan.
- 14.Krumholz HM, Currie PM, Riegel B, Phillips, CO, Peterson ED, Smith R, et al. A taxonomy for disease management: a scientific statement from the American Heart Association Disease Management Taxonomy Writing Group. Circulation. 2006;114:1432–45. [DOI] [PubMed]
- 15.Irvin JE, Bowers CA, Dunn ME, Wang MC. Efficacy of relapse prevention: a meta-analytic review. J Consult Clin Psychol. 1999;67:563–70. [DOI] [PubMed]
- 16.Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. J Consult Clin Psychol. 2003;71:843–61. [DOI] [PubMed]
- 17.Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. Applications to addictive behaviors. Am Psychol. 1992;47:1102–14. [DOI] [PubMed]
- 18.Britt E, Hudson SM, Blampied NM. Motivational interviewing in health settings: a review. Patient Educ Couns. 2004;53:147–55. [DOI] [PubMed]
- 19.Holtrop JS, Corser W, Jones G, Brooks G, Holmes-Rovner M, Stommel M. Health behavior goals of cardiac patients after hospitalization. Am J Health Behav. 2006;30:387–99. [DOI] [PubMed]
- 20.Boyle RG, O’Connor PJ, Pronk NP, Tan A. Stages of change for physical activity, diet, and smoking among HMO members with chronic conditions. Am J Health Promot. 1998;12:170–5. [DOI] [PubMed]
- 21.Cardinal BJ. Behavioral and biometric comparisons of the preparation, action, and maintenance stages of exercise. Wellness Perspect. 1995;11:37–43.
- 22.Hlatky MA, Boineau RE, Higginbotham MB, Lee KL, Mark DB, Califf RM, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol. 1989;64:651–4. [DOI] [PubMed]
- 23.Kind P, Dolan P, Gudex C, Williams A. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ. 1998;316:736–41. [DOI] [PMC free article] [PubMed]
- 24.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. [DOI] [PubMed]
- 25.Radloff L. The CES-D Scale: a self-report depression scale for research in the General Population. Appl Psychol Measuremt. 1997;1:385–401. [DOI]
- 26.Diggle P, Heagarty P, Lian K-Y, Zeger S. Analysis of Longitudinal Data, 2nd edn. New York: Oxford University Press; 2002.
- 27.Everitt PS, Pickles MP. Statistical Aspects of the Design and Analysis of Clinical Trials. London: Imperial College Press; 2004.
- 28.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale: Erlbaum; 1988.
- 29.Eagle KA, Montoye CK, Riba AL, DeFranco AC, Parrish R, Skorcz, S, et al. Guideline-based standardized care is associated with substantially lower mortality in medicare patients with acute myocardial infarction: the American College of Cardiology’s Guidelines Applied in Practice (GAP) Projects in Michigan. J Am Coll Cardiol. 2005;46:1242–8. [DOI] [PubMed]
- 30.Wadland WC, Holtrop JS, Weismantel D, Pathak PK, Fadel H, Powell J. Practice-based referrals to a tobacco cessation quit line: assessing the impact of comparative feedback vs general reminders. Ann Fam Med. 2007;5:135–42. [DOI] [PMC free article] [PubMed]
- 31.Holtrop JS, Wadland WC, Vansen S, Weismantel D, Fadel H. Recruiting health plan members receiving pharmacotherapy into smoking cessation counseling. Am J Manag Care. 2005;11:501–7. [PubMed]
- 32.Woolf SH, Glasgow RE, Krist A, Bartz, C, Flocke SA, Holtrop, JS, et al. Putting it together: finding success in behavior change through integration of services. Ann Fam Med. 2005;3(Suppl 2):S20–7. [DOI] [PMC free article] [PubMed]
- 33.Mistiaen P, Poot E. Telephone follow-up, initiated by a hospital-based health professional, for postdischarge problems in patients discharged from hospital to home. Cochrane Database of Systematic Reviews 2006, Issue 4. Art. No.:CD004510. doi:10.1002/14651858.CD004510.pub3. [DOI] [PMC free article] [PubMed]
- 34.O’Connor AM, Bennett C, Stacey D, Barry, MJ, Col, NF, Eden, KB, et al. Do patient decision aids meet effectiveness criteria of the international patient decision aid standards collaboration? A systematic review and meta-analysis. Med Decis Mak. 2007;27:554–74. [DOI] [PubMed]
- 35.Johnson A, Sandford J, Tyndall J. Written and verbal information versus verbal information only for patients being discharged from acute hospital settings to home. Cochrane Database of Systematic Reviews 2003, Issue 4. Art. No.: CD003716. doi:10.1002/14651858.CD003716. [DOI] [PMC free article] [PubMed]
- 36.Lewin SA, Skea ZC, Entwistle V, Zwarenstein M, Dick J. Interventions for providers to promote a patient-centred approach in clinical consultations. Cochrane Database of Systematic Reviews. 2001, Issue 4. Art. No.CD003267 doi:10.1002/14651858.CD003267. [DOI] [PubMed]
- 37.Wetzels R, Harmsen M, van Weel C, Grol R, Wensing M. Interventions for improving older patients’ involvement in primary care episodes. Cochrane Database of Systematic Reviews 2007, Issue 1. Art. No.: CD004273. doi:10.1002/14651858.CD004273.pub2. [DOI] [PMC free article] [PubMed]
- 38.Lorig KR, Ritter PL, Laurent DD, Fries JF. Long-term randomized controlled trials of tailored-print and small-group arthritis self-management interventions. Med Care. 2004;42:346–54. [DOI] [PubMed]
- 39.Foster G, Taylor SJC, Eldridge S, Ramsay J, Griffiths CJ. Self-management education programmes by lay leaders for people with chronic conditions. Cochrane Database of Systematic Reviews 2007, Issue 4. Art. No.: CD005108. doi:10.1002/14651858.CD005108.pub2. [DOI] [PubMed]
- 40.O’Connor AM, Stacey D, Entwistle V, Llewellyn-Thomas H, Rovner D, Holmes-Rovner M, Tait V, Tetroe J, Fiset V, Brry M, Jones J. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Systematic Reviews 2003, Issue 1. Art. No.: CD001431. doi:10.1002/14651858. CD001431. [DOI] [PubMed]
- 41.O’Connor AM, Rostom A, Fiset V, Tetroe J, Entwistle V, Llyewellyn-Thomas, H, et al. Decision aids for patients facing health treatment or screening decisions: systematic review. BMJ. 1999;319:731–4. [DOI] [PMC free article] [PubMed]


