Abstract
Background
Hospitalists improve efficiency, but little information exists regarding whether they impact quality of care.
Objective
To determine hospitalists’ effect on the quality of acute congestive heart failure care.
Design and Participants
Using data from the Multicenter Hospitalist Study, we retrospectively evaluated quality of care in patients admitted with congestive heart failure who were assigned to hospitalists (n = 120) or non-hospitalists (n = 252) among six academic hospitals.
Measurements
Quality measures included the percentage of patients who had ejection fraction (EF) measurement, received appropriate medications [i.e., angiotensin-converting enzyme inhibitor (ACE-I) or beta-blockers] at discharge, measures of care coordination (e.g., follow-up within 30 days), testing for cardiac ischemia (e.g., cardiac catheterization), as well as hospital length of stay, cost, and combined 30-day readmissions and mortality.
Results
Compared to non-hospitalist physicians, hospitalists’ patients had similar rates of EF measurement (85.3% vs. 87.5%; P = 0.57), ACE-I (91.5% vs. 88.0%; P = 0.52), or beta-blocker (46.9% vs. 42.1%; P = 0.57) prescriptions. Multivariable adjustment did not change these findings. Hospitalists’ patients had higher odds of 30-day follow-up (adjusted OR = 1.83, 95% CI, 1.44 – 2.93). There were no significant differences between the groups’ frequency of cardiac testing, length of stay, costs, or risk for readmission or death by 30-days.
Conclusion
Academic hospitalists and non-hospitalists provide similar quality of care for heart failure patients, although hospitalists are paying more attention to longitudinal care. Future efforts to improve quality of care in decompensated heart failure may require attention towards system-level factors.
KEY WORDS: health services research, congestive heart failure, quality of care, hospital medicine, hospitalists
INTRODUCTION
Congestive heart failure affects 5 million Americans and costs 30 billion dollars annually1, with much of these costs incurred during approximately 1 million yearly hospitalizations.2 Given the prevalence and costs of congestive heart failure, inpatient quality of care for heart failure has become a focus of a number of organizations (e.g., the Joint Commission, Center for Medicare and Medicaid Services).3,4 Despite the growing emphasis, there is still substantial room for improvement in many health-care delivery systems.5,6
Previous studies suggest that physicians’ practice patterns influence quality, with cardiologists tending to have better performance on acute congestive heart failure process and outcome measures compared to generalists.7,8 However, generalist physicians continue to care for the majority of decompensated heart failure patients, and increasingly, the generalists providing this care are hospitalists.8,9 Previous authors hypothesized that hospitalists may improve quality because of increased experience with acute medical illnesses, increased availability to address changes in clinical status, or because hospitalists are more involved in hospital quality initiatives.10,11 Most studies of hospitalist systems have suggested cost and length of stay reductions with no adverse effects on readmissions or patient satisfaction.12,13 Few studies have examined care quality, with only one reporting hospitalists’ effect on quality of heart failure care.13
To explore the effect of hospitalists on care of patients with acute congestive heart failure, we examined data from a cohort of patients within the Multicenter Hospitalist (MCH) study. Our primary hypothesis was that hospitalist physicians would have higher completion rates of acute congestive heart failure quality of care processes compared to non-hospitalists. Additionally, we hypothesized that hospitalists would have decreased lengths of stay, costs, and 30-day combined readmissions and mortality.
METHODS
Sites The Multicenter Hospitalist (MCH) study was a prospective multicenter observational study of the effect of hospitalist care on patients admitted to general medical services. Patient enrollment occurred between 1 July 2001 and 30 June 2003 at six geographically diverse academic medical centers: University of Chicago, University of Wisconsin, University of California at San Francisco, Brigham and Women’s Hospital, University of Iowa, and University of New Mexico. These sites were selected because hospitalist or non-hospitalist physicians (none of whom were cardiologists) cared for the majority of patients admitted to the general medical service at each center, and because they primarily used a system where patients were admitted to attending physicians essentially at random, according to day of the week. The MCH study was reviewed and approved by each centers’ institutional review board.
MCH Study Patients Patients were eligible if they were admitted by a hospitalist or non-hospitalist physician, were 18 years of age or older, and were able to give consent themselves or had an appropriate proxy. Patients were admitted to a hospitalist or non-hospitalist based on a pre-determined call schedule. The MCH study excluded patients admitted specifically under the care of their primary care physician or specialty physician (e.g., oncologist), and those with Mini-Mental Status Examination score of 17 out of 22 or lower.15 Informed consent for chart abstraction and interviews was obtained from eligible patients.
Congestive Heart Failure Patients Within the MCH-eligible patients, we retrospectively identified those with heart failure using International Classification of Diseases (ICD-9) diagnosis codes (Appendix 1) assigned at discharge. Patients identified by ICD-9 codes were excluded if they had severe chronic obstructive pulmonary disease (defined as being oxygen dependent, on oral steroids, or having a forced expiratory volume in 1 s of <0.8 l/s), sepsis, fluid overload secondary to renal failure, constrictive pericardial disease, a cardiac surgery planned within 24 h of admission, or having had a thoracotomy in the preceding 2 weeks16.
Data Collection Data were obtained from administrative sources, patient interviews, chart abstractions, and the National Death Index (NDI) database. Administrative data were used to obtain dates of admission and discharge, diagnosis codes (used to identify patients with heart failure, as above), insurance type, age, race, and gender. Intake interviews collected socioeconomic information not available in administrative data (such as education), functional status, and comorbidity data. One month follow-up telephone interviews assessed whether or not the patient had any follow-up appointments or rehospitalizations.17 A NDI search was used to ascertain 30-day mortality from the date of hospital discharge.18
Congestive heart failure process and risk adjustment data (such as use of angiotensin-converting enzyme inhibitors at discharge or ejection fraction) were collected by chart abstraction. Principal investigators at each site were responsible for training and overseeing interviews and chart abstraction activities, with central oversight of data quality provided by the coordinating center.
Identification of Hospitalist and Non-Hospitalist Physicians Hospitalists were defined as physicians whose primary focus is the care of general medical hospitalized patients, and whose activities include patient care, teaching, or research.19 Non-hospitalist physicians were most often outpatient general internal medicine faculty or non-cardiology subspecialists, who typically attended 1 month per year. Physicians were classified as hospitalists or non-hospitalists according to designations provided by each site. Physician designation was confirmed by site coordinators and linked to the attending physician at discharge using administrative data files.
Quality Measures We selected three process measures available in guidelines present at the time of the MCH, many of which are elements of current public-reporting initiatives.3,4,20 These processes included: (1) measurement of left ventricular ejection fraction (LVEF) anytime before or during hospital admission documented either by formal imaging study report, or within admission documentation, (2) prescription of an angiotensin-converting enzyme inhibitor (ACE-I) or angiotensin receptor blocker (ARB) at discharge for patients with left ventricular ejection fraction lower than 40%, highest creatinine <3.0 and highest potassium <5.5 in the 48 h prior to discharge, and (3) prescription of beta-blocker in patients with LVEF <40%. Patients were excluded from the two discharge medication measures if there was a documented allergy or adverse reactions. In addition, patients who expired, left against medical advice, or were discharged to hospice were also excluded.
We also examined two care processes highlighting coordination of care. First we assessed whether or not patients reported during the 30-day interview a physician visit within a month of discharge, a recommended element of the longitudinal care of heart failure patients.21 We limited the analysis to physician visits identified by patients as having been scheduled upon discharge. Next, we assessed whether formal inpatient cardiac consultation was obtained, based on literature suggesting improved outcomes with cardiology specialist consultation.22,23
Finally, as more stringent measures of quality, we determined the percentage of eligible patients who received all three care processes (LVEF assessment, ACE-I use, and beta-blocker use) and those who received all five measures (the first three plus care coordination measures).
Cardiac Test Utilization Because guidelines recommend evaluation for cardiac ischemia in selected heart failure patients,21 we examined use of exercise stress testing (with or without scintigraphy or echocardiography) as well as the use of pharmacologic stress testing (with scintigraphy or echocardiography). In addition, we examined whether or not coronary artery catheterization, with or without percutaneous coronary interventions, was performed.
Cost, Length of Stay, and 30-Day Combined Readmissions and Mortality Length of stay and cost data were obtained from administrative cost-accounting systems maintained at each site. Readmission within 30 days was defined using readmissions identified in administrative records combined with data collected at time of 30-day follow-up phone call. To guard against recall bias, self-report data were only included for non-site admissions.24 Additionally, patients who died within 30 days, or were discharged to hospice, were excluded from the readmission measure. The 30-day post-discharge mortality measure included all deaths identified by the NDI at discharge and up to 30-days, excluding patients discharged to hospice. For purposes of increasing statistical power, we combined both 30-day measures into a single outcome measure.
Statistical Analysis We first compared patient characteristics using chi-square tests for categorical variables and t- or Mann-Whitney rank sum tests for continuous variables. A p-value of ≤0.05 was considered statistically significant.
Next, we performed multivariable analyses to determine the independent association between hospitalist care and the odds of the patient receiving any process or outcome measures, after adjusting for confounding variables and accounting for clustering at the physician level in generalized estimating equation (GEE) regression models. Cost and length of stay regression models were also fitted using GEE, but the models were built on gamma distributions with log-link functions.
We tested socio-demographic, co-morbidities, and physiologic variables for inclusion in each model (see Appendix 2). To minimize any potential bias or loss of power that might result from limiting the analysis to patients with complete data, we used the multivariate imputation by chained equations method of multiple imputation, as implemented in STATA, to create ten imputed datasets.25 Imputation of missing values was restricted to confounding variables. Standard methods were then used to combine results over the ten imputed datasets and to compute summary standard errors, confidence intervals, and p-values taking account of the additional uncertainty due to imputation. We also performed a complete-case analysis as a check on the sensitivity of our results to the missing data.
Covariates were considered for inclusion in multivariable models if they were associated with the primary predictor or outcome of interest at a statistical significance of p ≤ 0.10 in unadjusted analysis. Backwards deletion was then used to select final adjusted models, retaining variables reaching statistical significance at p ≤ 0.05. To further guard against confounding, we included site of care, the strongest correlate of the primary predictor, as well as cardiac consultation (when not treated as an outcome itself) in models with a minimum of 50 events. All analyses were performed using STATA 9.0 (StataCorp, College Station, TX).
RESULTS
Patient Characteristics A total of 31,000 patients were screened for entry into MCH; 896 (2.9% overall) were admitted to a hospitalist or non-hospitalist and met ICD-9 criteria for congestive heart failure. Informed consent was obtained from 567 of heart failure patients of whom we excluded 147 patients because of clinical criteria and 48 patients because their records were unavailable or incomplete, yielding a final cohort of 372 (Fig. 1).
Figure 1.
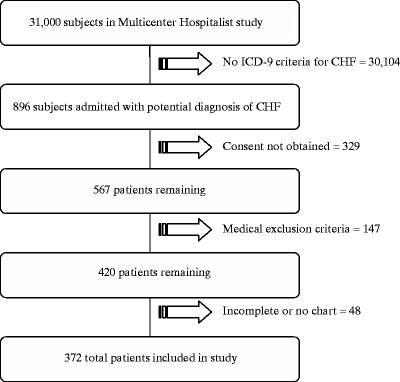
Eligible subjects for multicenter hospitalist study-congestive heart failure cohort.
Hospitalists cared for 120 (32%) patients and non-hospitalists for 252 (68%). Hospitalist and non-hospitalist physicians’ patients were similar (Table 1), although the proportions of heart failure patients in our study varied according to site.
Table 1.
Patient Characteristics
| Characteristic | Non-hospitalist, no. (%) or mean (SD) N = 252 | Hospitalist, no. (%) or mean (SD) N = 120 | P-value‡ |
|---|---|---|---|
| Age, years | |||
| <60 | 78 (30.9) | 45 (27.5) | |
| 60-74 | 75 (29.8) | 32 (26.7) | 0.45 |
| ≥75 | 99 (39.3) | 43 (35.8) | |
| Male sex | 97 (38.5) | 58 (48.3) | 0.07 |
| White race | 83 (32.9) | 49 (40.8) | 0.14 |
| >High school education (N = 301) | 60 (29.1) | 32 (33.7) | 0.43 |
| Self-reported salary (N = 171) | |||
| $0–25,000 | 58 (53.7) | 37 (58.7) | 0.52 |
| >$25,000 | 50 (46.3) | 26 (41.3) | |
| Insurance status (N = 347) | |||
| Medicare/privately insured | 173 (72.4) | 78 (72.2) | |
| Medicaid | 40 (16.7) | 18 (16.7) | 1.0 |
| Uninsured | 26 (10.9) | 12 (11.1) | |
| Site of enrollment | |||
| (A) | 149 (59.1) | 35 (29.2) | |
| (B) | 24 (9.5) | 35 (29.2) | |
| (C) | 13 (5.2) | 17 (14.2) | |
| (D) | 26 (10.3) | 13 (10.8) | <0.001 |
| (E) | 14 (5.6) | 9 (7.5) | |
| (F) | 26 (10.3) | 11 (9.2) | |
| Self-reported medical history | |||
| Heart disease* (N = 333) | 157 (70.1) | 87 (79.8) | 0.06 |
| COPD/asthma (N = 334) | 69 (30.8) | 31 (28.2) | 0.62 |
| Diabetes (N = 334) | 89 (39.7) | 41 (37.3) | 0.67 |
| Hypertension (N = 334) | 148 (66.1) | 71 (64.5) | 0.78 |
| Recent cancer, <3 years (N = 334) | 28 (12.5) | 7 (6.4) | 0.09 |
| Charlson comorbidity score | |||
| 0 | 80 (31.8) | 43 (35.8) | |
| 1–2 | 130 (51.6) | 53 (44.2) | 0.40 |
| ≥3 | 42 (16.7) | 24 (20.0) | |
| Medication prior to admission | |||
| Digoxin | 50 (19.8) | 29 (24.2) | 0.34 |
| Diuretics | 185 (73.4) | 76 (63.3) | 0.05 |
| ACE-inhibitor or ARB | 129 (51.2) | 62 (51.7) | 0.93 |
| Beta-blocker (N = 371) | 106 (42.1) | 48 (40.0) | 0.75 |
| Spironolactone | 26 (10.3) | 5 (4.2) | 0.05 |
| Aspirin | 125 (49.6) | 56 (46.7) | 0.60 |
| Heart failure severity | |||
| Hospitalized in past 12 months for congestive heart failure (N = 361) | 133 (54.7) | 63 (53.4) | 0.81 |
| NYHA Class III or IV (N = 208)† | 53 (37.6) | 28 (41.8) | 0.56 |
| LV function moderately to severely reduced or <40% (N = 320) | 103 (47.9) | 59 (56.2) | 0.16 |
| Vital signs and laboratory data, first 24 h | |||
| Highest creatinine, mg/dL (N = 371) | 1.7 (1.2) | 1.7 (0.9) | 0.40 |
| Lowest SBP, mmHg (N = 371) | 116.5 (20.0) | 115.9 (21.8) | 0.83 |
| Highest heart rate - bpm (N = 371) | 95.0 (17.9) | 97.2 (19.2) | 0.29 |
*Heart disease includes coronary artery disease, angina, or congestive heart failure
†NYHA, New York Heart Association
‡P-values based on chi-Square test of statistical independence for categorical data, Student’s t-test for parametric data, or Mann-Whitney rank sum test for non-parametric data. Totals may not add to 100% due to rounding
Quality of Care Physicians assessed LVEF prior to discharge for 320 (86%) of 372 patients. Among these, 279 (88%) had LVEF assessed between hospital discharge and a year prior to admission. In both unadjusted and adjusted analyses, patients treated by hospitalist and non-hospitalists were equally likely to have LVEF measurement prior to hospital discharge. Results were unchanged after excluding LVEF assessment more than 1 year from admission. Unadjusted and adjusted rates of ACE-I/ARB or beta-blocker prescription at discharge were similar between groups (Table 2).
Table 2.
Care Quality Measures
| Measure | No. of eligible patients | Non-hospitalist cases, no. (%) | Hospitalist cases, no. (%) | Unadjusted odds of hospitalist (OR, 95% CI) | Adjusted odds of hospitalist (adjusted OR, 95% CI) |
|---|---|---|---|---|---|
| LVEF assessment* | 372 | 215 (85.3) | 105 (87.5) | 1.26 (0.61–2.63) | 0.75 (0.35–1.63)‡ |
| ACE-inhibitor and/or ARB prescription | 132 | 75 (91.5) | 44 (88.0) | 0.66 (0.22–1.96) | 0.37 (0.09–1.45)§ |
| Beta-blocker prescription | 153 | 45 (46.9) | 24 (42.1) | 0.82 (0.41–1.65) | 0.64 (0.26–1.58)║ |
| 30-day follow-up† | 275 | 106 (56.1) | 64 (74.4) | 2.03 (1.23–3.35) | 1.83 (1.14–2.93)¶ |
| Cardiology consultation | 372 | 61 (24.2) | 45 (37.5) | 1.90 (1.12–3.23) | 1.56 (0.94–2.59)# |
| Three of three measures | 129 | 32 (40.5) | 21 (42.0) | 1.06 (0.52–2.18) | 1.02 (0.40–2.62)** |
| Five of five measures† | 129 | 5 (6.3) | 8 (16.0) | 2.85 (0.87–9.31) | 2.48 (0.69–8.89)†† |
*LVEF, left ventricular ejection fraction
†Scheduled follow-up data only available from 275 30-day follow-up interviews
‡Adjustments: enrollment site, cardiology consultation, LVEF assessment prior to admission
§Adjustments: enrollment site, cardiology consultation, age, ACE-I/ARB use prior to admission, beta-blocker use prior to admission, lowest systolic blood pressure ≤90 mmHg in the 48 h prior to discharge
║Adjustments: enrollment site, cardiology consultation, age, male sex, beta-blocker use prior to admission, aspirin use prior to admission
¶Adjustments: enrollment site, cardiology consultation
#Adjustments: enrollment site, LVEF <40%, history of coronary artery disease, angina, or congestive heart failure
**Adjustments: enrollment site, cardiology consultation, beta-blocker use prior to admission, aspirin use prior to admission, highest systolic blood pressure in the 48 h prior to discharge
††Adjustments: age
Patients cared for by hospitalists had almost two-fold higher adjusted odds of reporting scheduled follow-up within 30 days of hospital discharge and statistically similar odds for cardiology consultation. Adjustment for individual study site accounted for the largest proportion of the change between unadjusted and adjusted analyses for each process measure. There were no adjusted differences in adherence to more stringent composite measures of quality (three of three or five of five measures).
Cardiac Test Utilization Use of non-invasive tests for ischemia was similar between groups. There were higher unadjusted odds of cardiac catheterization in unadjusted analysis; however, this difference was no longer statistically significant after multivariable adjustment (Table 3).
Table 3.
Cardiac Test Utilization
| Measure | Non-hospitalist cases, no. (%) | Hospitalist cases, no. (%) | Unadjusted odds of hospitalist (OR, 95% CI) | Adjusted odds of hospitalist (adjusted OR, 95% CI) |
|---|---|---|---|---|
| Non-invasive stress test (N=369) | 18 (7.2) | 12 (10.2) | 1.45 (0.70–3.03) | 1.17 (0.54–2.55)* |
| Cardiac catheterization | 16 (6.4) | 18 (15.0) | 2.56 (1.21–5.42) | 2.35 (0.97–5.71)† |
*Adjustments: cardiology consultation, diuretic use prior to admission
†Adjustments: cardiology consultation, history of chronic kidney disease, highest serum creatinine (mg/dl) in the first 24 h after admission
Length of Stay, Costs, and 30-Day Combined Readmissions and Mortality There were no statistically significant differences in unadjusted or adjusted length of stay, costs, or combined 30-day readmissions and mortality rates between physician groups (Table 4).
Table 4.
Length of Stay, Costs, 30-Day Readmission, and 30-Day Mortality
| Measure | No. of eligible patients | Non-hospitalist mean (95% CI) or no. (%) | Hospitalist mean (95% CI) or no. (%) | Unadjusted % change or OR hospitalist (95% CI) | Adjusted % change or OR hospitalist (95% CI) |
|---|---|---|---|---|---|
| Hospital length of stay (days) | 372 | 4.57 days (4.07 to 5.07) | 4.95 days (4.32 to 5.58) | 9.2% (-8.9% to 31.0%) | 12.3% (-5.8 to 33.9)* |
| Cost (dollars) | 372 | 7082 (6204 to 7960) | 7883 (6576 to 9189) | 11.3% (-9.0% to 36.0%) | 9.3% (-7.2% to 28.8%)† |
| Combined readmissions and 30-day mortality rate | 369 | 95 (38.2) | 31 (25.8) | 0.61 (0.35 to 1.06) | 0.90 (0.49 to 1.66)‡ |
*Adjustments: enrollment site, cardiology consultation, history of chronic obstructive pulmonary disease or asthma, Charlson comorbidity index, lowest systolic blood pressure in the first 24 h after admission
†Adjustments: enrollment site, cardiology consultation, residence in a nursing home, ACE-I/ARB use prior to admission, lowest systolic blood pressure in the first 24 h after admission
‡Adjustments: enrollment site, cardiology consultation, beta-blocker use prior to admission, highest serum creatinine (mg/dl) in the first 24 h after admission
Sensitivity Analyses Likelihood of receiving an ACE-inhibitor or ARB was not modified by inclusion of interactions between the physician specialty and patients’ creatinine or blood pressure. Similarly, the interaction between physician specialty and pulse or blood pressure was not significantly associated with receipt of beta-blockers. We also tested for a potential relationship between 30-day follow-up rates and use of procedures (e.g., cardiac catheterization), receiving a new medication, or worse physiologic measures (e.g., lower blood pressure) at discharge, and continued to note higher odds for follow-up in the hospitalist patient group. Finally, results for our complete case sensitivity analysis were essentially unchanged, with one exception: the adjusted odds for patient-reported scheduled 1-month follow-up were no longer statistically significant (OR = 1.62, 95% CI 0.98–2.68) in the complete case analysis, with a reduced sample of 228 patients.
DISCUSSION
Results from our study suggest that across a broad set of quality measures, the performance of hospitalist and non-hospitalist physicians was equivalent among patients with acute congestive heart failure. While quality of care for patients in our study was similar to studies published during the period of the MCH,5,6 our data suggest a substantial opportunity for improvement. However, even if care processes in the hospital were not different, hospitalists’ patients actually had follow-up more frequently, suggesting that the academic hospitalist model may have a more positive impact on longitudinal care than traditional academic inpatient models.
Our results stand in contrast to a community-based study suggesting improved heart failure processes and decreased length of stay in hospitalist systems;13 this difference is perhaps because of the effect of residents in our study. For example, housestaff play an important patient care role in each of the study’s academic centers. Common cultural and clinical practices among housestaff may influence quality performance more than practices of supervising physicians. Alternatively, the hospital systems in our study were likely quite different during the MCH than they were during the time when initial studies of hospitalist programs were published,12,17,26 with more experienced non-hospitalists, more inexperienced hospitalists, growing use of multidisciplinary rounds, standardization of orders, and better case coordination all being potential influences.
Concern about handoff problems and loss of continuity are key criticisms of hospitalist systems.27 However, we were reassured that patients cared for by hospitalists reported higher rates of follow-up after discharge. While our analyses cannot discern whether follow-up resulted in better outcomes, follow-up is a recommended step to improving decompensated heart failure care and reducing the likelihood of readmission.20,21 However, our data cannot discern other more subtle measures of discharge coordination, such as whether the patient understood plans for post-discharge care; without adequate communication, notations of follow-up may not be sufficient to avoid readmission or other adverse outcomes.27
In contrast to previous studies suggesting no effect of hospitalist systems on rates of specialty consultation,26,28 we noted some evidence, though not conclusive, that MCH hospitalists consulted cardiologists more often and more frequently ordered invasive tests. Increases in referrals for congestive heart failure may be because hospitalists were less comfortable caring for heart failure patients or because they were more attentive to reasons why cardiologist care would be needed (e.g., evaluation for revascularization), or recognition that cardiologists may improve quality of care and potentially outcomes as well,22,23 particularly in patients with active cardiac ischemia. This latter mechanism is suggested by a trend towards more frequent invasive tests in hospitalists’ patients. However, the number of cardiac catheterizations is small, and the appropriateness of testing for cardiac ischemia cannot be ascertained with our data.
Our study has several limitations. First, the MCH took place in six academic medical centers, and our findings may not be applicable to hospitalists in community settings. Second, due to a small sample size, our study has limited statistical power to observe small differences in less common outcomes (e.g., cardiac procedures); this is reflected in relatively wide confidence intervals for hospitalist effects on many study outcomes. In addition, without complete ejection fraction information or a full range of laboratory data (such as B-type natriuretic peptide), we may not have fully adjusted for acute severity of illness. However, our cohort was very balanced in most other observable characteristics, making the threat of potential bias very small. Finally, beta-blocker prescription at discharge was not formally recommended in guidelines for inpatient heart failure care at the time of the MCH, potentially weakening the association between this measure of performance and overall quality of care. Despite this, guidelines are known to lag behind clinical evidence, and at the time of the study, evidence for pre-discharge beta-blockers was mounting.29,30
In this first multicenter study comparing hospitalists and non-hospitalists in the care of acute congestive heart failure, we did not observe a strong effect of hospitalists on key quality measures, but hospitalists more frequently arranged for follow-up care. Whether our observations would change in an era when acute congestive heart failure measures are publicly reported and whether specific types of physicians respond differently to these newer pressures are worthy subjects for future study.
Acknowledgment
The authors would like to thank Eric Vittinghoff, PhD, for providing expert advice on the statistical methods used in this analysis. Funding for the Multicenter Hospitalist Study was supported by grant R01 HS10597 AHRQ from the Agency for Healthcare Research and Quality.
Dr. Auerbach is supported by a K08 research and training grant (K080 HS11416-02) from the Agency for Healthcare Research and Quality. Dr. Kaboli is supported by a Research Career Development Award from the Health Services Research and Development Service, Department of Veterans Affairs (RCD 03-033-1). The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Dr. Wetterneck is supported by a Clinical Research Scholars Award from the National Institutes of Health (1 K12-RR01764-01). Data were presented at the Society of General Internal Medicine Annual Meeting on 26 April 2007.
The Multicenter Hospitalist Study was registered at Clinicaltrials.gov: NCT00204048.
Conflict of Interest None disclosed.
Appendix 1
Table 5
Table 5.
Congestive Heart Failure International Classification of Diseases (ICD-9) Codes and Descriptions
| ICD-9 code | ICD-9-CM description |
|---|---|
| 402.01 | Hypertensive heart disease, malignant, with heart failure |
| 402.11 | Hypertensive heart disease, benign, with heart failure |
| 402.91 | Hypertensive heart disease, unspecified, with heart failure |
| 404.01 | Hypertensive heart and chronic kidney disease, malignant, with heart failure and with chronic kidney disease stage I through stage IV, or unspecified |
| 404.03 | Hypertensive heart and chronic kidney disease, malignant, with heart failure and with chronic kidney disease stage V or end stage renal disease |
| 404.11 | Hypertensive heart and chronic kidney disease, benign, with heart failure and with chronic kidney disease stage I through stage IV, or unspecified |
| 404.13 | Hypertensive heart and chronic kidney disease, benign, with heart failure and chronic kidney disease stage V or end stage renal disease |
| 404.91 | Hypertensive heart and chronic kidney disease, unspecified, with heart failure and with chronic kidney disease stage I through stage IV, or unspecified* |
| 404.93 | Hypertensive heart and chronic kidney disease, unspecified, with heart failure and chronic kidney disease stage V or end stage renal disease |
| 428.0 | Congestive heart failure, unspecified CHF NOS |
| 428.1 | Left heart failure |
| 428.20 | Unspecified systolic heart failure |
| 428.21 | Acute systolic heart |
| 428.22 | Chronic systolic heart failure |
| 428.23 | Acute on chronic systolic heart failure |
| 428.30 | Unspecified diastolic heart failure |
| 428.31 | Acute diastolic heart failure AC |
| 428.32 | Chronic diastolic heart failure |
| 428.33 | Acute on chronic diastolic heart failure |
| 428.40 | Unspecified combined systolic and diastolic heart failure |
| 428.41 | Acute combined systolic and diastolic heart failure |
| 428.42 | Chronic combined systolic and diastolic heart failure |
| 428.43 | Acute on chronic combined systolic and diastolic heart failure |
| 428.9 | Heart failure, unspecified |
Appendix 2: Variables Tested for Inclusion for Multivariable Analyses
Enrollment site, six sites
Date of admission, dichotomized: 06/01/01–06/15/02 vs. 06/16/02–06/30/03
Cardiology consultation utilization*
Patient demographic and socioeconomic variables
Age, continuous
Sex
Race, dichotomized: white vs. non-white
Education, dichotomized: ≤high school vs. >high school
Salary, three categories: $0–25,000, 25,001–50,000, and >50,000
Insurance status, three categories: Medicare/private insurance, Medicaid, and uninsured
Residence status: private residence vs. group/nursing home
Self-reported medical history
Coronary artery disease, angina, or congestive heart failure
Chronic obstructive pulmonary disease/asthma
Stroke
Diabetes
Hypertension
Anemia
Cancer diagnosed within the last 3 years
Depression
Kidney disease
Administrative Charlson comorbidity score, continuous
Cardiac medications used prior to admission:
Digoxin
Diuretics
Angiotensin converting enzyme inhibitor or angiotensin receptor blocker
Beta-blocker
Spironolactone
Coumadin
Aspirin
Statin
Total number of cardiac medications, continuous
Baseline cardiac or heart failure characteristics
History of prior myocardial infarction
Hospitalized in the past 12 months for CHF
New York Heart Classifications, dichotomized: I and II vs. III and IV
Lowest documented left ventricular function, dichotomized: normal to mild vs. moderate to severe
Physiology parameters in the first 24 h of admission
Highest creatinine, continuous and dichotomized ≥1.5
Highest potassium
Lowest sodium
Lowest systolic blood pressure, continuous and dichotomized ≤90
Highest systolic blood pressure
Highest heart rate
Lowest hemoglobin
Physiology parameters in the final 48 h prior to discharge†
Highest creatinine, continuous and dichotomized ≥1.5
Highest potassium
Lowest sodium
Lowest systolic blood pressure, continuous and dichotomized ≤90
Highest systolic blood pressure
Highest heart rate
Lowest hemoglobin
Patient-identified prior utilization of a primary care provider
*Cardiology consultation: not used for multivariable model predicting cardiology consultation
†Used for multivariable models predicting the following: LV ejection fraction assessment, ACE-I/ARB at discharge, beta-blocker at discharge, 30-day scheduled follow-up
References
- 1.Miniño AM, Heron MP, Smith BL. Deaths: Preliminary Data for 2004. National vital statistics reports; vol 54 no 19. Hyattsville, MD: National Center for Health Statistics; 2006. [PubMed]
- 2.Krumholz HM, Parent EM, Tu N, et al. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med. 1997;157:99–104. [DOI] [PubMed]
- 3.Joint Commission on Accreditation of Healthcare Organizations. Ongoing Activities: 2000 to 2004 Standardization of Metrics. Available at: http://www.jointcommission.org/NR/rdonlyres/551576B9-4E5C-4C0D-ACA5-6FC5D788A5D4/0/OngoingActivities.pdf. Accessed April 14, 2008
- 4.Centers for Medicare and Medicaid Services. Hospital Quality Initiative Overview, December 2005. Available at: http://www.cms.hhs.gov/HospitalQualityInits/downloads/HospitalOverview200512.pdf. Accessed April 14, 2008.
- 5.Williams SC, Schmaltz SP, Morton DJ, Koss RG, Loeb JM. Quality of care in U.S. hospitals as reflected by standardized measures, 2002–2004. N Engl J Med. 2005;353:255–64. [DOI] [PubMed]
- 6.Fonarow GC, Yancy CW, Heywood JT. Adherence to heart failure quality-of-care indicators in US hospitals: analysis of the ADHERE Registry. Arch Intern Med. 2005;165:1469–77. [DOI] [PubMed]
- 7.Go AS, Rao RK, Dauterman KW, Massie BM. A systematic review of the effects of physician specialty on the treatment of coronary disease and heart failure in the United States. Am J Med. 2000;108:216–26. [DOI] [PubMed]
- 8.Jong P, Gong Y, Liu PP, Austin PC, Lee DS, Tu JV. Care and outcomes of patients newly hospitalized for heart failure in the community treated by cardiologists compared with other specialists. Circulation. 2003;108:184–91. [DOI] [PubMed]
- 9.Ko DT, Tu JV, Masoudi FA, et al. Quality of care and outcomes of older patients with heart failure hospitalized in the United States and Canada. Arch Intern Med. 2005;165:2486–92. [DOI] [PubMed]
- 10.Goldman L. The impact of hospitalists on medical education and the academic health system. Ann Intern Med. 1999;130:364–7. [DOI] [PubMed]
- 11.Wachter R. Hospitals and hospitalists can reach across the “quality chasm” by restructuring inpatient care. Cost Qual. 2001:21–2. [PubMed]
- 12.Wachter RM, Goldman L. The hospitalist movement 5 years later. JAMA. 2002;287:487–94. [DOI] [PubMed]
- 13.Lindenauer PK, Rothberg MB, Pekow PS, Kenwood C, Benjamin EM, Auerbach AD. Outcomes of Care by Hospitalists, General Internists, and Family Physicians. N Engl J Med. 2007;357:2589–600. [DOI] [PubMed]
- 14.Lindenauer PK, Chehabeddine R, Pekow P, Fitzgerald J, Benjamin EM. Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists. Arch Intern Med. 2002;162:1251–6. [DOI] [PubMed]
- 15.Roccaforte WH, Burke WJ, Bayer BL, Wengel SP. Validation of a telephone version of the mini-mental state examination. J Am Geriatr Soc. 1992;40:697–702. [DOI] [PubMed]
- 16.Auerbach AD, Hamel MB, Davis RB, et al. Resource use and survival of patients hospitalized with congestive heart failure: differences in care by specialty of the attending physician. Ann Intern Med. 2000;132:191–200. [DOI] [PubMed]
- 17.Meltzer D, Manning WG, Morrison J, et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137:866–74. [DOI] [PubMed]
- 18.National Death Index user’s manual. No. 03-0078. Hyattsville, Md.: National Center for Health Statistics, October 2000.
- 19.Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335:514–7. [DOI] [PubMed]
- 20.Lee DS, Tran C, Flintoft V, Grant FC, Liu PP, Tu JV. CCORT/CCS quality indicators for congestive heart failure care. Can J Card. 2003;19:357–64. [PubMed]
- 21.Heart Failure Society Of A. HFSA 2006 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2006;12:e1–2. [DOI] [PubMed]
- 22.Ahmed A, Allman RM, Kiefe CI, et al. Association of consultation between generalists and cardiologists with quality and outcomes of heart failure care. Am Heart J. 2003;145:1086–93. [DOI] [PubMed]
- 23.Polanczyk CA, Newton C, Dec GW, Di Salvo TG. Quality of care and hospital readmission in congestive heart failure: an explicit review process. J Card Fail. 2001;7:289–98. [DOI] [PubMed]
- 24.Ritter PL, Stewart AL, Kaymaz H, Sobel DS, Block DA, Lorig KR. Self-reports of health care utilization compared to provider records. J Clin Epidemiol. 2001;54:136–41. [DOI] [PubMed]
- 25.Schafer JL. Multiple imputation: a primer. Statistical Methods in Medical Research. 1999;8:3–15. [DOI] [PubMed]
- 26.Auerbach AD, Wachter RM, Katz P, Showstack J, Baron RB, Goldman L. Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes. Ann Intern Med. 2002;137:859–65. [DOI] [PubMed]
- 27.Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297:831–41. [DOI] [PubMed]
- 28.Rifkin WD, Conner D, Silver A, Eichorn A. Comparison of processes and outcomes of pneumonia care between hospitalists and community-based primary care physicians. Mayo Clin Proc. 2002;77:1053–8. [DOI] [PubMed]
- 29.Shekelle PG, Ortiz E, Rhodes S, et al. Validity of the agency for healthcare research and quality clinical practice guidelines: how quickly do guidelines become outdated? JAMA. 2001;286:1461–7. [DOI] [PubMed]
- 30.Fonarow GC. The role of in-hospital initiation of cardioprotective therapies to improve treatment rates and clinical outcomes. Rev Cardiovasc Med. 2002;3Suppl 3S2–10. [PubMed]


