Abstract
Chicken erythroid AE1 anion exchangers receive endoglycosidase F (endo F)-sensitive sugar modifications in their initial transit through the secretory pathway. After delivery to the plasma membrane, anion exchangers are internalized and recycled to the Golgi where they acquire additional N-linked modifications that are resistant to endo F. During recycling, some of the anion exchangers become detergent insoluble. The acquisition of detergent insolubility correlates with the association of the anion exchanger with cytoskeletal ankyrin. Reagents that inhibit different steps in the endocytic pathway, including 0.4 M sucrose, ammonium chloride, and brefeldin A, block the acquisition of endo F-resistant sugars and the acquisition of detergent insolubility by newly synthesized anion exchangers. The inhibitory effects of ammonium chloride on anion exchanger processing are rapidly reversible. Furthermore, AE1 anion exchangers become detergent insoluble more rapidly than they acquire endo F-resistant modifications in cells recovering from an ammonium chloride block. This suggests that the cytoskeletal association of the recycling anion exchangers occurs after release from the compartment where they accumulate due to ammonium chloride treatment, and prior to their transit through the Golgi. The recycling pool of newly synthesized anion exchangers is reflected in the steady-state distribution of the polypeptide. In addition to plasma membrane staining, anion exchanger antibodies stain a perinuclear compartment in erythroid cells. This perinuclear AE1-containing compartment is also stained by ankyrin antibodies and partially overlaps the membrane compartment stained by NBD C6-ceramide, a Golgi marker. Detergent extraction of erythroid cells in situ has suggested that a substantial fraction of the perinuclear pool of AE1 is cytoskeletal associated. The demonstration that erythroid anion exchangers interact with elements of the cytoskeleton during recycling to the Golgi suggests the cytoskeleton may be involved in the post-Golgi trafficking of this membrane transporter.
INTRODUCTION
Erythroid cells have served as the paradigm for understanding how interactions between the peripheral membrane cytoskeleton and integral membrane polypeptides of the plasma membrane can regulate cell shape. The N-terminal cytoplasmic domain of the AE1 anion exchanger provides a major attachment site for the membrane cytoskeleton with the plasma membrane of erythroid cells through its association with ankyrin (Bennett and Stenbuck, 1980), protein 4.1 (Pasternack et al., 1985), and protein 4.2 (Cohen and Korsgren, 1988). The critical role of these interactions in the maintenance of erythroid cell shape and stability is best illustrated by the recently described AE1 anion exchanger knockouts (Mutsumi et al., 1996; Peters et al., 1996). In addition to their severely altered morphology and fragility, erythroid cells from these animals have an ∼50% reduction in cellular ankyrin and completely lack protein 4.2 (Peters et al., 1996).
Molecular analyses have shown that multiple variant transcripts are derived from the AE1 anion exchanger gene in human (Tanner et al., 1988; Kollert-Jons et al., 1993), mouse (Kopito and Lodish, 1985; Brosius et al., 1989), rat (Kudrycki and Shull, 1993), and chicken (Cox and Cox, 1995; Cox et al., 1996). These transcripts, which primarily accumulate in erythroid cells and kidney, encode polypeptides that differ only in the sequences present at the N terminus of their cytoplasmic domains. In vitro binding studies have shown that the alternative N-terminal sequences of the variant AE1 anion exchangers affect their ability to associate with specific elements of the membrane cytoskeleton. The human (Wang et al., 1995) and murine (Ding et al., 1994) erythroid AE1 anion exchangers can associate with erythroid ankyrin in vitro. In contrast, the human and murine kidney AE1 variants, which lack the 65 and 79 N-terminal amino acids, respectively, of their erythroid counterparts, are unable to bind erythroid ankyrin.
The diversity observed among erythroid AE1 anion exchangers in chickens is greater than that seen in other species. Twelve transcripts are derived from two erythroid-specific promoters (Cox et al., 1996). These transcripts give rise to four structural variants with predicted molecular masses of ∼99, ∼102, ∼104, and ∼108 kDa. Two additional erythroid transcripts that are derived from a promoter that is active in both erythroid cells and kidney encode a single polypeptide with a predicted molecular mass of ∼94 kDa (Cox and Cox, 1995). Transfection studies have shown that the alternative N termini of the variant chicken anion exchangers can serve as signals to target these polypeptides to different membrane compartments within cells (Cox et al., 1996).
Pulse-chase studies have shown that newly synthesized AE1 anion exchangers slowly associate with the detergent-insoluble cytoskeleton of chicken erythroid cells over a period of hours (Cox et al., 1987). Although the association of AE1 with elements of the membrane cytoskeleton is critical for erythroid cell shape and stability (Mutsumi et al., 1996; Peters et al., 1996), the mechanisms that regulate these interactions are poorly understood. The studies described here have further investigated the steps involved in the cytoskeletal association of this electroneutral transporter. These analyses have shown that after delivery of newly synthesized AE1 to the plasma membrane, the majority of the polypeptides are internalized and recycled to the Golgi where they acquire additional N-linked modifications. During recycling, some of the anion exchangers associate with cytoskeletal ankyrin. These recycling anion exchangers are reflected in the steady-state distribution of the polypeptide. In addition to the cell surface population of anion exchangers, a substantial fraction of these electroneutral transporters are localized in a perinuclear compartment where they colocalize with cytoskeletal ankyrin. The observation that anion exchangers interact with cytoskeletal ankyrin during recycling to the Golgi suggests the cytoskeleton may be involved in the post-Golgi trafficking of anion exchangers in erythroid cells.
MATERIALS AND METHODS
Generation of Chicken Erythroid Ankyrin Peptide Antibodies
Polyclonal antibodies have been generated in rabbit against a peptide corresponding to a 16-amino acid sequence (TVERSADRLRDWNAEG) near the C terminus of chicken erythroid ankyrin. This peptide is homologous to amino acids 1692–1707 of the human erythroid ankyrin 2.1 polypeptide and amino acids 1530–1545 of the human erythroid ankyrin 2.2 polypeptide (Lux et al., 1990). The sequence of this 16-mer is based upon the deduced sequence of a chicken erythroid ankyrin cDNA (GenBank accession number AF107255) that was generated using a reverse transcriptase-PCR protocol. Briefly, poly A+ RNA isolated from 10-d embryonic erythroid cells was reverse transcribed using an oligo-dT primer. This first-strand cDNA was PCR amplified using oligonucleotides identical to nucleotides 5133–5148 and 5257–5272 of the human erythroid ankyrin 2.1 cDNA (Lux et al., 1990) as sense and antisense primers, respectively. The resulting amplification product was subcloned into a pGEM-3 vector and sequenced by the dideoxy chain termination method.
Immunoprecipitation
Circulating erythroid cells were isolated from 10-d-old chicken embryos as described previously (Cox et al., 1987) and washed once in methionine-free DMEM. After the cells were incubated in this media for 20 min at 37°C, 0.25 mCi/ml 35S-Translabel (ICN Biochemicals, Costa Mesa, CA) was added to the cells, and they were incubated an additional 15 min at 37°C. At this time, an aliquot of 107 cells was removed and processed for immunoprecipitation. The remainder of the cells were pelleted and resuspended in DMEM containing 10% FCS. After incubation for times ranging from 30 min to 4 h, additional aliquots of 107 cells were removed and processed for immunoprecipitation. At each time point, the cells were lysed by incubation in 150 mM NaCl, 10 mM Tris (pH 7.5), 5 mM MgCl2, 2 mM EGTA, 6 mM β-mercaptoethanol, and 1% (vol/vol) Triton X-100 for 5 min on ice. The lysate was centrifuged for 5 min in an Eppendorf centrifuge. The detergent-soluble supernatant was diluted twofold in 20 mM Tris (pH 7.5), 1% (wt/vol) NaCl, 5 mM EGTA, 5 mM EDTA, 0.1% (wt/vol) SDS, 1% (vol/vol) Triton X-100, and 1% (wt/vol) sodium deoxycholate (immunoprecipitation buffer). The detergent-insoluble pellet was resuspended in immunoprecipitation buffer and sonicated two times for 15 s. Insoluble material was pelleted by centrifugation and discarded. Immunoblotting analysis with chicken AE1 anion exchanger-specific (Cox and Cox, 1995) and chicken ankyrin-specific peptide antibodies has shown this discarded material to be devoid of AE1 and ankyrin. Protein A-agarose beads preloaded with AE1 anion exchanger-specific peptide antibodies (Cox and Cox, 1995) or with chicken ankyrin-specific peptide antibodies were added to the detergent-soluble and -insoluble fractions and incubated overnight at 4°C. The protein A-agarose beads were then washed three times in immunoprecipitation buffer, and the immune complexes were released by incubation in SDS sample buffer and analyzed on a 6% SDS polyacrylamide gel. In each instance, the gels were treated with 20% 2,5-diphenyl-oxazole in DMSO, dried, and exposed to Kodak XAR-5 x-ray film (Eastman Kodak, Rochester, NY) at −80°C.
Endoglycosidase and Neuraminidase Digestion of AE1 Anion Exchanger Immunoprecipitates
AE1 anion exchanger immunoprecipitates were prepared as described above. After washing of the protein A-agarose beads with immunoprecipitation buffer, the beads were washed three times with 10 mM Tris (pH 7.4) and 150 mM NaCl (TBS). The protein A-agarose beads were then resuspended in TBS containing 1% (vol/vol) Triton X-100 and 0.1% (wt/vol) SDS and digested either with 2.5 mU endoglycosidase H (endo H), 10 mU N-glycosidase-free endoglycosidase F (endo F), or 0.1 U N-glycosidase overnight at room temperature. Alternatively, the beads were resuspended in TBS containing 4 mM CaCl2, 1% (vol/vol) Triton X-100, and 0.1% (wt/vol) SDS, and digested with 10 mU neuraminidase overnight at room temperature. In some experiments, immune complexes were digested first with endo F followed by digestion with N-glycosidase. In each instance, the beads were washed one time in TBS following digestion, and immune complexes were released by incubation in SDS sample buffer and analyzed on a 6% SDS polyacrylamide gel. The observed digestion patterns were the same when digests were carried out at 37°C.
Metabolic Labeling and Cell Surface Biotinylation
Erythroid cells from 10-d-old chicken embryos were pulsed with 35S-Translabel for 15 min and chased for 1 h. At this time, the cells were washed three times in ice-cold 10 mM HEPES (pH 7.4), 154 mM NaCl, 7.2 mM KCl, and 1.8 mM CaCl2 (Ringer’s buffer), and then incubated in Ringer’s buffer containing 1 mg/ml NHS-SS-Biotin (Pierce Chemical, Rockford, IL) for 30 min at 4°C. The cells were then washed five times in Ringer’s buffer and detergent lysed by incubating in 150 mM NaCl, 10 mM Tris (pH 7.5), 5 mM MgCl2, 2 mM EGTA, and 1% (vol/vol) Triton X-100 for 5 min on ice. The detergent-soluble and -insoluble fractions were processed for immunoprecipitation as described above. Alternatively, the cells were resuspended in DMEM containing 10% FCS after biotinylation and washing in Ringer’s buffer and incubated an additional 1 or 3 h at 37°C. At each time point, immunoprecipitates were prepared. After washing of the protein A-agarose beads in immunoprecipitation buffer, the immune complexes were released by incubation in 0.2 M glycine (pH 2.5), and 1% (vol/vol) Triton X-100. This solution was neutralized and incubated overnight at 4°C with streptavidin-agarose beads (Pierce Chemical). After washing, the streptavidin beads were resuspended in TBS containing 4 mM CaCl2, 1% (vol/vol) Triton X-100, and 0.1% (wt/vol) SDS, and digested with 10 mU neuraminidase overnight at room temperature. Biotinylated anion exchangers were then released by incubation in SDS sample buffer and analyzed on a 6% SDS polyacrylamide gel. In control experiments, cells were surface biotinylated after a 15-min pulse. Under these conditions, we were unable to detect biotinylated forms of newly synthesized AE1. This result indicates that intracellular forms of AE1 are not biotinylated as a result of the cells becoming leaky during the biotinylation protocol.
Effect of Various Reagents on AE1 Anion Exchanger Processing
Erythroid cells from 10-d-old chicken embryos were pulsed with 35S-Translabel as described above. After a 45-min chase, the cells were pelleted and resuspended in media containing either 10 μg/ml brefeldin A (BFA), 25 mM ammonium chloride, 100 μM chloroquine, or 0.4 M sucrose. The cells were chased in media containing these reagents until a total chase period of 2 or 4 h had elapsed, and at each time point, the cells were detergent lysed and AE1 immunoprecipitates were prepared as described above. Immunoprecipitates were either untreated or digested with endo F and analyzed on a 6% SDS polyacrylamide gel. In some experiments, 10 μg BFA/ml were added to the cells 20 min before the initiation of the pulse and were included in the media throughout the experiment.
Additional experiments were performed to determine whether the effects of hypertonic medium and ammonium chloride on anion exchanger processing were reversible. Erythroid cells from 10-d-old embryos were pulsed and chased in the presence of 0.4 M sucrose or 25 mM ammonium chloride as described above. After a 4-h chase, the cells were washed and incubated an additional 10 or 30 min in media lacking the reagent. After 10- and 30-min reversals, cells were detergent lysed, and AE1 immunoprecipitates were prepared. Immunoprecipitates were digested with 10 mU of endo F before analysis on a 6% SDS polyacrylamide gel.
Steady-State Biotinylation and Immunoblotting Analysis
Erythroid cells from 10-d-old chicken embryos were surface biotinylated with NHS-SS-Biotin and detergent lysed as described above. The lysate was separated into detergent-soluble and -insoluble fractions by centrifugation. Each fraction was split in half, and one half was precipitated with streptavidin agarose. The detergent-soluble and -insoluble fractions, as well as the streptavidin-precipitable material from each fraction, were electrophoresed on a 6% SDS polyacrylamide gel and electrophoretically transferred to nitrocellulose. The filter was incubated overnight at room temperature with a 1:20,000 dilution of the AE1-specific peptide antibody in TBS containing 0.25% gelatin. The filter was then washed in TBS containing 0.05% Tween-20 and incubated with goat anti-rabbit IgG conjugated to horseradish peroxidase. After washing, immunoreactive species were detected by enhanced chemiluminescence.
Similar immunoblotting analysis of detergent-soluble and insoluble fractions from 10-d embryonic erythroid cells were carried out using the chicken erythroid ankyrin-specific peptide antibody at a 1:3000 dilution. The 225- and 205-kDa polypeptides detected with the ankyrin antibody were not observed when the peptide antigen used to generate the antibody was used as a competitor (10 μg/ml) in the immunoblotting analysis.
Quantitative Densitometry
X-ray films were scanned using DeskScan II 2.1 software (Hewlett-Packard, Palo Alto, CA), and quantitative densitometry was performed using NIH Image. All quantitation was done on exposures in which individual bands were not yet saturating.
Immunolocalization Analysis
Erythroid cells isolated from 10-d-chicken embryos were attached to glass slides and fixed by incubating in 1% paraformaldehyde in PBS for 15 min at room temperature. After fixation, the cells were permeabilized with acetone. The cells were washed with PBS and incubated with a 1:3000 dilution of the rabbit polyclonal AE1-specific peptide antibody in PBS for 2 h at room temperature. The cells were then washed and incubated with donkey anti-rabbit (DAR) IgG conjugated to lissamine. Alternatively, the cells were double stained with a 1:50 dilution of an AE1-specific monoclonal antibody (Jennings et al., 1986) and a 1:2000 dilution of the rabbit polyclonal ankyrin-specific peptide antibody. In this instance, the cells were washed and incubated with DAR-IgG conjugated to lissamine and donkey anti-mouse (DAM) IgG conjugated to fluorescein isothiocyanate (FITC). After washing, immunoreactive polypeptides were visualized using a Zeiss Axiophot microscope or a Bio-Rad laser scanning confocal microscope (Bio-Rad, Richmond, CA). Background levels of staining were observed in control experiments in which the peptide antigens used to generate the AE1-specific and ankyrin-specific peptide antibodies were included as a competitor. In some experiments, AE1-stained cells were incubated with 50 μg/ml NBD C6-ceramide (Molecular Probes, Eugene, OR) for 1 h at 37°C after antibody incubations. The slides were then washed three times for 15 min each with PBS containing 25 μg/ml fatty acid-free BSA. The distribution of AE1 was compared with that of ankyrin and NBD-ceramide in 0.5-μm optical sections collected on a Bio-Rad laser scanning confocal microscope .
The distribution of detergent-insoluble AE1 and ankyrin was examined by extracting erythroid cells from 10-d embryos with ice-cold 150 mM NaCl, 10 mM Tris (pH 7.5), 5 mM MgCl2, 2 mM EGTA, 6 mM β-mercaptoethanol, and 1% (vol/vol) Triton X-100 for 30 s. The preextracted cells were then fixed by incubation in 1% paraformaldehyde in PBS for 15 min at room temperature, and the intracellular localization of detergent-insoluble AE1 and ankyrin was assessed as described above.
RESULTS
Posttranslational Processing of Erythroid AE1 Anion Exchangers
Previous studies have shown that the extent to which newly synthesized chicken AE1 anion exchangers associate with the detergent-insoluble cytoskeleton of erythroid cells varies dramatically during embryonic development (Cox et al., 1987). These analyses also demonstrated that the acquisition of detergent insolubility by AE1 polypeptides correlated with posttranslational modifications of these electroneutral transporters. To define the nature of these modifications and determine their role in mediating the cytoskeletal association of AE1, additional pulse-chase studies have been performed.
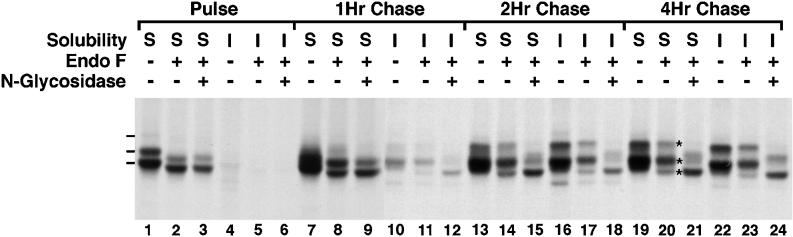
Erythroid cells isolated from 10-d-old chicken embryos were pulsed with 35S-Translabel for 15 min and chased for various periods ranging from 1 to 4 h. At each time point, the cells were lysed in isotonic buffer containing 1% Triton X-100 on ice and separated into soluble and insoluble fractions by centrifugation. AE1 anion exchanger immunoprecipitates were prepared from each fraction using AE1-specific peptide antibodies that recognize a sequence present in each of the five predicted chicken erythroid AE1 anion exchangers. Before gel analysis, immunoprecipitates were either untreated, digested with endo F, or digested with endo F followed by digestion with N-glycosidase. As shown previously (Cox et al., 1987), newly synthesized AE1 anion exchangers are primarily detergent soluble (∼90%) at the end of a 15-min pulse (Figure 1, lanes 1 and 4). These polypeptides, which have apparent molecular masses of 97, 100, and a faint species of 108 kDa, were not detected when the AE1 peptide antigen was used as a competitor in the analysis. These newly synthesized polypeptides gradually become detergent insoluble, until ∼50% are insoluble at the 4-h chase point (Figure 1, lanes 19 and 22).
Figure 1.
Posttranslational modification and detergent insolubility of AE1 anion exchangers. Erythroid cells from a 10-d-old chicken embryo were pulsed with 35S-Translabel in methionine-free DMEM for 15 min at 37°C. At the end of the pulse (lanes 1–6) an aliquot of cells was lysed in isotonic buffer containing 1% Triton X-100. The lysate was separated into soluble (S) and insoluble (I) fractions by centrifugation. The remainder of the cells were incubated at 37°C in DMEM containing 10% FCS for 1 h (lanes 7–12), 2 h (lanes 13–18), or 4 h (lanes 19–24), and at each time point the cells were fractionated as described above. Immunoprecipitates prepared from the soluble and insoluble fractions using AE1-specific peptide antibodies were either undigested, digested with endo F, or digested with endo F followed by digestion with N-glycosidase. Immune complexes were analyzed on a 6% SDS polyacrylamide gel, and labeled anion exchangers were detected by fluorography. Dashes adjacent to lane 1 mark the 97-, 100-, and 108-kDa AE1 polypeptides in the detergent-soluble fraction at the pulse point. Asterisks adjacent to lane 20 mark the detergent-soluble AE1 polypeptides of 95, 98, and 105 kDa detected at the 4-h chase point after endo F digestion.
A comparison of the glycosidase digestion profiles of the immunoprecipitated anion exchangers indicates that the endo F (Figure 1, lane 2) and N-glycosidase (Figure 1, lane 3) digests are indistinguishable at the pulse point, yielding a major species of 95 kDa and a less abundant species of 98 kDa. The relationship of these two digestion products to the five predicted chicken erythroid AE1 anion exchangers is not known at this time. The endo F digestion profile slowly changes during the chase. This shift is first apparent at the 1-h chase point, where diffuse species ranging from 102 to 105 kDa appear in the detergent-soluble fraction (Figure 1, lane 8), and a product of 105 kDa appears in the detergent-insoluble fraction (Figure 1, lane 11). After 4 h of chase, three digestion products of 95, 98, and 105 kDa are detected in both the soluble (Figure 1, lane 20) and insoluble (Figure 1, lane 23) fractions, and the 98-kDa polypeptide is the most abundant species. The endo F-resistant species that arise during the chase are, for the most part, still susceptible to digestion with N-glycosidase. However, an additional N-glycosidase digestion product of 99 kDa arises during the chase. The modification that gives rise to the 99-kDa species is first detectable in the soluble (Figure 1, lane 15) and insoluble (Figure 1, lane 18) pool of anion exchangers at the 2-h chase point, and further studies have shown this modification is insensitive to digestion with O-glycosidase (our unpublished results).
The initial N-glycosidase-sensitive modifications acquired by newly synthesized AE1 are also sensitive to digestion with endo F (Figure 1) and endo H (our unpublished results). This suggests that chicken AE1 polypeptides initially receive either high mannose or biantennary hybrid sugars on their single predicted N-linked site. However, by 1 h post synthesis, ∼15% of the polypeptides have acquired additional N-linked modifications that are resistant to endo F and sensitive to N-glycosidase, and by 4 h of chase ∼70% of the polypeptides have acquired these endo F-resistant, N-glycosidase-sensitive modifications. The appearance of endo F-resistant species probably results from the conversion of high mannose or biantennary hybrid sugars to tri- and/or tetra-antennary complex sugars (Tarentino et al., 1985). The formation of these more complex sugars requires the activity of α-mannosidase II and N-acetylglucosamine transferase, markers of the medial compartment of the Golgi (Kornfeld and Kornfeld, 1985). This suggests anion exchangers that acquire endo F-resistant modifications between 1 and 4 h of chase pass through the medial compartment of the Golgi. Although detergent-insoluble AE1 exhibits a mature endo F digestion profile earlier in the chase than detergent soluble AE1, the profile of endo F-resistant species is very similar in the soluble (Figure 1, lane 20) and insoluble (Figure 1, lane 23) fractions following a 4-h chase. This result indicates that factor(s) other than N-linked glycosylation must be involved in regulating the detergent solubility properties of the newly synthesized anion exchangers.
Association with Cytoskeletal Ankyrin Correlates with the Acquisition of Detergent Insolubility by Newly Synthesized AE1
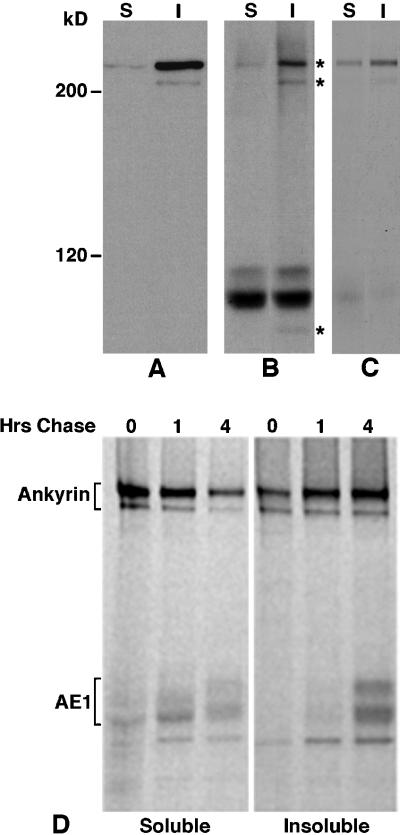
Examination of the total profile of proteins present in AE1 immunoprecipitates prepared from metabolically labeled 10-d embryonic erythroid cells revealed a single polypeptide of 225 kDa coprecipitated with detergent-soluble AE1, while polypeptides of 225, 205, and 85 kDa coprecipitated with detergent-insoluble AE1 (Figure 2B). Longer exposure of this autoradiogram revealed the 205-kDa polypeptide was also present in the detergent-soluble AE1 precipitate. The 225- and 205-kDa polypeptides present in these AE1 immunoprecipitates are similar in size and relative abundance to the human erythroid ankyrin 2.1 and 2.2 splice variants (Lux et al., 1990), which associate with human erythroid AE1 in vitro (Davis et al., 1992). To investigate whether these coprecipitating polypeptides corresponded to ankyrin, AE1 immunoprecipitates identical to those shown in Figure 2B were dissociated in SDS sample buffer, diluted 1:10 in immunoprecipitation buffer, and reprecipitated with a chicken erythroid ankyrin-specific peptide antibody. Immunoblotting analysis has shown this ankyrin-specific antibody recognizes a 225-kDa polypeptide in the detergent-soluble fraction and 225- and 205-kDa polypeptides in the detergent-insoluble fraction prepared from 10-d embryonic erythroid cells (Figure 2A). Longer exposure of this immunoblot revealed the 205-kDa ankyrin isoform is also present in the detergent-soluble fraction. Reprecipitation of AE1 immunoprecipitates with this ankyrin-specific antibody indicated the 225- and 205-kDa polypeptides that coprecipitated with AE1 could be reprecipitated with the ankyrin antibody, suggesting they are indeed ankyrin (Figure 2C). The identity of the additional polypeptide of 85 kDa that coprecipitates with detergent-insoluble AE1 is not known at this time.
Figure 2.
Association of erythroid AE1 anion exchangers with ankyrin. Detergent soluble (S) and insoluble (I) fractions from 105 erythroid cells from a 10-d chicken embryo were analyzed on a 6% SDS polyacrylamide gel, transferred to nitrocellulose, and probed with an ankyrin-specific antibody. After washing, the blot was incubated with goat anti-rabbit IgG conjugated to horseradish peroxidase, and immunoreactive species were detected by enhanced chemiluminescence (A). Erythroid cells from a 10-d-old chicken embryo were also pulsed for 15 min with 35S-Translabel and chased for 1 or 4 h in complete media. Aliquots of cells from the pulse and chase points were detergent lysed, and immunoprecipitates were prepared with the ankyrin-specific antibody from the detergent-soluble and insoluble fractions (D). Alternatively, AE1 anion exchanger immunoprecipitates were prepared from the detergent-soluble and -insoluble fractions of cells that had been pulsed for 15 min and chased for 4 h. The AE1 immunoprecipitates were either directly analyzed (B) or dissociated in SDS sample buffer and reprecipitated with the ankyrin-specific antibody (C). The immunoprecipitates were analyzed on a 6% SDS polyacrylamide gel, and labeled species were detected by fluorography. Asterisks to the right of panel B mark the 225-, 205-, and 85-kDa polypeptides that coprecipitate with AE1. Molecular weight markers to the left of panel A correspond to myosin (200 kDa), and β-galactosidase (120 kDa).
It cannot be determined whether the ankyrin molecules detected in AE1 immunoprecipitates are associated with the newly synthesized pool of anion exchangers monitored in pulse-chase analyses (Figure 1), or with a preexisting pool of unlabeled molecules. To address whether ankyrin is involved in the changing solubility properties observed for newly synthesized AE1, immunoprecipitates were prepared with the ankyrin-specific antibodies. These analyses revealed that the 225- and 205-kDa ankyrin polypeptides can be immunoprecipitated from both the detergent-soluble and -insoluble fractions after a 15-min pulse (Figure 2D). Furthermore, ∼60% of newly synthesized ankyrin fractionated in the detergent-soluble pool at the pulse point. This is in contrast to the immunoblotting analysis that indicated >95% of ankyrin is detergent insoluble at steady state (Figure 2A). After 4 h of chase, only ∼25% of labeled ankyrin fractionated in the detergent-soluble pool. Some of the soluble molecules were chased into the detergent-insoluble pool (Figure 2D, lanes 4–6). However, ∼40% of the detergent-soluble molecules turned over during the 4-h chase. In addition to ankyrin, polypeptides that comigrate with the variant AE1 anion exchangers were detected in the ankyrin immunoprecipitates (Figure 2D). These polypeptides were shown to be anion exchangers by virtue of their ability to be reprecipitated with AE1-specific antibodies after dissociation of ankyrin immunoprecipitates in SDS sample buffer (see Figure 7). The variant anion exchangers primarily precipitated with detergent-soluble ankyrin at the pulse and 1-h chase points (Figure 2D), even though this form of ankyrin only represents ∼5% of ankyrin at steady state. However, by 4 h of chase, ∼70% of the newly synthesized anion exchangers that coprecipitate with ankyrin are associated with the detergent-insoluble or cytoskeletal form of ankyrin (Figure 2D). This association of newly synthesized AE1 with cytoskeletal ankyrin mimics the kinetics with which these polypeptides become detergent insoluble during pulse-chase analyses (Figure 1).
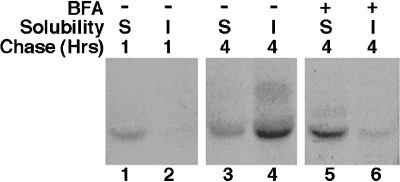
Figure 7.
Association of newly synthesized AE1 with cytoskeletal ankyrin is inhibited by BFA. Erythroid cells from a 10-d-old chicken embryo were pulsed for 15 min with 35S-Translabel and chased for 1 h (lanes 1 and 2) or 4 h (lanes 3 and 4) in complete media. Alternatively, BFA was added to the media at the 45-min chase point and included in the media for the duration of a 4-h chase (lanes 5 and 6). At each time point, the cells were detergent lysed, and ankyrin immunoprecipitates were prepared from the detergent-soluble (S) and -insoluble (I) fractions. The ankyrin immunoprecipitates were dissociated in SDS sample buffer and reprecipitated with AE1-specific antibodies. The AE1 immunoprecipitates were analyzed on a 6% SDS polyacrylamide gel, and labeled species were detected by fluorography.
Because AE1 and ankyrin have the capacity to interact in vitro, it was possible that the coprecipitation of anion exchangers with ankyrin resulted from interactions that occurred postlysis. To control for this possibility, the detergent-soluble and -insoluble fractions from cells pulsed for 15 min were mixed before immunoprecipitation with ankyrin antibodies. Even though ∼20-fold more ankyrin was available to bind the newly synthesized anion exchangers when the soluble and insoluble fractions were mixed before immunoprecipitation, the amount of anion exchanger that coprecipitated with ankyrin from these mixed fractions was no greater than that observed when ankyrin was precipitated from the detergent-soluble fraction alone (our unpublished results). This result indicates that postlysis interactions between detergent-insoluble ankyrin and the newly synthesized, detergent-soluble anion exchangers did not occur. These coprecipitation data suggest that association with cytoskeletal ankyrin is at least partially responsible for the acquisition of detergent insolubility observed for AE1 during pulse-chase studies.
Posttranslational Processing of AE1 Occurs after Delivery to the Plasma Membrane
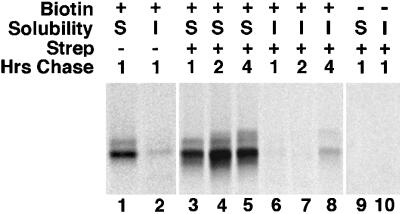
Previous studies demonstrated that the bulk of newly synthesized erythroid AE1 anion exchangers are susceptible to extracellular proteinase K digestion after a 10-min pulse and a 30-min chase (Cox et al., 1987). This observation, together with the results described above, suggests that the additional N-linked modifications and enhanced cytoskeletal binding observed for AE1 between 1 and 4 h of chase (Figure 1) occur to molecules that have been delivered to the plasma membrane. To verify that these changes occur to anion exchangers that have been delivered to the plasma membrane, we determined whether the newly synthesized polypeptides could be surface biotinylated with a membrane-impermeant biotinylating reagent, NHS-SS-Biotin, after a 15-min pulse and a 1-h chase. Following biotinylation, cells were detergent lysed, and anion exchangers were immunoprecipitated as described above. Immunoprecipitated proteins were released from protein A agarose beads and either directly analyzed or reprecipitated with streptavidin agarose before gel analysis. This experiment indicated that the population of newly synthesized AE1 that can be surface biotinylated after a 1-h chase (Figure 3, lanes 3 and 6) is similar in composition to the total pool of immunoprecipitable AE1 (Figure 3, lanes 1 and 2).
Figure 3.
Glycosylation and acquisition of detergent insolubility by AE1 anion exchangers occurs after delivery to the plasma membrane. Erythroid cells from a 10-d-old chicken embryo were pulsed for 15 min with 35S-Translabel and chased for 1 h in complete media. The cells were then washed in Ringer’s buffer and incubated in the presence (lanes 1–8) of 1 mg/ml NHS-SS-Biotin in Ringer’s buffer for 30 min at 4oC. At this time, an aliquot of cells (lanes 1, 2, 3, and 6) was detergent lysed, and AE1 immunoprecipitates were prepared from the detergent-soluble (S) and the detergent-insoluble (I) fractions. One-half of each precipitate was directly analyzed on a 6% SDS polyacrylamide gel (lanes 1 and 2), and the other half was reprecipitated with streptavidin agarose before gel analysis (lanes 3 and 6). The remainder of the cells were chased an additional 1 h (lanes 4 and 7), or 3 h (lanes 5 and 8) in complete media after biotinylation. At the latter time points, AE1 was immunoprecipitated followed by precipitation with streptavidin agarose. Streptavidin-precipitable material was analyzed on a 6% SDS polyacrylamide gel, and labeled AE1 anion exchangers were detected by fluorography. Lanes 9 and 10 are controls that were processed identically to lanes 3 and 6 except the cells were not biotinylated with NHS-SS-Biotin before immunoprecipitation and streptavidin precipitation. Precipitates were digested with neuraminidase before gel analysis.
The fate of the newly synthesized anion exchangers that were cell surface biotinylated after a 1-h chase was examined by incubating cells in media an additional 1 h (Figure 3, lanes 4 and 7) or 3 h (Figure 3, lanes 5 and 8) after the biotinylation step. At each time point, the anion exchangers were immunoprecipitated, followed by precipitation with streptavidin agarose. This analysis revealed that the biotinylated anion exchangers receive additional posttranslational modifications and undergo a gradual shift from the detergent-soluble to the detergent-insoluble pool. In two independent experiments, an average of 33% of the biotinylated molecules became detergent insoluble during 3 additional hours of incubation. The extent of cytoskeletal association in these experiments is lower than that observed in cells that are not biotinylated (Figure 1). This may be due to the biotin groups interfering with the normal processing of these polypeptides. Nonetheless, these data support the notion that erythroid AE1 anion exchangers become detergent insoluble and acquire additional modifications after their initial delivery to the plasma membrane. These results, together with previous studies (Cox et al., 1987), further suggest that the N-linked modifications acquired by newly synthesized polypeptides between 1 and 4 h of chase arise, in large part, via recycling of cell surface anion exchangers to the Golgi.
The Acquisition of Detergent Insolubility by Newly Synthesized AE1 Involves Recycling of Cell Surface Molecules
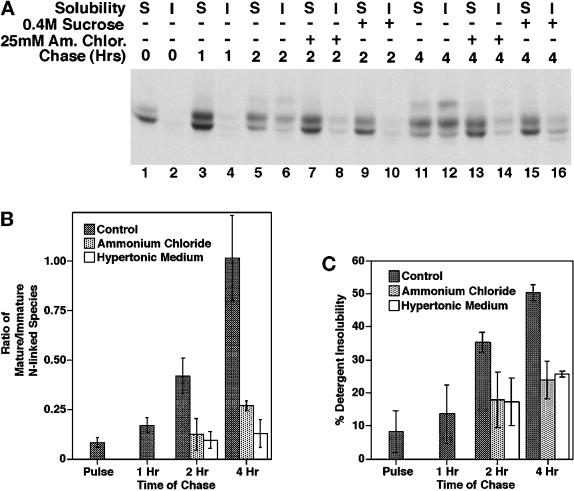
To investigate the role of recycling in anion exchanger processing, we have examined the effect of hypertonic medium and ammonium chloride, reagents known to block different steps in the endocytic pathway. Hypertonic medium has been shown to inhibit clathrin-dependent endocytosis by dissociating clathrin from the plasma membrane (Hansen et al., 1993), and ammonium chloride has been shown to inhibit the recycling of TGN38 and furin from the plasma membrane to the trans-Golgi network (TGN) by blocking endosomal acidification (Chapman and Munro, 1994). In these experiments, the reagent was added after a 15-min pulse and a 45-min chase, and the effect of the reagent on the acquisition of endo F-resistant modifications and the detergent insolubility of AE1 was monitored during a subsequent chase. These analyses revealed that both ammonium chloride (Figure 4A, lanes 13 and 14) and hypertonic medium (Figure 4A, lanes 15 and 16) block the acquisition of endo F-resistant modifications by newly synthesized anion exchangers that is observed in control cells (Figure 4A, lanes 11 and 12) during a 4-h chase. Quantitation of the inhibitory effect of hypertonic medium and ammonium chloride from three independent experiments has shown that both reagents almost completely block the acquisition of endo F-resistant sugars observed in control cells between 1 and 4 h of chase (Figure 4B). Similar studies have shown that chloroquine, another reagent that inhibits the recycling of TGN38 and furin from the plasma membrane to the TGN by blocking endosomal acidification (Chapman and Munro, 1994), also blocks the acquisition of endo F-resistant sugars by newly synthesized AE1 (our unpublished results). These results are consistent with the hypothesis that after delivery to the plasma membrane, AE1 anion exchangers are slowly internalized in a clathrin-dependent manner and delivered to the Golgi for additional N-linked modifications.
Figure 4.
Effect of ammonium chloride and hypertonic medium on the posttranslational processing of newly synthesized AE1. Erythroid cells from a 10-d-old chicken embryo were pulsed and chased as described in the legend of Figure 1. AE1 immunoprecipitates were prepared from the detergent-soluble (S) and -insoluble (I) fractions of cells that had been detergent lysed after 0 h (A, lanes 1 and 2), 1 h (A, lanes 3 and 4), 2 h (A, lanes 5–10), and 4 h (A, lanes 11–16) of chase. Each precipitate was digested with endo F before analysis on a 6% SDS polyacrylamide gel. For some of the cells, 25 mM ammonium chloride (A, lanes 7, 8, 13, and 14), or 0.4 M sucrose (A, lanes 9, 10, 15, and 16) was added to media after 45 min of chase and was present for the remainder of the chase period. Autoradiograms from three separate experiments identical to the one shown in panel A were scanned, and the abundance of the individual anion exchanger species after endo F digestion was determined using NIH image. The values shown in panel B reflect the ratio of the 105-kDa AE1 species to the 95-kDa AE1 species at each time point. The values shown in panel C reflect the percentage of newly synthesized AE1 that was detergent insoluble at each time point. Error bars in panels B and C reflect the SD from the three experiments.
A comparison of the detergent solubility properties of AE1 anion exchangers precipitated from control cells after a 4-h chase (Figure 4A, lanes 11 and 12) with those precipitated from cells treated with ammonium chloride (Figure 4A, lanes 13 and 14) or hypertonic medium (Figure 4A, lanes 15 and 16) revealed both reagents block the acquisition of detergent insolubility by newly synthesized AE1. Quantitation of three independent experiments has shown that these reagents significantly inhibit the shift of AE1 from the detergent-soluble to the detergent-insoluble pool (Figure 4C). Identical experiments have shown that chloroquine has a similar effect on the detergent solubility properties of newly synthesized AE1 (our unpublished results). There is a small, but reproducible, increase in detergent-insoluble AE1 between 1 and 4 h of chase in the presence of hypertonic medium and ammonium chloride (Figure 4C). However, these results suggest that the primary pathway for becoming detergent insoluble requires that anion exchangers are internalized from the cell surface and delivered to a membrane compartment downstream of the compartment where they accumulate as a result of incubating cells in hypertonic medium or ammonium chloride.
Recycling Anion Exchangers Become Detergent Insoluble before the Acquisition of Endo F-resistant Sugars
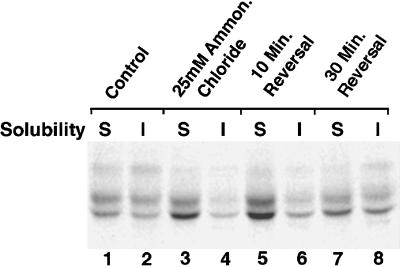
Additional experiments have examined whether the effects of hypertonic medium and ammonium chloride on the processing of AE1 are reversible. Erythroid cells were treated with hypertonic medium or ammonium chloride as described above. After a 4-h chase, the cells were washed and incubated an additional 10 or 30 min in media lacking the reagent. At each time point, AE1 polypeptides were assayed for the acquisition of endo F-resistant sugars and their detergent solubility properties. These analyses revealed that the inhibitory effects of hypertonic medium on the processing of AE1 anion exchangers were not reversible during the time course of the experiment. In contrast, AE1 rapidly became detergent insoluble and acquired endo F-resistant modifications after release from the ammonium chloride block (Figure 5). Assuming the effects of both reagents are reversible, these results suggest the ammonium chloride block is downstream of the block due to hypertonic medium, which is consistent with their proposed effects on different steps in the endocytic pathway. Interestingly, quantitation of two independent experiments has shown that following reversal of the ammonium chloride block, AE1 polypep-tides became detergent insoluble at a more rapid rate than they acquired endo F-resistant sugars. When these properties are expressed as a percent of that observed in control cells that were chased in the absence of ammonium chloride, the percent detergent-insoluble AE1 reached 89% of the value seen in control cells after a 30-min reversal. In contrast, endo F-resistant modifications only reached 42% of the value seen in untreated control cells after a 30-min reversal. These results suggest that the acquisition of detergent insolubility by recycling anion exchangers occurs after release from the compartment where these transporters accumulate due to ammonium chloride and before their transit through the Golgi.
Figure 5.
Fate of newly synthesized AE1 after removal of ammonium chloride from the medium. Erythroid cells from a 10-d-old embryo were pulsed for 15 min and chased for 45 min in complete media. At this time, 25 mM ammonium chloride was added to the media of some of the cells, and the cells were incubated until a total of 4 h of chase had elapsed. Treated cells (lanes 3 and 4) and untreated control cells (lanes 1 and 2) were then detergent lysed and separated into soluble (S) and insoluble (I) fractions, and AE1 immunoprecipitates were prepared from each fraction. After the 4-h chase period in 25 mM ammonium chloride, aliquots of cells were also pelleted and resuspended in complete media lacking the reagent. The cells were then incubated an additional 10 min (lanes 5 and 6), or 30 min (lanes 7 and 8) at 37°C in reagent-free media and processed for immunoprecipitation using AE1-specific antibodies. Immunoprecipitates were digested with endo F before analysis on a 6% SDS polyacrylamide gel.
The Recycling Pathway of AE1 Is Sensitive to BFA
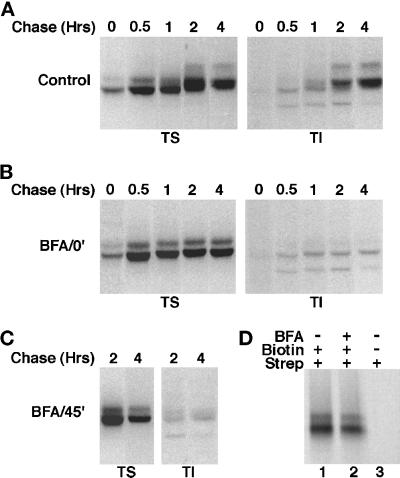
To further dissect the steps involved in the trafficking of AE1 anion exchangers, we have investigated the effect of the fungal metabolite BFA on AE1 processing. In these studies, BFA was added either 20 min before the 15-min pulse or after a 45-min chase when the bulk of the newly synthesized polypeptides have arrived at the plasma membrane. At each time point, the detergent solubility properties of newly synthesized AE1 was assessed. Addition of BFA before the pulse (Figure 6B) or after a 45-min chase (Figure 6C) had a similar effect on the detergent solubility properties of AE1. In each instance, ∼20% of the newly synthesized polypeptides fractionated in the detergent-insoluble pool after a 4-h chase, as compared with ∼50% in control cells (Figure 6A). Endoglycosidase digestion studies have shown that anion exchangers acquire their initial high mannose or biantennary hybrid sugars even when BFA is present throughout the experiment. However, BFA added before the pulse or after a 45-min chase blocks the acquisition of endo-F-resistant sugars by anion exchangers between 1 and 4 h of chase (our unpublished results).
Figure 6.
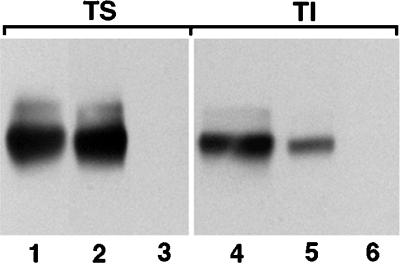
Effect of BFA on the posttranslational processing of newly synthesized AE1 anion exchangers. Erythroid cells from a 10-d-old chicken embryo were pulsed for 15 min with 35S-Translabel and chased in complete media for various periods ranging from 30 min to 4 h (A). To identical cultures of cells, 10 μg BFA/ml were added either 20 min before the pulse (B), or after a 45-min chase (C), and the drug was included in the media for the duration of the experiment. At each time point, cells were detergent lysed and separated into soluble (TS) and insoluble (TI) fractions. Immunoprecipitates were prepared from both fractions using AE1-specific antibodies. Alternatively, 10-d erythroid cells were pulsed for 15 min with 35S-Translabel and chased in complete media for 1 h in the absence (D, lanes 1 and 3), or presence (D, lane 2) of 10 μg BFA/ml. At this time the cells were washed in Ringer’s buffer and incubated in the presence (D, lanes 1 and 2), or absence (D, lane 3) of 1 mg/ml NHS-SS-Biotin for 30 min at 4°C. The cells were then lysed in immunoprecipitation buffer, and immunoprecipitates were prepared using AE1-specific antibodies. After release from protein A agarose beads, biotinylated polypeptides were precipitated with streptavidin agarose. Each precipitate was analyzed on a 6% SDS polyacrylamide gel, and labeled anion exchangers were detected by fluorography.
The observation that BFA added after 45 min of chase inhibits the processing of AE1 that occurs in control cells between 1 and 4 h of chase indicates that one or more of the vesicular sorting steps involved in the recycling of AE1 to the Golgi is sensitive to BFA. To determine whether BFA also affects the exocytosis of AE1, cell surface biotinylation studies have examined whether the addition of BFA to erythroid cells before the pulse prevents the cell surface delivery of newly synthesized polypeptides. Cells were pulsed for 15 min and chased for 1 h in the absence (Figure 6D, lane 1) or presence (Figure 6D, lane 2) of BFA. At this time the cells were surface biotinylated and lysed in immunoprecipitation buffer, and newly synthesized polypeptides were precipitated with AE1-specific antibodies followed by precipitation with streptavidin agarose. This analysis revealed that the cell surface delivery of AE1 in the presence of BFA is comparable to that seen in control cells. This suggests that the observed effects of BFA on anion exchanger processing are primarily due to its effect on events that occur after the initial delivery of AE1 to the plasma membrane.
Coimmunoprecipitation studies have directly examined whether the effect of BFA on the detergent solubility properties of newly synthesized AE1 correlates with a decrease in the association of AE1 with cytoskeletal ankyrin. Ankyrin immunoprecipitates were prepared from the detergent-soluble and -insoluble fractions of erythroid cells that were pulsed for 15 min and chased for either 1 or 4 h. These precipitates were dissociated in SDS sample buffer and reprecipitated with AE1-specific antibodies. Similar to what was observed in Figure 2, newly synthesized AE1 was primarily associated with detergent-soluble ankyrin at the 1-h chase point (Figure 7, lanes 1 and 2). During an additional 3 h of chase, this profile changed such that ∼70% of the newly synthesized anion exchangers that coprecipitate with ankyrin are associated with the cytoskeletal form of the polypeptide (Figure 7, lanes 3 and 4). However, when BFA was added to the media at the 45-min chase point and included in the media for the duration of the experiment, the association of AE1 with cytoskeletal ankyrin that was observed in control cells between 1 and 4 h of chase was almost completely blocked (Figure 7, lanes 5 and 6). These results indicate that the association of newly synthesized AE1 with cytoskeletal ankyrin is dependent upon a BFA-sensitive recycling step.
Steady-State Distribution of Erythroid AE1 Anion Exchangers
The data presented above indicate that the majority of newly synthesized AE1 anion exchangers are recycled from the plasma membrane to the Golgi where they acquire additional N-linked modifications. Cell surface biotinylation studies have examined whether this recycling pool of anion exchangers is reflected in the steady-state distribution of the protein. Erythroid cells from 10-d chicken embryos were surface biotinylated before detergent lysis. After separation into detergent-soluble and -insoluble fractions, half of each fraction was precipitated with streptavidin agarose. The detergent-soluble and -insoluble fractions, as well as the streptavidin-precipitable material from each fraction, were subjected to immunoblotting analysis using AE1-specific antibodies. This analysis revealed, unexpectedly, that >95% of the detergent-soluble anion exchangers were precipitable with streptavidin agarose (compare lanes 1 and 2 in Figure 8), while only ∼40% of the detergent-insoluble polypeptides were streptavidin precipitable (compare lanes 4 and 5 in Figure 8). Similar results have been obtained in two independent experiments. It is possible that the difference in the ability to streptavidin precipitate the detergent-soluble and detergent-insoluble anion exchangers is due to conformational changes in the insoluble polypeptides that render them less susceptible to cell surface biotinylation. However, the difference in the ability to streptavidin precipitate the soluble and insoluble anion exchangers could also be due to the existence of a large pool of intracellular AE1 that is associated with the detergent-insoluble cytoskeleton.
Figure 8.
Susceptibility of detergent-soluble and detergent-insoluble AE1 to surface biotinylation. Erythroid cells from a 10-d-old chicken embryo were cell surface biotinylated by incubating with 1 mg NHS-SS-Biotin/ml in Ringer’s buffer for 30 min at 4°C. The cells were then washed in Ringer’s buffer and detergent lysed by incubating in isotonic buffer containing 1% Triton X-100. The lysate was separated into soluble (TS) and insoluble (TI) fractions by centrifugation, and biotinylated polypeptides were precipitated using streptavidin agarose. Streptavidin-precipitable material was analyzed on a 6% SDS polyacrylamide gel, transferred to nitrocellulose, and probed with an AE1-specific antibody. After washing, the blot was incubated with goat anti-rabbit IgG conjugated to horseradish peroxidase, and immunoreactive species were detected by enhanced chemiluminescence. Lanes 1 and 4 correspond to the total detergent-soluble and detergent-insoluble fractions, respectively, from 105 erythroid cells. Lanes 2 and 5 correspond to the streptavidin-precipitable polypeptides from the soluble and insoluble fractions of an equivalent number of cells. Lanes 3 and 6 are controls that were processed identically to lanes 2 and 5 except the cells were not biotinylated before lysis and streptavidin precipitation.
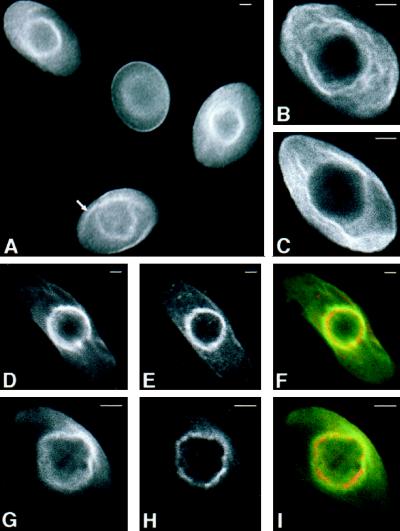
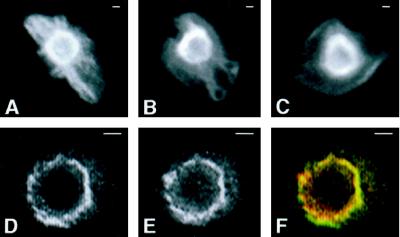
To further define the intracellular distribution of AE1, immunolocalization studies have determined its steady-state pattern of localization. These analyses revealed that AE1 accumulates both in the plasma membrane and in a perinuclear compartment in most, if not all, erythroid cells from 10-d chicken embryos (Figure 9A). Confocal microscopy has shown that both the cell surface (our unpublished results) and perinuclear pool (Figure 9, D–F) of AE1 in these embryonic erythroid cells colocalizes with ankyrin. The perinuclear compartment stained by anion exchanger antibodies also partially overlaps the staining pattern of NBD-ceramide (Pagano et al., 1991), a Golgi marker (Figure 9, G–I). In addition to perinuclear staining, AE1 antibodies stained filamentous membrane projections that extended from the perinuclear region of some cells (marked by arrow in Figure 9A). These filamentous membrane projections, which can be clearly seen in the higher magnification confocal images in Figure 9, B and C, do not appear to be present at the cell surface nor do they overlap the distribution of NBD-ceramide staining. To determine whether the intracellular pool of AE1 is associated with the detergent-insoluble cytoskeleton, erythroid cells were extracted in situ with 1% Triton X-100 before fixation and staining with AE1 antibodies. Although the cell surface staining was substantially reduced in these detergent-extracted cells, essentially all of the cells exhibited strong perinuclear staining for the anion exchanger (Figure 10, A–D). Furthermore, AE1 antibodies stained filamentous projections extending from the perinuclear compartment to the plasma membrane in some of the detergent-extracted cells (Figure 10, A and B). Double staining of these preextracted preparations with AE1 and ankyrin antibodies has shown that the detergent-resistant perinuclear pool of AE1 significantly overlaps the distribution of detergent-resistant ankyrin (Figure 10, D–F). These results suggest that a substantial fraction of the intracellular pool of AE1 in these embryonic erythroid cells is associated with the detergent-insoluble cytoskeleton, perhaps through interaction with ankyrin. These localization profiles also provide an explanation for why a large percentage of the detergent-insoluble AE1 in these cells is not susceptible to surface biotinylation.
Figure 9.
Intracellular localization of chicken erythroid AE1 anion exchangers. Erythroid cells from a 10-d-old chicken embryo were fixed in 1% paraformaldehyde in PBS for 15 min. The cells were permeabilized with acetone and incubated with a rabbit polyclonal antibody specific for AE1 (A, B, C, and G). Alternatively, cells were incubated with an AE1-specific mouse monoclonal antibody and a rabbit polyclonal antibody specific for ankyrin (D–F). Immunoreactive polypeptides were detected with DAR-IgG conjugated to lissamine and DAM-IgG conjugated to FITC and visualized on a Zeiss Axiophot microscope (Carl Zeiss, Thornwood, NY) (A), or on a Bio-Rad laser scanning confocal microscope (B–I). After antibody incubations, the cell shown in panels G–I was incubated with 50 μg/ml NBD-ceramide for 1 h at 37oC, and washed. AE1 accumulated both in the plasma membrane and in a perinuclear compartment of erythroid cells (A). Filamentous membrane projections extend from this perinuclear compartment in some cells (marked by arrow in panel A). These filamentous membrane projections, shown at higher magnification in the 0.5-μm confocal images in panels B and C, do not appear to be present at the level of the cell surface. The 0.5-μm confocal images showing the perinuclear distribution of AE1 (D) and ankyrin (E) were pseudocolored green and red, respectively, and merged (F) in Adobe Photoshop. Likewise, the 0.5-μm confocal images showing the perinuclear distribution of AE1 (G) and NBD-ceramide (H) were pseudocolored green and red, respectively, and merged (I). Bar, 2 μm.
Figure 10.
Perinuclear AE1 anion exchangers and ankyrin are resistant to detergent extraction. Erythroid cells from a 10-d-old chicken embryo were extracted with 1% Triton X-100 in isotonic buffer before fixation in 1% paraformaldehyde in PBS. The cells were incubated with rabbit polyclonal antibodies specific for AE1 (A–C). Alternatively, cells were incubated with an AE1-specific mouse monoclonal antibody and a rabbit polyclonal antibody specific for ankyrin (D–F). Immunoreactive polypeptides were detected with DAR-IgG conjugated to lissamine and DAM-IgG conjugated to FITC and visualized on a Zeiss Axiophot microscope. The images showing the perinuclear localization of AE1 (D) and ankyrin (E) were pseudocolored green and red, respectively, and merged (F) in Adobe Photoshop. Erythroid cells preextracted with Triton X-100 before fixation exhibited reduced cell surface staining and strong perinuclear staining for both AE1 (A–D) and ankyrin (E). In addition, AE1 antibodies stained filamentous projections extending from the perinuclear compartment to the plasma membrane in some of the preextracted cells (A and B). Bar, 1 μm.
Immunoblotting and immunolocalization studies have examined whether ammonium chloride, BFA, and hypertonic medium, which affect the processing of newly synthesized AE1, also alter the steady-state properties of these transporters. After treatment of 10-d embryonic erythroid cells for 2 h with each reagent, cells were detergent lysed, and the soluble and insoluble fractions were subjected to immunoblotting analysis using AE1-specific antibodies. Quantitation of two independent experiments revealed that ∼60% of AE1 is detergent insoluble in control cells. Although similar results were observed in cells treated with ammonium chloride and BFA, only ∼40% of the anion exchangers were detergent insoluble in cells treated with hypertonic medium. Immunolocalization analysis has revealed no obvious effect of these reagents on the intracellular distribution of AE1 (our unpublished results). These results suggest that at least the effects of ammonium chloride and BFA on the processing of newly synthesized anion exchangers are not due to a general redistribution of anion exchangers within erythroid cells.
DISCUSSION
The experiments described here have investigated the mechanisms through which chicken AE1 anion exchangers associate with the detergent-insoluble cytoskeleton of erythroid cells. These analyses have revealed that after their initial delivery to the plasma membrane, the bulk of newly synthesized AE1 anion exchangers are internalized and delivered to the Golgi where they acquire additional N-linked modifications. During this recycling process, a subset of the anion exchangers become detergent insoluble. Although multiple mechanisms may contribute to the acquisition of detergent insolubility, coprecipitation studies suggest that detergent insolubility is due, at least in part, to the association of newly synthesized AE1 with cytoskeletal ankyrin. Pulse-chase and inhibitor studies have shown that the acquisition of detergent insolubility by recycling anion exchangers occurs before their transit through the Golgi. This result suggests the possibility that the detergent-insoluble cytoskeleton plays an active role in the post-Golgi trafficking of anion exchangers in erythroid cells.
Endoglycosidase digestion studies have shown that newly synthesized anion exchangers acquire high-mannose or biantennary hybrid sugars on their single predicted N-linked site during their initial passage through the secretory pathway. After delivery to the cell surface, newly synthesized AE1 is recycled to the Golgi where it acquires additional N-linked modifications that are insensitive to digestion with endo F. The appearance of endo F-resistant forms of the anion exchanger suggests that the high-mannose or biantennary hybrid sugars of these polypeptides are converted to endo F-resistant tri- or tetra-antennary sugars (Tarentino et al., 1985). This conversion would require the activities of α-mannosidase II and N-acetylglucosamine transferase, markers of the medial compartment of the Golgi. Additional studies have shown that the initial N-linked modifications acquired by newly synthesized anion exchangers are insensitive to digestion with neuraminidase, whereas the endo F-resistant modifications that begin to appear after a 1-h chase are neuraminidase-sensitive (our unpublished results). Taken together, these data suggest that newly synthesized AE1 is recycled from the plasma membrane to the medial compartment of the Golgi. After delivery to this compartment, the poly-peptides transit through the remainder of the Golgi complex to the TGN, where the addition of sialic acid occurs.
Several polypeptides (Reichner et al., 1988; Volz et al., 1995) recycle from the plasma membrane to the TGN where they can acquire additional N-linked modifications. In addition, in some cell types the transferrin receptor recycles from the plasma membrane to the cis and medial compartments of the Golgi (Woods et al., 1986). However, in each instance it has been examined, polypeptides that undergo recycling acquire complex N-linked modifications in their initial transit through the secretory pathway (Reichner et al., 1988; Volz et al., 1995). It is unclear why newly synthesized AE1 must undergo recycling from the cell surface to acquire complex N-linked modifications. Although it is possible that the glycosylation machinery of the Golgi is saturated by the high levels of AE1 produced in erythroid cells from 10-d chicken embryos, this seems unlikely since the variant anion exchangers quantitatively acquire high-mannose sugars in the endoplasmic reticulum during a 15-min pulse. The conformation of anion exchangers may also render them inaccessible to the glycosylation machinery during their initial transit through the Golgi complex. Alternatively, the newly synthesized polypeptides could be compartmentalized such that they are segregated from the enzymes involved in the addition of complex sugars during their initial transit through the Golgi. The latter two possibilities would further suggest that modifications and/or protein interactions of the anion exchanger that occur during the recycling process may be necessary for access to the glycosylation machinery of the Golgi.
Pulse-chase studies have shown that the recycling of AE1 can be blocked with inhibitors that interfere with clathrin-dependent endocytosis (hypertonic medium), or endosomal acidification (ammonium chloride and chloroquine). Each of these reagents completely blocks the acquisition of endo F-resistant modifications by newly synthesized AE1. In addition, these reagents partially block the transition of newly synthesized AE1 from the detergent-soluble to the detergent-insoluble pool. Additional analyses have shown that the effect of ammonium chloride on anion exchanger processing is rapidly reversible. After release from an ammonium chloride block, AE1 anion exchangers become detergent insoluble and acquire endo F-resistant modifications much more rapidly than they do normally in untreated cells. This suggests that ammonium chloride treatment effectively concentrates anion exchangers in an endosomal compartment after their internalization from the cell surface. Upon reversal of the block, anion exchangers move synchronously from this compartment to the Golgi where additional N-linked modifications are added. The observation that AE1 anion exchangers become detergent insoluble at a more rapid rate than they acquire endo F-resistant sugars suggests that the association of AE1 with cytoskeletal polypeptides, such as ankyrin, occurs after exiting the membrane compartment where they accumulate as a result of the ammonium chloride block and before their transit through the Golgi. This implies that the initial association of recycling anion exchangers with the detergent-insoluble cytoskeleton occurs in an intracellular membrane compartment rather than at the plasma membrane. Furthermore, ∼10% of newly synthesized AE1 becomes detergent insoluble during a 15-min pulse (Figure 1). Cell surface biotinylation experiments have indicated that this is insufficient time to allow cell surface delivery of the newly synthesized polypeptides (our unpublished results). These results suggest the possibility that recycling anion exchangers and anion exchangers in their initial transit through the secretory pathway associate with the detergent-insoluble cytoskeleton in the same intracellular membrane compartment.
The effects of BFA on the processing of newly synthesized anion exchangers appear to be primarily due to its effect on events that occur after the initial delivery of AE1 to the plasma membrane. This suggests that one or more of the endosomal sorting steps during AE1 recycling is Arf dependent. Previous studies have shown that BFA inhibits a variety of steps involved in the internalization and sorting of endocytosed polypeptides. Although the recycling of TGN38 from the plasma membrane to the TGN is not blocked by BFA, the kinetics of endocytosis of TGN38 was altered by this drug (Reaves et al., 1993). In addition, BFA has been shown to inhibit the return of endocytosed transferrin receptor to the cell surface (Stoorgovel et al., 1996). This latter result is consistent with the observation that BFA blocks the formation of clathrin-coated buds on endosomes (Stoorgovel et al., 1996). The specific step(s) in the recycling pathway of AE1 that is inhibited by BFA remains to be elucidated.
Although the intensity of staining varied considerably, AE1 anion exchangers could be detected both at the plasma membrane and in a perinuclear compartment in most, if not all, of the erythroid cells from a 10-d chicken embryo. The erythroid cells from this stage embryo are comprised of ∼20% mature primitive lineage cells, ∼30% mature definitive lineage cells, and ∼50% mid- and late polychromatophilic erythroblasts and reticulocytes of the definitive lineage (Bruns and Ingram, 1973). Similar immunolocalization profiles have been observed with erythroid cells isolated from 15-d and 18-d chicken embryos (our unpublished results), which are almost entirely definitive lineage in origin. These results suggest that the ability of anion exchangers to accumulate in a perinuclear compartment is not dependent on the lineage or stage of differentiation of the erythroid cell, nor is it dependent upon the stage of embryonic development. Studies with human erythroid cells have also detected anion exchangers at the plasma membrane and in a perinuclear compartment in cells that have not yet undergone terminal differentiation (Nehls et al., 1993). The perinuclear staining observed in chicken erythroid cells may be due, in part, to the accumulation of specific AE1 variants in this membrane compartment. Previous transfection studies (Cox et al., 1996) have shown that ∼108-kDa chicken erythroid AE1 variant, which contains exons 5 and 6, accumulates in a perinuclear compartment of transfected human erythroleukemia (HEL) cells. In contrast, the ∼99 and ∼102-kDa variants, which lack exons 5 and 6, primarily accumulate in the plasma membrane of transfected HEL cells. It is possible that AE1 variants containing exons 5 and 6 , which represent ∼12% of the erythroid AE1 transcripts on day 10 of development (Cox et al., 1996), account for the majority of the perinuclear staining observed in embryonic chicken erythroid cells. Alternatively, the perinuclear staining observed in these cells may reflect all variant anion exchangers in their initial transit through the secretory pathway, as well as those in the process of recycling.
Previous studies have suggested that interaction of the anion exchanger with ankyrin is necessary for the cell surface delivery of newly synthesized anion exchangers (Gomez and Morgans, 1993). Additional analyses have suggested that the stable accumulation of the Na+,K+-ATPase in the basolateral membrane of polarized kidney epithelial cells involves the recruitment of intracellular complexes that contain the Na+,K+-ATPase and the membrane cytoskeletal polypeptides, ankyrin and fodrin (Nelson and Hammerton, 1989). In these studies, the complexes between these membrane transporters and components of the cytoskeleton are detergent soluble. The studies described here have demonstrated that detergent-soluble complexes containing AE1 and ankyrin also accumulate in chicken erythroid cells. Although these soluble complexes were detected throughout pulse-chase analyses, there is currently no evidence that association with detergent-soluble ankyrin is involved in either the cell surface delivery or recycling of chicken erythroid AE1. During the recycling process, a shift occurs such that newly synthesized AE1 primarily associates with the detergent-insoluble or cytoskeletal form of ankyrin rather than the detergent-soluble form of the polypeptide. The shift in the solubility properties of these complexes during recycling is consistent with localization studies that have shown detergent-resistant forms of both AE1 and ankyrin are primarily perinuclear. The features of these complexes that alter their detergent solubility properties remain to be determined. In addition, the mechanism of recruiting detergent-soluble forms of ankyrin and AE1 into the detergent-insoluble cytoskeleton is not known. One attractive possibility is that soluble complexes containing AE1 and ankyrin undergo internalization from the cell surface and recycling to the Golgi, where they are incorporated into the perinuclear cytoskeleton of erythroid cells en bloc.
The observations that ∼40% of the steady-state pool of detergent-insoluble AE1 is present on the cell surface, while cytoskeletal assembly of newly synthesized AE1 occurs in an intracellular membrane compartment suggest that the detergent-insoluble cytoskeleton is involved in the post-Golgi trafficking of a subset of AE1 polypeptides. The mechanism of translocating cytoskeletal AE1 from the perinuclear compartment to the cell surface is not known. However, it is possible the filamentous projections that extend from the perinuclear region of some detergent-extracted erythroid cells represent cytoskeletal forms of AE1 in the process of shuttling from the Golgi to the plasma membrane.
Immunolocalization studies have indicated that the perinuclear pool of anion exchangers in chicken erythroid cells substantially overlaps the distribution of ankyrin. Similar results have been observed in human erythroid cells that have not yet undergone terminal differentiation (Nehls et al., 1993). Golgi-specific isoforms of both ankyrin (Devarajan et al., 1996; Beck et al., 1997) and spectrin (Beck et al., 1994) have been detected in epithelial cells, where they have been implicated in the ER-to-Golgi transport of type I and type III membrane proteins (Devarajan et al., 1997). Although the role of ankyrin in the trafficking of erythroid AE1 is not known, the results presented here suggest that a perinuclear membrane compartment serves as the site for the initial interaction of the anion exchanger with cytoskeletal ankyrin. This interaction is dependent, in large part, upon the recycling of cell surface anion exchangers to the Golgi. Previous investigators have shown that recycling of the mannose 6-phosphate receptor to the TGN allows this receptor to associate with its cargo that is destined for the lysosome (Duncan and Kornfeld, 1988). Additional studies have shown that recycling from the plasma membrane to the TGN provides a mechanism for the return of TGN resident proteins to this compartment (Chapman and Munro, 1994). It is tempting to speculate that recycling from the plasma membrane to the Golgi may also provide a general mechanism through which cell surface transporters and channels associate with the membrane cytoskeleton in a variety of cell types. This association with the cytoskeleton may facilitate the delivery of these integral membrane polypeptides from the Golgi to specialized membrane domains within cells.
ACKNOWLEDGMENT
We thank Dr. Michael Jennings for generously providing monoclonal anti-AE1 antibodies. This research was supported by grants from the National Chapter of the American Heart Association (96-008610) and the American Cancer Society (IN-176-B) to J.V.C. S.G. was supported by the Alma and Hal Reagan Predoctoral Research Fellowship.
REFERENCES
- Beck K, Buchanan J, Nelson WJ. Golgi membrane skeleton: identification, localization, and oligomerization of a 195 kDa ankyrin isoform associated with the Golgi complex. J Cell Sci. 1997;110:1239–1249. doi: 10.1242/jcs.110.10.1239. [DOI] [PubMed] [Google Scholar]
- Beck KA, Buchanan JA, Malhotra V, Nelson WJ. Golgi spectrin: Identification of an erythroid β-spectrin homolog associated with the Golgi complex. J Cell Biol. 1994;127:707–723. doi: 10.1083/jcb.127.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V, Stenbuck PJ. The membrane attachment protein for spectrin is associated with band 3 in human erythrocyte membranes. Nature. 1980;280:468–473. doi: 10.1038/280468a0. [DOI] [PubMed] [Google Scholar]
- Brosius FC, Alper SL, Garcia AM, Lodish HF. The major kidney band 3 gene transcript predicts an amino-terminal truncated band 3 polypeptide. J Biol Chem. 1989;264:7784–7787. [PubMed] [Google Scholar]
- Bruns GAP, Ingram VM. The erythroid cells and the hemoglobins of the chick embryo. Phil Trans R Soc Lond B Biol Sci. 1973;266:225–305. doi: 10.1098/rstb.1973.0050. [DOI] [PubMed] [Google Scholar]
- Chapman RE, Munro S. Retrieval of TGN proteins from the cell surface requires endosomal acidification. EMBO J. 1994;13:2305–2312. doi: 10.1002/j.1460-2075.1994.tb06514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen CM, Korsgren C. Associations of human erythrocyte band 4.2. J Biol Chem. 1988;263:10212–10218. [PubMed] [Google Scholar]
- Cox JV, Stack JH, Lazarides E. Erythroid anion transporter assembly is mediated by a developmentally regulated recruitment onto a preassembled membrane cytoskeleton. J Cell Biol. 1987;105:1405–1416. doi: 10.1083/jcb.105.3.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox KH, Adair-Kirk TL, Cox JV. Four variant chicken erythroid AE1 anion exchangers. Role of the alternative N-terminal sequences in intracellular targeting in transfected human erythroleukemia cells. J Biol Chem. 1996;270:19752–19760. doi: 10.1074/jbc.270.34.19752. [DOI] [PubMed] [Google Scholar]
- Cox KH, Cox JV. Variant chicken AE1 anion exchangers possess divergent NH2-terminal cytoplasmic domains. Am J Physiol. 1995;268:F503–F513. doi: 10.1152/ajprenal.1995.268.3.F503. [DOI] [PubMed] [Google Scholar]
- Davis L, Davis JQ, Bennett V. Ankyrin regulation: An alternatively spliced segment of the regulatory domain functions as an intracellular modulator. J Biol Chem. 1992;267:18966–18972. [PubMed] [Google Scholar]
- Devarajan P, Stabach PR, DeMatteis MA, Morrow JS. Na+,K+-ATPase transport from endoplasmic reticulum to Golgi requires the Golgi spectrin-ankyrinG119 skeleton in Madin Darby canine kidney cells. Proc Natl Acad Sci USA. 1997;94:10711–10716. doi: 10.1073/pnas.94.20.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devarajan P, Stabach PR, Mann AS, Ardito T, Kashgarian M, Morrow JS. Identification of a small cytoplasmic ankyrin (AnkG119) in the kidney and muscle that binds βIΣ* spectrin and associates with the Golgi apparatus. J Cell Biol. 1996;133:818–830. doi: 10.1083/jcb.133.4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Casey JR, Kopito RR. The major kidney AE1 isoform does not bind ankyrin (ANK1) in vitro. J Biol Chem. 1994;269:32201–32208. [PubMed] [Google Scholar]
- Duncan JR, Kornfeld S. Intracellular movement of two mannose 6-phosphate receptors: Return to the Golgi apparatus. J Cell Biol. 1988;106:617–628. doi: 10.1083/jcb.106.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez S, Morgans C. Interactions between band 3 and ankyrin begins in early compartments of the secretory pathway and is essential for band 3 processing. J Biol Chem. 1993;268:19593–19597. [PubMed] [Google Scholar]
- Hansen SH, Sandvig K, van Deurs B. Clathrin and HA2 adaptors: Effects of potassium depletion, hypertonic medium, and cytosol acidification. J Cell Biol. 1993;121:61–72. doi: 10.1083/jcb.121.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings ML, Anderson MP, Monaghan R. Monoclonal antibodies against human erythrocyte band 3 protein: localization of proteolytic cleavage sites and stilbenedisulfonate-binding lysine residues. J Biol Chem. 1986;261:9002–9010. [PubMed] [Google Scholar]
- Kollert-Jons A, Wagner S, Hubner S, Appelhans H, Drenckhahn D. Anion exchanger 1 in human kidney and oncocytoma differs from erythroid AE1 in its NH2 terminus. Am J Physiol. 1993;265:F813–F821. doi: 10.1152/ajprenal.1993.265.6.F813. [DOI] [PubMed] [Google Scholar]
- Kopito RR, Lodish HF. Primary structure and transmembrane orientation of the murine anion exchange protein. Nature (Lond) 1985;316:234–238. doi: 10.1038/316234a0. [DOI] [PubMed] [Google Scholar]
- Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kudrycki KE, Shull GE. Rat kidney band 3 chloride/bicarbonate exchanger mRNA is transcribed from an alternative promoter. Am J Physiol. 1993;264:F540–F547. doi: 10.1152/ajprenal.1993.264.3.F540. [DOI] [PubMed] [Google Scholar]
- Lux SE, John KM, Bennett V. Analysis of cDNA for human erythrocyte ankyrin indicates a repeated structure with homology to tissue-differentiation and cell-cycle control proteins. Nature. 1990;344:36–42. doi: 10.1038/344036a0. [DOI] [PubMed] [Google Scholar]
- Mutsumi I, et al. Defective anion transport and marked spherocytosis with membrane instability caused by hereditary total deficiency of red cell band 3 in cattle due to a nonsense mutation. J Clin Invest. 1996;97:1804–1817. doi: 10.1172/JCI118610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehls V, Zeitler-Zapf P, Drenckhahn D. Different sequences of expression of band 3, spectrin, and ankyrin during normal erythropoiesis and erythroleukemia. Am J Pathol. 1993;142:1565–1573. [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ, Hammerton RW. A membrane cytoskeletal complex containing Na+,K+-ATPase, ankyrin, and fodrin in MDCK cells: implications for the biogenesis of epithelial cell polarity. J Cell Biol. 1989;108:893–902. doi: 10.1083/jcb.108.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano RE, Sepanski MA, Martin OC. Molecular trapping of a fluorescent ceramide analogue at the Golgi apparatus of fixed cells. J Cell Biol. 1991;109:2067–2079. doi: 10.1083/jcb.109.5.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternack GR, Anderson RA, Leto TL, Marchesi VT. Interaction between protein 4.1 and band 3: An alternative binding site for an element of the membrane cytoskeleton. J Biol Chem. 1985;260:3676–3683. [PubMed] [Google Scholar]
- Peters LL, et al. Anion exchanger 1 (band 3) is required to prevent erythrocyte membrane surface loss but not to form the membrane cytoskeleton. Cell. 1996;86:917–927. doi: 10.1016/s0092-8674(00)80167-1. [DOI] [PubMed] [Google Scholar]
- Reaves B, Horn M, Banting G. TGN38/41 recycles between the cell surface and the TGN: Brefeldin A affects its rate of return to the TGN. Mol Biol Cell. 1993;4:93–105. doi: 10.1091/mbc.4.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichner JS, Whiteheart SW, Hart GW. Intracellular trafficking of cell surface sialoglycoconjugates. J Biol Chem. 1988;263:16316–16326. [PubMed] [Google Scholar]
- Stoorgovel W, Ooschot V, Geuze HJ. A novel class of clathrin-coated vesicles budding from endosomes. J Cell Biol. 1996;132:21–33. doi: 10.1083/jcb.132.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner MJ, Martin PG, High S. The complete amino acid sequence of the human erythrocyte anion-transport protein deduced from the cDNA sequence. Biochem J. 1988;256:783–792. doi: 10.1042/bj2560703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino AL, Gomez CM, Plummer TH. Deglycosylation of asparagine-linked glycans by peptide:N-glycosidase F. Biochemistry. 1985;24:4665–4671. doi: 10.1021/bi00338a028. [DOI] [PubMed] [Google Scholar]
- Volz B, Orberger G, Porwoll S, Hauri HP, Tauber R. Selective reentry of recycling cell surface glycoproteins to the biosynthetic pathway in human hepatocarcinoma HepG2 cells. J Cell Biol. 1995;130:537–551. doi: 10.1083/jcb.130.3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CC, Moriyama R, Lombardo CR, Low PS. Partial characterization of the cytoplasmic domain of human kidney band 3. J Biol Chem. 1995;270:17892–17897. doi: 10.1074/jbc.270.30.17892. [DOI] [PubMed] [Google Scholar]
- Woods JW, Doriaux M, Farquhar MG. Transferrin receptors recycle to cis and middle as well as trans Golgi cisternae in Ig-secreting myeloma cells. J Cell Biol. 1986;103:277–286. doi: 10.1083/jcb.103.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]