Abstract
Background
Lower extremity peripheral arterial disease (PAD) is highly prevalent and strongly associated with cardiovascular morbidity and mortality. The ankle-brachial index used to screen for PAD is not routinely performed in primary care settings.
Objective
To determine if self-reported PAD is an independent predictor of combined vascular events (myocardial infarction, ischemic stroke, and vascular death).
Design
Ongoing population-based prospective cohort (the Northern Manhattan Study). Subjects enrolled between July 1993 and June 2001 with a mean follow-up time of 7.1 years.
Patients
Subjects (n = 2,977), aged 40 years or older and free of prior stroke or myocardial infarction, were classified as having self-reported PAD if they answered affirmatively to one of two questions regarding exercise-induced leg pain or a prior diagnosis of PAD.
Main Outcome Measures
Combined vascular outcome defined as incident myocardial infarction, incident ischemic stroke, or vascular death.
Results
The mean age of the cohort was 68.9 ± 10.4 years; 64% were women; 54% Hispanic, 25% African-American, 21% Caucasian; 15% reported having PAD. After a mean follow-up of 7.1 years, self-reported PAD was significantly predictive of combined events (n = 484) in the univariate model (HR 1.5, 95% CI, 1.2–1.9) and after adjustment for traditional cardiovascular risk factors (HR 1.3, 95% CI, 1.0–1.7).
Conclusion
Self-reported PAD is an independent risk factor for future vascular events in this predominantly non-white cohort. The addition of two simple PAD questions to the routine medical history in general medicine settings could identify high-risk patients who would benefit from further vascular evaluation and risk factor modification.
KEY WORDS: peripheral arterial disease, claudication, vascular events, myocardial infarction, ischemic stroke
INTRODUCTION
Peripheral arterial disease (PAD) is an atherosclerotic syndrome in which the lumen of the arteries in the lower extremities becomes progressively obstructed by plaque. Recent epidemiologic studies estimate a prevalence of PAD of 11 to 16% in the population aged 55 or older 1–3, and a prevalence as high as 20 to 30% in specific high-risk populations 4–6. Early detection of PAD is essential to prevent subsequent cardiovascular morbidity and mortality. Several prospective and cross-sectional studies have shown that PAD is a marker for arterial disease in other vascular beds 7–10 and is associated with a six-fold increase in fatal and nonfatal myocardial infarction 11–13 and a two- to three-fold increase in risk of ischemic stroke 14–16. In addition, the presence of PAD is a strong predictor of overall mortality 17–19.
Intermittent claudication, defined as reproducible pain in the lower limbs during exercise relieved by rest, is the most common manifestation of symptomatic PAD 20. The Framingham Heart Study initially described the association between intermittent claudication and both coronary heart disease and stroke and reported a two-fold age-adjusted increased risk of death in both men and women with claudication 21. However, up to one-third of patients do not even alert a physician to their leg symptoms 22, and fewer than half of general medicine physicians report routinely obtaining a history of claudication 23. Furthermore, in patients with an already established diagnosis of PAD, physician awareness of the diagnosis is low 5.
The ankle-brachial index (ABI), a non-invasive method used to assess the lower extremity arterial system, is considered the best PAD screening test 24,25. Recent PAD management guidelines recommend screening ABIs in high-risk patients in the primary care setting 26. However, major barriers currently prevent widespread implementation of this practice 27, and as a result, PAD remains under-diagnosed and under-treated in the general population. Therefore, we sought to determine whether self-reported PAD, defined as an affirmative response to one of two simple questions regarding exertional leg symptoms or a prior diagnosis of PAD, would help identify those patients who may be at higher risk for future vascular events.
METHODS
The Northern Manhattan Study (NOMAS) is an ongoing, prospective, population-based epidemiological study designed to determine stroke incidence, risk factors, and outcomes in a multi-ethnic, urban population of northern Manhattan, NY. In 1990, 71% of the 210,000 individuals who resided this area were ≥20 years of age; 22% were white, 13% were black, and 64% were Hispanic.
Participants
The methods of subject recruitment and enrollment into NOMAS have been described elsewhere 28,29. Briefly, random digit dialing of approximately 25,000 households was performed, and community participants were enrolled in NOMAS if they: (1) had never had a stroke, (2) were older than age 40, and (3) had resided in Northern Manhattan for ≥3 months in a household with a telephone. Ninety-one percent of those called participated in a telephone interview, and 75% of those who were eligible and invited to participate came to Columbia University Medical Center (CUMC) for an in-person evaluation (overall participation rate 68%). The study was approved by the Institutional Review Board at CUMC. All participants gave consent directly or through a surrogate when appropriate. The final cohort consisted of 3,298 participants, and 3,290 answered the self-reported PAD questions. Of those, 313 had a history of myocardial infarction (MI) and were thus excluded from this analysis. The final cohort included in this analysis consisted of 2,977 participants.
Procedures
Standardized questions were adapted from the Behavioral Risk Factor Surveillance System by the Centers for Disease Control 30 regarding hypertension, diabetes, cigarette smoking, and cardiac conditions. Blood pressure was measured with mercury sphygmomanometers and cuffs of appropriate size. Hypertension was defined as a blood pressure ≥140/90 mmHg (based on the average of two measurements during one sitting by a trained research assistant), the patient’s self-report of hypertension, or antihypertensive medication use. Diabetes mellitus was defined by the patient’s self-report of such a history, use of insulin or oral antidiabetic medication, or fasting glucose ≥126 mg/dl. Lipid profile was measured at the time of enrollment. Smoking was defined as having smoked more than 100 cigarettes in a lifetime or currently smoking. Moderate alcohol intake was defined as >0 drinks per week but ≤2 drinks per day in the past year as previously described 31. Prior coronary revascularization was defined as a history of balloon angioplasty, stenting, and/or coronary artery bypass surgery. Assessments were conducted in English or Spanish, depending on the primary language of the participant. Race-ethnicity was based on self-identification through a series of interview questions modeled after the US census and conformed to the standard definitions outlined by Directive 15.
Self-Reported Peripheral Arterial Disease
Peripheral arterial disease was self-reported and defined as an affirmative answer to either of the two following questions:
Do you get pain in the back of your legs when you walk that stops with rest?
Have you been told you have vascular (arterial) disease in the legs?
Annual Prospective Follow-Up
All subjects were prospectively followed annually by telephone. Subjects were interviewed and screened for changes in vital status, neurological and cardiac symptoms and events, and any interval hospitalizations. Any subject who screened positive by telephone was scheduled for an in-person assessment. All affirmative responses to neurological symptoms and conditions were reviewed and the subjects examined by a study neurologist. In addition, active hospital surveillance of admission and discharge International Classification of Diseases, Ninth Revision (ICD-9) codes at CUMC provided data on mortality and morbidity that may not have been captured during annual telephone follow-up. Review of discharge lists from outside hospitals and contacts with community physicians and visiting nurse services were also performed for surveillance of events. The overall loss to follow-up rate was minimal (<1%).
Outcome Classifications
Stroke was defined by the World Health Organization criteria as “rapidly developing clinical signs of focal (at times global) disturbance of cerebral function, lasting more than 24 h or leading to death with no apparent cause other than that of vascular origin.” 32 Ischemic stroke was defined as non-hemorrhagic cerebral infarction. Stroke subjects had a battery of standard diagnostic tests including brain imaging. Medical records of all hospitalizations were reviewed to verify details of suspected events. Two study neurologists classified the strokes independently after review of all available data. The principal investigator adjudicated any disagreements.
MI was defined by criteria adapted from the Cardiac Arrhythmia Suppression Trial 33 and the Lipid Research Clinics Coronary Primary Prevention Trial 34 requiring at least two of the three following criteria: (1) cardiac pain determined to be typical angina, (2) cardiac enzyme abnormalities defined as abnormal CPK-MB fraction or troponin values, and (3) electrocardiogram abnormalities. Cardiology co-investigators reviewed and classified all suspected events.
For subjects who died, the date of death was recorded along with cause of death. Deaths were classified as vascular or nonvascular based on information obtained from the family, medical records, and death certificate. Causes of vascular death included stroke, MI, heart failure, pulmonary embolus, cardiac arrhythmia, and other vascular causes. Nonvascular causes of death included accident, cancer, pulmonary (e.g., pneumonia or chronic obstructive pulmonary disease), and other nonvascular causes.
Statistical Analyses
The prevalence of self-reported PAD (srPAD) was calculated overall and by race-ethnic groups. The frequency of vascular risk factors was compared between those with and without srPAD using the chi-square test for categorical variables and the t-test for continuous variables. The Kaplan-Meier curves and log rank test were used for comparing the incidence of combined vascular events (ischemic stroke, MI, and vascular death) between those with and without srPAD. Cox proportional regression models [hazard ratios (HR) and 95% confidence intervals (CI)] were used to examine the association between srPAD and the incidence of combined vascular events after adjusting for vascular risk factors and other potential confounders. Time to the first event among combined vascular events was the failure time. The failure time was censored either at death from nonvascular cause or the last follow-up. The 1,000 person-year event rate was calculated and compared between those with and without srPAD using the Poisson regression model. All statistical analyses were performed with SAS version 9.0 software (SAS Institute, Cary, NC). A p-value of less than 0.05 was considered significant.
RESULTS
Baseline Characteristics by Self-Reported PAD Status
Among 2,977 individuals, the mean age was 68.9 ± 10.4 years; 64% were women; 54% were Hispanic, 25% were African-American, and 21% were Caucasian. The prevalence of srPAD was 15.0% (443/2,977): 16.4% in Hispanics, 14.8% in African-Americans, and 11.0% in whites. Baseline cohort characteristics by srPAD status are shown in Table 1. The mean age was similar in both groups. The srPAD group in comparison to the non-srPAD had a greater proportion of women (70.2% vs. 62.7%, p = 0.002), hypertension (80.1% vs. 64.9%, p < 0.0001), diabetes (35.9% vs. 18.3%, p < 0.0001), and prior coronary revascularization (3.8% vs. 1.8%, p = 0.003), but fewer had completed high school (37% vs. 46.5%, p = 0.0002) and reported moderate alcohol consumption (25.4% vs. 34.9%, p = 0.0002). There was no difference in mean LDL-C levels or smoking status between the two groups.
Table 1.
Characteristics Associated with Self-Reported PAD* Versus Without Self-Reported PAD Among Study Participants (N = 2,977)
| Characteristics | Self-reported PAD (n = 443) | No self-reported PAD (n = 2,534) | P-value |
|---|---|---|---|
| Age, mean ± SD, years | 68.4 ± 10.0 | 69.0 ± 10.4 | 0.29 |
| Women, no. (%) | 311 (70.2) | 1,588 (62.7) | 0.002 |
| Race-ethnicity | – | – | 0.006 |
| Hispanic, no. (%) | 265 (59.8) | 1,350 (53.3) | – |
| African-American, no. (%) | 110 (24.8) | 633 (35.0) | – |
| Caucasian, no. (%) | 8 (15.4) | 551 (21.7) | – |
| Completed high school, no. (%) | 164 (37.0) | 1,177 (46.5) | <0.001 |
| Hypertension, no. (%) | 355 (80.1) | 1644 (64.9) | <0.001 |
| Diabetes, no. (%) | 159 (35.9) | 464 (18.3) | <0.001 |
| LDL-C, mean ± SD, mg/dl | 130 ± 35 | 128 ± 37 | 0.32 |
| Cigarette smoking, no. (%) | 233 (52.6) | 1,337 (52.8) | 0.94 |
| Alcohol, moderate, no. (%) | 112 (25.4) | 882 (34.9) | <0.001 |
| Coronary revascularization, no. (%) | 17 (3.8) | 35 (1.4) | <0.001 |
*Abbreviations: PAD, peripheral arterial disease
Association Between Self-Reported PAD and Vascular Outcomes
There were 484 (16.3%) combined vascular events (157 ischemic strokes, 127 MIs, 200 vascular deaths) during a mean follow-up of 7.1 years. The incidence of vascular events was 31.1 per 1,000 person-years in participants with srPAD versus 26.6 per 1,000 person-years in those without srPAD (p = 0.002). The Kaplan-Meier event-free survival curves for time to combined vascular events, incident MI, incident ischemic stroke, and vascular death are displayed in Fig. 1A-D. There was a significant difference in combined event-free survival between subjects with srPAD versus those without (log rank test p = 0.0008) as well as for time to MI (log rank test p = 0.003) and vascular death (log rank test p = 0.018). However, no difference was detected in time to ischemic stroke between the two groups (log rank test p = 0.604).
Figure 1.
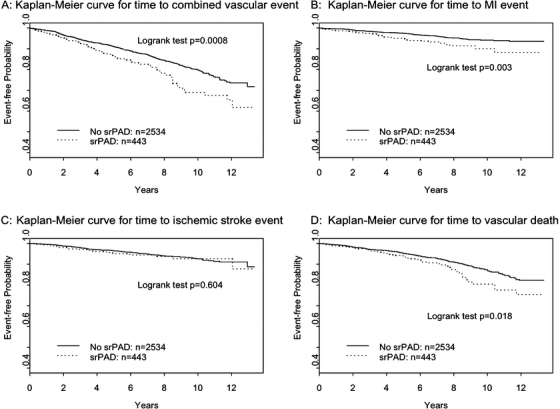
(A-D) The Kaplan-Meier event-free survival curves for time to combined vascular events (A), time to incident MI (B), time to incident ischemic stroke (C), and time to vascular death (D).
The relative risk of combined vascular events was increased by 48% in participants with srPAD (HR 1.48, 95% CI, 1.18–1.87) (Table 2). After adjusting for age, sex, race-ethnicity, education level, hypertension, diabetes, LDL-C, and smoking, the risk of vascular events remained statistically significant (adjusted HR 1.35, 95% CI 1.06 to 1.74), as well as after additional adjustment for prior coronary revascularization and moderate alcohol consumption (adjusted HR 1.30, 95% CI 1.01–1.67).
Table 2.
Unadjusted and Adjusted Models Displaying Hazard Ratios for Self-Reported PAD* and Combined Vascular Outcomes (Incident Myocardial Infarction, Incident Ischemic Stroke, and Vascular Death)
| Model | Hazard ratio | 95% Confidence interval |
|---|---|---|
| Model 1† | 1.48 | 1.18–1.87 |
| Model 2‡ | 1.70 | 1.35–2.15 |
| Model 3§ | 1.35 | 1.06–1.74 |
| Model 4║ | 1.30 | 1.01–1.67 |
*Abbeviations: PAD, peripheral arterial disease
†Model 1 = self-reported PAD
‡Model 2 = self-reported PAD adjusted for age, sex, race-ethnicity, and high school education
§Model 3 = Model 2 also adjusted for diabetes, hypertension, LDL-C, and smoking
║Model 4 = Model 3 also adjusted for moderate alcohol consumption and prior coronary revascularization
DISCUSSION
Our results demonstrate a clear and independent association between self-reported PAD and the risk of future vascular events. An affirmative response to either of two PAD questions was associated with an adjusted 30% increased relative risk of ischemic stroke, MI, or vascular death. Other independent predictors of vascular outcomes in our multivariate analysis included age, male sex, hypertension, diabetes, and smoking. With the exception of cholesterol levels, the predictors for combined vascular outcomes in our study were similar to the coronary heart disease predictors used in the Framingham Heart equation 35. Similarly, the Edinburgh Artery Study reported that the presence of PAD (defined by an ABI <0.9) was independently predictive of fatal MI after controlling for the same conventional risk factors 36. These results suggest that patients with PAD have an additional risk for adverse vascular events above and beyond that conferred by conventional risk factors alone.
Our results indicate that the main difference in combined vascular outcomes between those with and without srPAD was largely attributable to an increased risk of MI and vascular death. These results are consistent with Framingham data demonstrating a strong association between intermittent claudication and coronary heart disease 21. Our results, however, fail to show a clear relationship between srPAD and ischemic stroke. There are several possible explanations for this finding, including a lack of statistical power to detect differences in each outcome separately. Furthermore, PAD may be more closely associated with ischemic stroke due to large artery disease rather than other ischemic stroke subtypes, such as small vessel disease, which is more frequent in African-American and Hispanic populations 29. Finally, this finding could also suggest that adverse outcomes in PAD patients may be driven by coronary heart disease events more than by ischemic stroke.
As reported in the literature, intermittent claudication is ascertained using validated questionnaires such as the Rose Claudication Questionnaire 37; however, these questionnaires have limited applicability to the busy primary care setting. Furthermore, the Rose Questionnaire has been demonstrated to have high specificity, but very low sensitivity 4,5,38,39. The PARTNERS (PAD Awareness, Risk and Treatment: New Resources for Survival) program, which was conducted in primary care practices throughout the US, reported a positive Rose questionnaire in only 8.7% of patients with PAD as determined by ABI <0.9. Notably, over half of these patients exhibited leg symptoms other than classic Rose claudication 5. Aside from its ease of use, our Question 1 (Do you get pain in the back of your legs when you walk that stops with rest?) is designed to detect symptoms of intermittent claudication while also capturing atypical exertional leg symptoms. This would be expected to result in significantly increased sensitivity and hence be more appropriate for screening purposes in the primary care setting. Simply asking this question is particularly important given that one study revealed that up to one-third of patients do not even alert a physician to their leg symptoms as they attribute them to musculoskeletal pain, arthritis, or aging 22. In that same study, patients with objective PAD also demonstrated one or more co-morbidities, such as neuropathy, arthritis, and spinal stenosis. These co-morbidities can mask or alter the symptoms of classic claudication and, as a consequence, providers may be less likely to consider the diagnosis of PAD in these patients. Our Question 2 (Have you been told you have vascular (arterial) disease in the legs?) was designed to simply elicit the history of a prior PAD diagnosis directly from participants, a crucial first step in identifying those at higher risk. However, this question has yet to be incorporated into the routine general medicine history. A study aimed at assessing the factors affecting the diagnosis of PAD revealed that only 37% of internists reported taking a history of claudication; in contrast 92% reported taking a cardiac history most of the time (75–100% of the time) 23. Similarly, PARTNERS reported that while 83% of patients with prior PAD were aware of their diagnosis, only 49% of their physicians were aware of this diagnosis 5. Furthermore, prior studies have reported under-treatment of risk factors in PAD patients as compared to patients with CAD 5,40, highlighting the need for increased PAD awareness in primary care, where implementation of prevention measures could improve outcomes.
Prospective data linking the presence of PAD to adverse vascular events in large multi-ethnic populations remain scarce. This current study represents an opportunity to examine the association between PAD and vascular outcomes in a predominantly non-white population. In contrast to prior studies, we found that the prevalence of srPAD was significantly higher in Hispanics than in whites 41,42. The majority of Hispanics included in prior studies were Mexican-Americans 43, while the Hispanics in our study are mainly Caribbean Hispanics. The high prevalence of srPAD within this subgroup may be attributable to an excess of traditional cardiovascular risk factors, but also perhaps to other unknown factors. More data are needed to characterize PAD prevalence among different Hispanic subgroups.
The screening methods for detection of PAD are not standardized. PAD management guidelines recommend the routine use of ABIs in general medicine practices 26. However, the US Preventive Services Task Force currently recommends against routine screening for PAD by ABI testing 43. Moreover, in a recent survey, time constraints (56%), lack of reimbursement (45%), and staff availability (45%) were major barriers to the use of screening ABIs 27. Given the potential burden of performing ABIs in busy practices, we suggest using self-reported PAD as an initial screening tool to identify higher risk patients. Positive answers to the PAD questions should lead the physician to obtain more objective testing, such as ABIs. Confirmation of PAD by such testing would then in turn change treatment and monitoring goals for these patients.
The major limitation of our study is that ABI data were not available. The comparison study is currently underway and will provide an assessment of the relationship between self-reported PAD, ABIs, and vascular outcomes in this multi-ethnic cohort. The National Health and Nutrition Examination Survey (NHANES), a cross-sectional US survey of 2,174 individuals, reported a prevalence of PAD (defined as ABI <0.9) of 14.5% in those over age 70 44. Our cohort was of a similar age (mean age 68.7 years) as this NHANES subgroup and was found to have a similar overall prevalence of srPAD of 15%. This suggests that the prevalence of self-reported PAD may correlate reasonably well with the prevalence of PAD as determined by ABI.
Another limitation of our study is a possibility of missing or misclassifying the outcomes since the adjudication of events from the hospital records and death certificates is a difficult process that includes reviewing the records, which are not always complete or accurate. However, the incidence rates of events in our study are comparable to other US populations of similar demographics.
CONCLUSION
Our two simple questions evaluating self-reported PAD are practical and easy to implement. In the absence of an official recommendation by the US Preventive Services Task Force for routine screening of PAD in high-risk patients 43, the routine use of our questions by primary care physicians may represent a first step in identifying high-risk individuals who would benefit from objective testing for PAD and subsequent aggressive risk factor modification for prevention of MI, stroke, and vascular death.
Acknowledgments
This work was supported by grants from the National Institute of Neurological Disorders and Stroke (R01 NS 29993, T32 NS 07153) and the General Clinical Research Center (2 M01 RR00645). The data were presented in part in abstract form at the 18th Annual American Heart Association Scientific Sessions in November 2006.
Funding/Support This work was supported by grants from the National Institute of Neurological Disorders and Stroke (R01 NS 29993, T32 NS 07153) and the General Clinical Research Center (2 M01 RR00645). The funding organizations had no role in the design and conduct of the study, in the collection, analysis, or interpretation of the data, or in the decision to approve publication of the manuscript.
Conflict of Interest Dr. Tatjana Rundek is a member of the advisory boards for Pfizer and BMS-Sanofi Pharmaceutical Partnership and receives personal compensation from New Health Sciences, Inc., for consulting. Dr. Ralph Sacco discloses receiving personal compensation from Boehringer-Ingelheim, Inc., and BMS-Sanofi Pharmaceutical Partnership for lecturing, and personal compensation from Boehringer-Ingelheim, Inc., Merck, Wyeth, and GlaxoSmithKline for consulting.
References
- 1.Belch JJ, Topol EJ, Agnelli G, et al. Critical issues in peripheral arterial disease detection and management: a call to action. Arch Intern Med. 2003;163(8):884–92, Apr 28. [DOI] [PubMed]
- 2.Criqui MH, Denenberg JO, Langer RD, Fronek A. The epidemiology of peripheral arterial disease: importance of identifying the population at risk. Vasc Med. 1997;2:221–26. [DOI] [PubMed]
- 3.Fowkes FGR, Housley E, Cawood EHH, Macintyre CCA, Ruckley CV, Prescott RJ. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1991;20:384–92. [DOI] [PubMed]
- 4.Meijer WT, Hoes AW, Rutgers D, et al. Peripheral arterial disease in the elderly: the Rotterdam Study. Arterioscler Thromb Vasc Biol. 1998;18:185–92. [DOI] [PubMed]
- 5.Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–24. [DOI] [PubMed]
- 6.Murabito JM, Evans JC, Larson MG, et al. The ankle-brachial index in the elderly and risk of stroke, coronary disease, and death. Arch Intern Med. 2003;163:1939–42. [DOI] [PubMed]
- 7.Hertzer NR, Beven EG, Young JR, et al. Coronary artery disease in peripheral vascular patients: a classification of 1000 coronary angiograms and results of surgical management. Ann Surg. 1984;199:223–33. [DOI] [PMC free article] [PubMed]
- 8.CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 1996;348:1329–39. [DOI] [PubMed]
- 9.Ness J, Aronow WS. Prevalence of coexistence of coronary artery disease, ischemic stroke, and peripheral arterial disease in older persons, mean age 80 years, in an academic hospital-based geriatrics practice. J Am Geriatr Soc. 1999;47:1255–56. [DOI] [PubMed]
- 10.Klop RB, Eikelboom BC, Taks AC. Screening of the internal carotid arteries in patients with peripheral vascular disease by colour-flow duplex scanning. Eur J Vasc Surg. 1991;5:41–45. [DOI] [PubMed]
- 11.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–86. [DOI] [PubMed]
- 12.Newman AB, Shemanski L, Manolio TA, et al. Ankle-arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1999;19:538–45. [DOI] [PubMed]
- 13.Leng GC, Fowkes FG, Lee AJ, et al. Use of ankle brachial pressure to predict cardiovascular events and death: a cohort study. BMJ. 1996;313:1440–43. [DOI] [PMC free article] [PubMed]
- 14.Zheng ZJ, Sharrett AR, Chambless LE, et al. Associations of ankle-brachial index with clinical coronary heart disease, stroke and preclinical carotid and popliteal atherosclerosis: the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 1997;131:115–25. [DOI] [PubMed]
- 15.Leng GC, Lee AJ, Fowkes FG, et al. Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1996;25:1172–81. [DOI] [PubMed]
- 16.Abbott RD, Rodriguez BL, Pretrovich H, et al. Ankle-brachial blood pressure in elderly men and the risk of stroke: the Honolulu Heart Program. J Clin Epidemiol. 2001;54:973–78. [DOI] [PubMed]
- 17.McKenna M, Wolfson S, Kuller L. The ratio of ankle and arm arterial pressure as an independent predictor of mortality. Atherosclerosis. 1991;87:119–28. [DOI] [PubMed]
- 18.Vogt MT, Cauley JA, Newman AB, et al. Decreased ankle/arm blood pressure index and mortality in elderly women. JAMA. 1993;270:465–69. [DOI] [PubMed]
- 19.McDermott MM, Feinglass J, Slavensky R, et al. The ankle-brachial index as a predictor of survival in patients with peripheral vascular disease. J Gen Intern Med. 1994;9:445–49. [DOI] [PubMed]
- 20.Ouriel K. Peripheral arterial disease. Lancet. 2001;358:1257–64. [DOI] [PubMed]
- 21.Kannel WB, Skinner JJ, Schwartz MJ, Shurtleff D. Intermittent claudication: Incidence in the Framingham Study. Circulation. 1970;41:875–83. [DOI] [PubMed]
- 22.McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease. Associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–1606. [DOI] [PubMed]
- 23.McLafferty RB, Dunnington GL, Mattos MA, et al. Factors affecting the diagnosis of peripheral vascular disease before vascular surgery referral. J Vasc Surg. 2000;31(5):870–79. [DOI] [PubMed]
- 24.Ouriel K, McDonnell AE, Metz CE, Zarins CK. A critical evaluation of stress testing in the diagnosis of peripheral vascular disease. Surgery. 1982;91:686–93. [PubMed]
- 25.Yao ST, Hobbs JT, Irvine W. Ankle systolic pressure measurements in arterial disease affecting the lower extremities. B J Surg. 1969;59:676–79. [DOI] [PubMed]
- 26.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients with Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 2006;47:1239–1312. [DOI] [PubMed]
- 27.Mohler ER, Jacobson-Treat D, Reilly MP, et al. Utility and barriers to performance of the ankle-brachial index in primary care practice. Vasc Med. 2004;9(4):253–60. [DOI] [PubMed]
- 28.Sacco RL, Anand K, Lee HS, et al. Homocysteine and the risk of ischemic stroke in a triethnic cohort. The Northern Manhattan Study. Stroke. 2004;35:2263–69. [DOI] [PubMed]
- 29.White H, Boden-Albala B, Wang C, et al. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2005;111:1327–31. [DOI] [PubMed]
- 30.Gentry EM, Kalsbeek WD, Hegelin GC et al. The Behavioral Risk Factor Surveys, II: design, methods, and estimates from combined state data. Am J Prev Med. 1985;1:9–14. [PubMed]
- 31.Sacco RL, Elkind M, Boden-Albala B, et al. The protective effect of moderate alcohol consumption on ischemic stroke. JAMA. 1999;281:53–60. [DOI] [PubMed]
- 32.World Health Organization MONICA Project Principal Investigators. The World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease). J Clin Epidemiol. 1988;41:105–14. [DOI] [PubMed]
- 33.Greene HL, Richardson DW, Barker AH, et al. Classification of deaths after myocardial infarction as arrhythmic or nonarrhythmic (the Cardiac Arrhythmia Pilot Study). Am J Cardiol. 1989;63:1–6. [DOI] [PubMed]
- 34.Morris DL, Kritchevsky SB, Davis CE. Serum carotenoids and coronary heart disease. The lipid research clinics coronary primary prevention trial and follow-up study. JAMA. 1994; 272:1439–41. [DOI] [PubMed]
- 35.Wilson PWF, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. [DOI] [PubMed]
- 36.Lee AJ, Price JF, Russell MJ, Smith FB, vanWijk MCW, Fowkes FGR. Improved prediction of fatal myocardial infarction using the ankle-brachial index in addition to conventional risk factors. The Edinburgh Artery Study. Circulation. 2004;110:3075–80. [DOI] [PubMed]
- 37.Rose GA. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull World Health Organ. 1962;27:645–58. [PMC free article] [PubMed]
- 38.Leng GC, Fowkes FG. The Edinburgh Claudication Questionnaire: an improved version of the WHO/Rose Questionnaire for use in epidemiological surveys. J Clin Epidemiol. 1992;45:1101–09. [DOI] [PubMed]
- 39.Criqui MH, Fronek A, Barrett-Connor E, et al. The prevalence of peripheral arterial disease in a defined population. Circulation. 1985;71:510–15. [DOI] [PubMed]
- 40.McDermott MM, Mehta S, Ahn H, Greenland P. Atherosclerotic risk factors are less intensively treated in patients with peripheral arterial disease than in patients with coronary artery disease. J Gen Intern Med. 1997;12:209–15. [DOI] [PMC free article] [PubMed]
- 41.Collins TC, Petersen NJ, Suarez-Almazor M, Ashton C. The prevalence of peripheral arterial disease in a racially diverse population. Arch Intern Med. 2003;163:1469–74. [DOI] [PubMed]
- 42.Criqui MH, Vargas V, Denenberg JO, Ho E, Allison M, Langer RD, et al. Ethnicity and Peripheral Arterial Disease. The San Diego Population Study. Circulation. 2005;112:2703–07. [DOI] [PubMed]
- 43.United States Preventive Services Task Force. Recommendation Statement: Screening for Peripheral Arterial Disease. Washington, DC: Agency for Healthcare Research and Quality, 2005:1–8.
- 44.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States. Results from the National Health and Nutrition Examination Survey 1999–2000. Circulation. 2004;110:738–43. [DOI] [PubMed]


