Abstract
Background
Failure to reconcile medications across transitions in care is an important source of potential harm to patients. Little is known about the predictors of unintentional medication discrepancies and how, when, and where they occur.
Objective
To determine the reasons, timing, and predictors of potentially harmful medication discrepancies.
Design
Prospective observational study.
Patients
Admitted general medical patients.
Measurements
Study pharmacists took gold-standard medication histories and compared them with medical teams’ medication histories, admission and discharge orders. Blinded teams of physicians adjudicated all unexplained discrepancies using a modification of an existing typology. The main outcome was the number of potentially harmful unintentional medication discrepancies per patient (potential adverse drug events or PADEs).
Results
Among 180 patients, 2066 medication discrepancies were identified, and 257 (12%) were unintentional and had potential for harm (1.4 per patient). Of these, 186 (72%) were due to errors taking the preadmission medication history, while 68 (26%) were due to errors reconciling the medication history with discharge orders. Most PADEs occurred at discharge (75%). In multivariable analyses, low patient understanding of preadmission medications, number of medication changes from preadmission to discharge, and medication history taken by an intern were associated with PADEs.
Conclusions
Unintentional medication discrepancies are common and more often due to errors taking an accurate medication history than errors reconciling this history with patient orders. Focusing on accurate medication histories, on potential medication errors at discharge, and on identifying high-risk patients for more intensive interventions may improve medication safety during and after hospitalization.
KEY WORDS: medication errors; medication systems, hospital; continuity of patient care; inpatients
INTRODUCTION
Recent efforts to improve the quality and safety of healthcare have included attention to medication discrepancies, defined as unexplained differences among documented regimens across different sites of care.1 Discrepancies are highly prevalent, with up to 67% of inpatients having at least one error in their prescription medication history at the time of admission.2 Medication discrepancies are an important contributor to adverse drug events (ADEs) among hospitalized and recently discharged patients,3–5 and for this reason, the Joint Commission designated inpatient medication reconciliation as a National Patient Safety Goal in 2005.6
Hospitals have undertaken diverse approaches to comply with the Joint Commission’s mandate. Some studies support medication reconciliation as a means to reduce ADEs7,8, but for reconciliation efforts to be as effective as possible, institutions need a more thorough understanding of the nature of the discrepancies that reconciliation is intended to prevent. Previous studies of medication discrepancies at hospital admission, discharge, and post-discharge4,9,10 provide insufficient guidance regarding where reconciliation efforts should be focused. This study, using a modification of an existing typology,11 aimed to classify potentially serious medication discrepancies according to timing (admission vs. discharge), reason (obtaining the medication history vs. reconciling the history with patient orders), and type (omission vs. commission); and, to explore patient, hospital, and physician predictors of such errors.
METHODS
Design Overview, Setting, and Participants
Participants and data for this prospective observational study are derived from the control group of the Partners Medication Reconciliation Study, a cluster-randomized controlled trial conducted from May 1 through June 20, 2006 at two large academic hospitals in Boston, Massachusetts. The parent study examined the impact of a novel, computer-aided intervention for reconciling medications. Control patients were admitted to one of several general medicine service teams on specific floors of each hospital, and were cared for by physicians and nurses separate from patients who received the intervention in the larger study. These services generally excluded oncology patients, but otherwise cared for a wide variety of medical patients; residents were involved in the care of all patients. If a study pharmacist, working weekdays, had time to obtain a preadmission medication history during the hospitalization, then a patient could be included in the study. Patients discharged from a non-study team or floor and patients transferred at any time from a control team to an intervention team were excluded. The study was approved by the Institutional Review Board of Partners HealthCare; patient consent was deemed not necessary. Physicians and nurses were informed of the nature of the study by email prior to study initiation.
Outcomes
The main outcome for this study was the number of unintentional medication discrepancies with potential for causing harm (potential adverse drug events or PADEs) per patient. PADEs have been previously described as “incidents with potential for injury related to a drug12.”
A two-step process was employed to identify PADEs. First, a “gold-standard” preadmission medication history was taken by one of two study pharmacists at each site, following a strict protocol and using all available sources of information, including subject and family/caregiver interviews, prescription pill bottles, outpatient electronic medical records, previous hospital discharge orders, outpatient providers, and outpatient pharmacies (see Appendix 1 for complete protocol). The resulting preadmission medication list was then compared with the medical team’s preadmission medication list in the admission note and with all admission and discharge medication orders. Any discrepancies between the gold-standard history and medication orders were identified and reasons for these changes sought from the medical record. Pharmacists also communicated directly with the medical team after discharge orders were written to clarify reasons for discrepancies, as needed. Medication discrepancies that were not clearly intentional were then recorded.
Second, recorded discrepancies were shown to rotating adjudication teams consisting of two physicians (from a pool of six) blinded to intervention status. Physician adjudicators were from both study sites and included four hospitalists, a geriatrician with inpatient and outpatient responsibilities, and a chief medical resident. Together with the study pharmacist, adjudicators discussed each medication discrepancy and reviewed the discharge summary for each patient. Additional electronic sources of patient information such as ordered medications and laboratory test results were also reviewed as needed. Using an expert-derived classification scheme,11 the two physicians each recorded details of the medication discrepancy, including whether it was intentional, the time of the discrepancy (admission vs. discharge), and the type of discrepancy (e.g., omission, change in dose). We modified the scheme to also capture the reason for the discrepancy: an error in the preadmission medication history was recorded as a “history error” (e.g., not including aspirin on the preadmission medication list, thus explaining why it is not ordered at discharge); conversely, an error of reconciling the medication history with medication orders was recorded as a “reconciliation error” (e.g., aspirin held at admission but not restarted at discharge despite being present on the preadmission medication list and clinically indicated at discharge). Independently, the two reviewers judged each unintentional discrepancy as having potential for patient harm and the potential severity of the error12 (see Appendix 2 for complete adjudication protocol). All disagreements were resolved by discussion and by a third adjudicator if necessary.
Weekly meetings were conducted to ensure consistency between the two sites and among study pharmacists and physician adjudicators. To evaluate inter-rater reliability of the gold-standard medication histories, 19 randomly selected medication histories were collected independently by two study pharmacists. Among all the medications recorded for each patient, there was complete agreement in medication, dose, route, and frequency for 147 of 192 medications (77%). Inter-rater reliability for physician adjudicator evaluation was also calculated, with a kappa of 0.95 for potential for harm and a kappa of 0.94 for potential severity.
Predictors of PADEs
To explore the relationship between PADEs and various patient and system factors, we collected information on a number of patient characteristics, including age, whether a subject had a primary care provider (PCP) from within the hospital network, the source of the hospital admission, whether the discharging physician was the patient’s PCP, the number of outpatient visits in the prior year and any inpatient visits in the prior month. The level of training of the physician documenting the medication history was determined from the medical record. Data were also collected on the total number and classes of preadmission medications (from the gold-standard list) and number of medication changes from the gold-standard preadmission list to the discharge orders; these medication counts excluded as-needed medications and topical agents. Study pharmacists also provided information regarding patients’ level of understanding of their preadmission medication lists (subjectively categorized as high, medium, or low, depending on whether patients could name their medications and provide dose, route and frequency information; could provide the names of their medications but not directions for use; or neither, respectively) and the sources used to obtain the gold-standard medication list.
Statistical Analysis
Patient characteristics and study results were calculated using proportions, means with standard deviations, and medians with interquartile ranges. Multivariable Poisson regression was used to determine the association between the number of PADEs per patient and the covariates described above. Model fit was assessed based on aggregates of residuals13 using the ASSESS statement in SAS, with a value computed based on 10,000 simulated paths ( = 0.43, suggesting good model fit). A similar model was constructed using only variables available at admission (e.g., excluding number of medication changes). Based on the beta coefficients of this second model, a scoring system was created to identify patients at highest risk for PADEs. Generalized estimating equations were used to adjust for clustering of results by discharging physician. Analyses were implemented using SAS statistical software, version 9.1 (SAS Institute Inc, Cary, NC).
RESULTS
Description of Study Sample
We identified 379 potential study subjects at the two sites. Research pharmacists did not have time to obtain preadmission medication histories prior to discharge for 179 patients, an additional eight patients were not admitted to study teams and floors, and 12 patients were transferred to non-study teams or floors. The final study population comprised 180 patients, including 94 patients at site 1 and 86 patients at site 2. Subject characteristics are shown in Table 1. Compared with excluded subjects, study patients were older, had longer lengths of stay, and more medications at discharge (Table 1).
Table 1.
Characteristics of Study Sample and Excluded Subjects
| Characteristic | Study sample ( = 180) | Excluded subjects ( = 199) |
|---|---|---|
| Age, (%)* | ||
| <50 years | 38 (21) | 68 (34) |
| 50–60 years | 37 (20) | 30 (16) |
| 60–75 years | 39 (22) | 49 (24) |
| >75 years | 66 (37) | 52 (26) |
| Female sex, (%) | 109 (61) | 97 (49) |
| Median income by zip code, (%) | ||
| <$39,001 | 43 (24) | 66 (33) |
| $39,001-$47,000 | 48 (27) | 41 (20) |
| $47,001-$63,000 | 41 (23) | 45 (23) |
| >$63,000 | 47 (26) | 47 (24) |
| Insurance, (%) | ||
| Private | 52 (29) | 62 (31) |
| Medicare with secondary insurance | 91 (51) | 94 (47) |
| Medicare alone | 8 (4) | 2 (1) |
| Free care/Medicaid | 15 (8) | 35 (18) |
| Other/self pay | 14 (8) | 6 (3) |
| Length of stay (days), (%)* | ||
| 0–2 | 41 (24) | 75 (38) |
| 3–4 | 48 (27) | 47 (24) |
| 5–8 | 44 (25) | 35 (18) |
| ≥9 | 42 (24) | 38 (20) |
| Top 10 diagnosis-related groups, (%) | ||
| Simple pneumonia and pleurisy | 7 (4) | 9 (5) |
| Heart failure and shock | 9 (5) | 5 (3) |
| Gastrointestinal hemorrhage | 6 (3) | 7 (4) |
| Chronic obstructive pulmonary disease | 9 (5) | 3 (2) |
| Renal failure | 6 (3) | 5 (3) |
| Respiratory infections and inflammations | 4 (2) | 6 (3) |
| Nutritional and miscellaneous metabolic disorders | 3 (2) | 7 (4) |
| Chest pain | 5 (3) | 4 (2) |
| Cardiac arrhythmia and conduction disorders | 4 (2) | 4 (2) |
| Other circulatory system diagnoses | 6 (3) | 2 (1) |
| Cumulative | 59 (33) | 52 (26) |
| Admission DRG weight, median (IQR) | 1.03 (0.83 - 1.28) | 1.03 (0.81–1.33) |
| Number of medications prescribed at discharge, median (IQR)* | 11 (6–14) | 9 (5–13) |
* < 0.05 for comparison between groups
Frequency of Discrepancies and PADEs
Among the 2066 medication discrepancies, 939 (45%) were determined to be unintentional. Of these 939 errors, 682 (73%) were deemed not to have potential for patient harm, but 257 (27%) had potential for harm, an average of 1.4 PADEs per patient. The rates were similar at the two study sites (1.37 and 1.48, = 0.46). Approximately 54% of patients had at least one PADE, 37% had two or more PADEs, and 9% had five or more. Fifty-nine of the PADEs (23%) were considered serious, i.e., to have potential to cause serious harm such as re-hospitalization or persistent alteration in health function.
Classifying PADEs
Figure 1 shows the classification of PADEs. Many more PADEs were due to errors in taking the preadmission medication history (72%) than to errors in reconciling preadmission medications with admission or discharge orders (30%; 2% were due to both causes). Most PADEs occurred at discharge (75%) rather than at admission (25%). Of unintended discrepancies 60% were due to omissions of medications, 21% to discrepancies in dose, 10% to discrepancies in frequency, 5% to additional medications, and 4% to substitutions.
Figure 1.
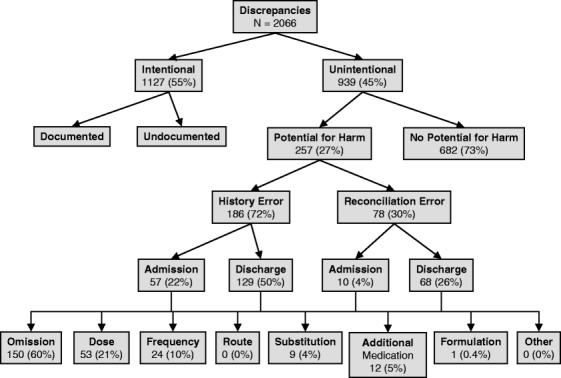
Classification of medication discrepancies.
The most common medication classes involved in PADEs were cardiovascular (20% of all 257 PADEs), respiratory (9%), gastrointestinal (8%), lipid-lowering (6%), and antidepressant medications (5%). Because certain types of medications are prescribed more frequently, we also calculated event rates based on prevalence of use. The five most common “high-risk” classes were gout medications (7 of 13 prescriptions resulted in a PADE, or 54%), muscle relaxants (3/8, 38%), lipid-lowering (14/70, 20%), antidepressant (14/76, 18%), and respiratory medications (20/118, 17%).
Predicting PADEs
In the multivariable model, several variables independently predicted a higher number of PADEs (Table 2): four or more “high-risk” medications (as described above) prescribed at admission, six or more medication changes during the hospitalization, low or medium patient understanding of preadmission medications, medication history supplied by a family member or caregiver, 13 or more outpatient visits during the previous year, and admission history taken by an intern. Age older than 85 years was associated with fewer PADEs.
Table 2.
Predictors of Number of PADEs Per Patient: Adjusted Results ( = 180)
| Characteristic | Adjusted relative risk (95% CI) | |
|---|---|---|
| Patient age | ||
| <50 years | 38 | Ref. |
| 50–59 years | 37 | 1.05 (0.65–1.72) |
| 60–74 years | 39 | 1.37 (0.81–2.30) |
| 75–84 years | 51 | 0.94 (0.52–1.68) |
| ≥85 years | 15 | 0.34 (0.16–0.73)* |
| Total number of high-risk preadmission medications† | ||
| 0 | 41 | Ref. |
| 1 | 52 | 1.70 (0.75–3.86) |
| 2–3 | 56 | 1.45 (0.56–3.79) |
| ≥4 | 31 | 3.00 (1.29–7.00)* |
| Number of medication changes from preadmission to discharge | ||
| 1–5 | 55 | Ref. |
| 6–9 | 50 | 3.22 (1.76–5.89)* |
| 10–13 | 35 | 3.21 (1.58–6.49)* |
| 14–28 | 40 | 4.06 (2.13–7.74)* |
| Patient understanding of preadmission medications‡ | ||
| High | 60 | Ref. |
| Medium or low | 117 | 1.65 (1.14–2.39)* |
| Family member or caregiver as source of preadmission medication information | ||
| No | 134 | Ref. |
| Yes | 46 | 1.62 (1.10–2.38)* |
| Number of outpatient visits within past 12 months | ||
| 0–1 | 56 | Ref. |
| 2–5 | 40 | 1.22 (0.75–1.98) |
| 6–12 | 40 | 0.75 (0.42–1.33) |
| ≥13 | 44 | 1.75 (1.16–2.65)* |
| Admitting physician experience | ||
| Intern (PGY 1) | 102 | Ref. |
| Resident (PGY 2–3) | 24 | 0.51 (0.31–0.82)* |
| Attendings/Fellows | 54 | 0.70 (0.43–1.14) |
| Partners PCP | ||
| Yes | 107 | Ref. |
| No | 73 | 0.95 (0.72–1.27) |
| Preadmission source | ||
| Emergency department | 114 | Ref. |
| Transfer from other service | 16 | 1.41 (0.81–2.44) |
| Transfer from other institution | 23 | 0.76 (0.39–1.47) |
| Scheduled from home | 11 | 0.92 (0.42–2.01) |
| Clinic/Other | 16 | 0.86 (0.42–1.80) |
| Total number of preadmission medications§ | ||
| Quartile 1 (0–9) | 55 | Ref. |
| Quartile 2 (10–15) | 43 | 0.92 (0.39–2.13) |
| Quartile 3 (16–21) | 44 | 1.13 (0.50–2.55) |
| Quartile 4 (22–36) | 38 | 1.13 (0.43–3.00) |
| Inpatient admissions within the past 31 days | ||
| No | 149 | Ref. |
| Yes | 31 | 0.78 (0.55–1.11) |
| Discharge physician is PCP | ||
| No | 149 | Ref. |
| Yes | 31 | 0.98 (0.61–1.58) |
Abbreviations: Ref. = reference group
* < 0.05
†In 5 medication classes most likely to cause PADEs when prescribed: gout medications, muscle relaxants, hyperlipidemic agents, anti-depressants, and respiratory medications
‡Based on pharmacist assessment
§Excluding PRN medications and topical agents
PADE Risk Score
To develop an exploratory PADE risk score, we built a model including only variables available at the time of admission (Table 3): patients younger than 85 years of age, low or medium understanding of preadmission medications, 16 or more preadmission medications, four or more medications from high-risk classes, family member or caregiver as source of preadmission medication information, and 13 or more outpatient visits during the previous year. The 21% of patients with the highest scores, 5–7 points, had an 85% chance of having at least one PADE, had a mean of 2.9 PADEs per patient, and accounted for 44% of the PADEs in the population (Table 4).
Table 3.
PADE Risk Score – Using Predictor Variables Available at Admission
| Characteristic | Points |
|---|---|
| Low or medium patient understanding of preadmission medications | 1 |
| Age under 85 | 2 |
| Having 16 or more preadmission medications | 1 |
| Having 4 or more high risk preadmission medications* | 1 |
| Having 13 or more outpatient visits in the previous year | 1 |
| Having a family member or caregiver as a source of preadmission medication information | 1 |
*Gout medications, muscle relaxants, hyperlipidemic medications, antidepressants, and respiratory medications
Table 4.
Distribution of PADE Risk Scores
| Score range | (%) in score range | (%) with any PADEs | PADEs per patient, mean (SD) | Total PADEs accounted for by group, (%)* |
|---|---|---|---|---|
| 0–2 | 30 (17) | 5 (17) | 0.26 (0.64) | 8 (3) |
| 3 | 56 (31) | 23 (41) | 0.71 (1.26) | 40 (16) |
| 4 | 55 (31) | 36 (65) | 1.72 (1.92) | 95 (37) |
| 5–7 | 39 (21) | 33 (85) | 2.92 (2.50) | 113 (44) |
* % of all PADEs in entire study population
DISCUSSION
In our study of potential adverse drug events related to the usual medication reconciliation process, we found a high prevalence of unintentional medication discrepancies with potential for patient harm: an average of 1.4 per patient. Most PADEs were due to errors taking a medication history rather than errors of reconciling the medication history with patient orders. The majority of PADEs occurred at discharge rather than admission, and most errors were ones of omission. Predictors of the number of PADEs per patient included low patient understanding of their preadmission medications, number of high-risk medications, number of differences between preadmission and discharge medication regimens, and medication histories taken by an intern.
Our findings are consistent with those of other studies despite different conceptualization, definitions, and methods. Most studies corroborate that at least half of all patients have at least one PADE during the reconciliation process.4,14–16 In a single community hospital in Ontario, Canada, the rate of unintentional medication discrepancies was lower, but almost all admission discrepancies were corrected by study pharmacists before discharge orders were written.17 Previous studies have also shown that omissions are the most common type of medication discrepancy, with up to 61% of hospitalized patients having at least one drug omitted from their regimen.2,4,15
Our finding that PADEs are more often caused by errors of medication history-taking than by errors of reconciliation is not surprising when considering the difficulty of taking an accurate medication history in today’s healthcare environment. Multiple outpatient providers may each prescribe a subset of a patient’s medications, and none may take responsibility for ensuring the accuracy of the regimen as a whole. Incomplete data sources and inadequate communication among providers and patients may exacerbate this problem. In addition, patients may not have adequate health literacy to fully understand their medication regimens.18 The effort required to obtain an accurate list may therefore be substantial, including communication with community pharmacists, outpatient physicians, family members and caregivers, and time spent reviewing pill bottles with patients. A recent meta-analysis estimated that 27%-54% of patients suffer at least one unintentional medication discrepancy due to medication history errors.2 Conversely, the process of reconciling preadmission medications with discharge orders requires attention to detail but is a less complex activity than taking an accurate medication history. Errors of reconciling preadmission medications with admission orders, which are usually written by the same physician who took the medication history and performed shortly thereafter, are less common. The Joint Commission places equal weight on medication history-taking and admission and discharge reconciliation in its National Patient Safety Goals, yet reconciling medications at admission was the source of only 10 out of 257 PADEs in our study.
That more PADEs occurred with discharge than admission orders makes sense in light of the differences between inpatient and outpatient environments. The hospitalization itself is often brief and highly monitored in contrast to the post-discharge setting. Therefore, the same error (e.g., mild warfarin overdose) may have little potential for patient harm when written at admission but much greater potential for harm when written at discharge. In fact, in 313 cases, the same unintentional medication discrepancy occurred at admission and discharge, and in 79 of these (25%), the error was adjudicated as not being a PADE at admission but was considered a PADE at discharge.
Our finding that patients older than 85 were at lower risk for PADEs was surprising.19,20 This effect persisted when adjusted for number and classes of medications, sources used to generate the preadmission medication list, and several PCP characteristics. Outpatient physicians may be more careful about maintaining accurate medication lists in very old patients. There also may be unmeasured differences in the degree of medical and social supports these patients receive. In contrast, relying on family members or caregivers as a source of medication information in this study was a risk factor for PADEs. This factor may correlate with low functional status and high medical complexity. Family members may also represent a source of accurate medication information utilized by study pharmacists but not by medical teams.
That interns’ taking the medication history was an independent risk factor for PADEs, compared to more senior physicians, suggests either that medication history-taking improves with experience or that interns spend less time taking medication histories, perhaps as a result of competing demands or interruptions. Lack of time, training, or prioritization of medication history-taking could all serve as potential targets for future interventions.
The list of “high-risk” medications most associated with PADEs (muscle relaxants, lipid-lowering, antidepressant, gout, and respiratory medications) was somewhat different from that found in general studies of ADEs21–23 and from a recent study of discrepancies after discharge, which found cardiovascular, gastrointestinal, and pulmonary medications to be most common.24 Our high-risk medication list was adjusted for frequency of prescription, which made it more predictive and may account for some of these differences.
Limitations and Strengths
This study has several limitations. The study was conducted on general medical services at academic medical centers, and the results may not be generalizable to other settings. Patients with very short lengths of stay may have been disproportionately discharged prior to enrollment, thus leading to selection of a patient population on more medications and an overestimation of the number of PADEs per patient. The study measured potential and not actual ADEs. Our adjudication process was sometimes hindered by the lack of documentation of reasons for medication discrepancies. However, one could argue that intentional but undocumented medication discrepancies represent “latent” medical errors that could lead to downstream patient harm as subsequent providers try to determine why certain medication changes occurred. Finally, our use of pharmacists to establish a “gold-standard” preadmission medication regimen could be questioned. However, studies have shown that pharmacists perform this process better than other medical personnel,25 and we demonstrated moderately high reliability when the process was conducted independently by two pharmacists.
Our study also has several strengths. To our knowledge, our study is the first to distinguish errors of history-taking from those of reconciling that history with orders, which is highly relevant to the Joint Commission medication reconciliation mandate. To our knowledge, it is also the first study to propose a scoring system to identify patients at high risk for PADEs. Other strengths include a rigorous adjudication process of all discrepancies and conduct of the study at two different academic medical centers.
Based on the results of this study, interventions to improve medication safety at transitions in care should focus first and foremost on gathering accurate preadmission medication information, and secondly on preventing reconciliation errors at discharge. Comparatively less effort can be spent preventing reconciliation errors at admission. If our risk score is prospectively validated in other populations, it could be useful to identify patients who may need more than the minimum medication reconciliation standard, for example, greater pharmacist involvement in taking medication histories and/or counseling patients at discharge. Studies of medication reconciliation interventions of various types and in different populations are needed to demonstrate benefits to patients during transitions in patient care.
Acknowledgements
We would like to acknowledge the tremendous efforts of the Partners Information Systems personnel involved in developing the medication reconciliation intervention (Barry Blumenfeld, MD; Carol Broverman, PhD; Eric Poon, MD, MPH; Cheryl Van Putten, PMP; Eric Godlewski, BA; Linda Moroni, MBA; Michael McNamara, BA; Sandra Smith, BA; Marilyn Paterno, MBI; Daniel Fuchs, BS; Oliver James, BS; Greg Rath, BA), the BWH medication reconciliation implementation team (Erin Graydon-Baker, MS, RRT; Christine McCormack, BA; John Poikonen, BA; Christina Pelletier, BA; Emily Maher, MD; Ellen Bergeron, RN, MSN; Jennie Kuzemchak, BA; Michael Cotugno, RPh; Andrea Giannattasio, BA), the MGH medication reconciliation implementation team (George Baker, MD; Sally Millar, RN; Margaret Clapp, BA), and BWH personnel John Orav, PhD, and Stuart Lipsitz, ScD, for biostatistical assistance, Elisabeth Burdick, MS, for statistical programming, Amy Bloom, MPH, for project management, and Emily Barsky, BA, and Emily Dattwyler, BA, for research assistance. We also thank Erin Hartman, MS (University of California, San Francisco) for generous in-kind editorial assistance.
Grant Suppport This study was funded in part by an investigator-initiated grant from the Harvard Risk Management Foundation, including compensation for Elisabeth Burdick, Amy Bloom, and Emily Barsky, as well as internal funding from BWH, MGH, and Partners Healthcare. Dr. Pippins was supported by a National Research Service Award from the Health Resources and Services Administration (T32 HP11001–18). Dr. Schnipper was supported by a Mentored Clinical Scientist Award from the National Heart, Lung, and Blood Institute (K08 HL072806).
Conflict of Interest None disclosed.
Appendix 1: Protocol for Collecting Gold Standard Medication History
Who: Study pharmacist
When: As soon after admission as possible
- Use all available sources:
- Patient (introduce yourself, get informal consent)
- Family
- Have someone bring in pill bottles and/or med lists from home, review with patient/family.
- Available outpatient electronic medical records (EMRs)
- Previous discharge summaries
- Primary care providers (PCPs) or other doctor’s office
- Community pharmacy
- Time-saving tips
- Review previous discharge summaries only if there is one from the previous year, unless there is no information in the available outpatient EMRs (in which case look at older discharge summaries).
- In outpatient EMRs, if last note is comprehensive, then no need to look at previous notes.
- In outpatient EMRs, just scan lists of inactive meds and non-meds, but don’t spend a lot of time on them. Focus on lists of active medications.
Begin by gathering all easily accessible sources: outpatient EMR medication lists, hospital discharge summaries, transfer orders, and physician’s admission note.
When reviewing these data sources with the patient/family, specifically ask about differences among these different lists and clarify what the patient is actually taking.
Encourage patients to use more than just their memory, i.e., use a paper list, pill bottles, etc.
If patients use a list or pill bottles and seem completely reliable (and the data are not that dissimilar from the other sources, and differences can be explained), then other sources are not needed.
If patients are not sure or are relying on memory only, or cannot clearly “clean up” the other sources of medication information, then it’s time to rely on other sources: community pharmacies, outpatient physician offices, having the family bring in pill bottles, etc.
Pill bottles, reviewed with patient/family, are preferable to pharmacist refill information if available and if the review with patient/family seems reliable.
It is not enough to rely on the physician’s preadmission medication list as the main source of additional information.
Use an interpreter with non-English speaking patients unless you are fluent in the other language.
- How to document non-adherence:
- If completely non-adherent (on purpose or because didn’t know to take medication), then leave off list and note it in general comments.
- If sporadically non-adherent, give general assessment of adherence in comments.
- If systematically non-adherent (e.g., always takes medicine once a day instead of 3 times a day), then note actual frequency taken in dose/route/frequency section and make note of discrepancy from prescribed frequency in comments.
If patient denies knowledge of a medication that is on another list (i.e., doesn’t know why not taking it), keep track of these in comments. If these occur often, you will need to call the PCP’s office to see if the patient is really supposed to be on it.
Appendix 2: Potential ADE Adjudication Instructions
- Overview of medication discrepancies
- Definition: any difference between medications taken by a patient prior to admission and medications ordered in the hospital
- Typology:
- Intentional vs. Unintentional
- If intentional (not an error): documented or not
- If unintentional: medication error
- Location: Admission orders vs. Discharge orders
- Type:
- Omission (not written for at all)
- Dose (per administration, e.g., 100 mg bid vs. 200 mg bid)
- Frequency (e.g., 100 mg bid vs. 100 mg tid)
- Note that 100 mg bid vs. 200 mg qd would be a discrepancy in dose and frequency even though the total daily dose is the same.
- Route
- Substitution (i.e., with a medication in the same class)
- Additional medication (not taken at home, but ordered in the hospital)
- Reason:
- History error: this means the admitting physician made an error in taking the medication history, but then faithfully perpetuated this error when writing orders in the hospital.
- Reconciliation error: this means the medication history taken by the physician was correct, but the error occurred when writing the orders (this occurs at discharge much more often than at admission).
- Theoretically, both (1) and (2) could occur simultaneously.
- Potential for harm
- Definition: in your opinion, what is your confidence that the unintentional medication discrepancy described above has the potential to cause at least significant patient harm if the order is not corrected? Assume a reasonable patient (e.g., if an OTC prn medication is not prescribed, assume the patient can get access to it and resume it; but if a prescription medication is not prescribed, assume the patient does not have access to it).
- Scale
- Little or no confidence (e.g., omission of multivitamin)
- Slight to modest confidence (e.g., colace 200 mg qam instead of 100 mg bid)
- Less than 50–50 but close call (e.g., omission of prn fleets enema at discharge)
- More than 50–50 but close call (e.g., omission of flovent bid at discharge)
- Strong confidence (e.g., omission of prn haldol in nursing home patient)
- Virtually certain confidence (e.g., valium 10 mg instead of 1 mg prn insomnia)
- Potential Severity
- Definition: this is the degree of patient harm that could be caused by the above unintentional medication discrepancy.
- Significant: an error that can cause patient symptoms that, while harmful to the patient, pose little or no threat to the patient’s life function.
- Serious: an error than can cause signs/symptoms that are associated with a serious level of risk that is not high enough to be life-threatening. In addition, a potential ADE is serious if it can cause persistent alteration of daily function.
- Life-threatening: an error that can cause signs/symptoms that if not treated would put the patient at risk of death.
Examples of Potential ADE Severity Categories
(Assuming the discrepancy is unintentional)
LIFE THREATENING
Incorrect dose of anti-rejection medication is prescribed in patient with kidney transplant
Omission of amiodarone at discharge when given for prevention of VT
Patient with a prior penicillin anaphylaxis reaction and ordered penicillin at admission
Incorrect APAP dose prescribed at discharge with a total daily dose >15 grams
Omission of warfarin at admission in patient with St. Jude’s mitral valve replacement
SERIOUS
Patients’ correct dose is 2 mg diazepam, MD writes for 10 mg on admission
Patient with CHF flare discharged on 1/4 preadmission dose of lasix
Omission of beta-blocker at discharge in patient with CAD
Warfarin 5 mg QD prescribed at discharge instead of 3 mg QD (prescribed for atrial fibrillation)
Indomethacin for gout prescribed at discharge to patient concurrently taking Ibuprofen
Two concurrent APAP prescriptions at discharge with a total daily dose of >10 grams but ≤15 grams
Omission of lactulose bid in patient with history of hepatic encephalopathy
SIGNIFICANT
Omission of diazepam prn insomnia at discharge
Change from dulcolax prn to dulcolax bid standing
Omission of lisinopril in patient without CAD, CHF, or valve disease
Two concurrent APAP prescriptions with a total daily dose >4 grams but ≤10 grams
Omission of ultram prn headaches
Additional Examples of Potential ADEs and their severity
Errors that may lead to hypotension or over-treatment of hypertension are considered to be serious potential ADEs.
Errors that may lead to under-treatment of hypertension, angina, or ischemia are considered to be significant potential ADEs.
Errors that may lead to significant over-anticoagulation or under-coagulation are considered to be serious potential ADEs.
Errors that lead to under-treatment of asthma are considered to be significant potential ADEs.
- Errors that lead to under-treatment with antibiotics:
- If IV antibiotics were originally prescribed, consider the errors to be serious potential ADEs.
- If oral antibiotics were originally prescribed, consider the errors to be significant potential ADEs.
- Errors that lead to over-treatment with antibiotics:
- If either IV or oral antibiotics were prescribed, consider the errors to be significant potential ADEs, unless the antibiotic is directly toxic to end organs in a highly dose-sensitive fashion (e.g., gentamicin), in which case, the severity will be higher (usually serious).
Footnotes
Portions of this work were presented as a poster at the 2007 Summer Meeting of the American Society of Health-System Pharmacists, June 24–27, 2007, San Francisco, CA and as a poster at the 2008 Annual Meeting of the Society of General Internal Medicine, April 9–12, 2008, Pittsburgh, PA. This study was supported in part by an investigator-initiated grant from the Harvard Risk Management Foundation, and by internal support from Massachusetts General Hospital, Brigham and Women’s Hospital, and Partners Healthcare. Dr. Pippins was supported by a National Research Service Award from the Health Resources and Services Administration (T32 HP11001–18). Dr. Schnipper is supported by a Mentored Clinical Scientist Award from the National Heart, Lung, and Blood Institute (K08 HL072806).
REFERENCES
- 1.Coleman EA, Smith JD, Raha D, Min SJ. Posthospital medication discrepancies: prevalence and contributing factors. Arch Intern Med. 2005;165:1842–7. [DOI] [PubMed]
- 2.Tam VC, Knowles SR, Cornish PL, Fine N, Marchesano R, Etchells EE. Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review. CMAJ. 2005;173:510–5. [DOI] [PMC free article] [PubMed]
- 3.Using medication reconciliation to prevent errors. Jt Comm J Qual Patient Saf. 2006;32:230–2. [DOI] [PubMed]
- 4.Cornish PL, Knowles SR, Marchesano R, et al. Unintended medication discrepancies at the time of hospital admission. Arch Intern Med. 2005;165:424–9. [DOI] [PubMed]
- 5.Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166:565–71. [DOI] [PubMed]
- 6.Joint Commission on Accreditation of Healthcare Organizations. 2005 Hospital National Patient Safety Goals. http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/05_hap_npsgs.htm Accessed May 20, 2008.
- 7.Bartick M, Baron D. Medication reconciliation at Cambridge Health Alliance: experiences of a 3-campus health system in Massachusetts. Am J Med Qual. 2006;21:304–6. [DOI] [PubMed]
- 8.Haig K. Medication reconciliation. Am J Med Qual. 2006;21:299–303. [DOI] [PubMed]
- 9.King JL, Schommer JC, Wirsching RG. Patients’ knowledge of medication care plans after hospital discharge. Am J Health Syst Pharm. 1998;55:1389–93. [DOI] [PubMed]
- 10.Rodehaver C, Fearing D. Medication reconciliation in acute care: ensuring an accurate drug regimen on admission and discharge. Jt Comm J Qual Patient Saf. 2005;31:406–13. [DOI] [PubMed]
- 11.Etchells E. Reconcilable differences: enhancing medication safety at times of transition. Paper presented at: University of Calgary 4th Annual Quality Improvement Forum; January 27, 2006, 2006; Calgary.
- 12.Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA. 1995;274:29–34. [DOI] [PubMed]
- 13.Lin DY, Wei LJ, Ying Z. Model-checking techniques based on cumulative residuals. Biometrics. 2002;58:1–12. [DOI] [PubMed]
- 14.Gleason KM, Groszek JM, Sullivan C, Rooney D, Barnard C, Noskin GA. Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients. Am J Health Syst Pharm. 2004;61:1689–95. [DOI] [PubMed]
- 15.Lau HS, Florax C, Porsius AJ, De Boer A. The completeness of medication histories in hospital medical records of patients admitted to general internal medicine wards. Br J Clin Pharmacol. 2000;49:597–603. [DOI] [PMC free article] [PubMed]
- 16.Rozich JD, Howard RJ, Justeson JM, Macken PD, Lindsay ME, Resar RK. Standardization as a mechanism to improve safety in health care. Jt Comm J Qual Saf. 2004;30:5–14. [DOI] [PubMed]
- 17.Vira T, Colquhoun M, Etchells E. Reconcilable differences: correcting medication errors at hospital admission and discharge. Qual Saf Health Care. 2006;15:122–6. [DOI] [PMC free article] [PubMed]
- 18.Kripalani S, Henderson LE, Chiu EY, Robertson R, Kolm P, Jacobson TA. Predictors of medication self-management skill in a low-literacy population. J Gen Intern Med. 2006;21:852–6. [DOI] [PMC free article] [PubMed]
- 19.Schmader KE, Hanlon JT, Pieper CF, et al. Effects of geriatric evaluation and management on adverse drug reactions and suboptimal prescribing in the frail elderly. Am J Med. 2004;116:394–401. [DOI] [PubMed]
- 20.Simon SR, Chan KA, Soumerai SB, et al. Potentially inappropriate medication use by elderly persons in U.S. Health Maintenance Organizations, 2000–2001. J Am Geriatr Soc. 2005;53:227–32. [DOI] [PubMed]
- 21.Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. Adverse drug events occurring following hospital discharge. J Gen Intern Med. 2005;20:317–23. [DOI] [PMC free article] [PubMed]
- 22.Gandhi TK, Weingart SN, Borus J, et al. Adverse drug events in ambulatory care. N Engl J Med. 2003;348:1556–64. [DOI] [PubMed]
- 23.Gandhi TK, Weingart SN, Seger AC, et al. Outpatient prescribing errors and the impact of computerized prescribing. J Gen Intern Med. 2005;20:837–41. [DOI] [PMC free article] [PubMed]
- 24.Moore C, Wisnivesky J, Williams S, McGinn T. Medical errors related to discontinuity of care from an inpatient to an outpatient setting. J Gen Intern Med. 2003;18:646–51. [DOI] [PMC free article] [PubMed]
- 25.Dawson P, Gray S. Clinical significance of pharmacist-obtained drug histories. Pharm J. 1981;227:120.


