Abstract
Background
The double-blind placebo-controlled design is commonly considered the gold standard in research methodology; however, subject expectation bias could subvert blinding.
Objective
The primary aim of this study was to examine the impact of expectation bias. Specifically, we examined perceived treatment assignment on smoking cessation outcome rates among participants enrolled in a clinical trial of bupropion (150 mg SR, BID).
Design
Analyses were conducted on data collected during “Kick It at Swope,” a double-blind, placebo-controlled, randomized trial of 600 African-American smokers. Chi-square and multiple logistic regression analyses were used to examine the impact of perception of assignment on treatment effect and cotinine-verified smoking abstinence rates.
Participants
Participants were predominantly middle-aged (mean 44.7, SD 11.2), African-American women (68.6%), who smoked 19 CPD (SD = 8.1). Most had completed at least a high school education or GED (51.6%), and 55% had a monthly family income <$1,800.
Measurements
At week 6 (end of treatment) and week 26 (end of study), participants were asked to report their perceived treatment group assignment. Self-reported abstinence (weeks 6 and 26) was confirmed using CO and cotinine biochemical verification.
Results
After adjusting for actual treatment assignment, age and baseline cotinine, participants who perceived being assigned to bupropion vs. placebo were more likely to be abstinent at weeks 6 (OR = 2.07, 95% CI: 1.29 to 3.33, p = 0.002) and 26 (OR = 1.85, 95% CI: 1.05 to 3.24, p = 0.032).
Conclusions
Results support previous research that expectation bias associated with judgment of treatment assignment is a strong predictor of outcome and confirms this relationship in a smoking cessation trial using bupropion SR among African-American smokers.
Key Words: placebo effect, African American, smoking cessation, bupropion, expectation bias
INTRODUCTION
The double-blind placebo-controlled design is commonly considered the gold standard in research methodology; however, the integrity of the blind may be compromised by subject expectation bias regarding perceived treatment assignment. In the standard blinded trial, participants are told that they have an equal chance of receiving the active drug or placebo. Seemingly harmless, this statement often creates a “guessing set” in which participants attempt to recognize their condition.1 Typically, participants provide one of three guesses or judgments (i.e., active drug, placebo drug, or unsure) and, consistent with expectancy theory,2 most tend to behave according to the perceived effectiveness of their judged treatment.3,4 The strength of the expectation may be acquired through direct personal experience, suggestion, observational learning, classical conditioning, or treatment-related variables, such as the therapeutic relationship or physiologic changes associated with the agent (e.g., side effects, lack of therapeutic effect).3,5
If correct and incorrect judgments are balanced, any expectancy effects should be equally distributed between active and placebo groups. However, if participants are able to correctly identify their treatment assignment, expectancies may be unequally distributed across treatment arms, and the internal validity of the design may be threatened. Furthermore, internal validity is also at risk as any differences observed between the groups cannot be attributed to the pharmacologic agent alone. Therefore, use of the placebo-control design may lead to an overemphasis of the pharmacologic agent’s impact, while ignoring the possibility that expectancies may have mediated the relationship.1
To investigate partiality due to perception of treatment received (i.e., systematic bias), Hughes and Krahn6 examined the magnitude of drug effect among those who correctly identified their treatment assignment (i.e., blinding failure) and compared them to participants who made an incorrect identification. They conclude that evidence for the integrity of the blind can be documented only if the drug effect between those making correct vs. incorrect judgments is the same.
Mooney and colleagues7 conducted a meta-analysis examining blinding failure (i.e., participants or experimenters judged treatment assignment better than by chance) in the smoking cessation literature. Of 73 double-blind, placebo-controlled nicotine replacement therapy (NRT) studies, only 17 analyzed the integrity of the blind. Of these, 12 reported blinding failure. These results indicate that the integrity of the blind may be compromised in NRT trials.
To date, only a handful of smoking cessation trials utilizing bupropion have reported on blinding integrity8–12. Results of these trials indicate that 31–72% of participants are able to correctly perceive assignment to bupropion, while 30–47% correctly perceive placebo. However, no published reports have evaluated the effect of perceived assignment on cessation outcomes and treatment effect in ethnic minority populations.
Reducing tobacco use among underserved populations is a national priority.13,14 Despite an overall decline in cigarette smoking prevalence in the last few decades, smoking rates are not declining at a pace sufficient to meet the 2010 national health objective, which is to reduce the smoking rate among adults to ≤12%.14 Among reasons for the slow decline in smoking rates are documented differences in smoking patterns and treatment effectiveness across subpopulations of smokers.15 For example, although African Americans on average smoke fewer cigarettes per day and are more likely to attempt to quit smoking than European Americans,16–21 the cessation success rate is lower for African Americans.18,20–22 A number of hypothesized reasons exist to explain these differences, including the observation that African Americans show preference for mentholated and high tar/nicotine cigarettes, inhale more deeply, have higher cotinine levels,23–25 and begin smoking later in life compared to European Americans.23,26 Because African Americans have lower smoking cessation rates with pharmacological treatments, a better understanding of expectation bias among this high-risk group could provide insight into reasons for differential treatment response in this population.
To further this goal, we analyzed data collected during a randomized clinical trial of bupropion vs. placebo for smoking cessation among African-American smokers.27 Specifically, we tested the integrity of blinding among participants, examined demographic and tobacco-related factors related to participants’ perception (i.e., judgment) of treatment assignment (bupropion vs. placebo vs. uncertain), analyzed the impact of judgment of assignment on week-6 and week-26 cotinine-verified quit rates, and examined the potential impact of judgment on treatment effect.
METHODS
Study Design
Briefly, “Kick it at Swope” was a double-blind, placebo-controlled, randomized trial of 600 African-American smokers.27 Participants either received 150 mg of bupropion SR (n = 300) or placebo (n = 300) twice daily for 7 weeks starting from 1 week prior to quit date. To mask medication identity, identical-appearing tablets were used. All enrolled participants received brief motivational counseling at quit day, day 3, and at weeks 1, 3, 5, 6, and 7. The primary outcome variable for the study was 7-day point prevalence abstinence at week 26, defined as having smoked “no cigarettes-not even a puff” in the previous 7 days; however, outcomes were also assessed at the end of the treatment phase, week 6. Participants provided written informed consent, and the trial procedures were approved and monitored by the Human Subjects Committee at the University of Kansas Medical Center.
Participants, Screening, and Randomization
Eligible persons described themselves as either “African American” or “Black,” were at least 18 years of age, smoked at least ten cigarettes per day, and were interested in quitting in the next 30 days. Those with a contraindication for bupropion SR (e.g., seizure history, excessive alcohol use, bulimia or anorexia nervosa), pregnancy, or use of other forms of tobacco, bupropion, or NRT products in the past 30 days were excluded. Of the 1,498 potential participants screened, 981 were eligible and invited to participate. Sequential enrollment continued until 600 participants were randomized. Randomized participants did not differ in gender, number of years smoked or other demographic, tobacco-related or psychosocial characteristics. They were however, older than those who were eligible but did not return for randomization (44.2 vs. 38.8 years).
Measures
The baseline assessment included measures of demographic and health information, smoking behaviors, and psychosocial variables.
At weeks 6 (end of treatment) and 26 (end of study), participants were asked, “Do you think you were given the actual medication Zyban or the sugar pill (placebo)?” Response options included: “Zyban,” “Placebo,” “some Zyban and some placebo,” and “don’t know.” Given the potential ambiguity inherent in the wording of the final two answer choices (i.e., some of both or unsure), the 92 participants who responded with either of these answer choices were combined into a group labeled “uncertain.”
Self-reported abstinence at weeks 6 and 26 were biochemically confirmed with expired carbon-monoxide assessment and discrepancies between self-reported abstinence and CO levels (>10 ppm) were resolved with saliva cotinine analysis (<20 ng/ml).
Data Analysis
Participant Characteristics Chi-square test and t-test analyses were conducted to identify significant demographic, psychosocial, and tobacco-related differences between those who answered the week-6 judgment question (n = 512) and those who did not (n = 88).
Integrity of the Blind Chi-square tests of independence were conducted to determine whether there was a relationship between participants’ randomized treatment arm and their judgment of received treatment.
Impact of Judgment on Cessation Outcome and Treatment Effect Multiple logistic regression (MLR) was used to examine whether judgment of treatment at week 6 was associated with verified cessation rates. The first model included actual treatment assignment only; the second included judged assignment only as the predictor variable; the third included both judged assignment and actual assignment; and the fourth model included actual and judged assignments, as well as baseline age and cotinine as potential confounds. For ease of interpretation, age was scaled to 10 years, and cotinine was scaled to 150 ng/ml. We further examined potential heterogeneous treatment effects across perceived treatment groups by adding interaction terms between actual and judged assignments to the third and fourth models. Finally, we examined the consistency of judgment of treatment assignment between weeks 6 and 26.
RESULTS
Participants
Overall, participants were predominantly middle-aged (44.7 years, SD 11.2) females (68.6%), who completed at least a high school education (48.4%) and smoked mentholated cigarettes (77.3%) at an average of 19.1 (SD = 8.1) cigarettes a day. Chi-square and t-tests comparing those responding to the judgment question (n = 512) with those who did not (n = 88) found significant group differences in age only. That is, those who answered the judgment question at week 6 were older [44.7 (SD 11.2) vs. 41.6 years (SD = 9.8), p = 0.017].
Judgment of Treatment Assignment at Week 6
As detailed in Table 1, of the 512 participants included in the analyses, 253 participants judged their assignment to be bupropion, 167 judged their assignment to be placebo, and 92 were “uncertain.” Chi-square and ANOVA analyses identified significant differences among these three groups on income (p < 0.0001), marital status (p = 0.032), and cotinine levels (p = 0.047).
Table 1.
Baseline Demographic, Psychological, and Smoking-Related Characteristics for Total Sample and Among Treatment Judgment Groups
| Total (n = 512) | Judged bupropion (n = 253) | Judged placebo (n = 167) | Judged uncertain (n = 66) | |
|---|---|---|---|---|
| Age, mean (SD) | 44.7 (11.2) | 45.2 (12.0) | 43.9 (9.7) | 44.6 (11.7) |
| Female (n, %) | 351(68.6%) | 170 (67.2%) | 115 (68.9%) | 66 (71.7%) |
| Weight (kg), mean (SD) | 183.9 (46.6) | 182.5 (47.4) | 186.8 (47.4) | 82.8 (19.5) |
| ≤High school graduate, n (%) | 248 (48.4%) | 120 (47.4%) | 79 (47.3%) | 49 (53.3%) |
| aMonthly income <$1,800, n (%) | 276 (54.8%) | 139 (55.9%)a | 74 (45.1%)a | 63 (69.2%)a |
| Married or cohabitating, n (%) | 193 (37.7%) | 95 (37.6%)b | 73 (43.7%)b | 25 (27.2%)b |
| Menthol, n (%) | 396 (77.3%) | 193 (76.3%) | 135 (80.8%) | 68 (73.9%) |
| CPD, mean (SD) | 19.0 (8.1) | 19.0 (8.1) | 19.3 (7.8) | 18.8 (8.3) |
| bSalivary cotinine (ng/ml), mean (SD) | 288.9 (144.2) | 277.0 (137.5)c | 311.4 (150.7)c | 280.3 (147.1)c |
| FTND, mean (SD) | 4.6 (2.0) | 4.5 (2.0) | 4.5 (2.1) | 4.8 (2.0) |
| Previous quits, mean (SD) | 2.5 (4.9) | 2.7 (5.0) | 2.4 (5.4) | 2.0 (3.1) |
| Previous use of sustained-release bupropion, n (%) | 40 (7.8%) | 18 (7.1%) | 10 (6.0%) | 12 (13.0%) |
ap < 0.001;
bp = 0.032;
cp < 0.047
CPD = cigarettes per day
FTND = Fagerstrom Test for Nicotine Dependence score33
Integrity of the Blind
As detailed in Table 2, among those who received active bupropion (n = 259), 57.9% (n = 150) correctly judged receiving active drug; 24.3% (n = 63) incorrectly judged receiving placebo agent and 17.8% were uncertain (n = 46). Among those who received placebo (n = 253), 40.7% (n = 103) correctly judged receiving placebo; 41.1% (n = 104) incorrectly judged receiving bupropion and 18.2% (n = 46) were uncertain. Participants receiving bupropion were significantly more likely to correctly judge their treatment assignment than those receiving placebo (150/259 vs.103/253, p < 0.0001).
Table 2.
Judgment of Treatment Assignment at Week 6 by Actual Treatment Assignment
| Total sample N (%) | Actual bupropion n (%, CI) | Actual placebo n (%) | |
|---|---|---|---|
| Judged bupropion | 253 (49%) | 150 (58%) | 103 (41%) |
| Judged placebo | 167 (33%) | 63 (24%) | 104 (41%) |
| Judged uncertain | 92 (18%) | 46 (18%) | 46 (18%) |
| Total | 512 (100%) | 259 (51%) | 253 (49%) |
Results of chi-square analysis which examined judgment of treatment assignment by actual treatment assignment (p < 0.0001)
Association of Judgment and Abstinence Outcomes
Among the 512 participants, 7-day point prevalence abstinence rates at week 26 were 24% in the bupropion group and 16% in the placebo group (p = 0.016). Differences in quit rates emerged when judgment of treatment assignment was analyzed. That is, among those on active drug, 7-day point prevalence abstinence rates were 29.3% (44/150) for those who correctly judged bupropion treatment; 14.3% (9/63) for those who incorrectly judged placebo and 21.7% (10/46) among those uncertain. When the uncertain group was ungrouped into “don’t know” vs. “some of bupropion and some of both,” abstinence rates were 20.6% vs. 25.0%, respectively.
Among those on placebo, abstinence rates were 16.5% (17/103) for those who incorrectly judged bupropion; 11.5% (12/104) for those who correctly judged placebo and 23.9% (11/46) among those uncertain of assignment (p = 0.30). When the uncertain group was ungrouped into “don’t know” vs. “some of bupropion and some of both,” abstinence rates were 25% vs. 21.4%, respectively, (i.e., opposite direction of bupropion above).
Figure 1
Figure 1.
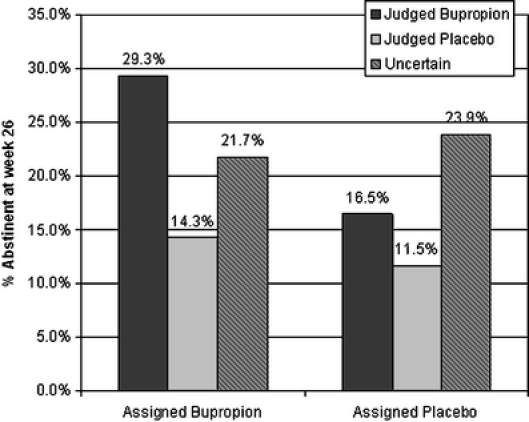
Quit rates (proportion of group achieving cotinine-verified 7-day abstinence at week 26) by actual treatment assignment and judged treatment assignment. For participants receiving buproprion, those who correctly judged bupropion were more likely to have quit than those that incorrectly judged placebo or were uncertain (29.3% vs. 14.3% vs. 21.7%, respectively). Further, among participants receiving placebo, those who incorrectly judged bupropion or correctly judged placebo were less likely to quit than those who were uncertain (16.5% vs. 11.5% vs. 23.9%).
Impact of Judgment on Cessation and Treatment Effect
Univariate analysis (Tables 3 and 4; Models 1 and 2) found that both actual treatment assignment and judgment of treatment assignment were significantly associated with week-26 cessation outcomes. That is, the odds of cessation for participants who were assigned bupropion were greater than among participants assigned placebo (OR = 1.71, 95% CI: 1.10 to 2.26, p = 0.017). The odds of cessation for participants who judged bupropion were greater than participants who judged placebo (OR = 2.21, 95% CI: 1.29 to 3.79, p = 0.004) and those who were uncertain of their assignment (OR = 2.06, 95% CI: 1.05 to 4.01, p = 0.034). After controlling for actual assignment (Model 3), judgment of treatment assignment was significantly associated with cessation (OR = 2.02, 95% CI: 1.17 to 3.50, p = 0.012). We found no interaction between actual assignment and perceived treatment assignment (p = 0.31). But perception did mildly attenuate the treatment effect (decreased by 16%). As depicted in Model 4, after adjusting for actual treatment assignment, age and baseline cotinine levels, participants who judged bupropion were more likely to achieve successful cessation at week 26 than participants who judged placebo (OR = 1.85, 95% CI: 1.05 to 3.24, p = 0.032). Again, there was not a significant interaction between actual assignment and perceived treatment assignment (p = 0.39).
Table 3.
Results Of Logistic Regression Modeling which Examined Quit Rates at Week 26 by Judged and Actual Treatment Assignment (N = 512)
| Variable | Odds ratio | 95% confidence interval | p-value | |
|---|---|---|---|---|
| Model 1 | Actual assignment (bupropion vs. placebo) | 1.71 | 1.10–2.66 | .017 |
| Model 2 | Judged assignment (bupropion vs. placebo) | 2.21 | 1.29–3.79 | 0.004 |
| Judged assignment (uncertain vs. placebo) | 2.06 | 1.05–4.01 | .034 | |
| Model 3 | Actual assignment (bupropion vs. placebo) | 1.55 | 0.98–2.42 | .058 |
| Judged assignment (bupropion vs. placebo) | 2.02 | 1.17–3.50 | .012 | |
| Judged assignment (uncertain vs. placebo) | 1.96 | 1.00–3.83 | .050 | |
| Model 4* | Actual assignment (bupropion vs. placebo) | 1.52 | 0.96–2.41 | .076 |
| Judged assignment (bupropion vs. placebo) | 1.85 | 1.05–3.24 | .032 | |
| Judged assignment (uncertain vs. placebo) | 1.79 | 0.90–3.55 | .097 | |
| Age (10 years) | 1.28 | 1.05–1.55 | .013 | |
| Salivary cotinine (150 ng/ml) | 0.61 | 0.47–0.80 | .0003 |
Table 4.
Judgment Variables Cross Tabulation (Week 6 vs. Week 26)
| Week 26 | ||||
|---|---|---|---|---|
| Week 6 | Total N | Judged bupropion n | Judged placebo n | Judged uncertain n |
| Judged bupropion | 222 | 175 | 24 | 23 |
| Judged placebo | 150 | 18 | 120 | 12 |
| Judged uncertain | 78 | 29 | 19 | 30 |
| Total | *450 | 222 | 163 | 65 |
P < 0.0001
*Judgment response missing from 62 participants at week 26
We repeated the above analyses on week-6 outcomes and found similar results except higher cessation rates and odds ratios (OR = 2.07, 95% CI: 1.29 to 3.33, p = 0.002) comparing participants who judged bupropion with participants who judged placebo adjusting for actual treatment assignment, age, and baseline cotinine.
Change in Judgment of Treatment Condition at Week 26
We cross-tabulated the frequencies between judgment of assignment at week 6 and week 26 and found out that 78.8% of participants who judged bupropion at week 6 still believed they were given bupropion at week 26 and 80.0% of participants who judged placebo at week 6 still believed they were given placebo at week 26. However, among those who were uncertain at week 6 (n = 78), only 38.5% continued to judge uncertain at week 26. Chi-square test of independence indicated a strong association between judgment of assignment at week 6 and week 26 (p < 0.0001).
DISCUSSION
This study’s first aim was to determine whether the integrity of the blind design was maintained in this randomized, controlled trial among AA smokers. In our original trial enrolling 600 African-American smokers, we concluded that blinding was successful.27 That is, at the end of treatment, of participants who returned for the week-26 visit (n = 512), 58% (150/259) correctly guessed that they received bupropion SR, and 41% (104/253) correctly guessed they received placebo or were uncertain. However, according to the definition published by Desbiens,28 if the blind is successful, the rate at which participants correctly judge their treatment arm should be 50%. Among participants believing they had been assigned to either bupropion or placebo, those in the active treatment arm were able to correctly judge that they were receiving active bupropion at a higher rate than by chance (58%). In our trial, fewer participants in the placebo arm correctly judged that they were taking an inactive agent (41%). Thus, participants were equally likely to correctly identify their treatment assignment (41% vs. 41%) but were more likely to correctly guess their assignment to bupropion (58% vs. 24%, respectively).
Although participants’ ability to accurately discern treatment condition among those in the bupropion arm were similar to that reported by Mooney and colleagues7 (i.e., 57%), our findings indicate that participants were less likely to correctly identify that they were receiving placebo than prior studies (i.e., 63.6%). These findings indicate that participants who received active bupropion may be more likely to correctly detect treatment condition, thereby suggesting that the integrity of blinding may be compromised in studies utilizing bupropion vs. placebo for smoking cessation. As discussed by Mooney et al.7 and Kaptchuk,29 the processes underlying these assumptions remain poorly understood, and more studies need to be conducted before conclusions are made. In our study, it is clear that participants generally formed strong beliefs about which treatment they had received (i.e., only 18% of participants in both groups were uncertain (i.e., thought they were receiving either agents or “did not know”). However, we lack the data to further explore reasons behind these beliefs.
We next examined the impact of the blinding failure on our study outcomes. We found no interaction between the treatment effect of bupropion and perceived treatment group, both before and after adjusting for baseline age and cotinine. However, we found that treatment effect of bupropion was attenuated by 16% when judgment was included in the model. Thus, participant expectancy appears to influence actual treatment response. If expectancy attenuates treatment effectiveness, as indicated by our results, perhaps clinical practice guidelines might be designed to encourage patients to believe in therapy.
The study of the indirect and direct placebo effects produced by the physician’s attitude toward the patient, treatment and results of treatment has been coined “placebogenics and iatroplacebogenics.”30,31 The physician’s attitude toward treatment (e.g., enthusiasm, conviction, optimism) is a nonspecific factor in most therapies. However, there is ample evidence documenting the important influence the provider can have in encouraging patient optimism for treatment. In their review of placebo effects during pain treatment, Turner and colleagues 32 concluded…“The quality of the interaction between physician and patient can be extremely influential in patient outcomes, and…patient and provider expectations may be more important than specific treatment (p. 1613).”
This study’s third aim was to examine the impact of judged treatment assignment on smoking cessation outcome rates. Our findings indicate that at week 26, those who judged bupropion had 71% greater odds of quitting than those who judged placebo, regardless of actual medication assignment and after controlling for potential confounds. These findings and those reported in the Mooney7 meta-analysis suggest that expectations regarding bupropion and placebo may be associated with treatment outcomes and indicate that expectancy bias may be associated with treatment group judgment. Thus, although we can conclude that judgment has an important role in cessation outcome, several factors limit our ability to conclude that judgment of bupropion treatment increased the likelihood of smoking cessation.
First, participants were originally informed that they would be randomly assigned to the placebo or active agent. Thus, it is possible that the response option of “some placebo and some bupropion” may have confused participants and contributed to uncertainty when asked about their judgment. Second, we did not assess preconceived notions about cessation likelihood associated with each condition or participants’ belief in the integrity of the study design. As discussed by Mooney and colleagues,7 the factors thought to influence participants’ judgment of treatment assignment remain unclear. However, smoking cessation researchers are advised to carefully script verbiage used to deliver active and placebo agents. Ideally, drug delivery should be accompanied by optimism, enthusiasm, and positive expectancies regarding the effectiveness of the agent. Our failure to assess the exact verbiage used by each counselor dispensing medication and additional factors likely to influence judgment of treatment assignment (e.g., medication side effects, affective response) is also a study limitation. Finally, future studies are encouraged to employ longitudinal designs better equipped to assess expectancy causality.
In conclusion, our results support previous research that expectation bias associated with judgment of treatment assignment is a strong predictor of outcome and confirms this relationship in a smoking cessation trial using bupropion SR among African-American smokers. It is our hope that these findings influence future research investigating mechanisms underlying intervention expectancy among diverse populations. Further, future research might investigate methods by which providers might instill positive expectancy of medical treatment to enhance adherence and clinical outcomes.
Acknowledgements
This study was funded by grant R01CA77856 from the National Cancer Institute. GlaxoSmithKline provided study medication (both active and placebo) but played no role in the design, conduct of the study, or interpretation and analysis of the data. We thank the staff and participants at Swope Parkway Health Center for making this research possible.
Conflict of Interest None disclosed.
Footnotes
Presented at the 12th Annual Meeting of the Society of Nicotine and Tobacco Research, February, 2006, Orlando, Florida.
References
- 1.Sutton SR. Great expectations: some suggestions for applying the balanced placebo design to nicotine and smoking. [Review] [8 refs]. Br J Addict.. 1991;86:659–62. [DOI] [PubMed]
- 2.Stewart-Williams S, Podd J. The placebo effect: dissolving the expectancy versus conditioning debate.[see comment]. Psychol Bull.. 2004;130:324–40. [DOI] [PubMed]
- 3.Kirsch I. Specifying nonspecifics: Psychological mechanisms of placebo effects. In: Harrington A, ed. The placebo effect: an interdisciplinary exploration. Cambridge: Harvard University Press; 1997:166–86.
- 4.Perkins K, Sayette M, Conklin C, Caggiula A. Placebo effects of tobacco smoking and other nicotine intake. Nicotine Tob Res. 2003;5:695–709. [DOI] [PubMed]
- 5.Stewart-Williams S. The placebo puzzle: putting together the pieces. Health Psychol. 2004;23:198–206. [DOI] [PubMed]
- 6.Hughes JR, Krahn D. Blindness and the validity of the double-blind procedure. J Clin Psychopharmacol. 1985;53138–42. [DOI] [PubMed]
- 7.Mooney M, White T, Hatsukami D. The blind spot in the nicotine replacement therapy literature: assessment of the double-blind in clinical trials. [Review] [49 refs]. Addict Behav. 2004;294673–84. [DOI] [PubMed]
- 8.Hall SM, Humfleet GL, Reus VI, et al. Psychological intervention and antidepressant treatment in smoking cessation. Arch Gen Psychiatry. 2002;59:930–6. [DOI] [PubMed]
- 9.Killen JD, Fortmann SP, Murphy GM Jr., et al. Extended treatment with bupropion SR for cigarette smoking cessation. J Consult Clin Psychol. 2006;74:286–94. [DOI] [PubMed]
- 10.Killen JD, Robinson TN, Ammerman S, et al. Randomized clinical trial of the efficacy of bupropion combined with nicotine patch in the treatment of adolescent smokers. J Consult Clin Psychol. 2004;72:729–35. [DOI] [PubMed]
- 11.Wagena EJ, Knipschild PG, Huibers MJ, Wouters EF, van Schayck CP. Efficacy of bupropion and nortriptyline for smoking cessation among people at risk for or with chronic obstructive pulmonary disease. Arch Intern Med. 2005;165:2286–92. [DOI] [PubMed]
- 12.Simon JA, Duncan C, Carmody TP, Hudes ES. Bupropion for smoking cessation: A randomized trial. Arch Intern Med. 2004;164:1797–803. [DOI] [PubMed]
- 13.USDHHS. Tobacco use among U.S. racial ethnic minority groups African Americans, American Indians, and Alaskan Natives, Asian Americans, and Pacific Islanders and Hispanics: A report of the surgeon general (Washington, D.C., Government Printing Office). 1998. [PubMed]
- 14.USDHHS. Healthy people 2010: Objectives for improving health (Washington, DC). 2000.
- 15.Lerman C, Kaufmann V, Rukstalis M, et al. Individualizing nicotine replacement therapy for the treatment of tobacco dependence: a randomized trial. Ann Intern Med. 2004;140:426–33. [DOI] [PubMed]
- 16.Pederson LL, Ahluwalia JS, Harris KJ, McGrady GA. Smoking cessation among African Americans: what we know and do not know about interventions and self-quitting. Prev Med. 2000;31:23–38. [DOI] [PubMed]
- 17.Royce JM, Hymowitz N, Corbett K, Hartwell TD, Orlandi MA. Smoking cessation factors among African Americans and Whites. Am J Public Health. 1993;83:220–6. [DOI] [PMC free article] [PubMed]
- 18.National Cancer Institute. Population Based Smoking Cessation: Proceedings of a Conference on What Works to Influence Cessation in the General Population. Smoking and Tobacco Control Monograph No. 12. (Bethesda, MD., Department of Health And Human Services, National Institutes of Health, National Cancer Institute). 2000
- 19.Hahn LP, Folsom AR, Sprafka JM, Norsted SW. Cigarette smoking and cessation behaviors among urban blacks and whites. Public Health Rep. 1990;105:290–5. [PMC free article] [PubMed]
- 20.Giovino GA, Schooley MW, Zhu BP, Chrismon JH, Tomar SL, Peddicord JP, Merritt RK, Husten CG, Eriksen MP. Surveillance for selected tobacco-use behaviors–United States, 1900–1994. Morb Mortal Wkly Rep CDC Surveill Summ. 1994;43:1–43. [PubMed]
- 21.Fiore MC, Novotny TE, Pierce JP, et al. Trends in cigarette smoking in the U. S. – the changing influence of gender and race. JAMA. 1989;261:49–55. [DOI] [PubMed]
- 22.Trinidad DR, Gilpin EA, White MM, Pierce JP. Why does adult African-American smoking prevalence in California remain higher than for non-Hispanic whites? Ethn Dis. 2005;15:505–11. [PubMed]
- 23.Kabat GC, Morabia A, Wynder EL. Comparison of smoking habits of blacks and whites in a case-control study. Am J Public Health. 1991;81:1483–6. [DOI] [PMC free article] [PubMed]
- 24.Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18:188–204. [DOI] [PubMed]
- 25.Clark PI, Gautam S, Gerson LW. Effect of menthol cigarettes on biochemical markers of smoke exposure among black and white smokers. Chest. 1996;110:1194–8. [DOI] [PubMed]
- 26.Okuyemi KS, Ahluwalia JS, Richter KP, Mayo MS, Resnicow K. Differences among African American light moderate and heavy smokers. Nicotine Tob Res. 2001;3:49–54. [DOI] [PubMed]
- 27.Ahluwalia JS, Harris KJ, Catley D, Okuyemi KS, Mayo MS. Sustained-release bupropion for smoking cessation in African Americans: a randomized controlled trial. J Am Med Assoc. 2002;288:468–74. [DOI] [PubMed]
- 28.Desbiens NA. In randomized controlled trials, should subjects in both placebo and drug groups be expected to guess that they are taking drug 50% of the time? Med Hypotheses. 2002;59:227–32. [DOI] [PubMed]
- 29.Kaptchuk TJ. Powerful placebo: the dark side of the randomised controlled trial. Lancet. 1998;351:1722–5. [DOI] [PubMed]
- 30.Shapiro AK. Iatroplacebogenics. Int Pharacopsychiatry. 1969;2:215.
- 31.Shapiro AK. Placebogenics and iatroplacebogenics. Med Times. 1964;92:1037–43. [PubMed]
- 32.Turner JA, Deyo RA, Loeser JD, Von Korff M, Fordyce WE. The importance of placebo effects in pain treatment and research. Jama. 1994;271:1609–14. [DOI] [PubMed]
- 33.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The fagerstrom test for nicotine dependence: a revision of the fagerstrom tolerance questionnaire. Br J Addict. 1991;86:1119–27. [DOI] [PubMed]


