Abstract
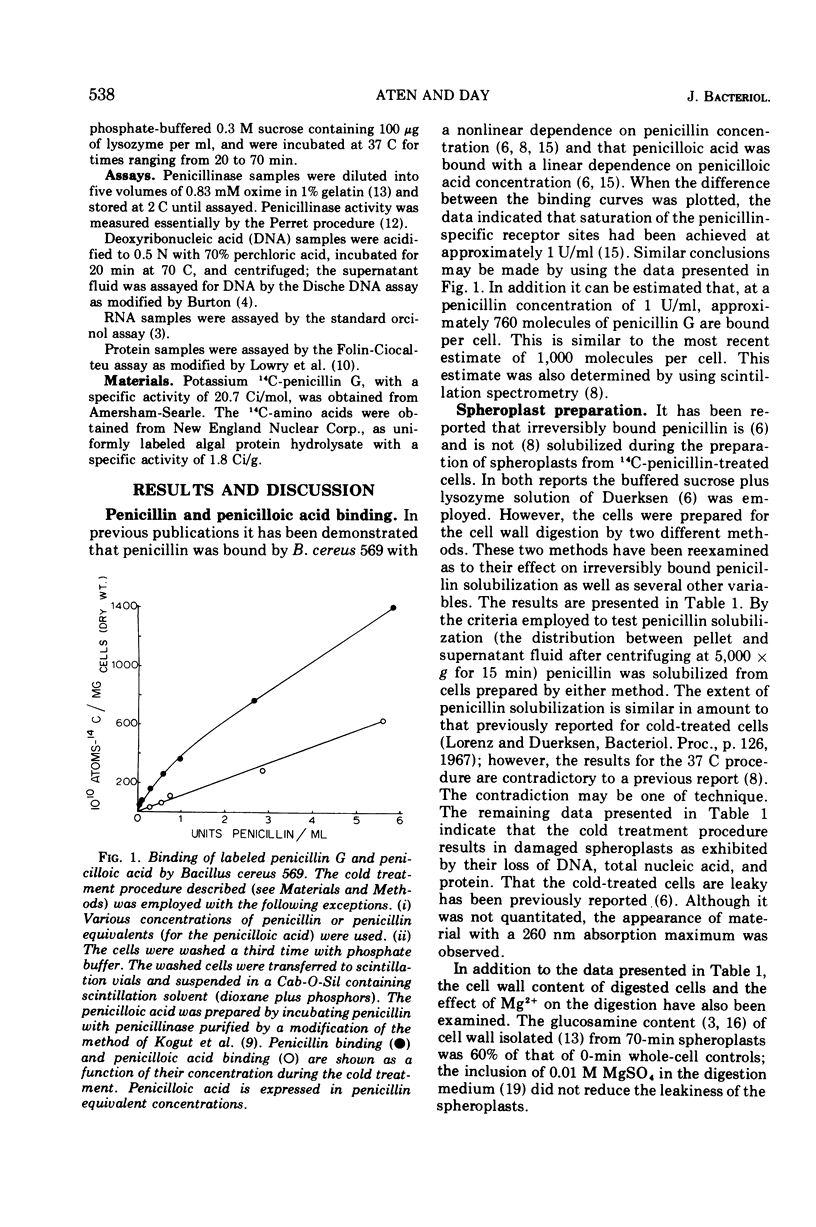
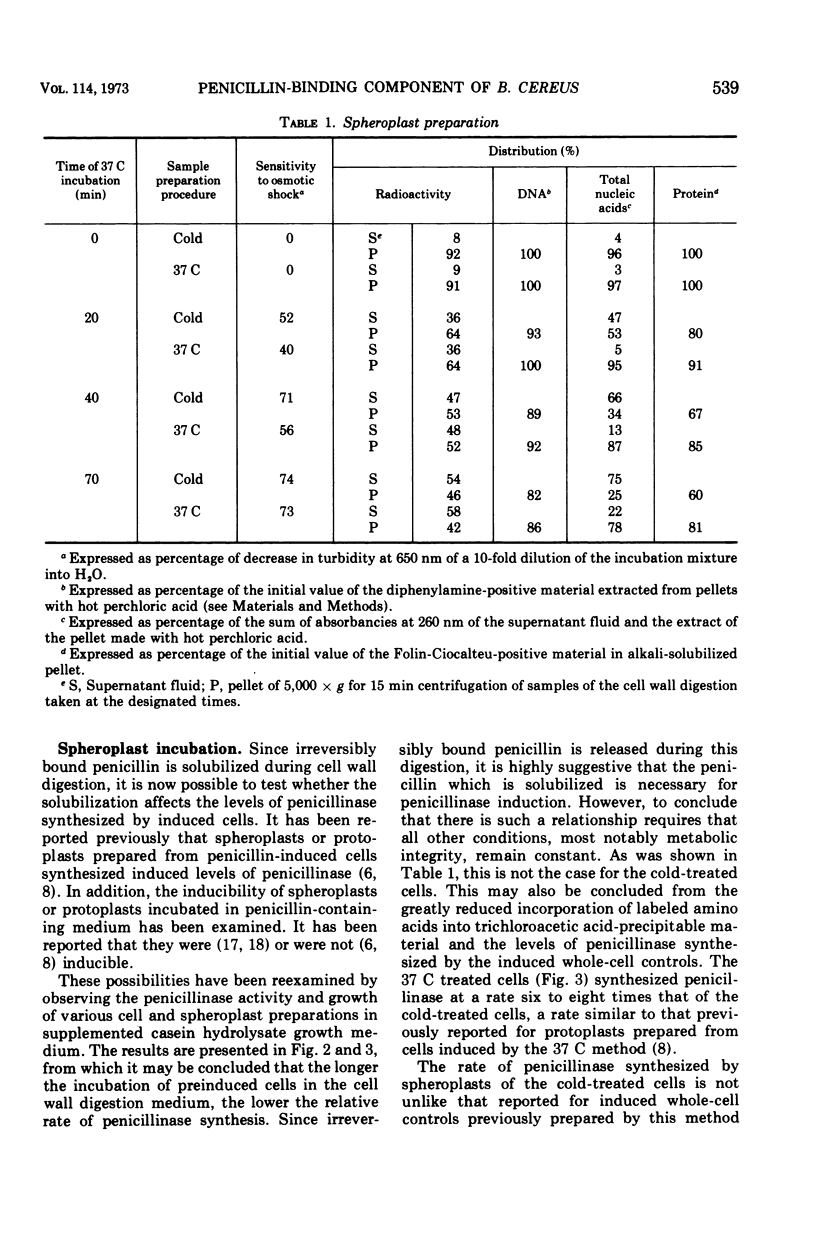
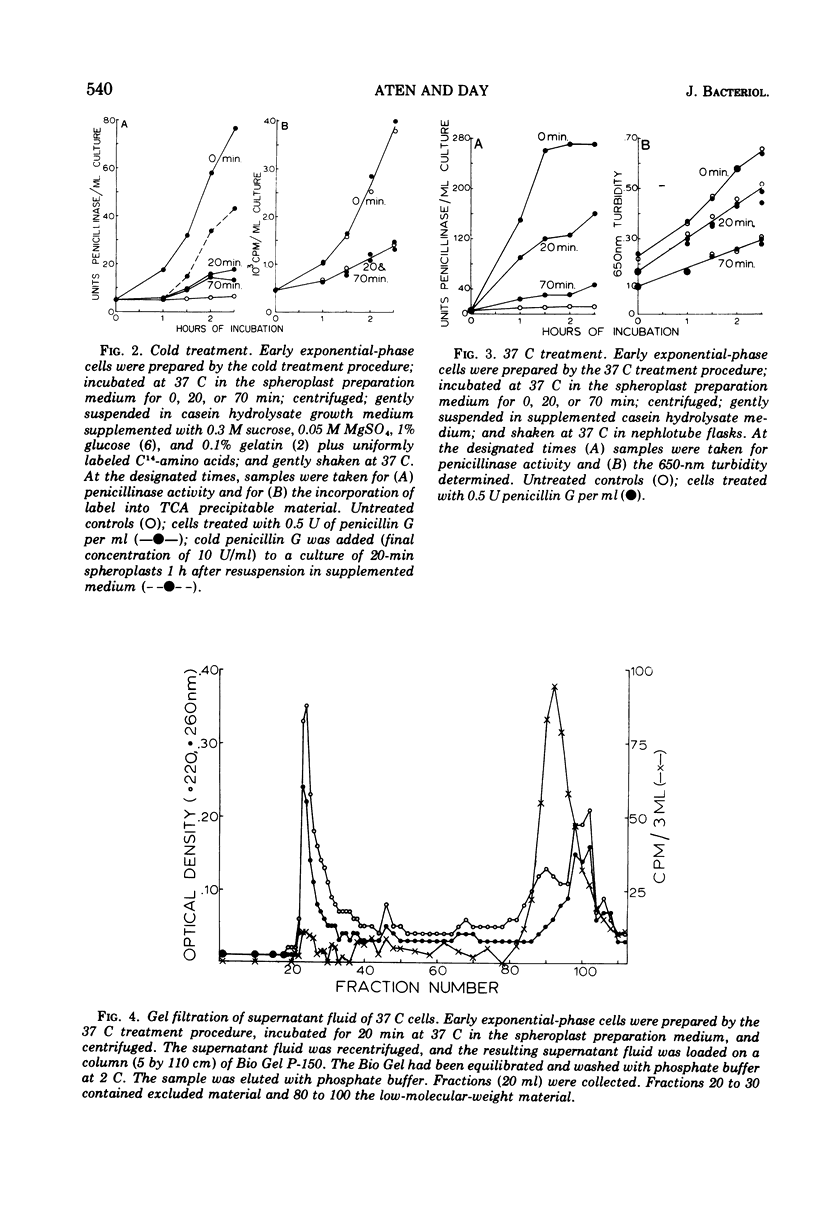
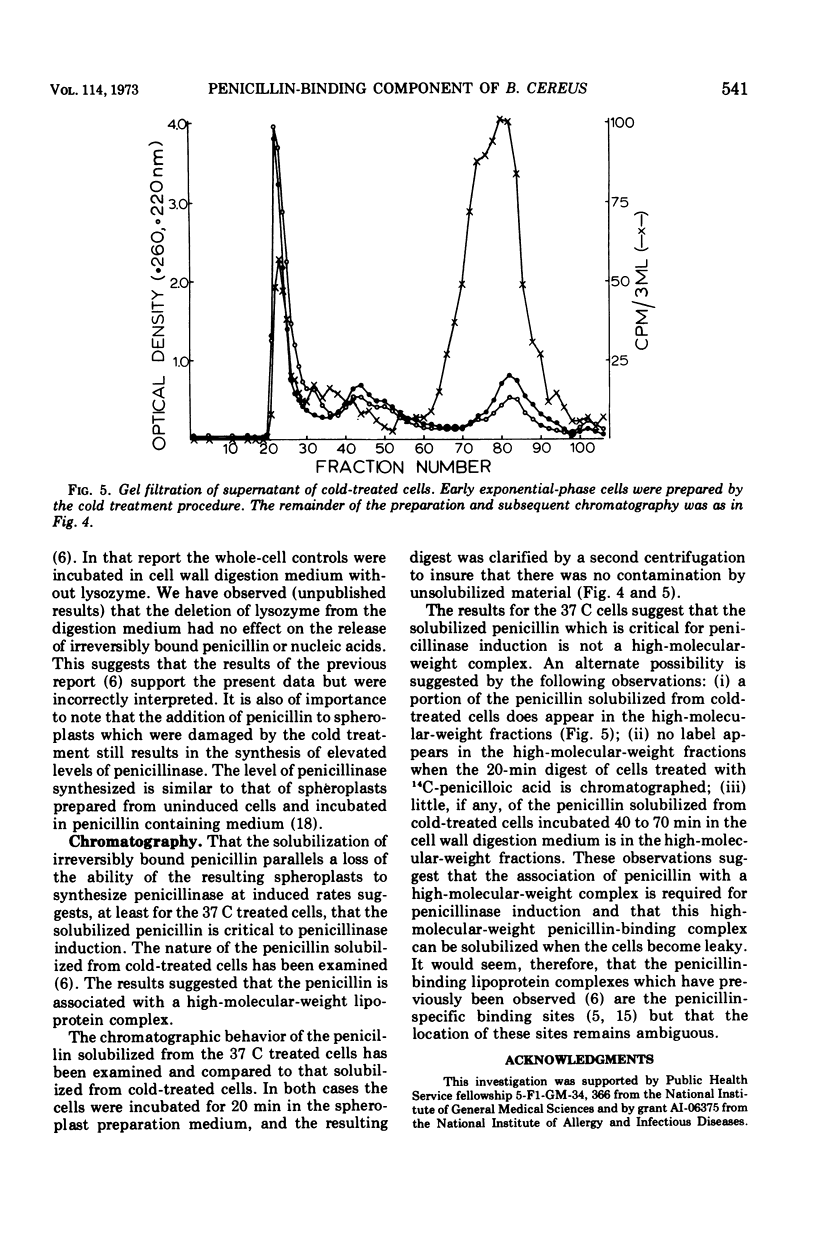
14C-penicillin is irreversibly bound by Bacillus cereus 569. Incubation of penicillin-treated cells in a cell wall digestion medium results in solubilization of approximately 60% of the irreversibly bound lable. The extent of the solubilization is the same when cells are prepared by either a cold or 37 C treatment procedure. However, spheroplasts prepared by the cold treatment are leaky. When the resulting spheroplasts are incubated in supplemented medium, reduced rates and levels of penicillinase synthesis, relative to induced whole-cell controls, are observed. Spheroplasts from both cold and 37 C prepared cells exhibit this phenomena, although the spheroplasts from 37 C prepared cells synthesized approximately sixfold higher levels of penicillinase. The size distribution of the label solubilized during the preparation of spheroplasts was examined by using Bio Gel P-150 columns. Although no label appeared in the exclusion volume fractions when the cell wall digest of the 37 C treated cells was chromatographed, approximately 10% of the label from cold-treated cells did appear. These results suggest that the presence of irreversibly bound penicillin is required for the synthesis of induced levels of penicillinase and that the irreversibly bound penicillin can be solubilized as a labile complex with material which is excluded from BioGel P-150. It may be concluded that the penicillin-binding lipoprotein complex which has been previously observed is the penicillin-specific binding site. However, the location of this complex in relation to the cell membrane could not be determined.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOAS N. F. Method for the determination of hexosamines in tissues. J Biol Chem. 1953 Oct;204(2):553–563. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein A., Nickerson K. W., Day R. A. Thermal penicillinasderepression and temperature dependence of penicillinase production inducible and constitutive strains of Bacillus cereus. Arch Biochem Biophys. 1967 Mar;119(1):50–54. doi: 10.1016/0003-9861(67)90427-4. [DOI] [PubMed] [Google Scholar]
- Csányi V., Jacobi G., Straub B. F. The regulation of penicillinase synthesis. Biochim Biophys Acta. 1967 Sep 26;145(2):470–484. doi: 10.1016/0005-2787(67)90065-2. [DOI] [PubMed] [Google Scholar]
- DUERKSEN J. D. LOCALIZATION OF THE SITE OF FIXATION OF THE INDUCER, PENICILLIN, IN BACILLUS CEREUS. Biochim Biophys Acta. 1964 May 18;87:123–140. doi: 10.1016/0926-6550(64)90053-2. [DOI] [PubMed] [Google Scholar]
- Imsande J. Regulation of penicillinase synthesis: evidence for a unified model. J Bacteriol. 1970 Jan;101(1):173–180. doi: 10.1128/jb.101.1.173-180.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOGUT M., POLLOCK M. R., TRIDGELL E. J. Purification of penicillin-induced penicillinase of Bacillus cereus NRRL 569: a comparison of its properties with those of a similarly purified penicillinase produced spontaneously by a constitutive mutant strain. Biochem J. 1956 Mar;62(3):391–401. doi: 10.1042/bj0620391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PARK J. T., HANCOCK R. A fractionation procedure for studies of the synthesis of cell-wall mucopeptide and of other polymers in cells of Staphylococcus aureus. J Gen Microbiol. 1960 Feb;22:249–258. doi: 10.1099/00221287-22-1-249. [DOI] [PubMed] [Google Scholar]
- PERRET C. J. Iodometric assay of penicillinase. Nature. 1954 Nov 27;174(4439):1012–1013. doi: 10.1038/1741012a0. [DOI] [PubMed] [Google Scholar]
- POLLOCK M. R. A simple method for the production of high titre penicillinase. J Pharm Pharmacol. 1957 Sep;9(9):609–611. doi: 10.1111/j.2042-7158.1957.tb12316.x. [DOI] [PubMed] [Google Scholar]
- POLLOCK M. R., PERRET C. J. The relation between fixation of penicillin sulphur and penicillinase adaptation in B cereus. Br J Exp Pathol. 1951 Oct;32(5):387–396. [PMC free article] [PubMed] [Google Scholar]
- POLLOCK M. R. Penicillinase adaptation in B. cereus; adaptive enzyme formation in the absence of free substrate. Br J Exp Pathol. 1950 Dec;31(6):739–753. [PMC free article] [PubMed] [Google Scholar]
- SHEININ R. The localizatio of the cell-bound penicillinase of Bacillus cereus in protoplasts. J Gen Microbiol. 1959 Aug;21:124–134. doi: 10.1099/00221287-21-1-124. [DOI] [PubMed] [Google Scholar]
- WEIBULL C. The nature of the ghosts obtained by lysozyme lysis of Bacillus megaterium. Exp Cell Res. 1956 Feb;10(1):214–221. doi: 10.1016/0014-4827(56)90087-8. [DOI] [PubMed] [Google Scholar]



