Abstract
Airway epithelial cells provide mechanical and immune protection against pathogens and allergens. Following activation, these cells produce a wide range of cytokines including thymic stromal lymphopoietin (TSLP). Recently it was established that a high level of TSLP is associated with asthma in mice and in humans. These findings suggest that interfering with the ability of cells to respond to TSLP might prevent the development of airway inflammation. Our review presents current knowledge on mediators that induce TSLP production and on the actions of TSLP on different populations of cells that are related to airway inflammation. TSLP affects dendritic cells, T cells, NKT cells, and mast cells, indicative of the broad role of TSLP in the regulation of inflammatory/allergic processes.
Introduction
Asthma is a disease characterized by chronic airway inflammation that is mediated by T-helper 2 (TH2) cells [1, 2]. TH2 cells release interleukin-4 (IL-4), IL-5, IL-9, and IL-13, driving IgE production by B cells, stimulating basophils and eosinophils, enhancing mast cell differentiation, and increasing mucus production [1, 2]. Allergens trigger the cross-linking of IgE on mast cells, leading to the activation and degranulation of these cells. The inflammatory mediators released by mast cells cause bronchial smooth muscle contraction, vascular permeability, inflammatory cell infiltrate, increased mucus in airways, epithelial cell loss, and goblet cell hyperplasia [1, 3].
Airway epithelial cells provide the initial barrier against pathogens (allergens) invading the lung. In addition to mechanical protection, epithelial cells provide immune defense against harmful materials. Following their activation, these cells produce an array of cytokines and chemokines, including IL-1, IL-6, IL-8, granulocyte-macrophage colony-stimulating factor (GM-CSF), interferons-α and β, tumor necrosis factor -α(TNF-α, and RANTES (regulated upon activation, normal T cell expressed and secreted), eotaxin and others, which promote the recruitment and activation of immune and inflammatory cells [4–6]. Among the mediators produced by epithelial cells is thymic stromal lymphopoietin (TSLP) [7]. Recent studies have established that high levels of TSLP are associated with airway inflammatory disease in humans and mice [8*, 9*]. Strikingly, mice lacking TSLPR fail to develop inflammatory allergic response in the lung [10**]. Conversely, the over-expression of TSLP in transgenic mice induces spontaneous airway inflammation and atopic dermatitis, consistent with an important role for this cytokine in allergy/inflammation [11**, 12].
TSLP/TSLPR signaling
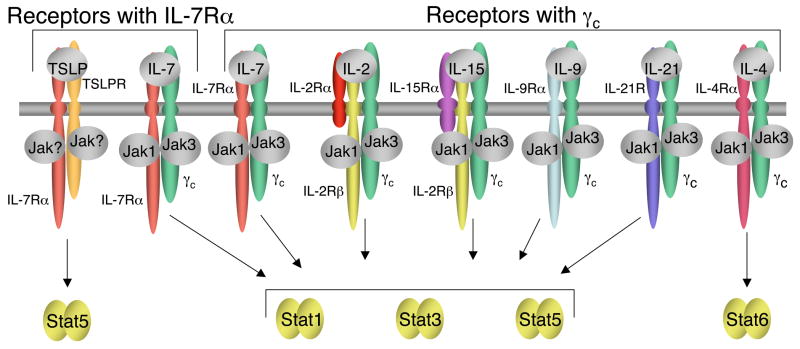
TSLP was originally identified as a growth factor in the supernatant of Z210R.1 thymic stromal cells that support proliferation and survival of the NAG8/7 pre-B cell line [13]. TSLP is a short-chain four α-helical bundle type I cytokine that is closely related to interleukin-7 (IL-7), another stromal factor [14, 15]. IL-7 signals via IL-7Rα and the common cytokine receptor γ chain (γc), a protein that is also a critical component of the receptors for IL-2, IL-4, IL-9, IL-15, and IL-21 [16]. TSLP also signals via IL-7Rα, but instead of γc, uses a specific TSLPR subunit that is highly related to γc [17, 18] (see Figure 1). TSLPR contains a Box 1 region that is common to all type I cytokine receptor family proteins and is important for the binding of Janus family tyrosine kinases (JAKs). Although it has been suggested that TSLP failed to activate any JAK [19], TSLP mediates Stat5 activation [19, 20], and this activation requires the Box1 region of both TSLPR and IL-7Rα [20]. In addition to its activation of Stat5, TSLP presumably might activate other pathways as well, including perhaps those often activated by other cytokines such as PI 3-K/Akt, MAPK and/or Src family kinase pathways [9*]. A better comprehension of the signaling pathways activated by this cytokine will be useful to the understanding of the mechanisms by which TSLP exerts its effects, and may lead to additional ways of regulating its actions.
Figure 1. Schematic showing TSLP and IL-7 receptors sharing IL-7Rα and IL-7, IL-2, IL-15, IL-9, IL-21, and IL-4 receptors sharing γc.
The receptors are grouped to show TSLP on the far left, IL-7 complexes adjacent in both groups and IL-4 at the far right, as it activates Stat6. For each γc family cytokine receptor, Jak1 associates with the more “distinctive” type I cytokine receptor chain, whereas Jak3 associates with γc. TSLP, IL-2, IL-7, IL-9, and IL-15 primarily activate Stat5a and Stat5b, IL-4 primarily activates Stat6, and IL-21 primarily activates Stat3 and Stat1.
Induction of TSLP in allergic disorders
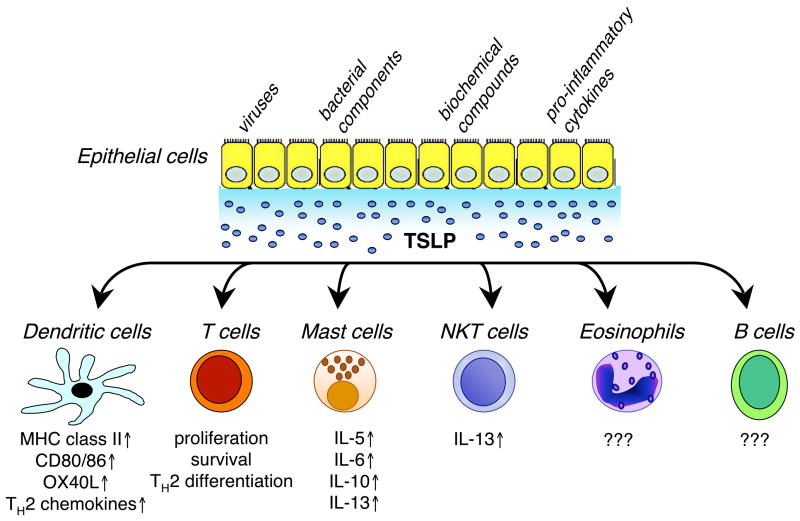
Studies on TSLP have indicated that epithelial cells, keratinocytes, and stromal cells are major producers of TSLP [7]. Recently, it was demonstrated that allergen-activated basophils also produce TSLP and thus also may be important in the initiation of TH2 responses [21]. TSLP is implicated in allergic inflammation, and a number of mediators have been defined, with the potential to promote TSLP expression that might be associated with airway inflammation and atopic dermatitis (Figure 2) [22–25].
Figure 2. Schematic showing production of TSLP by epithelial cells in response to a range of stimuli.
These include viruses, bacterial components such as peptidoglycan, lipoproteins, lipoteichoic acid, LPS and bacterial DNA, biochemical compounds like vitamin D and low-calcemic analogs [22], and pro-inflammatory cytokines TNFα and IL-1β. In turn, TSLP can act on cells of multiple lineages, inducing the production of cytokines and also having effects on proliferation and differentiation.
A key molecular mechanism that links mediators and TSLP production is the NF-κB signaling pathway, which is activated by many pro-inflammatory cytokines and Toll-like receptor (TLR) ligands [23*, 26]. Recent findings reveal that pro-inflammatory cytokines, such as TNFα and IL-1, synergize with TH2 cytokines (IL-4 and IL-13) in increasing TSLP production by human airway epithelial cells and human keratinocytes [23*, 24]. Similarly, engagement of TLR3 by viral double-strand RNA (dsRNA) of rhinoviruses or by synthetic dsRNA can augment production of TSLP by epithelial cells [25, 27*]. Although it has not been proven that respiratory viruses induce asthma, they are known to induce airway hyper-responsiveness (AHR) and amplify the response to allergen exposure [28]. Therefore, TSLP may at least in part explain this observation. Similarly, a role of TLR2, TLR8, and TLR9 in the release of TSLP has been suggested [23*, 27*]. Although it was proposed that the activation of airway epithelial cells via TLR4 does not affect TSLP expression, it was found that LPS-stimulated intestinal epithelial cells up-regulate TSLP mRNA expression [26]. Together these findings suggest that TSLP may be important in a range of inflammatory processes.
The role of TSLP in the development of immune cells
TSLP, like IL-7, has effects in T- and B-cell lymphopoiesis [9*, 29, 30, 33]. In mice, disruption of IL-7Rα signaling leads to T- and B-cell lymphopenia and an absence of γδ T cells [31]. In humans, mutation of the IL7R gene results in a form of severe combined immunodeficiency in which T cells are profoundly diminished in number while other lineages are intact [31, 32]. It is reasonable to predict that TSLP might partially replace the role of IL-7 in T cell development because IL-7Ra is also shared by TSLP. Indeed, although the lack of TSLP signaling does not affect hematopoiesis, TSLPR/γc double knockout mice have more impaired T-cell development than do γc-deficient mice, and recovery of T cells is defective in sub-lethally irradiated TSLPR knockout mice [33]. Similarly, transgenic overexpression of TSLP in Il7−/− lymphopenic mice promotes generation of functional B- and T-cells, consistent with the ability of TSLP to compensate for IL-7 deficiency [30].
Whereas lymphoid and myeloid progenitors are the major targets for TSLP in the development of the immune system, relatively limited information is available regarding the cells that respond to TSLP in the periphery.
TSLP exerts actions on multiple lineages
TSLP has effects on a range of immune cells, including dendritic cells (DCs), T cells, natural killer T (NKT) cells, and mast cells. These effects will be summarized, focusing on the relationship to asthma (Figure 2).
Dendritic cells
DCs are professional antigen presenting cells (APCs) that bridge between innate and adaptive immunity. DCs can be activated directly by pathogens via Toll-like receptors or by mediators produced by epithelial cells. Activated DCs up-regulate co-stimulatory molecules including MHC class II antigens, and release cytokines and chemokines that together induce recruitment, activation, and differentiation of T cells. Human DCs express TSLP receptors [34] and rapidly respond to TSLP, as evaluated by the up-regulation of MHC class II, CD40, CD80, CD86, OX40L, TARC, and MDC molecules (Figure 2) [9*]. Following TSLP stimulation, DCs mediate homeostasis and differentiation of human CD4+ T cells to inflammatory TH2 cells [9*] and CD8+ T cells to pro-allergic cytotoxic cells with IL-13 production [35]. In mice, TSLP also is able to induce the maturation of DCs, and TSLP-activated DCs regulate the differentiation of naive CD4+ T cells to TH2 cells [10**, 11**]. These results suggest that in both humans and mice, TSLP-activated DCs promote the differentiation of T cells to pro-allergic cells.
T cells
Much discussion has focused on possible species-specific actions of TSLP on T cells. TSLP was reported to have certain specific T cell-related actions in mice, whereas human TSLP was shown to activate DCs, with only an indirect effect on T cells [9*]. However, when we investigated the action of TSLP on human and mouse T cells, we found that CD4+ T cells in both species can directly respond to this cytokine [33, 36**], indicating that there is not a significant species-specific variation in the actions of this cytokine in humans and mice.
Careful analysis of human CD4+ T cells showed that pre-activated but not naïve CD4+ T cells express TSLPR and that TSLP can rapidly activate Stat5 and induce expression of Stat5 target genes, indicating the presence of functional TSLP receptors on activated human T cells. Consistent with this, TSLP augments the proliferation rate of T cell receptor (TCR)-activated human CD4+ T cells [36**], analogous to the effect previously observed in mice [33]. In addition, TSLP also increases IL-2Rα expression, thus increasing the sensitivity of CD4+ T cells to low doses of IL-2; this indicates a possible mechanism for regulating the proliferation of these cells following TCR engagement [36**].
In mice, TSLPR deficiency has been associated with defective development of an inflammatory allergic response to ovalbumin in the lung, but this can be reversed by the addition of wild type (WT) CD4+ T cells [10**]. Interestingly, a comparison of the four possible combinations of DCs and CD4+ T cells from WT versus TSLPR KO mice in a proliferative assay revealed that the absence of TSLPR on CD4+ T cells appeared to be even more deleterious than the loss of TSLPR on DCs [10**]. Moreover, it was shown that TSLP can regulate differentiation of pre-activated mouse CD4+ T cells towards the TH2 phenotype in a DC-independent fashion [37]. Together these data demonstrate a unique role of TSLP in the regulation of CD4+ T cell action (Figure 2).
Natural Killer T cells
NKT cells represent a unique sub-population of T cells that have properties of both conventional T cells and NK cells. Like T cells, NKT cells develop from thymocyte progenitors, migrate to the same organs as T cells, and rapidly produce IL-4 and IFN-γ upon TCR stimulation. NKT cells play an important role in the rejection of malignant tumors and in the regulation of infections and autoimmune diseases [38]. The percent of NKT cells appears to be enriched in bronchoalveolar lavage fluid, as compared with whole peripheral blood in asthmatic patients [39]. Recent evidence has established a role for NKT cells in the development of allergen-induced AHR by producing IL-4 and IL-13 [40]. Although NKT cells are not required for the development of TH2 cells, the lack of NKT cells or inability of NKT cells to release IL-4 and/or IL-13 could potentially prevent development of AHR [40]. In this regard, TSLP was proposed to increase production of IL-13 by TCR-activated NKT cells based on studies using TSLP transgenic mice [41]. Interestingly, the percent of eosinophils in the lung and level of serum IgE are much higher in TSLP transgenic mice than in wild type animals [11**, 41]. Thus, further studies on the effect of TSLP on the differentiation of eosinophils and maturation of B cells may provide a better understanding of the mechanism(s) of increased airway inflammation in asthma.
Mast cells
The infiltration of mast cells to mucosal gland stroma and airway smooth muscle in asthma subsequently leads to mucous gland hypertrophy, mucus hypersecretion, and smooth muscle dysfunction, which are hallmarks of asthma [3, 42]. Mast cells release numerous mediators, including histamine, enzymes, TNF-α, pro-inflammatory TH2 cytokines and chemokines in response to different stimuli including IgE, cytokines, Toll-like receptor ligands and complement. It also has been established that mast cells not only express high levels of TSLP mRNA [7, 8*], but also they can respond to TSLP [27*]. Immunostaining of a bronchial biopsy of an asthmatic patient has revealed TSLP receptor expression on infiltrating mast cells. Furthermore, stimulation of human mast cells in vitro with IL-1β and TNF-α in the presence of TSLP strongly augments production of pro-inflammatory TH2 cytokines, including IL-5, IL-6, IL-10, and IL-13, as well as chemokines that are involved in allergic diseases. Consistent with this, blocking endogenous TSLP that is released by primary activated human epithelial cells completely inhibits production of IL-13 by mast cells [27*]. This suggests that TSLP may facilitate crosstalk between epithelial cells and mast cells.
Old and new therapeutic strategies
The general approach in the treatment of asthma is to diminish symptoms by using bronchodilators and corticosteroids that can prevent and reduce airway swelling and decrease the amount of mucus in the lungs [1]. However, these medications can have side effects, and tolerance can arise during long-term treatment. Moreover, because each case of asthma is different, treatment needs to be modified for each person. A novel approach for asthma is to develop immunological therapies to prevent its development instead of solely treating symptoms of the disease [1, 43, 44]. Anti-IgE (Omalizumab) is one such treatment [44, 45]. However, whereas IgE binding to FcεRI activates mast cells, anti-IgE treatment alone is not enough to prevent AHR [46]. Another innovative approach is to block TH2 cytokines or their receptors [43]. For example, treatment using humanized monoclonal antibodies to IL-5 reduces eosinophil levels in asthmatic patients [47]. Moreover, inhalation of soluble forms of IL-4 receptor α chain (sIL4-4Rα) in humans and chemically modified IL-4Rα antisense oligonucleotide (IL-4Rα ASO) in mice can diminish asthma symptoms and AHR [48, 49]. Importantly, administration of TSLPR-Fc fusion protein can prevent lung allergic inflammation in a mouse model [10**]. This beneficial response in such models of asthma has suggested that inhibiting TSLP may have potential as a therapeutic approach to asthma in humans.
Conclusion
TSLP is a cytokine of tremendous interest that is implicated as playing a pathophysiologic role in allergic inflammatory disease, including asthma and atopic skin disease. As such, it is a potential target for the modulation of inflammation/allergy. Although highly related to IL-7, including the sharing of a receptor component, TSLP is distinctive in having a greater role in inflammation whereas IL-7 appears to be more important for lymphoid development and survival. Additional data on the biology and signaling pathways of TSLP should provide a more comprehensive understanding of its function in the immune system. More information about the mechanisms controlling TSLP production and the actions of this cytokine will hopefully also allow the design of new therapeutic approaches for the control of pathological immune responses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Corry DB. Emerging immune targets for the therapy of allergic asthma. Nat Rev Drug Discov. 2002;1:55–64. doi: 10.1038/nrd702. [DOI] [PubMed] [Google Scholar]
- 2.Bloemen K, Verstraelen S, Van Den Heuvel R, Witters H, Nelissen I, Schoeters G. The allergic cascade: review of the most important molecules in the asthmatic lung. Immunol Lett. 2007;113:6–18. doi: 10.1016/j.imlet.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Hart PH. Regulation of the inflammatory response in asthma by mast cell products. Immunol Cell Biol. 2001;79:149–153. doi: 10.1046/j.1440-1711.2001.00983.x. [DOI] [PubMed] [Google Scholar]
- 4.Cookson W. The immunogenetics of asthma and eczema: a new focus on the epithelium. Nat Rev Immunol. 2004;4:978–988. doi: 10.1038/nri1500. [DOI] [PubMed] [Google Scholar]
- 5.Message SD, Johnston SL. Host defense function of the airway epithelium in health and disease: clinical background. J Leukoc Biol. 2004;75:5–17. doi: 10.1189/jlb.0703315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kato A, Schleimer RP. Beyond inflammation: airway epithelial cells are at the interface of innate and adaptive immunity. Curr Opin Immunol. 2007 doi: 10.1016/j.coi.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 8*.Ying S, O'Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, Robinson D, Zhang G, Zhao J, Lee TH, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174:8183–8190. doi: 10.4049/jimmunol.174.12.8183. This paper provides evidence of elevated TSLP expression in the bronchial epithelium of asthmatics, as compared to healthy patients. Epithelial cells, endothelial cells, and mast cells show a significant elevation of TSLP mRNA expression in bronchial biopsies of asthmatic patients. [DOI] [PubMed] [Google Scholar]
- 9*.Liu YJ, Soumelis V, Watanabe N, Ito T, Wang YH, Malefyt Rde W, Omori M, Zhou B, Ziegler SF. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. This is an excellent general review on TSLP, including TSLP-induced signaling and the action of this cytokine on dendritic cells. [DOI] [PubMed] [Google Scholar]
- 10**.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005;202:829–839. doi: 10.1084/jem.20050199. The authors demonstrate that TSLP increases CD4+ T cells responses to TCR activation. Moreover, the defect in TSLPR signaling can prevent the development of airway inflammation in mouse model of asthma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11**.Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. This paper illustrates the major role of TSLP in allergic airway inflammation. Introduction of a TSLP transgene into the lungs of animals increases release of TH2 cytokines and augments IgE production. [DOI] [PubMed] [Google Scholar]
- 12.Yoo J, Omori M, Gyarmati D, Zhou B, Aye T, Brewer A, Comeau MR, Campbell DJ, Ziegler SF. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005;202:541–549. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friend SL, Hosier S, Nelson A, Foxworthe D, Williams DE, Farr A. A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp Hematol. 1994;22:321–328. [PubMed] [Google Scholar]
- 14.Sims JE, Williams DE, Morrissey PJ, Garka K, Foxworthe D, Price V, Friend SL, Farr A, Bedell MA, Jenkins NA, et al. Molecular cloning and biological characterization of a novel murine lymphoid growth factor. J Exp Med. 2000;192:671–680. doi: 10.1084/jem.192.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quentmeier H, Drexler HG, Fleckenstein D, Zaborski M, Armstrong A, Sims JE, Lyman SD. Cloning of human thymic stromal lymphopoietin (TSLP) and signaling mechanisms leading to proliferation. Leukemia. 2001;15:1286–1292. doi: 10.1038/sj.leu.2402175. [DOI] [PubMed] [Google Scholar]
- 16.Leonard WJ. Cytokines and immunodeficiency diseases. Nat Rev Immunol. 2001;1:200–208. doi: 10.1038/35105066. [DOI] [PubMed] [Google Scholar]
- 17.Park LS, Martin U, Garka K, Gliniak B, Di Santo JP, Muller W, Largaespada DA, Copeland NG, Jenkins NA, Farr AG, et al. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med. 2000;192:659–670. doi: 10.1084/jem.192.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pandey A, Ozaki K, Baumann H, Levin SD, Puel A, Farr AG, Ziegler SF, Leonard WJ, Lodish HF. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol. 2000;1:59–64. doi: 10.1038/76923. [DOI] [PubMed] [Google Scholar]
- 19.Isaksen DE, Baumann H, Trobridge PA, Farr AG, Levin SD, Ziegler SF. Requirement for stat5 in thymic stromal lymphopoietin-mediated signal transduction. J Immunol. 1999;163:5971–5977. [PubMed] [Google Scholar]
- 20.Isaksen DE, Baumann H, Zhou B, Nivollet S, Farr AG, Levin SD, Ziegler SF. Uncoupling of proliferation and Stat5 activation in thymic stromal lymphopoietin-mediated signal transduction. J Immunol. 2002;168:3288–3294. doi: 10.4049/jimmunol.168.7.3288. [DOI] [PubMed] [Google Scholar]
- 21.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2007 doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li M, Hener P, Zhang Z, Kato S, Metzger D, Chambon P. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci U S A. 2006;103:11736–11741. doi: 10.1073/pnas.0604575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23*.Lee HC, Ziegler SF. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFkappaB. Proc Natl Acad Sci U S A. 2007;104:914–919. doi: 10.1073/pnas.0607305104. Different inflammatory stimuli, such as pro-inflammatory cytokines and Toll-like receptors ligands, can promote expression of TSLP through induction of the NF-κB signaling pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bogiatzi SI, Fernandez I, Bichet JC, Marloie-Provost MA, Volpe E, Sastre X, Soumelis V. Cutting Edge: Proinflammatory and Th2 cytokines synergize to induce thymic stromal lymphopoietin production by human skin keratinocytes. J Immunol. 2007;178:3373–3377. doi: 10.4049/jimmunol.178.6.3373. [DOI] [PubMed] [Google Scholar]
- 25.Kato A, Favoreto S, Jr, Avila PC, Schleimer RP. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol. 2007;179:1080–1087. doi: 10.4049/jimmunol.179.2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaph C, Troy AE, Taylor BC, Berman-Booty LD, Guild KJ, Du Y, Yost EA, Gruber AD, May MJ, Greten FR, et al. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446:552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 27*.Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, Paquette N, Ziegler SF, Sarfati M, Delespesse G. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204:253–258. doi: 10.1084/jem.20062211. This paper demonstrates synergistic effects of TSLP with IL-1 and TNF-α on human mast cells, which produce high levels of pro-allergic cytokines and chemokines in the presence of TSLP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papadopoulos NG, Xepapadaki P, Mallia P, Brusselle G, Watelet JB, Xatzipsalti M, Foteinos G, van Drunen CM, Fokkens WJ, D'Ambrosio C, et al. Mechanisms of virus-induced asthma exacerbations: state-of-the-art. A GA2LEN and InterAirways document. Allergy. 2007;62:457–470. doi: 10.1111/j.1398-9995.2007.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osborn MJ, Ryan PL, Kirchhof N, Panoskaltsis-Mortari A, Mortari F, Tudor KS. Overexpression of murine TSLP impairs lymphopoiesis and myelopoiesis. Blood. 2004;103:843–851. doi: 10.1182/blood-2003-05-1557. [DOI] [PubMed] [Google Scholar]
- 30.Chappaz S, Flueck L, Farr AG, Rolink AG, Finke D. Increased TSLP availability restores T- and B-cell compartments in adult IL-7 deficient mice. Blood. 2007;110:3862–3870. doi: 10.1182/blood-2007-02-074245. [DOI] [PubMed] [Google Scholar]
- 31.Puel A, Leonard WJ. Mutations in the gene for the IL-7 receptor result in T(−)B(+)NK(+) severe combined immunodeficiency disease. Curr Opin Immunol. 2000;12:468–473. doi: 10.1016/s0952-7915(00)00122-9. [DOI] [PubMed] [Google Scholar]
- 32.Giliani S, Mori L, de Saint Basile G, Le Deist F, Rodriguez-Perez C, Forino C, Mazzolari E, Dupuis S, Elhasid R, Kessel A, et al. Interleukin-7 receptor alpha (IL-7Ralpha) deficiency: cellular and molecular bases. Analysis of clinical, immunological, and molecular features in 16 novel patients. Immunol Rev. 2005;203:110–126. doi: 10.1111/j.0105-2896.2005.00234.x. [DOI] [PubMed] [Google Scholar]
- 33.Al-Shami A, Spolski R, Kelly J, Fry T, Schwartzberg PL, Pandey A, Mackall CL, Leonard WJ. A role for thymic stromal lymphopoietin in CD4(+) T cell development. J Exp Med. 2004;200:159–168. doi: 10.1084/jem.20031975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reche PA, Soumelis V, Gorman DM, Clifford T, Liu M, Travis M, Zurawski SM, Johnston J, Liu YJ, Spits H, et al. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J Immunol. 2001;167:336–343. doi: 10.4049/jimmunol.167.1.336. [DOI] [PubMed] [Google Scholar]
- 35.Gilliet M, Soumelis V, Watanabe N, Hanabuchi S, Antonenko S, de Waal-Malefyt R, Liu YJ. Human dendritic cells activated by TSLP and CD40L induce proallergic cytotoxic T cells. J Exp Med. 2003;197:1059–1063. doi: 10.1084/jem.20030240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36**.Rochman I, Watanabe N, Arima K, Liu YJ, Leonard WJ. Cutting edge: direct action of thymic stromal lymphopoietin on activated human CD4+ T cells. J Immunol. 2007;178:6720–6724. doi: 10.4049/jimmunol.178.11.6720. This paper resolves a major controversy by demonstrating that human pre-activated CD4+ T cells, like mouse activated T cells, express functional TSLP receptors, indicating that there is not a major species difference in the actions of TSLP in humans and mice, in contrast to the prior dogma in the field. TSLP rapidly activates Stat5 in these cells, inducing IL-2 receptor expression and augmenting their sensitivity to IL-2. TSLP also increases TCR-dependent proliferation of human CD4+ T cells. [DOI] [PubMed] [Google Scholar]
- 37.Omori M, Ziegler S. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J Immunol. 2007;178:1396–1404. doi: 10.4049/jimmunol.178.3.1396. [DOI] [PubMed] [Google Scholar]
- 38.Godfrey DI, Hammond KJ, Poulton LD, Smyth MJ, Baxter AG. NKT cells: facts, functions and fallacies. Immunol Today. 2000;21:573–583. doi: 10.1016/s0167-5699(00)01735-7. [DOI] [PubMed] [Google Scholar]
- 39.Thomas SY, Banerji A, Medoff BD, Lilly CM, Luster AD. Multiple chemokine receptors, including CCR6 and CXCR3, regulate antigen-induced T cell homing to the human asthmatic airway. J Immunol. 2007;179:1901–1912. doi: 10.4049/jimmunol.179.3.1901. [DOI] [PubMed] [Google Scholar]
- 40.Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9:582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 41.Nagata Y, Kamijuku H, Taniguchi M, Ziegler S, Seino K. Differential role of thymic stromal lymphopoietin in the induction of airway hyperreactivity and Th2 immune response in antigen-induced asthma with respect to natural killer T cell function. Int Arch Allergy Immunol. 2007;144:305–314. doi: 10.1159/000106319. [DOI] [PubMed] [Google Scholar]
- 42.Bradding P, Walls AF, Holgate ST. The role of the mast cell in the pathophysiology of asthma. J Allergy Clin Immunol. 2006;117:1277–1284. doi: 10.1016/j.jaci.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 43.Lewis DB. Allergy immunotherapy and inhibition of Th2 immune responses: a sufficient strategy? Curr Opin Immunol. 2002;14:644–651. doi: 10.1016/s0952-7915(02)00388-6. [DOI] [PubMed] [Google Scholar]
- 44.Tarantini F, Baiardini I, Passalacqua G, Braido F, Canonica GW. Asthma treatment: 'magic bullets which seek their own targets'. Allergy. 2007;62:605–610. doi: 10.1111/j.1398-9995.2007.01390.x. [DOI] [PubMed] [Google Scholar]
- 45.Kuhn R. Immunoglobulin E blockade in the treatment of asthma. Pharmacotherapy. 2007;27:1412–1424. doi: 10.1592/phco.27.10.1412. [DOI] [PubMed] [Google Scholar]
- 46.Djukanovic R, Wilson SJ, Kraft M, Jarjour NN, Steel M, Chung KF, Bao W, Fowler-Taylor A, Matthews J, Busse WW, et al. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am J Respir Crit Care Med. 2004;170:583–593. doi: 10.1164/rccm.200312-1651OC. [DOI] [PubMed] [Google Scholar]
- 47.Leckie MJ, ten Brinke A, Khan J, Diamant Z, O'Connor BJ, Walls CM, Mathur AK, Cowley HC, Chung KF, Djukanovic R, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356:2144–2148. doi: 10.1016/s0140-6736(00)03496-6. [DOI] [PubMed] [Google Scholar]
- 48.Borish LC, Nelson HS, Corren J, Bensch G, Busse WW, Whitmore JB, Agosti JM. Efficacy of soluble IL-4 receptor for the treatment of adults with asthma. J Allergy Clin Immunol. 2001;107:963–970. doi: 10.1067/mai.2001.115624. [DOI] [PubMed] [Google Scholar]
- 49.Karras JG, Crosby JR, Guha M, Tung D, Miller DA, Gaarde WA, Geary RS, Monia BP, Gregory SA. Anti-inflammatory activity of inhaled IL-4 receptor-alpha antisense oligonucleotide in mice. Am J Respir Cell Mol Biol. 2007;36:276–285. doi: 10.1165/rcmb.2005-0456OC. [DOI] [PubMed] [Google Scholar]