Abstract
Exercise training (ET) and hormone replacement therapy (HRT) are both recognized influences on insulin action, but the influence of HRT on responses to ET has not been examined. In order to determine if HRT use provided additive benefits for the response of insulin action to ET, we evaluated the impact of HRT use on changes in insulin during the course of a randomized, controlled, aerobic ET intervention. Subjects at baseline were sedentary, dyslipidemic, and overweight. These individuals were randomized to six months of one of three aerobic ET interventions or continued physical inactivity. In 206 subjects, an insulin sensitivity index (SI) was obtained with a frequently sampled intravenous glucose tolerance test pre- and post-ET. Baseline and post-intervention fitness, regional adiposity, general adiposity, skeletal muscle biochemistry and histology, and serum lipoproteins were measured as other putative mediators influencing insulin action. Two-way analyses of variance were used to determine if gender or HRT use influenced responses to exercise training. Linear modeling was used to determine if predictors for response in SI differed by gender or HRT use. Women who used HRT (HRT+) demonstrated significantly greater improvements in SI with ET than women not using HRT (HRT−). In those HRT+ women, plasma triglyceride change best correlated with change in SI. For HRT− women, capillary density change, and for men, subcutaneous adiposity change, best correlated with change in SI. In summary, in an ET intervention, HRT use appears associated with more robust responses in insulin action. Also, relationships between ET-induced changes in insulin action and potential mediators of change in insulin action are different for men, and for women on or off HRT. These findings have implications for the relative utility of ET for improving insulin action in middle-aged men and women, particularly in the setting of differences in HRT use.
Keywords: exercise training, insulin action, hormone replacement therapy, gender, subcutaneous adiposity
With the increasing prevalence of obesity and type 2 diabetes, efforts are focused on understanding the underlying pathogenesis of insulin resistance and how to improve insulin action with lifestyle efforts[1]. It is well recognized that physical activity improves insulin action, reduces adiposity, enhances cardiorespiratory fitness, and induces a favorable lipoprotein particle distribution[2–5]. Also, exercise training has been found to alter a number of skeletal muscle parameters that may impact insulin action and glucose disposal[6–8].
In addition to exercise training, in post-menopausal women, HRT use can improve insulin action as well as adiposity, LDL and HDL cholesterol, and lean body mass[9] despite paradoxical, yet clear evidence that chronic HRT use promotes adverse cardiovascular events[10–12]. Previously, we reported observations regarding the impact of specific exercise prescription effects on insulin sensitivity[2], where improvements with aerobic training were most dependent on the time spent training rather than the intensity of training in men and post-menopausal women. Here, in the context of a randomized controlled aerobic exercise intervention, STRRIDE (Studies of Targeted Risk Reduction Through Defined Exercise), our objective was to determine the impact of HRT use on insulin action response to exercise training. A secondary objective was to determine if physiologic characteristics unique to each group might account for any gender or HRT use differences in response in insulin action. Given that both exercise training and HRT use are associated with improvements in insulin action, we hypothesized that the combination of both would produce more robust responses in insulin sensitivity.
Research Design and Methods
Study Design
Detailed descriptions of the STRRIDE design, including subject recruitment, randomization, exercise training, and outcome variable measurements have been published elsewhere[13]. Relevant institutional review boards approved the research protocol, and informed consent was obtained from all subjects.
Recruitment
Subjects were recruited with newspaper advertisements and through a network of communication in the institutions involved. Subjects were nominally reimbursed for participation in data collection, but the main inducement for participation was the opportunity to participate in exercise training at no cost.
Subjects
Inclusion criteria were physical inactivity (not participating in regular exercise), overweight to mild obesity (BMI 25–35 kg/m2), dyslipidemia (LDL-cholesterol of 130 to 190 mg/dl or HDL-cholesterol < 40 mg/dl for men and < 45 mg/dl for women), and age between 40 and 69. All women were post-menopausal with verification by subject confirmation of no menses in the six months prior to study enrollment. Self-reported HRT use was assessed at study enrollment, but details of HRT routes and doses were not collected. To avoid effects of medication changes, those using HRT at enrollment were confirmed to have been doing do for at least six months, and those not using were asked to not begin during the study period. Subjects were excluded if they had diabetes mellitus (fasting glucose > 140 mg/dl), hypertension (blood pressure > 160/90 mmHg), known cardiovascular disease, or a musculoskeletal condition that would prohibit exercise training. Additional, exclusion criteria included cigarette smoking, the use of medications known to affect carbohydrate or lipid metabolism (including insulin, oral anti-diabetic agents, HMG CoA reductase inhibitors or statins, fibric acid derivatives, bile acid sequestrants, and nicotinic acid), and participation in a dietary regimen designed to induce weight loss.
Exercise Training
Subjects were randomized to six months of continued inactivity or one of three aerobic exercise groups: a) low-amount-moderate-intensity (caloric equivalent of approximately 12 miles/week or 1200 kcal/week at 40 to 55% peak VO2), b) low-amount-vigorous-intensity (caloric equivalent of approximately 12 miles/week or 1200 kcal/week at 65 to 80% peak VO2), or c) high-amount-vigorous-intensity (caloric equivalent of approximately 20 miles/week or 2000 kcal/week at 65 to 80% peak VO2). Subjects randomized to exercise completed a two to three month ramp period in order to minimize musculoskeletal injuries prior to the six months of prescribed training. Subjects were counseled to maintain and not alter dietary intake throughout the study. Dietary stability was confirmed with three-day food records and 24-hour dietary recall interviews performed at baseline, during the intervention, and at the conclusion of the study. Subject flow through STRRIDE and a detailed analysis of response in insulin sensitivity by exercise training group have been previously published[2, 14]. The original analysis of the effect of exercise volume and intensity on insulin sensitivity was performed prior to the conclusion of the study but once the accrued sample size was felt sufficient (n=154) for the outcome of interest. Here, our primary analysis of interest was to identify the impact of HRT use on change in insulin action with exercise training, and we included in these analyses all subjects who completed six months of exercise training or inactivity and had insulin action measurements at baseline and after completion of training (n=206).
Fitness, body composition, lipid, skeletal muscle, and insulin action
Each of these were evaluated at baseline (prior to ramp period) and after six months of inactivity or prescribed exercise training in each subject. Exercise treadmill testing was used to assess cardiorespiratory fitness. Adiposity and body composition measurements were performed as previously described[2, 13] – percent body fat was assessed as the sum of skin fold caliper measurements; visceral adiposity (VAT) and subcutaneous adiposity (SAT) were measured with a single slice abdominal CT scan at the level of the L4 vertebra[13, 15]. Lipoprotein analyses were performed via nuclear magnetic resonance spectroscopy using fasted plasma samples (LipoScience, Cary, NC)[3].
Skeletal muscle biopsies were obtained from the vastus lateralis using a percutaneous needle sampling technique.[16] Immunohistochemical techniques were used to determine skeletal muscle capillary density by counting anti-CD31 stained endothelial cells in a minimum of three 100X magnification fields and a minimum of 100 muscle fibers as previously described[17]. Capillary density was expressed as capillaries per mm2 and capillaries per muscle fiber. Skeletal muscle fiber area was also measured on a minimum of 100 fibers per sample. Mitochondrial derived citrate synthase activity was determined with a fluorescent based enzymatic assay using homogenized skeletal muscle[18].
Insulin action was assessed as previously described[2]. A three hour frequently sampled intravenous glucose tolerance test (IVGTT)[19] was performed at baseline and 16 to 24 hours after the final exercise bout. After establishing intravenous access, fasting samples were obtained and then glucose (50% at 0.3 g/kg body mass) was infused. At minute 20, insulin (0.025 U/kg body mass) was injected. Blood samples were collected at minutes 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 19, 22, 25, 30, 40, 50, 60, 70, 80, 90, 100, 120, 140, 160, and 180. After centrifugation, plasma was stored at −80°C until measurements of insulin and glucose were performed. Insulin was measured by immunoassay (Access Immunoassay System, Beckman Coulter, Fullerton, CA) and glucose with an oxidation reaction (YSI Model 2300 Stat Plus, Yellow Springs Instruments, Yellow Springs, OH). Using the minimal model of Bergman[19], an insulin sensitivity index (SI) was calculated and reflects the ability of insulin to promote glucose disposal and suppress glucose secretion. Normal values for SI have been reported as 2.62 ± 2.21 mU/L/min, and values below this normal range indicate insulin resistance[20].
Data Analysis
The main objective of this analysis was to identify whether gender and HRT use impact exercise training-induced responses in insulin action. Variables not approximating a normal distribution were logarithmically transformed prior to statistical analysis. Change scores were created for each variable by subtracting the baseline value from the post-training value. Analyses including HRT use incorporated a categorical variable with identifiers for each of women using HRT (HRT+), women not using HRT (HRT−), and men. Gender and HRT use differences in insulin action were assessed with analyses of variance followed by Tukey’s adjustment for multiple comparisons.
Our secondary objective was to determine whether potential mediators of exercise training-induced responses differ with respect to gender and HRT use. Linear modeling was preceded by a variable reduction step whereby we a priori defined each of the following domains of potential mediators: gender/HRT use, cardiorespiratory fitness, regional adiposity, general adiposity, lipids, and skeletal muscle. Within each of these domains, the variable with the largest significant correlation coefficient in the relationship with change in SI was selected as the representative variable for that domain. Next, these six variables were used in linear modeling. Given a concern for interactions between gender or HRT use and each of the potential mediators, a full model containing each of the intermediates and each HRT use*intermediate interaction term was fit. If none of the gender interaction terms was found significant, these terms were removed, and the main effects model containing each of the six potential mediators was refit. Final models were validated on 100 bootstrap replicates of the original sample to investigate the robustness of the model and to address concerns about possible overfitting.
Statistics were performed using SAS Enterprise Guide v8.2 (Cary, NC). Prior to analysis, we established statistical significance as P<0.05 for all main effects and P<0.10 for interaction terms, which require more power for detection.
Results
Baseline demographic and metabolic characteristics of this study population have been reported previously[2, 3, 5], and there were no statistically significant differences in demographic or baseline metabolic characteristics between exercise groups. Briefly, 67% of subjects completed the investigation. Of these, 80% of participants were Caucasian, 17% were African-American, 2% were Asian, and 1% was Hispanic. At baseline, there were no statistically significant differences in metabolic characteristics between HRT+ and HRT− women (Table 1). In contrast, men differed from both HRT+ and HRT− women in that they were slightly younger, with greater cardiorespiratory fitness, and greater lean body mass, but were less insulin sensitive, more dyslipidemic and had increased amounts of VAT (Table 1). Additionally, relative to men, HRT− women had slightly lower BMIs and capillary density.
Table 1.
Baseline Demographic and Metabolic Characteristics*
| Men n=113 | Women HRT − n=47 | Women HRT + n=46 | ||
|---|---|---|---|---|
| Demographics | Age | 50.7±6.9† | 54.7±5.3 | 53.7±5.6 |
|
| ||||
| Exercise training | Adherence (%) | 88.2±14.6 | 86.4±10.2 | 86.4±16.0 |
|
| ||||
| Insulin Sensitivity (SI) (mU/L/min) | 2.31±2.00† | 3.25±1.95 | 3.28±1.69 | |
|
| ||||
| Cardiorespiratory fitness | ||||
| Time to exhaustion (sec) | 868±167† | 644±179 | 615±140 | |
| Absolute Peak VO2 (ml/min) | 2.98±0.52† | 1.90±0.27 | 1.78±0.24 | |
| Relative Peak VO2 (ml/kg/min) | 31.4±4.9† | 23.7±3.7 | 23.3±3.6 | |
|
| ||||
| Regional adiposity | ||||
| Visceral adiposity (cm2) | 193.6±64.9† | 139.7±53.4 | 126.7±53.9 | |
| Subcutaneous adiposity (cm2) | 284.8±93.7 | 321.8±105.7 | 302.0±100.2 | |
| Minimal waist (cm) | 101.5±7.3† | 91.3±8.9 | 87.7±7.2 | |
| Umbilical waist (cm) | 106.2±7.9† | 102.0±11.4 | 98.7±9.5 | |
|
| ||||
| General adiposity | ||||
| Body mass index (kg/m2) | 30.1±2.8 | 30.2±3.2 | 28.8±2.9** | |
| Lean body mass (kg) | 67.3±7.8† | 49.7±6.1 | 47.9±6.3 | |
|
| ||||
| Serum lipoproteins | ||||
| LDL particle concentration (nmol/L) | 1431±1 | 1315±1 | 1371±1 | |
| LDL particle size (nm) | 20.4±1.0† | 21.2±1.1 | 21.2±1.0 | |
| HDL- cholesterol (mmol/L) | 37.4±1.3† | 48.3±1.3 | 54.7±1.3 | |
| Triglyceride (mmol/L) | 158.2±1.7† | 112.3±1.6 | 114.4±1.6 | |
|
| ||||
| Skeletal muscle parameter | ||||
| Capillary density (endothelial cells:fiber) | 1.63±1.26 | 1.52±1.19 | 1.41±1.17** | |
| Capillary density (#/mm2) | 345.0±1.1 | 319.7±1.2 | 298.0±1.2** | |
| Fiber area (μm2) | 4557.5±1.1† | 3707.0±1.1 | 3539.9±1.1 | |
| Citrate synthase activity (μmol/min/μg) | 0.20±1.9 | 0.17±2.5 | 0.21±1.8 | |
Means ± standard deviations presented; for those without normal distributions, geometric means and standard deviations are presented.
Exercise training adherence = Completed/prescribed miles per week*100
Differs from other groups with Tukey’s post-hoc testing p<0.05
Differs from men with Tukey’s post-hoc testing p<0.05
Our primary objective was to determine whether HRT use influenced the response in SI with exercise training. While no gender difference in SI response was noted (Figure 1A, P <0.71), independent of group assignment, HRT+ women improved SI more than did HRT− women (Figure 1B, P <0.003). We observed no significant group*HRT use interaction (P<0.96); however, when exercise groups were analyzed separately, HRT− women demonstrated greater improvements in SI with low-amount-moderate-intensity exercise training than with low-amount-vigorous intensity training (P<0.04) or inactivity (P<0.007).
Figure 1. Gender and Hormone Replacement Therapy (HRT) Use and Exercise Training Responses in Insulin Sensitivity.
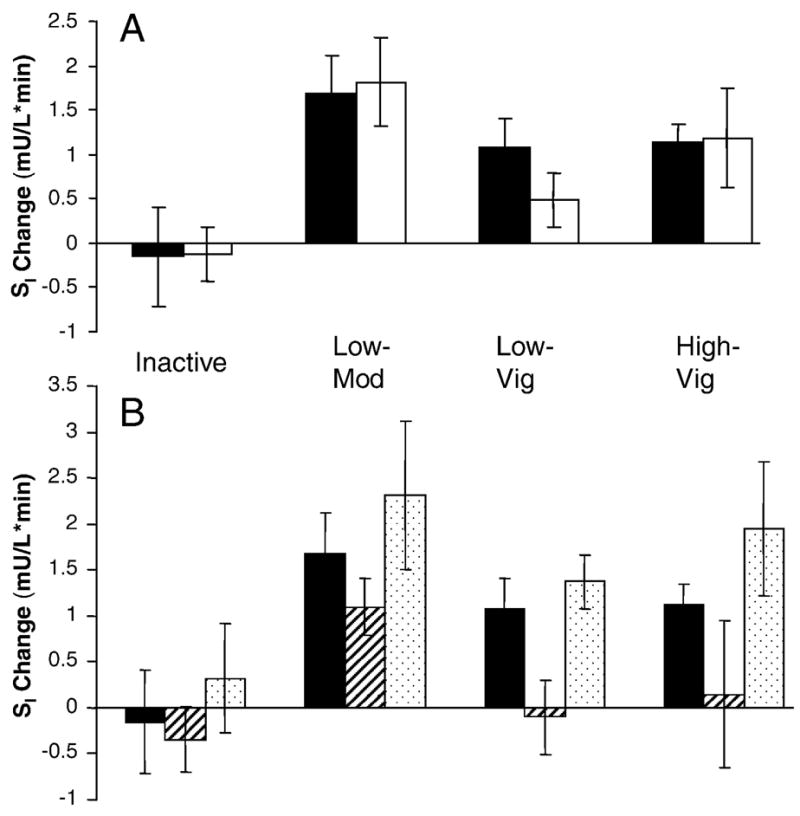
Subjects (n=206) were randomized for six months to inactivity (nmen= 12, nwomen HRT−=10; nwomen HRT+=5) or one of three aerobic exercise training groups: Low-Amount Moderate Intensity (Low-Mod; nmen= 30, nwomen HRT−=11; nwomen HRT+=16); Low Amount-Vigorous Intensity (Low-Vig; nmen= 33, nwomen HRT−=15; nwomen HRT+=10); High-Amount Vigorous Intensity (High-Vig; nmen= 38, nwomen HRT−=11; nwomen HRT+=15)
A. Change in insulin sensitivity by group and gender was assessed with a two-way analysis of variance (P<0.71). Black bars depict men, and white bars depict women (HRT− and HRT+ combined).
B. Change in insulin sensitivity by group and hormone therapy use was assessed with a two-way analysis of variance. Black bars depict men; white bars with diagonal lines depict HRT− women; and white bars with dots depict HRT+ women. Both group (P<0.005) and HRT use (P<0.005) were independently related to change in insulin sensitivity. Error bars represent standard error.
Given the previous finding, our secondary aim was to identify whether potential mediators of response in insulin action would differ by gender and/or HRT use. We evaluated the relationships between the change in SI other variables felt likely to influence response in SI (Table 2). Using the variable reduction strategy and linear modeling described above, we observed that when considered together gender/HRT use, SAT change, body mass change, serum triglyceride change, and skeletal muscle capillary density (endothelial cells per fiber) change, and cardiorespiratory fitness (relative peak VO2) change explained 18% of the variance in the change in SI (P<0.005). By including gender/HRT interaction terms, the amount of variance in change in SI explained by these potential mediators increased from 18% to 35% (P<0.0001). In this model, HRT use*triglyceride change (P<0.03), HRT use*SAT change (P<0.03), and HRT use*capillary density change (P<0.07) were each independently related to change in SI. In the 100 bootstrap replicates of the sample, the mean ± standard deviation R2 for this model was 0.42 ± 0.10. The frequency of each of the interaction terms as independently related to change in SI in the bootstrap procedure was as follows: HRT*SAT change 69%; HRT*capillary density change 63%; HRT*triglyceride change 66%.
Table 2.
Correlation Coefficients for Change in Insulin Sensitivity (SI [mU/L/min])
| Variable correlated with SI change | All subjects n=206 | Men n=113 | Women HRT − n=47 | Women HRT + n=46 | |
|---|---|---|---|---|---|
| Baseline variables | |||||
| HDL-cholesterol baseline (log) (mmol/L) | 0.05 | 0.24* | −0.18 | −0.17 | |
| SI baseline (log) (mU/L/min) | −0.12 | 0.03 | −0.59† | −0.13 | |
|
| |||||
| Cardiorespiratory fitness change | |||||
| Time to exhaustion change (sec) | 0.03 | 0.08 | −0.10 | −0.05 | |
| VO2max change (ml/min) | 0.09 | 0.15 | −0.05 | −0.04 | |
| rVO2max change (ml/kg/min) | 0.09 | 0.15 | −0.06 | −0.04 | |
|
| |||||
| Regional adiposity change | |||||
| Visceral adiposity change (cm2) | −0.13 | −0.10 | 0.04 | −0.27 | |
| Subcutaneous adiposity change (cm2) | −0.06 | −0.32† | −0.07 | 0.18 | |
| Minimal waist change (cm) | −0.10 | −0.28* | −0.17 | 0.15 | |
| Umbilical waist change (cm) | −0.10 | −0.29* | −0.07 | 0.10 | |
|
| |||||
| General adiposity change | |||||
| Mass change (kg) | −0.18* | −0.24* | 0.03 | −0.10 | |
| Lean body mass change (kg) | −0.02 | −0.08 | 0.03 | 0.03 | |
|
| |||||
| Serum lipoprotein change | |||||
| LDL particle concentration change (nmol/L) | −0.14 | −0.04 | −0.08 | −0.35* | |
| LDL particle size change (nm) | 0.18* | 0.13 | 0.17 | 0.25 | |
| HDL-cholesterol change (mmol/L) | 0.06 | 0.10 | 0.17 | −0.008 | |
| Triglyceride change (mmol/L) | −0.22† | −0.14 | −0.17 | −0.52† | |
|
| |||||
| Skeletal muscle parameter change | |||||
| Capillary density change (endothelial cells:fiber) | 0.09 | 0.01 | 0.49† | 0.05 | |
| Capillary density change (#/mm2) | 0.11 | 0.02 | 0.44* | 0.09 | |
| Fiber area change (μm2) | 0.01 | 0.06 | −0.11 | 0.02 | |
| Citrate synthase activity change (μmol/min/μg) | 0.01 | −0.02 | −0.18 | 0.10 | |
Data are presented as correlation coefficients (r) for the univariate relationship between
the indicated variable and insulin sensitivity change.
P<0.05
P<0.005
The above analyses indicate that HRT use and/or gender affect the relationship between the change in SI and triglyceride change, capillary density change, and SAT change. In other words, the relationships between change in SI and each of triglyceride change, capillary density change, and SAT change were different depending on HRT use and gender. In order to illustrate how HRT use and gender modified these effects, we depicted these relationships as scatter plots that contain unique identifiers for men, HRT+ and HRT− women (Figure 2). As shown in Figure 2A, SI change related to triglyceride change for HRT+ women but not for HRT− women or men. For HRT− women, SI change related to capillary density, but no relationship was evident for HRT+ women or for men. The relationship between SI change and SAT change was limited to men; no relationship was present for women, irrespective of HRT use. These results were verified with linear modeling performed separately for each gender. For women, we were able to explain 40% of the variance in change in SI using HRT use, triglyceride change and capillary density change. In contrast, for men, SAT change, the strongest single potential mediator in men, explained only 10% of the variance in change in SI.
Figure 2. Relationships for Insulin Sensitivity Change by Gender and Hormone Replacement Therapy (HRT) Use.
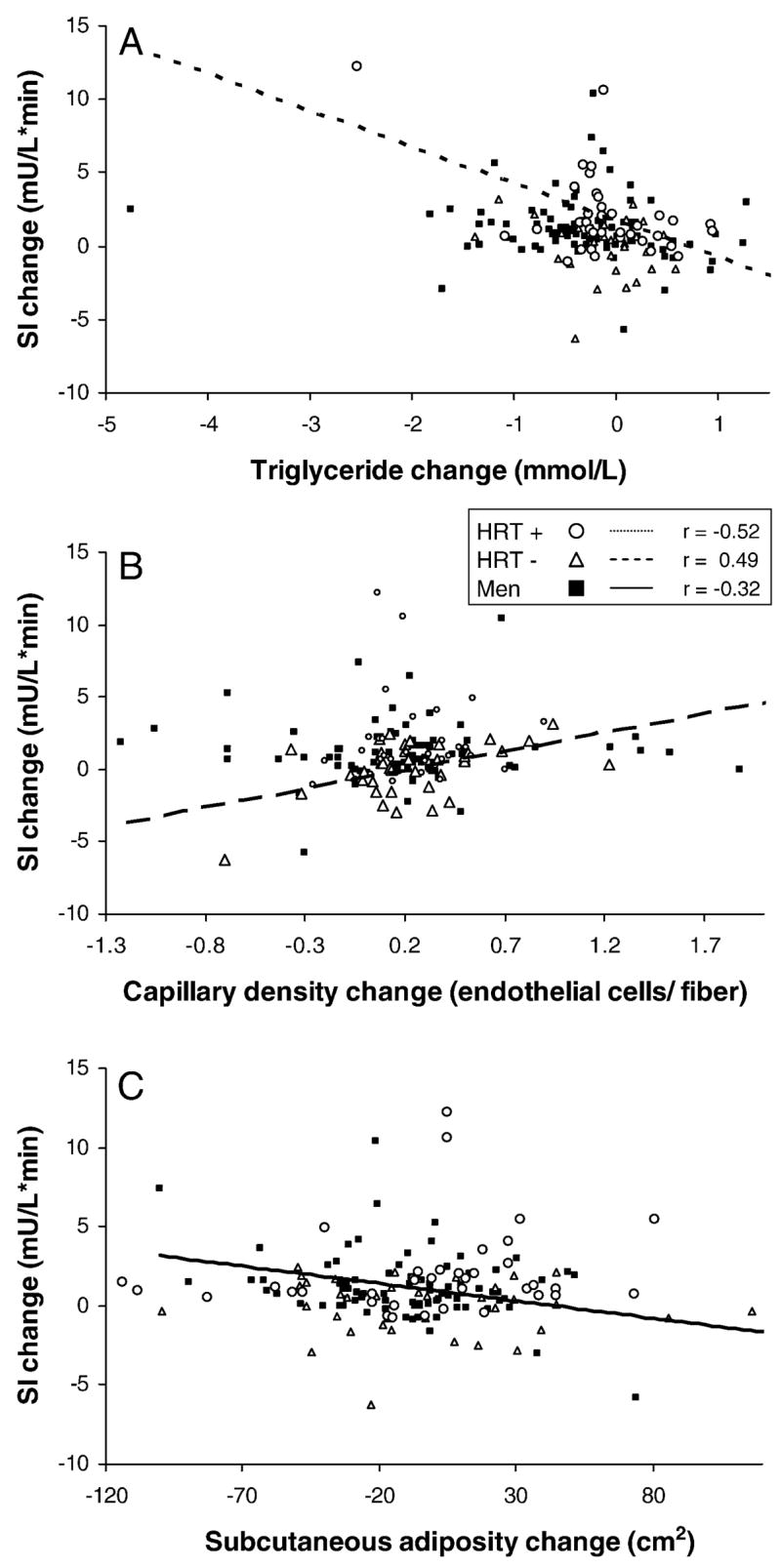
Standard correlations were performed for men (n=113), HRT− women (n=47), and HRT+ women (n=46). Men are identified with solid squares and a solid regression line. HRT− women are depicted with open triangles and a heavy dashed regression line. HRT+ women are depicted with open circles and a light dashed regression line. Regression lines are illustrated for only those relationships found significant (P<0.05).
A. Triglyceride change is related to change in insulin sensitivity change for HRT+ women (P<0.05).
B. Capillary density change is related to change in insulin sensitivity for HRT− women (P<0.05).
C. Subcutaneous adiposity change is related to change in insulin sensitivity for men (P<0.05).
Discussion
Here, in a group of sedentary, dyslipidemic, overweight to mildly obese, middle-aged men and women, we observed that the response of insulin sensitivity to exercise training differed for men, post-menopausal women using HRT, and post-menopausal women not using HRT. Most remarkably, we observed much more robust improvements in insulin sensitivity for women using HRT than those not using HRT.
To our knowledge, this is the first report examining the effect of HRT use on insulin sensitivity responses to a randomized controlled exercise intervention. The observation that post-menopausal HRT+ women show significantly greater improvements in insulin sensitivity with exercise training than do post-menopausal HRT− women has important implications for scientific investigation and clinical care. Our observation highlights the need for careful attention to exogenous hormone use in investigations relating exercise training and responses in insulin action. Alternatively, our findings suggest that in investigations relating HRT use and surrogates for cardiovascular risk, physical activity needs to be carefully assessed.
Since the increased risks of cardiovascular disease, stroke, and breast cancer with the use of exogenous estrogen/progesterone combinations in women were revealed, there has been a substantial reduction in HRT use[10–12]. For the majority of post-menopausal women not currently using HRT, our observations suggest that an exercise prescription designed to improve insulin sensitivity, such as those in the present study, might be optimized by following a low-amount-moderate intensity regimen rather than a regimen of higher intensity but with less time spent exercising. Moreover, our observations represent yet another example of the paradox between the potentially beneficial effects of HRT on metabolic cardiovascular risk markers in women, but the well established detrimental effects of HRT use on hard cardiovascular outcomes in post menopausal women.
Estrogen has been shown to exhibit multiple effects which may drive enhanced improvements in insulin sensitivity with exercise training. Since insulin sensitivity change related to triglyceride change for HRT+ but not for HRT− women, it is possible that at least a proportion disparity in change in the insulin sensitivity for HRT users and nonusers results from the first pass hepatic effects of oral HRT on triglyceride concentrations. Interestingly, in HRT+ women, triglyceride concentrations have been found to both decrease[21, 22] and increase[23] with exercise training in previous studies. The discordant findings among these studies is likely related to significant differences in exercise training protocols, doses and routes of HRT among these investigations[23], and genetic determinants. Additionally, HRT-driven exercise-induced insulin sensitivity changes may be due to effects of estrogen metabolites on skeletal muscle glucose uptake signaling pathway component such as AMP kinase[24], Akt[24, 25], and insulin receptors[26].
For HRT− women, capillary density change was the potential mediator related most strongly with insulin sensitivity change. Since capillaries deliver insulin and glucose to skeletal muscle cells and skeletal muscle is the principal site for glucose uptake[27], it is physiologically relevant that changes in capillary density were indicative of insulin sensitivity response. In contrast, we were perplexed to find that this potential mediator was not related to insulin sensitivity change in HRT+ women or men.
Central adiposity changes were best related to change in insulin sensitivity for men. Of these, SAT change, specifically, abdominal SAT change, demonstrated the best relationship with insulin sensitivity change. Given the recent emphasis on intra-abdominal VAT, we found it notable that SAT change was related more strongly than VAT change to the insulin sensitivity response to exercise training. While we believe that VAT is an important and metabolically active reservoir, our data and those of others[28, 29] remind us that SAT is also a very important contributor to metabolic health, particularly in men. The superior relationship between SAT and insulin sensitivity may be attributed to the contribution of SAT to a larger proportion of total central adiposity[29, 30], but may also reflect the critical importance of the saturation of SAT, both the superficial and deep reservoirs, as a mediator of insulin resistance. Further, this observation emphasizes the utility of surrogate measures, such as waist circumference, as an indication of metabolically important central adiposity stores that can be easily implemented in the clinic and used to monitor response to exercise training, at least in men.
One recognized weakness of this work is that HRT use was not blinded or placebo controlled. Additionally, we have limited information about the doses, routes and duration of HRT use in these individuals. However, given the current controversy surrounding HRT use, it is unlikely that an intervention optimally designed to answer this question will be feasible. Nonetheless, our observations strongly imply that physiologic responses to exercise differ with respect to gender and HRT use. Additionally, in this investigation, we cannot account for genetic determinants of responses to HRT and exercise training. Future attempts to dissect mechanisms of alterations in insulin action with exercise training should consider genetic contributions and address such differences by gender and estrogen status.
In sum, in this investigation of middle aged, moderately overweight to obese men and women, we observed that women using HRT improve insulin sensitivity with exercise training more robustly than do women not using HRT. In fact, only moderate intensity exercise led to improvements in insulin sensitivity in women not using HRT. We observed that predictors for exercise-induced responses in insulin sensitivity were different for men (subcutaneous central adiposity), for women not using HRT (skeletal muscle capillary density), and for women using HRT (serum triglycerides). These observations represent yet another example of the paradox between the potentially beneficial effects of HRT on metabolic cardiovascular risk markers in women and the well established detrimental effects of HRT use on hard cardiovascular outcomes in post menopausal women. All of these observations command attention when generating exercise training prescriptions for sedentary middle-aged to elderly men and women when anticipating improvements in insulin action and a delay in development of overt type 2 diabetes.
Acknowledgments
We thank the rest of the STRRIDE research team at East Carolina University and Duke University. We appreciate thoughtful input from Drs. Svati Shah and Andrew Goldberg. Preliminary findings relevant to this manuscript were presented in abstract form at the 2006 American College of Sports Medicine Integrative Physiology Meeting, Indianapolis, IN. This work was supported by the NHLBI (NIH) R01HL-57354 (Kraus, PI) and NIA (NIH) P30 AGO28716-01 (Cohen, PI) and AG028930-01 (Muoio, PI).
This work was supported by the NHLBI (NIH) grant number R01HL-57354 and NIA (NIH) grant numbers P30 AGO28716-01 and AG028930-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Houmard JA, Tanner CJ, Slentz CA, Duscha BD, McCartney JS, Kraus WE. Effect of the volume and intensity of exercise training on insulin sensitivity. J Appl Physiol. 2004;96(1):101–6. doi: 10.1152/japplphysiol.00707.2003. [DOI] [PubMed] [Google Scholar]
- 3.Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347(19):1483–92. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- 4.Slentz CA, Aiken LB, Houmard JA, Bales CW, Johnson JL, Tanner CJ, et al. Inactivity, exercise, and visceral fat. STRRIDE: a randomized, controlled study of exercise intensity and amount. J Appl Physiol. 2005;99(4):1613–8. doi: 10.1152/japplphysiol.00124.2005. [DOI] [PubMed] [Google Scholar]
- 5.Slentz CA, Duscha BD, Johnson JL, Ketchum K, Aiken LB, Samsa GP, et al. Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE--a randomized controlled study. Arch Int Med. 2004;164(1):31–9. doi: 10.1001/archinte.164.1.31. [DOI] [PubMed] [Google Scholar]
- 6.Zierath JR. Invited review: Exercise training-induced changes in insulin signaling in skeletal muscle. J Appl Physiol. 2002;93(2):773–81. doi: 10.1152/japplphysiol.00126.2002. [DOI] [PubMed] [Google Scholar]
- 7.Houmard JA, Shinebarger MH, Dolan PL, Leggett-Frazier N, Bruner RK, McCammon MR, et al. Exercise training increases GLUT-4 protein concentration in previously sedentary middle-aged men. Am J Physiol. 1993;264(6 Pt 1):E896–901. doi: 10.1152/ajpendo.1993.264.6.E896. [DOI] [PubMed] [Google Scholar]
- 8.Youngren JF, Keen S, Kulp JL, Tanner CJ, Houmard JA, Goldfine ID. Enhanced muscle insulin receptor autophosphorylation with short-term aerobic exercise training. Am J Physiol Endocrinol Metab. 2001;280(3):E528–33. doi: 10.1152/ajpendo.2001.280.3.E528. [DOI] [PubMed] [Google Scholar]
- 9.Salpeter SR, Walsh JM, Ormiston TM, Greyber E, Buckley NS, Salpeter EE. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab. 2006;8(5):538–54. doi: 10.1111/j.1463-1326.2005.00545.x. [DOI] [PubMed] [Google Scholar]
- 10.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 11.Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) JAMA. 2002;288(1):49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- 12.Hing E, Brett KM. Changes in U.S. prescribing patterns of menopausal hormone therapy, 2001–2003. Obstet Gynecol. 2006;108(1):33–40. doi: 10.1097/01.AOG.0000220502.77153.5a. [DOI] [PubMed] [Google Scholar]
- 13.Kraus WE, Torgan CE, Duscha BD, Norris J, Brown SA, Cobb FR, et al. Studies of a targeted risk reduction intervention through defined exercise (STRRIDE) Med Sci Sports Exerc. 2001;33(10):1774–84. doi: 10.1097/00005768-200110000-00025. [DOI] [PubMed] [Google Scholar]
- 14.Huffman KM, Samsa GP, Slentz CA, Duscha BD, Johnson JL, Bales CW, et al. Response of high-sensitivity C-reactive protein to exercise training in an at-risk population. Am Heart J. 2006;152(4):793–800. doi: 10.1016/j.ahj.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 15.Ellis KJ. Human body composition: in vivo methods. Physiol Rev. 2000;80(2):649–80. doi: 10.1152/physrev.2000.80.2.649. [DOI] [PubMed] [Google Scholar]
- 16.Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35(7):609–16. [PubMed] [Google Scholar]
- 17.Duscha BD, Kraus WE, Keteyian SJ, Sullivan MJ, Green HJ, Schachat FH, et al. Capillary density of skeletal muscle: a contributing mechanism for exercise intolerance in class II-III chronic heart failure independent of other peripheral alterations. J Am Coll Cardiol. 1999;33(7):1956–63. doi: 10.1016/s0735-1097(99)00101-1. [DOI] [PubMed] [Google Scholar]
- 18.Chi MM, Hintz CS, Coyle EF, Martin WH, 3rd, Ivy JL, Nemeth PM, et al. Effects of detraining on enzymes of energy metabolism in individual human muscle fibers. Am J Physiol. 1983;244(3):C276–87. doi: 10.1152/ajpcell.1983.244.3.C276. [DOI] [PubMed] [Google Scholar]
- 19.Bergman RN, Finegood DT, Ader M. Assessment of insulin sensitivity in vivo. Endocr Rev. 1985;6(1):45–86. doi: 10.1210/edrv-6-1-45. [DOI] [PubMed] [Google Scholar]
- 20.Bergman RN. Orchestration of glucose homeostasis: from a small acorn to the California oak. Diabetes. 2007;56(6):1489–501. doi: 10.2337/db07-9903. [DOI] [PubMed] [Google Scholar]
- 21.Lindheim SR, Notelovitz M, Feldman EB, Larsen S, Khan FY, Lobo RA. The independent effects of exercise and estrogen on lipids and lipoproteins in postmenopausal women. Obstet Gynecol. 1994;83(2):167–72. [PubMed] [Google Scholar]
- 22.Wegge JK, Roberts CK, Ngo TH, Barnard RJ. Effect of diet and exercise intervention on inflammatory and adhesion molecules in postmenopausal women on hormone replacement therapy and at risk for coronary artery disease. Metabolism. 2004;53(3):377–81. doi: 10.1016/j.metabol.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 23.Green JS, Stanforth PR, Rankinen T, Leon AS, Rao Dc D, Skinner JS, et al. The effects of exercise training on abdominal visceral fat, body composition, and indicators of the metabolic syndrome in postmenopausal women with and without estrogen replacement therapy: the HERITAGE family study. Metabolism. 2004;53(9):1192–6. doi: 10.1016/j.metabol.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Schulz E, Anter E, Zou MH, Keaney JF., Jr Estradiol-mediated endothelial nitric oxide synthase association with heat shock protein 90 requires adenosine monophosphate-dependent protein kinase. Circulation. 2005;111(25):3473–80. doi: 10.1161/CIRCULATIONAHA.105.546812. [DOI] [PubMed] [Google Scholar]
- 25.Ren J, Hintz KK, Roughead ZK, Duan J, Colligan PB, Ren BH, et al. Impact of estrogen replacement on ventricular myocyte contractile function and protein kinase B/Akt activation. Am J Physiol Heart Circ Physiol. 2003;284(5):H1800–7. doi: 10.1152/ajpheart.00866.2002. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez C, Alonso A, Grueso NA, Diaz F, Esteban MM, Fernandez S, et al. Role of 17beta-estradiol administration on insulin sensitivity in the rat: implications for the insulin receptor. Steroids. 2002;67(13–14):993–1005. doi: 10.1016/s0039-128x(02)00073-9. [DOI] [PubMed] [Google Scholar]
- 27.DeFronzo RA, Gunnarsson R, Bjorkman O, Olsson M, Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest. 1985;76(1):149–55. doi: 10.1172/JCI111938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abate N, Garg A, Peshock RM, Stray-Gundersen J, Adams-Huet B, Grundy SM. Relationship of generalized and regional adiposity to insulin sensitivity in men with NIDDM. Diabetes. 1996;45(12):1684–93. doi: 10.2337/diab.45.12.1684. [DOI] [PubMed] [Google Scholar]
- 29.Abate N, Garg A, Peshock RM, Stray-Gundersen J, Grundy SM. Relationships of generalized and regional adiposity to insulin sensitivity in men. J Clin Invest. 1995;96(1):88–98. doi: 10.1172/JCI118083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab. 2000;278(5):E941–8. doi: 10.1152/ajpendo.2000.278.5.E941. [DOI] [PubMed] [Google Scholar]


