Abstract
Objectives
Particulate matter (PM) air pollution is associated with alterations in cardiac conductance and sudden cardiac death in epidemiological studies. Traffic-related air pollutants, including diesel exhaust (DE) may be at least partly responsible for these effects. In this experimental study we assessed whether short-term exposure to DE would result in alterations in heart rate variability (HRV), a non-invasive measure of autonomic control of the heart.
Methods
In a double-blind, crossover, controlled-exposure study, 16 adult volunteers were exposed (at rest) in randomized order to filtered air (FA) and two levels of diluted DE (100 or 200 μg/m3 of fine particulate matter) in two-hour sessions. Before, and at four time-points after each exposure we assessed HRV. HRV parameters assessed included both time domain statistics (standard deviation of N-N intervals (SDNN), and the square root of the mean of the sum of squared differences between successive N-N intervals (RMSSD)) and frequency domain statistics (high frequency power (HF), low frequency power (LF), and the LF/HF ratio).
Results
We observed an effect at 3-hours after initiation of DE inhalation on the frequency domain statistics of HRV. DE at 200 μg/m3 elicited an increase in HF power compared to FA (Δ=0.33; 95% CI: 0.01 to 0.7) and a decrease in LF/HF ratio (Δ=-0.74; 95% CI: -1.2 to -0.2). The effect of DE on HF power was not consistent among study participants. There was no DE-effect on time domain statistics and no significant DE effect on HRV in later time-points.
Conclusions
We did not observe a consistent DE effect on the autonomic control of the heart in a controlled exposure experiment in young participants. Efforts are warranted to understand discrepancies between epidemiological and experimental studies of air pollution’s impact on HRV.
Keywords: Air pollution, Heart rate variability, Autonomic nervous system, Diesel exhaust
INTRODUCTION
Acute cardiovascular morbidity and mortality have been consistently associated with ambient fine particulate matter air pollution (PM2.5, particles with aerodynamic diameter 2.5μm or less) in epidemiological studies (Dominici, et al. 2006; Ostro, et al. 2007). PM-induced alterations in the balance of autonomic control of the heart may partly contribute to these associations. Recent epidemiological studies have demonstrated association between PM2.5 and a shift of balance in the autonomic control of the heart (Liao, et al. 2004; Park, et al. 2005; Pope, et al. 2004; Romieu, et al. 2005). These associations may be particularly strong for vehicle-related pollutants (Adar, et al. 2007; Riediker, et al. 2004; Schwartz, et al. 2005).
By mass, diesel exhaust (DE) is the primary source of vehicle-derived PM2.5 (USEPA 2000). DE is also a substantial contributor to poor air quality in urban environments, accounting for the majority of ultrafine particulate matter (<100 nm). These small particles have a very large surface area, are highly respirable and are excellent carriers for adsorbed inorganic and organic compounds. Therefore, we hypothesized that controlled exposure to DE would result in early alterations in heart rate variability (HRV) measured at 1 and 3 hours from the onset of exposure. Because previous studies have also shown effects of PM2.5 on autonomic balance on the order of several hours to a day (Liao, et al. 1999; Park, et al. 2005), we also explored the hypothesis that exposure to DE would effect HRV parameters measured at 6 and 22 hours from initiation of exposure.
METHODS
We conducted a double-blind crossover experiment of DE and filtered air (FA) exposures. In this experiment, each participant was exposed on three different days to each of three conditions: FA, DE calibrated to generate 100μg/m3 of PM2.5 (DE100), and DE calibrated to generate 200μg/m3 of PM2.5 (DE200). DE was derived from a 2002 model turbocharged direct-injection 5.9 liter Cummins B-series engine (6BT5.9G6, Cummins, Inc., Columbus, IN). During exposures, PM2.5 concentrations were continuously measured and adjusted to maintain steady-state conditions (TEOM 1400a PM2.5, Rupprecht & Patashnick Co., Albany, NY) (for a detailed description of the DE exposure system see the online data supplement. Exposures were randomized by order and separated in time by at least 2 weeks. All participants gave written informed consent. The University of Washington Human Subjects Division approved the consent form and study protocol.
Study participants
Recruited subjects were 18-49 years old with no history of ongoing medical care for heart disease, hypertension, asthma, diabetes, hypercholesterolemia, or other chronic conditions. All subjects were nonsmokers for at least six months and had normal spirometry. Since we were interested in susceptibility by disease status, we separately recruited “healthy” and “metabolic syndrome” participants. Healthy participants had a body mass index (BMI) below 30kg/m2, fasting blood sugar below 126mg/dL, resting blood pressure below 130/85 mmHg, and no signs of arrhythmia or ischemia on a screening electrocardiogram (ECG). Metabolic syndrome participants fulfilled any three of the following five criteria: waist circumference ≥102 cm in males and ≥ 88 cm in females; triglycerides ≥ 150 mg/dL; HDL cholesterol < 40 mg/dL in males and < 50 mg/dL in females; systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥ 85 mmHg; and fasting glucose ≥ 100 mg/dL.(Grundy, et al. 2005)
Study protocol
Exposures began at 9am and were two hours in duration; participants were at rest throughout. We used a three-channel ambulatory Holter recorder (Del Mar, Irvine, CA, USA) to monitor continuous ECG tracings. The electrodes were placed in a modified V1-V5 position. ECG recordings started approximately 1 hour before the onset of exposures and continued throughout the exposure session days (~8-9 hours). ECG recordings were also collected over the entire duration of the follow-up visit (~1 hour) on the morning after exposure days.
Heart rate variability parameters
In order to prevent interference by participant activity, we limited the data for this analysis to standardized 10-minute periods during which subjects were resting supine. These resting periods were conducted at various time-points during the exposure day: before exposure started, at one hour into exposure (1h), and at three hours (3h), six hours (6h), and 22 hours (22h) from the onset of exposure. A single trained analyst, blinded to exposure levels, reviewed and edited the automatically determined readings of QRS complexes in these 10-minute intervals using Del Mar Dartscan software (Model DS-90, Irvine, CA, USA) to obtain all normal-normal (N-N) intervals. Any 10-minute recording with more than 10% ectopic beats and/or artifacts was excluded from the analysis.
For the analysis of HRV we used the middle five minutes of each 10-minute recording period, including only N-N intervals with a successive ratio within 0.8-1.2. We analyzed HRV using software created by The Biomedical Signal Analysis Group, Department of Applied Physics, University of Kuopio, Finland (Niskanen J-P 2004). Generated time domain statistics included the standard deviation of N-N intervals (SDNN), and the square root of the mean squared differences between successive N-N intervals (RMSSD). For frequency domain analysis we used a Fast Fourier Transform (FFT) algorithm. The signal was analyzed with Hanning window for segment lengths of 256 samples with 50% overlapping (Singh, et al. 2004). Spectral data obtained from N-N intervals were analyzed according to the following frequency bands: very-low frequency (VLF, 0.00–0.04 Hz), low frequency (LF, 0.04–0.15 Hz), and high frequency (HF, 0.15–0.40 Hz). Since very-low (and lower) frequency modulation of N-N interval data has no interpretable significance in short ECG recordings (Task-Force 1996), we report the frequency domain measures of LF and HF power in ms2, and the LF/HF ratio. To address any irregularity in the N-N interval time series, cubic interpolation at a rate of 4Hz was used before spectrum estimation. To remove the influence of a large low-frequency baseline trend component, a de-trending algorithm was used based on a smoothness priors method (Tarvainen MP 2002).
Statistical Analysis
All statistical testing was based on two-tailed tests with α =0.05. Descriptive data are presented as a mean ± standard error to the mean (SEM) unless specified otherwise. Statistical analyses were performed using STATA 9.1 (StataCorp LP, College Station TX).
All HRV parameters were log-transformed prior to statistical analysis as they were highly skewed. Changes in HRV were normalized as the difference between each HRV parameter at a given time-point after exposure onset relative to the same HRV parameter, pre-exposure. Exposure-related changes in HRV at each time point were computed for DE200 or DE100 by contrasting to the FA levels using paired t- tests. Due to our small study population, we computed the percent difference at 1h and 3h from the pre-exposure non-transformed levels of the HRV parameters that were significantly affected by DE exposure, to assess whether the effect was consistent among study participants. We also tested our results for effect modification by subject-related characteristics, and for period and carryover effects, using interaction terms in an ANOVA model.
RESULTS
A total of 23 participants (6 healthy and 17 metabolic syndrome) completed all three levels of exposure. Seven were excluded from further analyses: one participant did not meet the inclusion criteria for N-N intervals taken from the ECG measurement at pre-exposure to DE200; six participants had missing ECG measurements at pre-exposure to either FA, or DE200. Characteristics of the 16 participants (3 healthy and 13 metabolic syndrome) with acceptable HRV measurements are presented in Table 1.
Table 1.
Study participants’ characteristics by health status
| Characteristic | Healthy | Metabolic Syndrome |
|---|---|---|
| Participants (n) | 3 | 13 |
| Age, mean in yr (range) | 32 (24-39)a | 41 (31-48)a |
| Gender (F) (n) | 0 | 5 |
| Race | ||
| Caucasian (n) | 2 | 10 |
| Other (n) | 1 | 3 |
| Body Mass Index (kg/m2) | 24.7 (0.9)a | 39.2 (1.8)a |
| Total Cholesterol (mg/dl) | 157.3 (23.9)a | 183.2 (6.1)a |
| Triglycerides (mg/dl) | 90.3 (19.8) | 168.9 (34.6) |
| Glucose (mg/dl) | 90.3 (2)a | 101.9 (3)a |
| Smoking history (n) (P/Y)b | 0 | 5 (3.5) |
Statistically significant differences between healthy and metabolic subjects at p<0.05;
P/Y- average pack-year calculated as (number of cigarettes per day X number of years smoked)/20; where not specified, values are mean (SEM)
The average exposure levels to PM2.5 approximated the target concentrations. In addition, we maintain low concentrations of the gases carbon monoxide (CO) and nitrogen dioxide (NO2) (Table 2).
Table 2.
Average PM2.5 mass concentrations* and gas concentrations† measured during two-hour exposure sessions of 16 participants.
| Pollutant | Filtered air | Diesel exhaust at 100μg/m3 | Diesel exhaust at 200μg/m3 |
|---|---|---|---|
| PM2.5 (μg/m3) | 4.16 | 101.95 | 206.03 |
| NO2 (ppb) | 15.44 | 20.57 | 28.29 |
| NO (ppb) | 38.29 | 947.89 | 1629.31 |
| CO (ppm) | 0.29 | 0.47 | 0.74 |
From TEOM - discrete 10-min averaging intervals;
1-min averaging intervals.
Pre-exposure HRV statistics were not significantly different between exposure sessions or between healthy and metabolic syndrome participants within exposure session (Table 3). Although metabolic syndrome participants generally exhibited slightly lower HRV measures than healthy individuals within the same exposure session, we analyzed the DE effect on HRV statistics without sorting by health status due to the small number of healthy participants with available data. As a sensitivity analysis we also conducted analysis limited to the metabolic syndrome participants. Results were similar to those from both study populations combined.
Table 3.
Geometric means (95% CI) of HRV measurements prior to exposure of healthy (n=3) and metabolic syndrome (n=13) participants.
| HRV parameter | Filtered Air | 100μg/m3 Diesel exhaust | 200μg/m3 Diesel exhaust |
|---|---|---|---|
| SDNN (ms) | |||
| All participants | 29.6(25.4-34.5) | 28.5(24-33.8) | 30.0 (24.4-36.8) |
| Healthy | 33.1(26.2-41.6) | 34.5(21-56.9) | 30.4(20.3-45.5) |
| Metabolic syndrome | 28.8(23.8-34.9) | 27.2(22.3-33.3) | 29.9(23-38.7) |
| RMSSD (ms) | |||
| All participants | 26.7(21-34) | 27.0(21-34.6) | 26.9(21.6-33.5) |
| Healthy | 34.2(28.3-41.3) | 30.9(16.6-57.4) | 28.7(16.9-48.7) |
| Metabolic syndrome | 25.2(18.8-33.9) | 26.1(19.2-35.6) | 26.5(20.2-34.8) |
| LF (ms2) | |||
| All participants | 200.4(141.3-284.1) | 181.6(127.8-258.1) | 222.7(132.8-373.5) |
| Healthy | 230.7(48.5-1097.3) | 284.0(41.9-1924.1) | 266.5(67.9-1045.9) |
| Metabolic syndrome | 193.9(128.5-292.8) | 163.8(112.7-238.1) | 213.7(112.9-404.7) |
| HF (ms2) | |||
| All participants | 120(69.5-207.2) | 106(65.7-171.1) | 114.8(71-185.6) |
| Healthy | 188.3(81.8-433.6) | 150.5(27.7-816.9) | 120(52.9-272) |
| Metabolic syndrome | 108.2(55.3-211.6) | 97.8(55.3-173.1) | 113.7(62.1-207.8) |
| LF/HF ratio | |||
| All participants | 1.7(1-2.9) | 1.7(1-2.9) | 1.9(1.2-3.2) |
| Healthy | 1.2(0.2-9.2) | 1.9(0.1-33.8) | 2.2(0.7-6.9) |
| Metabolic syndrome | 1.8(0.9-3.5) | 1.7(0.9-3.1) | 1.9(1-3.4) |
Analysis of exposure-related changes of HRV parameters at all time periods are presented in Figure 1. These results revealed no consistent differences at 1h between DE and FA. Table 4 shows the effect of exposure to DE100 and DE200 on HRV Parameters at 3h. While there was no significant effect for any HRV parameter from DE100, we noted a significant increase in HF power over pre-exposure levels after DE200 (0.26) as compared to FA (-0.07) (Δ= 0.33; 95% CI: 0.01 to 0.7). At this same time-point, there was a decrease in LF/HF ratio from pre-exposure levels following DE200 (-0.54) as compared to an increase after exposure to FA (0.2) (Δ= -0.74; 95% CI: -1.2 to -0.2).
Figure 1.
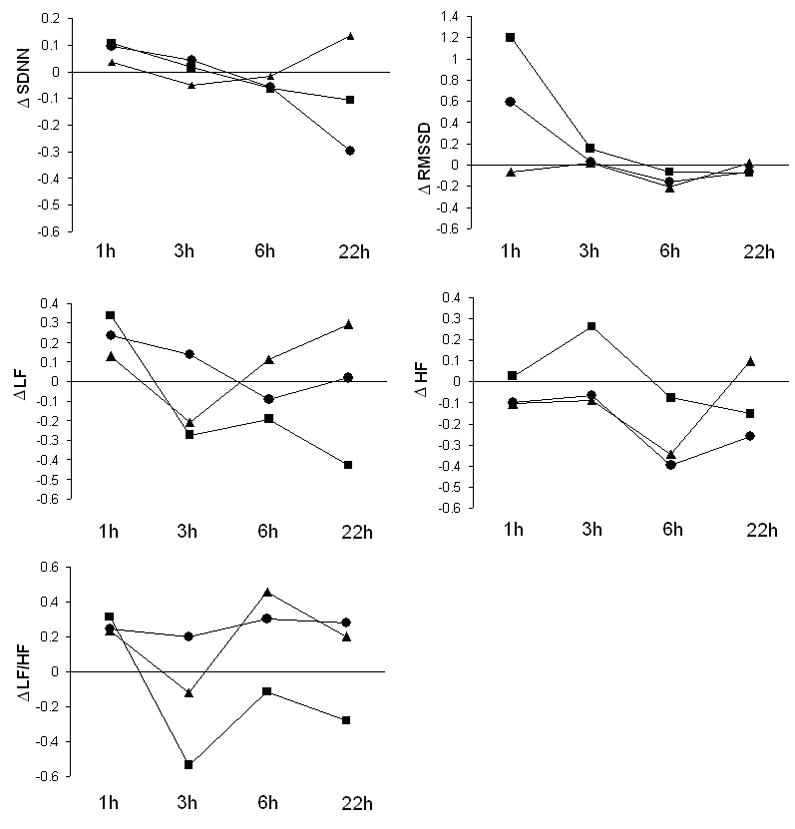
Mean change in log transformed HRV parameters at each time point from pre-exposure levels for exposure to filtered air (FA) (
 ), diesel exhaust at 100μg/m3 (
), diesel exhaust at 100μg/m3 (
 ), or diesel exhaust at 200μg/m3 (
), or diesel exhaust at 200μg/m3 (
 ).
).
Table 4.
Changesa in Heart rate variability (HRV) from pre-exposure to 3 hours after initiating exposure, log transformed, mean (SEM)
| HRV parameter | Filtered air | 100μg/m3 Diesel exhaust | 200μg/m3 Diesel exhaust | Average DE effectb (95% CI) |
|---|---|---|---|---|
| SDNN | 0.04 (0.05) | -0.05 (0.07) | 0.01 (0.09) | DE100: -0.09 (-0.27 to 0.84)
DE200: -0.03 (-0.2 to 0.2) |
| RMSSD | 0.03 (0.04) | 0.02 (0.1) | 0.15 (0.1) | DE100: -0.01 (-0.23 to 0.22)
DE200: 0.13 (-0.07 to 0.3) |
| LF | 0.14 (0.12) | -0.21 (0.15) | -0.27 (0.2) | DE100: -0.35 (-0.71 to 0.02)
DE200: -0.41 (-0.9 to 0.1) |
| HF | -0.07 (0.08) | -0.09 (0.22) | 0.26 (0.19) | DE100: -0.02 (-0.45 to 0.4)
DE200: 0.33 (0.01 to 0.7)c |
| LF/HF ratio | 0.2 (0.12) | -0.12 (0.24) | -0.54 (0.18) | DE100: -0.32 (-0.81 to 0.17)
DE200: -0.74 (-1.2 to -0.2)d |
HRV (post-exposure) − HRV (pre-exposure);
difference between changes at filtered air and changes at 100μg/m3 diesel exhaust (DE100) or 200μg/m3 diesel exhaust (DE200);
p<0.05;
p<0.01
Changes in the frequency domain HRV parameters observed at 3h remained generally consistent for DE200 across the later time points with evidence of increased HF, decreased LF, and a decreased LF/HF ratio after DE as compared to FA (Figure 1). At 22h following exposure to FA, frequency domain parameters showed a trend toward pre-exposure baseline values that was not observed after exposure to DE200. However, exposure-related changes in frequency domain statistics at 6h and 22h were not statistically different between exposures to DE200 and FA. In addition, no dose response was observed in these later time points. No significant effect of DE was observed on heart rate (data not shown) and on time domain measurements at any time-points. Age, gender, BMI, fasting plasma lipid levels, smoking history, carryover, and period effects did not modify the association between DE and frequency domain statistics in all time points.
To assess whether the increased HF power was consistent among study participants, we plotted the percent difference of HF power from the pre-exposure level for each participant for both DE200 and DE100 at 1h and 3h (Figure A online data supplement). Only 7 (1 healthy and 6 metabolic syndrome) out of 16 participants showed an increased HF power at DE200 as compared to FA. Six showed an increased HF at DE100 as compared to FA. No consistent dose response relationship was observed. The same approach used for LF/HF ratio, revealed that only 9 out of 16 participants showed a decreased ratio at DE200 as compared to FA.
DISCUSSION
In this study we did not find a consistent effect of DE inhalation on the autonomic modulation of the heart as assessed by heart rate variability measures. Although we showed a significant increment of HF power at 3h following DE200 compared to FA, only 7 of 16 participants demonstrated this effect (supplementary Figure A). No dose-response relationship was found for increasing concentrations of DE, and the effect was opposite of the most typically observed effect of air pollutants. Moreover, the significant decrease in LF/HF ratio at 3h following DE200 compared to FA was driven by a small number of participants. Our inconsistent effect of DE on HF power and LF power does not allow inference regarding a shift in the balance of the autonomic control of the heart.
HRV has been used frequently in air pollution research as a signal of cardiovascular effect, and to explore for potential pathophysiological mechanisms by which air pollution may lead to cardiovascular mortality and morbidity. Epidemiological studies in susceptible populations-especially panel study designs-have shown a relationship between air pollution and decreased HRV (Adar, et al. 2007; Chan, et al. 2004; Gold, et al. 2000; Liao, et al. 1999; Liao, et al. 2004; Park, et al. 2005) as well as increased cardiac arrhythmia with exposure to components of ambient particulate air pollution (Dockery, et al. 2005; Peters, et al. 2000; Rich, et al. 2006; Sarnat, et al. 2006). The previously observed effects of air pollutants on HRV and arrhythmia are not entirely consistent across studies. Potential factors that may explain these discrepancies include age and health status of study participants, composition of particles and gases, and use of different methodologies for HRV analysis.
Compared to observational approaches such as panel study designs, an experimental approach as used in this study-with rigorous control over exposure situations and potentially confounding factors-would be expected to be highly tuned to finding an exposure-related effect if one exists. Our inability to find a decrease in HRV after DE inhalation in young individuals without known cardiovascular disease is consistent with prior controlled exposure studies to concentrated ambient particles (CAPS) (Devlin, et al. 2003; Gong, et al. 2004) and ultrafine carbon particles (Frampton, et al. 2004). Moreover, while HRV was decreased in the lion’s share of published epidemiological studies that involved elderly participants, HRV effects of air pollution in young healthy individuals did not consistently demonstrate this decline in HRV (Chan, et al. 2004; Devlin, et al. 2003; Frampton, et al. 2004; Gong, et al. 2004; Magari, et al. 2001; Riediker, et al. 2004; Scharrer, et al. 2007). It has long been recognized that autonomic function changes with increasing age, as demonstrated by a decrease in HRV even in individuals without pre-existing cardiovascular or autonomic disease (Liao, et al. 1995; O’Brien, et al. 1986). It is possible that the response of the autonomic nervous system to a stimulus such as pollutant inhalation may be different between elders who are healthy or possess underlying unknown cardiovascular disease and our study population. Although our population included individuals with metabolic syndrome, the absence of pre-existing cardiovascular disease or autonomic neuropathy in our population may decrease the utility of HRV measures. The significant range of HRV responses in routine cardiac modulation in our study population may have overwhelmed the small adverse DE effect on autonomic modulation.
The composition of the inhaled pollutants may also contribute to the discordance of our findings with previously published data. DE is not the only source of pollution and co-pollutants such as ozone may potentially make diesel particles more injurious to biological tissues (Bosson, et al. 2007; Madden, et al. 2000). Therefore, although our system creates a realistic combustion-derived exposure, which is highly relevant to urban air pollution, especially traffic-related exposure, it may not replicate the situations seen in observational studies of HRV.
The different methods of HRV analyses in prior air pollution studies (long-term (24 hours) (Adar, et al. 2007; Magari, et al. 2002; Pope, et al. 2004; Pope, et al. 1999) and short-term (5 minutes)) (Devlin, et al. 2003; Frampton, et al. 2004; Gold, et al. 2000; Gong, et al. 2004; Liao, et al. 2004; Park, et al. 2005; Sullivan, et al. 2005) can lead to different inferences for PM effect on HRV (Task-Force 1996). To allow for maximal comparability between exposures, we opted for short-term standardized HRV analysis rather than long-term analysis, thus eliminating influence by factors such as variation in posture, physical and mental activity, and sleep. As a result, our results cannot be compared directly to all other published studies of HRV and pollution.
The timing of outcome assessments also differs between studies, and reflects uncertainty regarding the time course of the cascade of effects which might culminate in the health effects of PM. While PM appear to exert health effects within hours of exposure (Mills, et al. 2005; Peters, et al. 2001), we sought to find early effects of DE on HRV. Alternatively, PM exposure may lead to an initial pulmonary inflammatory response with subsequent cascade of delayed effects (hours or even days) on the autonomic nervous system (Haefeli, et al. 1993; Nel, et al. 2001; van Eeden, et al. 2001; Weisensee, et al. 1993). We did not demonstrate a late effect of DE on HRV; however, we noticed long-term trends in frequency domain parameters compared to pre-exposure values. Although this trend lacked statistical significance, it may represent the onset of a late autonomic effect. In fact, Park et al. demonstrated the strongest effect on HRV (decreased HRV) was with a 48-hour moving average of PM2.5 (Park, et al. 2005). If future experimental studies are conducted, it would be prudent to explore late autonomic effects.
Strengths and limitations
This study’s strength is in the experimental design, unlike most published studies that have used an observational design from which it is much more difficult to determine causal relationships. Nonetheless, several limitations may hinder inference from this study. First, after applying our exclusion criteria on HRV data, only a small and nonhomogenous study population was analyzed. We did not find significant baseline differences in HRV statistics between the two sub-populations of the study. Second, we assessed HRV in short recording intervals. This may have affected our ability to identify any difference in time domain statistics between the exposure sessions (Task-Force 1996), and we may have sacrificed some power to detect associations by limiting the number of outcome measures in our dataset. In addition, this approach may have limited our ability to directly compare our results with those of other studies.
CONCLUSIONS
In a controlled exposure experiment, we were not able to show a consistent effect of diesel exhaust inhalation on heart rate variability. To better understand and confirm prior observations of PM effects on HRV principally derived from non-experimental designs, controlled exposure experimental studies in different susceptible populations, using relevant exposures and standardized analysis of ECG recordings, are needed.
Supplementary Material
Acknowledgments
We thank Jim Stewart, Tim Gould, and Karen Jansen for conducting and controlling the exposures to diesel exhaust, the staff in the General Clinical Research Center, University of Washington for their invaluable contributions to this study, and all the study participants who contributed their time to the study.
FUNDING: Support for this study was provided by grant R830954 and R827355 from the Environmental Protection Agency and grants K24ES013195, K23ES011139, ES013195, P30ES07033, and M01RR-00037 from the National Institutes of Health and from the National Institute of Environmental Health Sciences.
The study protocol was approved by the University of Washington Human Subjects Division.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adar SD, Gold DR, Coull BA, Schwartz J, Stone PH, Suh H. Focused Exposures to Airborne Traffic Particles and Heart Rate Variability in the Elderly. Epidemiology. 2007;18:95–103. doi: 10.1097/01.ede.0000249409.81050.46. [DOI] [PubMed] [Google Scholar]
- Bosson J, Pourazar J, Forsberg B, Adelroth E, Sandstrom T, Blomberg A. Ozone enhances the airway inflammation initiated by diesel exhaust. Respir Med. 2007;101:1140–6. doi: 10.1016/j.rmed.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Chan CC, Chuang KJ, Shiao GM, Lin LY. Personal exposure to submicrometer particles and heart rate variability in human subjects. Environ Health Perspect. 2004;112:1063–7. doi: 10.1289/ehp.6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin RB, Ghio AJ, Kehrl H, Sanders G, Cascio W. Elderly humans exposed to concentrated air pollution particles have decreased heart rate variability. Eur Respir J Suppl. 2003;40:76s–80s. doi: 10.1183/09031936.03.00402403. [DOI] [PubMed] [Google Scholar]
- Dockery DW, Luttmann-Gibson H, Rich DQ, Link MS, Mittleman MA, Gold DR, Koutrakis P, Schwartz JD, Verrier RL. Association of air pollution with increased incidence of ventricular tachyarrhythmias recorded by implanted cardioverter defibrillators. Environ Health Perspect. 2005;113:670–4. doi: 10.1289/ehp.7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, Samet JM. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. Jama. 2006;295:1127–34. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton MW, Utell MJ, Zareba W, Oberdorster G, Cox C, Huang LS, Morrow PE, Lee FE, Chalupa D, Frasier LM, Speers DM, Stewart J. Effects of exposure to ultrafine carbon particles in healthy subjects and subjects with asthma. Res Rep Health Eff Inst. 2004:1–47. discussion 49-63. [PubMed] [Google Scholar]
- Gold DR, Litonjua A, Schwartz J, Lovett E, Larson A, Nearing B, Allen G, Verrier M, Cherry R, Verrier R. Ambient pollution and heart rate variability. Circulation. 2000;101:1267–73. doi: 10.1161/01.cir.101.11.1267. [DOI] [PubMed] [Google Scholar]
- Gong H, Jr, Linn WS, Terrell SL, Clark KW, Geller MD, Anderson KR, Cascio WE, Sioutas C. Altered heart-rate variability in asthmatic and healthy volunteers exposed to concentrated ambient coarse particles. Inhal Toxicol. 2004;16:335–43. doi: 10.1080/08958370490439470. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith J, S C, Spertus JA, Costa F. Diagnosis and Management of the Metabolic Syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- Haefeli WE, Bargetzi MJ, Starnes HF, Blaschke TF, Hoffman BB. Evidence for activation of the sympathetic nervous system by recombinant human interleukin-1 beta in humans. J Immunother. 1993;13:136–40. doi: 10.1097/00002371-199302000-00009. [DOI] [PubMed] [Google Scholar]
- Liao D, Barnes RW, Chambless LE, Simpson RJ, Jr, Sorlie P, Heiss G. Age, race, and sex differences in autonomic cardiac function measured by spectral analysis of heart rate variability--the ARIC study. Atherosclerosis Risk in Communities. Am J Cardiol. 1995;76:906–12. doi: 10.1016/s0002-9149(99)80260-4. [DOI] [PubMed] [Google Scholar]
- Liao D, Creason J, Shy C, Williams R, Watts R, Zweidinger R. Daily variation of particulate air pollution and poor cardiac autonomic control in the elderly. Environ Health Perspect. 1999;107:521–5. doi: 10.1289/ehp.99107521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Duan Y, Whitsel EA, Zheng ZJ, Heiss G, Chinchilli VM, Lin HM. Association of higher levels of ambient criteria pollutants with impaired cardiac autonomic control: a population-based study. Am J Epidemiol. 2004;159:768–77. doi: 10.1093/aje/kwh109. [DOI] [PubMed] [Google Scholar]
- Madden MC, Richards JH, Dailey LA, Hatch GE, Ghio AJ. Effect of ozone on diesel exhaust particle toxicity in rat lung. Toxicol Appl Pharmacol. 2000;168:140–8. doi: 10.1006/taap.2000.9024. [DOI] [PubMed] [Google Scholar]
- Magari SR, Hauser R, Schwartz J, Williams PL, Smith TJ, Christiani DC. Association of heart rate variability with occupational and environmental exposure to particulate air pollution. Circulation. 2001;104:986–91. doi: 10.1161/hc3401.095038. [DOI] [PubMed] [Google Scholar]
- Magari SR, Schwartz J, Williams PL, Hauser R, Smith TJ, Christiani DC. The association between personal measurements of environmental exposure to particulates and heart rate variability. Epidemiology. 2002;13:305–10. doi: 10.1097/00001648-200205000-00011. [DOI] [PubMed] [Google Scholar]
- Mills NL, Tornqvist H, Robinson SD, Gonzalez M, Darnley K, MacNee W, Boon NA, Donaldson K, Blomberg A, Sandstrom T, Newby DE. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation. 2005;112:3930–6. doi: 10.1161/CIRCULATIONAHA.105.588962. [DOI] [PubMed] [Google Scholar]
- Nel AE, Diaz-Sanchez D, Li N. The role of particulate pollutants in pulmonary inflammation and asthma: evidence for the involvement of organic chemicals and oxidative stress. Curr Opin Pulm Med. 2001;7:20–6. doi: 10.1097/00063198-200101000-00004. [DOI] [PubMed] [Google Scholar]
- Niskanen J-P, T M, Ranta-aho PO, Karjalainen PA. Software for advanced HRV analysis. Comput Methods Programs Biomed. 2004 doi: 10.1016/j.cmpb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- O’Brien IA, O’Hare P, Corrall RJ. Heart rate variability in healthy subjects: effect of age and the derivation of normal ranges for tests of autonomic function. Br Heart J. 1986;55:348–54. doi: 10.1136/hrt.55.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostro B, Feng WY, Broadwin R, Green S, Lipsett M. The effects of components of fine particulate air pollution on mortality in california: results from CALFINE. Environ Health Perspect. 2007;115:13–9. doi: 10.1289/ehp.9281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, O’Neill MS, Vokonas PS, Sparrow D, Schwartz J. Effects of air pollution on heart rate variability: the VA normative aging study. Environ Health Perspect. 2005;113:304–9. doi: 10.1289/ehp.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Dockery DW, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103:2810–5. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- Peters A, Liu E, Verrier RL, Schwartz J, Gold DR, Mittleman M, Baliff J, Oh JA, Allen G, Monahan K, Dockery DW. Air pollution and incidence of cardiac arrhythmia. Epidemiology. 2000;11:11–7. doi: 10.1097/00001648-200001000-00005. [DOI] [PubMed] [Google Scholar]
- Pope CA, 3rd, Hansen ML, Long RW, Nielsen KR, Eatough NL, Wilson WE, Eatough DJ. Ambient particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environ Health Perspect. 2004;112:339–45. doi: 10.1289/ehp.6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA, 3rd, Verrier RL, Lovett EG, Larson AC, Raizenne ME, Kanner RE, Schwartz J, Villegas GM, Gold DR, Dockery DW. Heart rate variability associated with particulate air pollution. Am Heart J. 1999;138:890–9. doi: 10.1016/s0002-8703(99)70014-1. [DOI] [PubMed] [Google Scholar]
- Rich DQ, Mittleman MA, Link MS, Schwartz J, Luttmann-Gibson H, Catalano PJ, Speizer FE, Gold DR, Dockery DW. Increased risk of paroxysmal atrial fibrillation episodes associated with acute increases in ambient air pollution. Environ Health Perspect. 2006;114:120–3. doi: 10.1289/ehp.8371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riediker M, Cascio WE, Griggs TR, Herbst MC, Bromberg PA, Neas L, Williams RW, Devlin RB. Particulate matter exposure in cars is associated with cardiovascular effects in healthy young men. Am J Respir Crit Care Med. 2004;169:934–40. doi: 10.1164/rccm.200310-1463OC. [DOI] [PubMed] [Google Scholar]
- Romieu I, Tellez-Rojo MM, Lazo M, Manzano-Patino A, Cortez-Lugo M, Julien P, Belanger MC, Hernandez-Avila M, Holguin F. Omega-3 fatty acid prevents heart rate variability reductions associated with particulate matter. Am J Respir Crit Care Med. 2005;172:1534–40. doi: 10.1164/rccm.200503-372OC. [DOI] [PubMed] [Google Scholar]
- Sarnat SE, Suh HH, Coull BA, Schwartz J, Stone PH, Gold DR. Ambient particulate air pollution and cardiac arrhythmia in a panel of older adults in Steubenville, Ohio. Occup Environ Med. 2006;63:700–6. doi: 10.1136/oem.2006.027292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharrer E, Hessel H, Kronseder A, Guth W, Rolinski B, Jorres RA, Radon K, Schierl R, Angerer P, Nowak D. Heart rate variability, hemostatic and acute inflammatory blood parameters in healthy adults after short-term exposure to welding fume. Int Arch Occup Environ Health. 2007;80:265–72. doi: 10.1007/s00420-006-0127-2. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Litonjua A, Suh H, Verrier M, Zanobetti A, Syring M, Nearing B, Verrier R, Stone P, MacCallum G, Speizer FE, Gold DR. Traffic related pollution and heart rate variability in a panel of elderly subjects. Thorax. 2005;60:455–61. doi: 10.1136/thx.2004.024836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D, Vinod K, Saxena SC, Deepak KK. Effects of RR segment duration on HRV spectrum estimation. Physiol Meas. 2004;25:721–35. doi: 10.1088/0967-3334/25/3/012. [DOI] [PubMed] [Google Scholar]
- Sullivan JH, Schreuder AB, Trenga CA, Liu SL, Larson TV, Koenig JQ, Kaufman JD. Association between short term exposure to fine particulate matter and heart rate variability in older subjects with and without heart disease. Thorax. 2005;60:462–6. doi: 10.1136/thx.2004.027532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarvainen MP, R-A P, K P. An advanced detrending method with application to HRV analysis. IEEE Trans Biomed Eng. 2002;49:172–175. doi: 10.1109/10.979357. [DOI] [PubMed] [Google Scholar]
- Task-Force. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- USEPA. National Air Pollutant Emission Trends, 1900-1998 2000 [Google Scholar]
- van Eeden SF, Tan WC, Suwa T, Mukae H, Terashima T, Fujii T, Qui D, Vincent R, Hogg JC. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10)) Am J Respir Crit Care Med. 2001;164:826–30. doi: 10.1164/ajrccm.164.5.2010160. [DOI] [PubMed] [Google Scholar]
- Weisensee D, Bereiter-Hahn J, Schoeppe W, Low-Friedrich I. Effects of cytokines on the contractility of cultured cardiac myocytes. Int J Immunopharmacol. 1993;15:581–7. doi: 10.1016/0192-0561(93)90075-a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


