Abstract
The pathologic changes of Alzheimer disease (AD) evolve very gradually over decades before the disease becomes clinically manifest. Thus, it is not uncommon to find substantial numbers of Aβ plaques and neurofibrillary tangles in autopsy brains of older subjects with documented normal cognition, a state that we define as asymptomatic AD (ASYMAD). The goal of this study is to understand the morphometric substrate of ASYMAD subjects compared with mild cognitive impairment and definite AD cases. We used designed-based stereology to measure the volumes of neuronal cell bodies, nuclei, and nucleoli in 4 cerebral regions: anterior cingulate gyrus, posterior cingulate gyrus, primary visual cortex, and CA1 of hippocampus. We examined and compared autopsy brains from 4 groups (n = 15 each) of participants in the Baltimore Longitudinal Study of Aging: ASYMAD, mild cognitive impairment, AD, and age-matched controls. We found significant hypertrophy of the neuronal cell bodies, nuclei, and nucleoli of CA1 of hippocampus and anterior cingulate gyrus neurons in ASYMAD subjects compared with control and mild cognitive impairment cases. In the posterior cingulate gyrus and primary visual cortex, the hypertrophy was limited to the nuclei and nucleoli. The hypertrophy of cortical neurons and their nuclei and nucleoli in ASYMAD may represent an early reaction to the presence of neurotoxic Aβ or tau, or a compensatory mechanism that prevents the progression of the disease into dementia.
Keywords: β-Amyloid, Alzheimer disease pathogenesis, Mild cognitive impairment, Nuclear hypertrophy, Nucleolar hypertrophy, Neuronal resistance, Tau
INTRODUCTION
The neuropathologic hallmarks of Alzheimer disease (AD) are β-amyloid deposits, neuritic plaques (Aβ plaques), neurofibrillary tangles (NFTs), neuropil threads, and the degeneration of vulnerable neurons and their synapses (1–7). These pathologic changes accumulate gradually over decades before becoming clinically evident. Thus, it is not uncommon to find substantial numbers of Aβ plaques and NFTs in the autopsy brains of older subjects with normal cognition documented shortly before death (8–13). These observations have suggested 2 explanations: 1) that AD has a “silent phase” preceding by years or decades the appearance of any cognitive impairment until the functional threshold for effective neuronal functioning is overwhelmed by the AD pathology (14); or, alternatively, 2) the “cognitive reserve hypothesis,” which postulates that some individuals have brains that are able to compensate for or resist the neuronal and synaptic derangements of AD (15–17). In the present study, we defined asymptomatic AD (ASYMAD) as the status of subjects with preserved cognition assessed less than a year before death but with identifiable AD pathology at autopsy, that is, a Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) neuritic plaque score of A or higher (18) and a Braak NFT score of III or higher (19). Subjects with similar status have been described in the past as “preclinical” AD stage (20) or “high pathology control” state (21). We prefer the term ASYMAD because we do not know a priori whether these subjects would have remained cognitively intact or eventually would have progressed to mild cognitive impairment (MCI) or AD dementia if they had lived longer.
In a previous study (22), we showed that ASYMAD is characterized by nuclear hypertrophy of the CA1 of hippocampus (CA1) and anterior cingulate gyrus (ACG) neurons compared with age-matched controls (C), and that the nuclear hypertrophy of neurons was not present in the brains of patients with MCI or definite AD. In the present study, one goal was to determine whether the hypertrophy of neuronal nuclei is limited to the ACG and hippocampus or is also present in other regions by studying a larger sample of brains. We tested the hypothesis that the nuclear hypertrophy of cortical neurons has a hierarchic distribution consistent with the morphologic progression of AD, that is, as demonstrated by neuropathologic (18) and neuroimaging studies (23–28), there is early involvement of anterior cingulate and late involvement of occipital cortex in AD. A second goal was to examine whether the neuronal changes in ASYMAD are limited to the nucleus or involve other neuronal compartments such as the cell body and nucleolus. Therefore, we extended our analysis to the posterior cingulate gyrus (PCG) and the primary visual cortex (PVC) of age-matched C’s, ASYMAD, MCI, and definite AD subjects. Specifically, we examined the ACG (Brodmann Area 24), the PCG (Brodmann Area 23), the PVC (Brodmann Area 17), and the CA1. In each of these regions, we used designed-based stereology to measure the mean volumes of neuronal cell bodies, nuclei, and nucleoli throughout the entire thickness of the cortical mantle. Our measurements did not address changes in specific cortical layers or cytoarchitectonic subregions such as those described in the cingulate cortex (29, 30).
MATERIALS AND METHODS
Baltimore Longitudinal Study of Aging and Autopsy Program
The subjects examined in this study were all participants in the autopsy program of the Baltimore Longitudinal Study of Aging (BLSA) (31). Participants in this study agree to follow up at specific intervals until their death and give consent for eventual autopsy. The ongoing mean age at the time of the enrollment in the BLSA is 62.9 years. The local institutional review board approved BLSA protocols and the associated autopsy program, and all participants gave written informed consent prior to each assessment. The larger proportion of men in the sample reflects the composition of the BLSA participant cohort that was limited to men until 1978.
Clinical and Neuropsychological Evaluations of the BLSA
Baseline evaluations for each subject entering in the BLSA study were performed by a neurologist to document a history of cerebrovascular disease, focal neurologic abnormalities, and impairment of cognitive or behavioral functions due to secondary causes or medical treatments. After the age of 75 years, physical and cognitive assessments were performed annually. The evaluation included a neuropsychological battery (32), neurologic examination, medication review, and informant-/subject-structured interview. The latter was based on the Clinical Dementia Rating scale (33, 34) for the years between 1998 and 2005 and the Dementia Questionnaire (35) prior to 1998. All subjects were followed annually and were reviewed at a diagnostic consensus conference if their Blessed Information Memory Concentration score (36) was 3 or greater, if their informant or subject Clinical Dementia Rating score was 0.5 or greater, or if their Dementia Questionnaire was abnormal. All subjects, regardless of the screening tests, were evaluated at a diagnostic conference at the time of entry into the autopsy program and at withdrawal or death. The full panel of neuropsychological diagnostic tests and clinical data were available for review at the diagnostic conference. Diagnosis of dementia was based on Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition criteria (37). A diagnosis of cognitive impairment not meeting criteria for dementia, defined as MCI, was based on the Mayo Clinic criteria (38). Accordingly, the diagnosis of MCI was given to subjects who had deficits limited to 1 or 2 areas of cognition (usually memory), with preservation of normal activities of daily living compared with other people of similar age.
Neuropathology Methods and Diagnostic Criteria
All brains were examined in the Division of Neuropathology of the Johns Hopkins University. After weighing and external examination, the right hemibrain was cut in 1-cm coronal slabs and frozen at −80°C. The left hemibrain was fixed in 10% buffered formaldehyde for at least 2 weeks and then cut coronally. For diagnostic purposes, tissue blocks were dissected from middle frontal gyrus, superior and middle temporal gyri, inferior parietal cortex, occipital cortex, entorhinal cortex, amygdala, thalamus, basal ganglia, and cerebellum. For the specific aims of the present study, we also examined the ACG, PCG, PVC, and CA1. Tissue blocks were processed and embedded in paraffin, cut at 10 µm, and stained with hematoxylin and eosin and Hirano silver method (39) for diagnostic purposes. The severity of neuritic plaques was assigned a semiquantitative and age-adjusted score (0, A, B, or C) according to CERAD (18). The NFT stage was assigned a score (0–VI) according to Braak (19) (Table 1). Vascular lesions (infarcts, lacunes, and hemorrhages) were evaluated on all hematoxylin and eosin–stained sections. We excluded brains with infarcts, lacunes, intraparenchymal hemorrhages, and primary or metastatic brain tumors. Moreover, using immunostains, we excluded brains with α-synuclein lesions (Lewy bodies or neurites; anti–α-synuclein antibody from BD Transduction Laboratories, Palo Alto, CA; dilution, 1:500) in brainstem or cerebral cortex, tauopathies (anti-phosphorylated tau, paired helical .lament 1 clone; a gift of Dr. P. Davies, Albert Einstein College of Medicine, Bronx, NY; dilution, 1:100), and other possible etiologies of neurodegenerative disease, including Lewy body diseases, Parkinson disease, and tauopathies.
TABLE 1.
Demographic, Cognitive, and Neuropathologic Features for the 4 Groups
| Subject | Age, year | Sex | MMSE | Path Dx | CERAD* | Braak† |
|---|---|---|---|---|---|---|
| Age-Matched C | ||||||
| 1 | 92 | M | 30 | C | 0 | II |
| 2 | 79 | M | 29 | C | 0 | 0 |
| 3 | 93 | F | 30 | C | 0 | II |
| 4 | 81 | M | 30 | C | 0 | II |
| 5 | 69 | M | 29 | C | 0 | II |
| 6 | 86 | M | 30 | C | 0 | II |
| 7 | 80 | M | 29 | C | 0 | II |
| 8 | 80 | M | 29 | C | 0 | 0 |
| 9 | 71 | F | 28 | C | 0 | II |
| 10 | 84 | M | 30 | C | 0 | II |
| 11 | 99 | M | 28 | C | 0 | IV |
| 12 | 86 | M | 28 | C | 0 | IV |
| 13 | 95 | M | 30 | C | 0 | III |
| 14 | 77 | M | 30 | C | 0 | II |
| 15 | 88 | M | 30 | C | 0 | II |
| Mean ± SE | 84.0 ± 2.2 | 29.2 ± 0.2 | ||||
| ASYMAD | ||||||
| 16 | 93 | M | 30 | poAD | B | 0 |
| 17 | 83 | M | 29 | poAD | B | II |
| 18 | 91 | M | 30 | poAD | B | III |
| 19 | 79 | M | 28 | poAD | B | II |
| 20 | 96 | M | 29 | poAD | B | IV |
| 21 | 93 | M | 30 | poAD | C | IV |
| 22 | 94 | M | 30 | poAD | B | III |
| 23 | 85 | F | 29 | poAD | B | III |
| 24 | 95 | F | 28 | poAD | B | IV |
| 25 | 75 | M | 30 | poAD | C | II |
| 26 | 92 | M | 30 | poAD | B | IV |
| 27 | 94 | M | 29 | poAD | B | IV |
| 28 | 71 | M | 30 | poAD | C | III |
| 29 | 75 | M | 29 | poAD | C | IV |
| 30 | 92 | F | 30 | poAD | B | IV |
| Mean ± SE | 87.2 ± 2.1 | 29.4 ± 0.1 | ||||
| MCI | ||||||
| 31 | 77 | M | 26 | prAD | B | 0 |
| 32 | 69 | M | 27 | prAD | A | II |
| 33 | 81 | M | 29 | prAD | B | IV |
| 34 | 89 | F | 23 | prAD | B | III |
| 35 | 96 | M | 28 | prAD | B | II |
| 36 | 89 | M | 26 | prAD | B | IV |
| 37 | 87 | M | 25 | prAD | B | IV |
| 38 | 90 | M | 29 | prAD | B | IV |
| 39 | 99 | M | 22 | prAD | B | IV |
| 40 | 89 | M | 27 | poAD | A | IV |
| 41 | 90 | M | 26 | prAD | B | II |
| 42 | 88 | F | 26 | poAD | A | III |
| 43 | 82 | M | 29 | prAD | B | IV |
| 44 | 78 | M | 27 | prAD | B | IV |
| 45 | 101 | F | 19 | poAD | B | IV |
| Mean ± SE | 87.0 ± 2.2 | 25.9 ± 0.7 | ||||
| AD | ||||||
| 46 | 89 | M | 17 | deAD | C | IV |
| 47 | 96 | M | 24 | deAD | C | VI |
| 48 | 97 | M | 26 | deAD | C | II |
| 49 | 98 | M | 23 | deAD | C | IV |
| 50 | 90 | F | 17 | deAD | B | IV |
| 51 | 85 | M | 17 | deAD | B | IV |
| 52 | 95 | F | 5 | deAD | B | IV |
| 53 | 88 | M | 24 | deAD | C | VI |
| 54 | 85 | M | 10 | deAD | B | III |
| 55 | 95 | M | 26 | deAD | B | IV |
| 56 | 82 | F | 22 | deAD | C | VI |
| 57 | 92 | M | 4 | deAD | C | VI |
| 58 | 94 | F | 9 | deAD | C | VI |
| 59 | 94 | F | 23 | deAD | C | VI |
| 60 | 101 | F | 10 | deAD | B | IV |
| Mean ± SE | 92.0 ± 1.3 | 17.1 ± 2.1 |
CERAD age-adjusted neuritic plaque score.
Braak scoring for neurofibrillary tangles.
AD, Alzheimer disease; ASYMAD, asymptomatic AD; C, control; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; dead, definite AD; F, female; M, male; MCI, mild cognitive impairment; MMSE, Mini Mental State Examination; Path Dx: Pathologic diagnosis according to CERAD; poAD, possible AD; prAD, probable AD; SE, standard error.
Definition of Study Groups
Based on the clinical diagnosis within the last year of life and the neuropathologic evaluation, the brains were divided into 4 groups.
Age-Matched C Subjects
The C subjects (n = 15) had no history of cognitive and/or behavioral deficits, cerebrovascular disease, or alcohol/ drug abuse. On neuropathologic evaluation, the brains showed no diffuse Aβ plaques or neuritic plaques on Hirano silver stains. Accordingly, the CERAD neuritic plaque score was 0. Neurofibrillary changes were confined to transentorhinal, entorhinal cortex, and hippocampus; thus, their Braak NFT scores ranged between 0 and II.
ASYMAD Subjects
The ASYMAD subjects (n = 15) had no history of behavioral deficits, cerebrovascular disease, or alcohol/drug abuse, and were cognitively intact through the last cognitive and physical evaluation within 1 year before death. On neuropathologic evaluation, the brains showed diffuse and neuritic Aβ plaques, and CERAD neuritic plaque scores were B or C. The NFT Braak scores ranged from 0 to IV.
MCI Subjects
The MCI subjects (n = 15) had no history of cerebrovascular disease or alcohol/drug abuse but had impairment in either a single cognitive domain (typically memory) or 2 domains. They did not, however, manifest functional loss in activities of daily living. All MCI cases showed neuritic Aβ plaques with CERAD scores in the A to B range and NFT in Braak stages of 0 to IV. The brains were free of other potential causes of cognitive decline.
AD Subjects
The AD subjects (n = 15) were patients who had received a clinical diagnosis of dementia according to the Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition criteria and of probable AD according to the National Institute of Neurological Diseases and. Stroke–Alzheimer’s Disease and Related Disorders Association criteria (40). The neuropathologic examination of these subjects showed neuritic Aβ plaques with CERAD scores of B to C and NFTs with Braak stages of II to VI. The brains were free of other potential causes of cognitive decline.
Based on the variability of the neuronal cell bodies and nuclear volumes observed in our previous study (22), we examined 15 brains per group. To avoid a selection bias, we selected the first 15 consecutive BLSA autopsy brains that fulfilled the group criteria previously described. Demographic data for the 4 groups, -including clinical and neuropathologic information, are summarized in Table 1. Table 2 lists the cause of death for subject in all groups.
TABLE 2.
Causes of Death
| Subject | Cause of Death |
|---|---|
| Age-Matched C’s | |
| 1 | Aspiration pneumonia |
| 2 | Arrhythmia |
| 3 | Extensive acute bronchopneumonia |
| 4 | Complications of metastatic renal cell carcinoma* |
| 5 | Lung carcinoma* |
| 6 | Subdural hemorrhage |
| 7 | Atherosclerotic coronary vascular disease |
| 8 | Pneumonia |
| 9 | Metastatic breast carcinoma* |
| 10 | Multiple injuries secondary to vehicle accident |
| 11 | Cardiomegaly |
| 12 | Unknown (brain-only autopsy) |
| 13 | Ruptured aortic aneurysm |
| 14 | Suicide |
| 15 | Unknown (brain autopsy only) |
| ASYMAD | |
| 16 | Streptococcal pneumonia and sepsis |
| 17 | Prostate adenocarcinoma* |
| 18 | Prostate adenocarcinoma* |
| 19 | Unknown (brain-only autopsy) |
| 20 | Congestive heart failure |
| 21 | Acute pulmonary congestion |
| 22 | Dilated cardiomyopathy |
| 23 | Bowel obstruction, colon adenocarcinoma* |
| 24 | Pulmonary embolus |
| 25 | Pancreatic carcinoma* |
| 26 | Chronic heart disease |
| 27 | Acute cardiovascular disease |
| 28 | Gastrointestinal hemorrhage |
| 29 | Bronchopneumonia |
| 30 | Unknown (brain-only autopsy) |
| MCI | |
| 31 | Coronary artery disease |
| 32 | Colorectal adenocarcinoma* |
| 33 | Unknown (brain-only autopsy) |
| 34 | Pulmonary thromboembolism |
| 35 | Prostate carcinoma* |
| 36 | Unknown (brain-only autopsy) |
| 37 | Acute bronchopneumonia, infarct spleen |
| 38 | Acute pyelonephritis with papillary necrosis |
| 39 | Pulmonary emboli, congestive heart failure |
| 40 | Acute myocardial infarct |
| 41 | Acute bronchopneumonia |
| 42 | Pulmonary edema |
| 43 | Gastrointestinal hemorrhage |
| 44 | Congestive heart failure, pulmonary edema |
| 45 | Aspiration pneumonia |
| AD | |
| 46 | Acute pneumonia |
| 47 | Unknown (brain-only autopsy) |
| 48 | Bronchopneumonia |
| 49 | Congestive heart failure |
| 50 | Myocardial infarction |
| 51 | Congestive heart failure |
| 52 | Aspiration pneumonia |
| 53 | Acute bronchopneumonia and pulmonary emboli |
| 54 | Coronary artery arteriosclerosis |
| 55 | Purulent tracheobronchitis, bronchopneumonia |
| 56 | Pneumonia |
| 57 | Bronchopneumonia |
| 58 | Aspiration pneumonia |
| 59 | Acute bronchopneumonia |
| 60 | Bronchopneumonia |
Brain autopsy excluded the presence of metastases.
The table shows the primary cause of death as ascertained by complete autopsy. For Subjects 12 and 15 in C’s, 19 and 30 in ASYMAD, 33 and 36 in MCI, and 47 in the AD group, the immediate cause of death is unknown because autopsies in these subjects were limited to the brain.
AD, Alzheimer disease; ASYMAD, asymptomatic AD; C, control; MCI, mild cognitive impairment.
Histology Protocols for Unbiased Stereological Analyses
ACG, PCG, and PVC
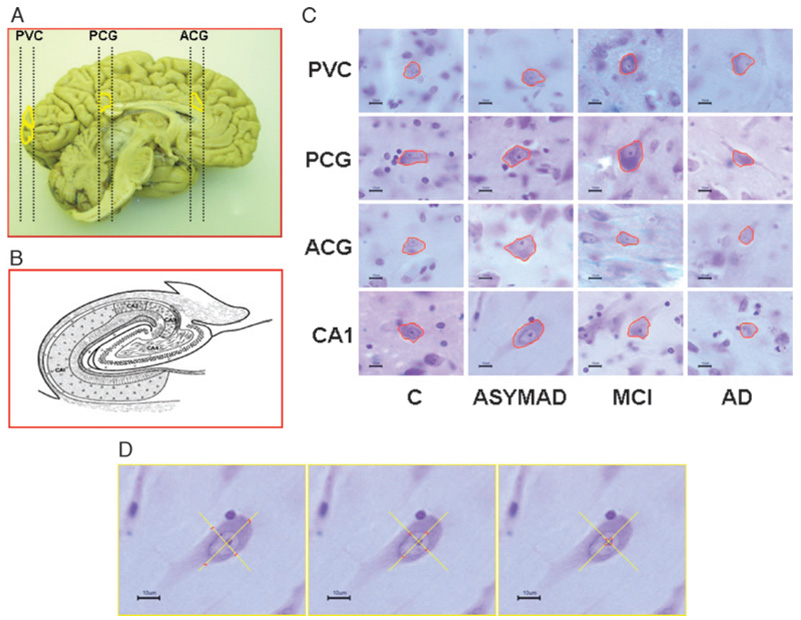
Cylindrical punches of formalin-fixed tissue (4-mm diameter and 10-mm long), including gray and subjacent white matter, were taken perpendicular to the brain surface from Brodmann Area 24 at the level of the genu of the corpus callosum (ACG), from Brodmann Area 23 at the level of the splenium of the corpus callosum (PCG), and from Brodmann Area 17 at the calcarine fissure (PVC) recognized by visualization of the stria of Gennari (Fig. 1). Vertical histologic sections, in which the orientation around the vertical axis is isotropic and the position is random, were cut according to Howard (41) and Baddeley et al (42). The tissue cylinders were longitudinally cut in 2 halves, processed and embedded in paraffin, cut serially at 50 µm (yielding ~20 sections), and stained with cresyl violet (43).
FIGURE 1.
(A) Left hemibrain from a control subject showing where tissue punches were collected from anterior cingulate gyrus (ACG), posterior cingulate gyrus (PCG), and primary visual cortex (PVC). (B) Schematic representation of hippocampal regions. (C) Cell body contours of representative neurons for each group and cerebral region in the study. To demonstrate the differences among the ACG, PCG, and PVC, all neurons depicted are from the same cortical layer (V). The scale in each image at the lower left corner is 10 µm. (D) Images showing 4 intersecting rays and the corresponding markers (from left to right) measuring the cell body, the nucleus, and the nucleolus in the same neuron.
CA1 Region
The hippocampus was already serially sectioned on the coronal plane at 50-µm thickness. For this study, we selected 2 sections at the level of the lateral geniculate body, 500 µm apart, and stained them with cresyl violet. The CA1 region was defined according to Lorente De Nó (44).
Unbiased Stereological Measurements
For the stereological analysis, we examined a subset of 10 alternate sections for ACG, PCG, and PVC, and 2 sections (500 µm apart) of CA1 with a light microscope using a 100×, NA 1.30, oil Uplan FL ∞/0.17 objective. The images were captured with a Hitachi (Tokyo, Japan) HV-C20 3CCD video camera. Using the StereoInvestigator Optical Dissector software from MicroBrightField, Inc. Biosciences (Williston, VT) (45), we outlined a contour of cortical gray matter and selected sampling sites with a 700 × 700-µm grid for the ACG, PCG and PVC, and a 250 × 250-µm grid for the CA1. The dissector height was 35 µm with a 2-µm guard zone. Using the Vertical Nucleator probe (46), we placed 4 rays through the center of the nucleolus and marked the intersections of these rays with the edge of the nucleolus, the nuclear membrane, and the cell membrane. Based on these measurements, the software calculates the volumes of the nucleolus, nucleus, and cell body, respectively (47). If a ray extended into a dendrite, the ray was cut at the base of the dendrite. Neurons were measured irrespective of their shape and cortical layer localization, that is, the entire cortical thickness, including all cortical layers and all types of neurons (pyramidal and nonpyramidal), was sampled. We measured between 201 and 280 neurons per region in each brain. Neurons were measured if their nuclear membrane intersected or touched the inclusion (green) line and excluded if their nuclear membrane intersected or touched the exclusion (red) line. The neurons measured were selected with optical dissectors so that the selection was number-weighted, not size-weighted.
Statistical Analytic Methods
Analyses were performed using SAS 9.1 software (Cary, NC) (48). One-way analyses of variance were conducted for the mean volumes of the neuronal cell body, nucleus, and nucleolus in the examined regions to compare the C group with ASYMAD, MCI, and AD groups. Comparisons between the ASYMAD and MCI groups were also made. In addition, we calculated the volumetric percentage change of the neuronal body, nucleus, and nucleolus of each group with respect to the C. One-way ANOVA was also performed to compare the neuronal cell body volumes among the 4 different anatomic regions in the C. One-way ANOVA was performed to compare demographic variables and brain weights.
To compare the severity of pathologic lesions of AD among the groups, we used categoric CERAD neuritic plaque and Braak NFT scores. The Fisher exact test was used to examine differences in the proportions of pathologic stages among the different groups.
RESULTS
Statistical analyses did not show significant differences among groups for mean age, brain weight, postmortem delay, or interval of time between last cognitive assessment and death (Fig. 2). In particular, the means for these intervals expressed in years were 1.6 for AD, 0.5 for MCI, 0.7 for ASYMAD, and 0.6 for C. These intervals are short and are similar for the last 3 groups and allow rigorous clinicopathologic correlations.
FIGURE 2.
Histograms show the mean values of age (AGE), brain weight (BW), postmortem delay (PMD), and interval between the last cognitive evaluation and death (ICD) for control (C), asymptomatic Alzheimer disease (ASYMAD), mild cognitive impairment (MCI), and definite AD (AD) groups. and definite AD (AD) The mean age ± SE was 84.0 ± 2.2 for C’s, 87.2 ± 2.1 for ASYMAD, 87.0 ± 2.2 for MCI, and 92.0 ± 1.3 for AD. The mean PMD ± SE was 17.2 ± 1.4 for C’s, 15.3 ± 0.9 for ASYMAD, 15.9 ± 1.9 for MCI, and 16.9 ± 2.1 for AD. The only significant difference was for BW between the C and AD groups.
Severity of AD Lesions in ASYMAD and MCI
Consortium to Establish a Registry for Alzheimer’s Disease and Braak scores are shown in Table 1, and comparisons among groups are shown in Table 3. The neuritic plaque scores (CERAD) in the ASYMAD group were not significantly different from the AD group, but they were significantly higher compared with MCI subjects (p < .02). The NFT scores, however, were similar in ASYMAD and MCI. Based on these scores, the levels of AD neuropathology in ASYMAD seem comparable to those in MCI.
TABLE 3.
Comparisons of CERAD NP and Braak NFT Staging Among Groups
| CERAD Classification (NP) |
||||
|---|---|---|---|---|
| A | B | C | Fisher Exact Test | |
| Comparison of ASYMAD vs MCI | ||||
| ASYMAD | 0 | 11 | 4 | p = 0.0238 |
| MCI | 3 | 12 | 0 | |
| Total | 3 | 23 | 4 | |
| Comparison ASYMAD vs AD | ||||
| ASYMAD | 0 | 11 | 4 | p = 0.1394 |
| AD | 0 | 6 | 9 | |
| Total | 0 | 17 | 13 | |
| Braak Staging (neurofibrillary tangles) | ||||
| 0–II | III–V | V–VI | ||
| Comparison ASYMAD vs MCI | ||||
| ASYMAD | 4 | 11 | 0 | Equal distribution between the 2 groups |
| MCI | 4 | 11 | 0 | |
| Total | 8 | 22 | 0 | |
| Comparison ASYMAD vs AD | ||||
| ASYMAD | 4 | 11 | 0 | p = 0.0113 |
| AD | 1 | 8 | 6 | |
| Total | 5 | 19 | 6 | |
The statistical analysis of the CERAD and Braak NFT scores using the Fisher test shows that the ASYMAD group is not significantly different from the AD group in CERAD neuritic plaque classification, whereas it is different in the Braak NFT staging. In contrast, the ASYMAD group is significantly different from the AD group for the Braak stages but shows the same scores distribution as the MCI group. Notably, the ASYMAD group, although comparable to MCI on Braak NFT staging, shows a more severe neuritic plaque score. Thus, it seems that the severity of AD lesions in ASYMAD is comparable to or even more severe than in MCI.
AD, Alzheimer disease; ASYMAD, asymptomatic AD; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; MCI, mild cognitive improvement; NFT, neurofibrillary tangle; NP, neuritic plaque.
Regional Differences in the Expansion of Neuronal Volumes in ASYMAD and MCI
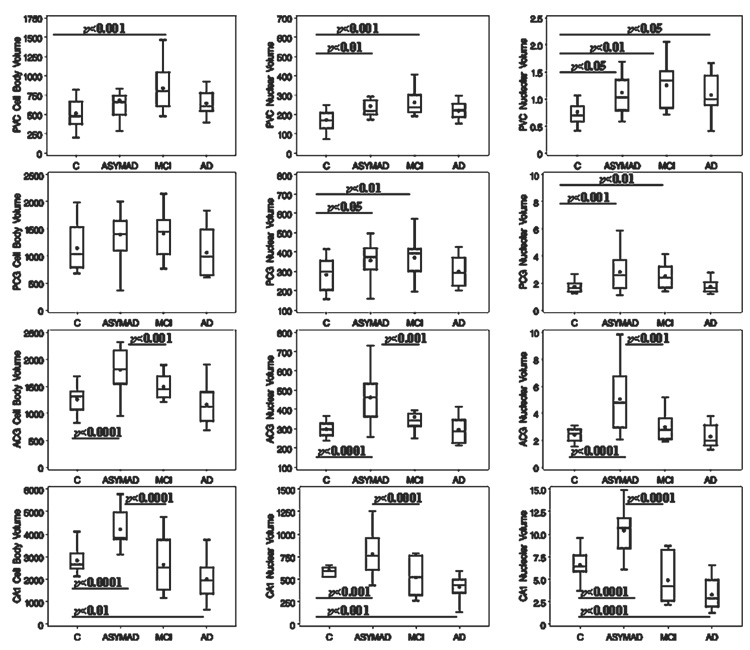
Table 4 displays the percentage variations of the volumes for the cell body, nucleus, and nucleolus in ASYMAD, MCI, and AD groups compared with C. In general, in the ASYMAD group, there was a significant increase in the volume of neuronal cell bodies, nuclei, and nucleoli of the anterior regions (CA1 and ACG) compared with C (p < 0.0001 for cell bodies in CA1 and ACG; p < 0.0001 for nuclei in CA1 and ACG; p < 0.0001 for nucleoli in CA1 and ACG). In the MCI group, expanded volumes relative to C’s were present only in the posterior regions (PCG and PVC). Accordingly, in ASYMAD, we observed a significant increase in the volumes of neuronal cell bodies, nuclei, and nucleoli in ACG and CA1 compared both with C and MCI groups. In more posterior regions (i.e. PCG and PVC), significant increases in volume were present only in the nuclei (p < 0.01 for PCG; p < 0.01 for PVC) and nucleoli (p < 0.001 for PCG; p < 0.01 for PVC) of ASYMAD compared with C’s but were absent in the cell bodies. When we compared ASYMAD versus MCI, no volumetric differences were noted in PCG or PVC. All neuronal volumes were, however, significantly increased in the PVC of MCI compared with C’s (p < 0.001 for cell body; p < 0.001 for nucleus; p < 0.01 for nucleolus; Fig. 3).
TABLE 4.
Mean ± SD and (Percentage of Control Values) for the Volumes of Cell Body, Nucleus, and Nucleolus in the 4 Groups
| Diagnosis | Cell Body | Nucleus | Nucleolus | Neurons | |
|---|---|---|---|---|---|
| CA1 | AD | 2,000.7 ± 881.1 (−29.4%) | 414.9 ± 124.9 (−30.7%) | 3.2 ± 1.6 (−50.0%) | 205.4 |
| MCI | 2,648.5 ± 1,197.0 (−6.5%) | 520.2 ± 191.2 (−13.1%) | 4.8 ± 2.4 (−25.6%) | 205.6 | |
| ASYMAD | 4,215.4 ± 700.7 (+48.7%) | 780.9 ± 231.3 (+30.4%) | 10.4 ± 1.7 (+58.6%) | 203.8 | |
| C | 2,834.07 ± 576.9 | 598.8 ± 79.3 | 6.5 ± 2.1 | 207 | |
| ACG | AD | 1,159.9 ± 356.8 (−7.7%) | 294.3 ± 65.44 (−0.8%) | 2.2 ± 0.7 (−2.27%) | 207.5 |
| MCI | 1,488.5 ± 216.3 (+18.4%) | 360.8 ± 86.22 (+21.5%) | 2.9 ± 1.2 (+23.3%) | 204.4 | |
| ASYMAD | 1,804.4 ± 413.2 (+43.5%) | 461.7 ± 128.6 (+55.5%) | 5.0 ± 2.3 (+109.2%) | 210.2 | |
| C | 1,256.7 ± 258.6 | 296.8 ± 38.17 | 2.4 ± 0.7 | 207.2 | |
| PCG | AD | 1,062.4 ± 415.3 (−7.1%) | 298.5 ± 72.7 (+5.5%) | 1.7 ± 0.4 (−0.9%) | 216.6 |
| MCI | 1,396.6 ± 397.4 (+22.0%) | 368.6 ± 91.2 (+30.2%) | 2.4 ± 0.8 (+42.2%) | 218.0 | |
| ASYMAD | 1,402.2 ± 540.6 (+22.5%) | 357.3 ± 103.4 (+26.2%) | 2.8 ± 1.9 (+63.4%) | 212.9 | |
| C | 1,144.2 ± 423.2 | 282.9 ± 82.7 | 1.7 ± 0.4 | 207.8 | |
| PVC | AD | 641.9 ± 158.1 (+29.9%) | 219.3 ± 40.8 (+31.3%) | 1.0 ± 0.5 (+44.7%) | 209.4 |
| MCI | 836.2 ± 280.9 (+69.2%) | 262.2 ± 65.6 (+57.1%) | 1.2 ± 0.4 (+68.9%) | 209.5 | |
| ASYMAD | 680.7 ± 152.3 (+37.7%) | 242.7 ± 51.9 (+45.4%) | 1.1 ± 0.8 (+50.7%) | 211.4 | |
| C | 494.0 ± 170.5 | 166.8 ± 47.1 | 0.7 ± 0.2 | 208.3 |
Mean ± SD values and percentages of control values for the cell body, nuclear, and nucleolar volumes in each group for each region: CA1, ACG, PCG, and PVC. Number of neurons is the mean number of neurons measured for each category. Significant differences relative to the control group are in bold (p < 0.01).
AD, Alzheimer disease; ACG, anterior cingulate gyrus; ASYMAD, asymptomatic AD; CA1, CA1 of hippocampus; MCI, mild cognitive improvement; PCG, cingulate gyrus; PVC, primary visual cortex
FIGURE 3.
Box plots showing the cell body, nuclear, and nucleolar volumes for each group (control [C], asymptomatic Alzheimer disease [ASYMAD], mild cognitive impairment [MCI], Alzheimer disease [AD]) in CA1, ACG, PCG, and PVC. The boxes enclose the middle half of the values bounded by upper and lower quartiles. The dots represent the means and the lines the medians. p values are indicated when statistical significance is present for any group compared with the C group and between ASYMAD and MCI groups.
Neuronal Atrophy in AD Subjects Compared With C’s
In the AD group, there was significant atrophy of neuronal cell body (p < 0.01), nucleus (p < 0.001), and nucleolus (p < 0.0001) only in CA1. Surprisingly, the nucleolar volume was still significantly enlarged in AD compared with C’s in the PVC (p < 0.05).
Analysis of Regional Neuronal Volumes in C’s
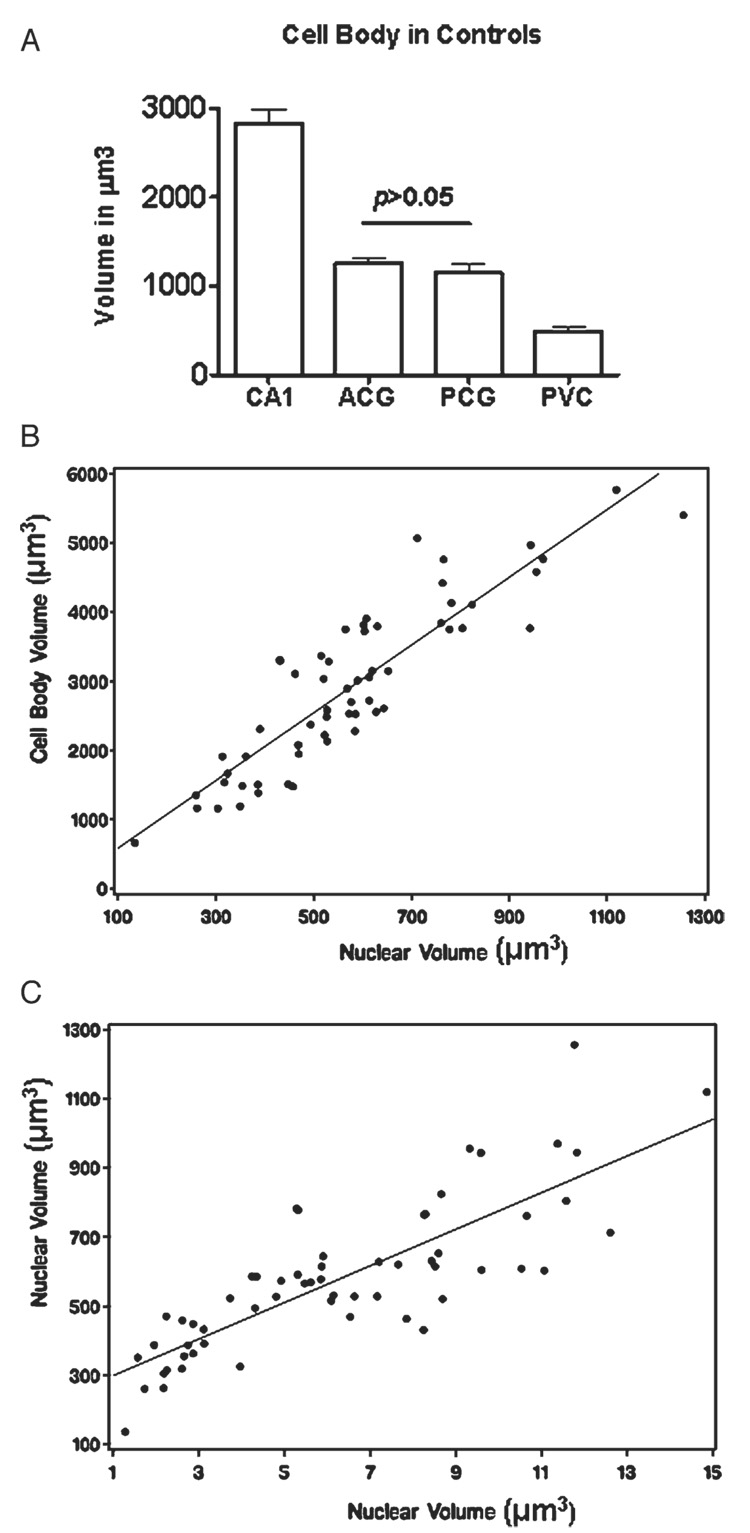
To establish a baseline on the volumes of neurons across the different regions examined, we compared the mean volumes of neuronal soma in CA1, ACG, PCG, and PVC in C. As expected, the largest volume corresponded to the pyramidal neurons of CA1, whereas the smallest volume was that of PVC neurons. The volumes of ACG and PCG were similar and intermediate between those of CA1 and PVC (Fig. 4A).
FIGURE 4.
(A). Histograms comparing the mean cell body volumes in all 4 regions examined in the control cases. Notice the absence of a significant difference between anterior (ACG) and posterior cingulate (PCG) gyri. (B) Correlation (R = 0.89) between the volumes of cell bodies and nuclei of CA1 neurons, including all subjects from the 4 groups combined. (C) Correlation (R = 0.88) between the volumes of nuclei and nucleoli, including all subjects from the 4 groups combined. CA1, CA1-hippocampus area; PVC, primary visual cortex.
Analysis of the Relationships Among the Volumes of the 3 Neuronal Compartments
To determine whether the changes in the volumes of cell bodies, nuclei, and nucleoli were interrelated, we compared the volumetric changes of these 3 compartments in the CA1 neurons of all study groups. We chose this brain region because it displayed the most prominent volumetric changes. We first analyzed the correlations between the volumes of cell bodies versus nuclei (R = 0.89) and of nuclei versus nucleoli (R = 0.88) for all subjects from the 4 study groups (Figs. 4B, C). We also analyzed the individual groups separately (data not shown). The latter analyses revealed linear correlations similar to those observed in the analyses of subjects from the 4 combined groups.
DISCUSSION
Previous morphometric studies of neurons in AD using either conventional (49) or stereological (50) methods have focused on the contrasts between the brains of demented and C subjects. In the present study, we focus on morphometric differences between ASYMAD and MCI. Our observations demonstrate striking differences in neuronal volumes in ASYMAD and MCI. These findings occur in different brain regions at different stages, however, in a manner that is consistent with the progression of AD lesions throughout the cerebral cortex (18, 19). In this context, it is important to emphasize that, although the ASYMAD and MCI groups diverge in cognitive performance, their pathologic changes of AD are similar with respect to neuritic plaques and neurofibrillary tangles (Table 1). Moreover, these 2 groups are comparable in age, and the time lapse between the last clinical evaluation and death was less than 8 months in both groups.
Group Differences in Cell Body Volume
Our data confirm that in ASYMAD, there is hypertrophy of the nuclei in neurons of the ACG and CA1 compared with age-matched C and MCI cases (22). The most remarkable observation in differences in cell body volumes was the marked hypertrophy of the neuronal somata in ASYMAD compared both with MCI and C. This finding may reflect the neuronal adaptations that allow subjects with ASYMAD to maintain normal cerebral function despite comparable numbers of AD lesions as in MCI cases. In ASYMAD, there are larger volumes of the neuronal somata both in ACG and CA1 but not in PCG or PVC. In contrast, a significant enlargement of the neuronal soma was only detected in PVC in MCI brains.
One possible interpretation of an increase in the mean volume of cell bodies in ACG and CA1 neurons in ASYMAD is that there is a selective loss of small neurons. However, because we know that the number of neurons in these subjects is stable at least in CA1 (51), true neuronal hypertrophy seems more likely. A caveat in the interpretation of the regional differences in neuronal hypertrophy, however, is the baseline size of neurons. To address this issue, we measured and compared the cell bodies of neurons in the 4 regions examined in C’s. The mean volumes of neurons in ACG and PCG were not significantly different. Therefore, the presence of neuronal hypertrophy found in ACG, but not in PCG, cannot be attributed to differences in baseline neuronal size, and a more likely explanation is that differences in neuronal size reflect the regional progression of the pathologic processes of AD.
We also observed a significant atrophy of the neuronal cell bodies in CA1 in AD but not in any of the other regions. The lack of atrophy in 3 of the regions examined is surprising, but it may be attributable to the relatively early stage of AD cases in our sample, that is, the mean period between diagnosis of dementia and death was 3 years. Notably, although most AD cases had Braak NFT scores between IV and VI, the brain weights were not significantly different compared with C’s. Our observations are consistent with a previous stereological study that found no significant difference between the sizes of the neuronal perikaryon in the neocortex of AD cases compared with C’s (50).
Group Differences in Nuclear Volume
Our results indicate that in ASYMAD, the hypertrophy of neuronal nuclei is not limited to CA1 and ACG, as we reported previously (22), but is also present in more posterior cortical regions (i.e. the PCG and PVC). In contrast, in MCI, there is significant nuclear hypertrophy only of the PCG and PVC but not in the ACG or CA1. Notably, in ASYMAD, the neuronal nuclei are significantly larger not only compared with C’s but also to MCI. Different patterns in MCI and ASYMAD are remarkable because both groups have comparable AD pathologic changes. Thus, it seems that in ASYMAD, the nuclear enlargement is somehow related to the preservation of cognitive function despite the presence of Aβ plaques and neurofibrillary changes. Moreover, the difference in the regional expression of nuclear hypertrophy among ASYMAD, MCI, and C’s is consistent with the notion that the pathologic changes of AD occur earlier in the ACG and CA1 than in the more posterior cortical regions (18).
Group Differences in Nucleolar Volume
Although larger in magnitude, the differences in the volume of nucleoli parallel those of the nuclei and cell bodies. In ASYMAD, the nucleoli were significantly larger in ACG and CA1 but not in the posterior cortical regions. In MCI, a different pattern was observed, with nucleolar hypertrophy limited to PCG and PVC. The only instance of nucleolar atrophy was in cases of AD, in which there was significant atrophy of all neuronal compartments in CA1 but not in other regions.
Relations Among the Volumes in Neuronal Compartments
Consistent with a previous study of nuclear size in cortical neurons in AD (50), we observed a general concordance among the group differences in volumes of neuronal cell bodies, nuclei, and nucleoli. In ASYMAD and MCI, we identified expansion of these volumes, whereas in AD, we observed the opposite in the hippocampus. Changes in the 3 compartments, however, may not occur at the same time. For example, the hypertrophy of nuclei and nucleoli seems to precede the enlargement of the cell bodies. This is illustrated in the PCG and PVC in ASYMAD, in which we detected hypertrophy of neuronal nuclei and nucleoli in the absence of differences in cell body size. There is growing evidence in the literature that in neurons, there is a positive linear correlation among the size of the nucleolus, nucleus, and cell body (52). Furthermore, the nucleolar volume appears as a sensitive indicator of the transcriptional and metabolic activities (53, 54). Because we found a strong positive linear correlation among the volumes of the 3 neuronal compartments (Figs. 4B, C), our analyses are consistent with these notions. In particular, nucleoli had the highest percent differences in volumes. Because the nucleolus is a key organelle for the synthesis, assembly, and production of ribosomes (55), the nucleolar hypertrophy may reflect enhanced transcriptional and biosynthetic activities. Therefore, our observations of enlarged neuronal nucleoli, nuclei, and cell bodies in ASYMAD suggest that neurons in these subjects might have been able to enhance their metabolism and delay the pathologic effects of AD. The exact nature of this adaptation and its resilience over time is uncertain. In the relation among specific AD lesions and changes in neuronal morphometry, the hypertrophy of nuclei and nucleoli in PVC cortex neurons occurs at a time when Aβ deposition and neurofibrillary/tau changes in that region are minimal or nonexistent. This apparent discrepancy can be explained by the exposure of these neurons to AD changes not directly by their perikarya in the PVC but by their projections in other cortical regions where Aβ and tau changes are already severe. The enlargement of the neuronal nucleolus, nucleus, and cell body in ASYMAD can be interpreted as the morphologic counterpart of cellular mechanisms that prevent the development of cognitive decline in some individuals despite the neurotoxic/tau effects of AD lesions.
Neurobiologic Perspectives
The neurobiologic mechanisms underlying the observed hypertrophy of neuronal cell bodies, nuclei, and nucleoli in ASYMAD are unclear and are beyond the scope of this study. We can, nevertheless, formulate some possible explanations drawing from previous studies in experimental animals and humans. Studies in animal models have shown that the cell body sizes of neurons and their nuclei and nucleoli are plastic and can be influenced by exposure to sexual hormones (56, 57), chemotherapeutic drugs (58), and regeneration after axonotomy (59) or brain trauma (60), as well as by learning and enriched environmental experiences (61). Based on these observations, we postulate that in ASYMAD and MCI, the enlargement of nuclei and nucleoli is the expression of enhanced DNA and RNA synthesis necessary to sustain the repair of injured neurons and their processes and synapses. This latter notion is further supported by a recent study showing that in the frontal cortex of patients with MCI, there is a paradoxical upregulation of presynaptic boutons (62). To sustain such synaptic upregulation, neurons may have " to enhance their overall metabolism and expand the various compartments. Once neurons cannot sustain synaptic remodeling or upregulation, functional and clinical deficits may then emerge. A second possibility is that in ASYMAD, some neurons and circuits are damaged by the AD pathology, and alternative circuits become operative (63–67). The development of these new circuits would also require additional biosynthetic activities. In this way, the circuitry of the cerebral cortex may remain functional despite the presence of AD lesions. Another alternative explanation for the enlarged neuronal compartments in ASYMAD is that these changes are a reaction to the neurotoxic effects of intracellular or extracellular Aβ-amyloid (68), or hyperphosphorylated-tau protein (69). A final, although less likely, explanation for the observed morphometric findings can be that neurons are reentering the cell cycle, a phenomenon that has been proposed in AD (70, 71).
CONCLUSIONS
We have shown that in ASYMAD, cortical and hippocampal neurons undergo significant hypertrophy of their cell bodies, nuclei, and nucleoli. Furthermore, these hypertrophic changes have a topographic gradient that follows the accepted progression of pathologic changes of AD throughout portions of the cerebral cortex. Hypertrophy of neurons in ASYMAD may represent a reaction to Aβ or tau long before the onset of cognitive decline or may constitute a surrogate for the activation of cellular processes in an attempt to forestall the progression of AD. Future understanding of the changes in gene or protein expression underlying the neuronal hypertrophy in ASYMAD may provide valuable clues to the pathogenesis and eventual therapy of AD.
ACKNOWLEDGMENTS
The authors thank Karen Wall for preparation of the article, the BLSA participants, families, and National Institute of Aging and Johns Hopkins University staff, in particular to David Dolan and Hillary Norton.
This work was supported by the Johns Hopkins University Alzheimer’s Disease Research Center (National Institutes of Health Grant No. AG 05146), the Burroughs Wellcome Fund for Translational Research, and the Intramural Research Program of the National Institute of Aging and National Institutes of Health.
REFERENCES
- 1.Masliah E, Mallory M, Hansen L, et al. Synaptic and neuritic alterations during the progression of Alzheimer’s disease. Neurosci Lett. 1994;174:67–72. doi: 10.1016/0304-3940(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 2.Tomlison BE, Blessed G, Roth M. Observations on the brains of non-demented old people. J Neurol Sci. 1968;7:331–356. doi: 10.1016/0022-510x(68)90154-8. [DOI] [PubMed] [Google Scholar]
- 3.Tomlison BE, Blessed G, Roth M. Observations on the brains of demented old people. J Neurol Sci. 1970;11:205–242. doi: 10.1016/0022-510x(70)90063-8. [DOI] [PubMed] [Google Scholar]
- 4.Bancher C, Jellinger K, Lassmann H, et al. Correlations between mental state and quantitative neuropathology in the Vienna Longitudinal Study on Dementia. Eur Arch Psychiatry Clin Neurosci. 1996;246:137–146. doi: 10.1007/BF02189115. [DOI] [PubMed] [Google Scholar]
- 5.Sze CI, Troncoso JC, Kawas C, et al. Loss of the presynaptic vesicle protein synaptophysin in hippocampus correlates with cognitive decline in Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:933–944. doi: 10.1097/00005072-199708000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Braak H, Alafuzoff I, Arzberger T, et al. Staging of Alzheimer disease–associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheff SW, Price DA, Schmitt FA, et al. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology. 2007;68:1501–1508. doi: 10.1212/01.wnl.0000260698.46517.8f. [DOI] [PubMed] [Google Scholar]
- 8.Katzman R, Terry R, DeTeresa R, et al. Clinical, pathological, and neurochemical changes in dementia: A subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1998;23:138–144. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- 9.Crystal H, Dickson D, Fuld P, et al. Clinico-pathologic studies in dementia: Nondemented subjects with pathologically confirmed Alzheimer’s disease. Neurology. 1988;38:1682–1687. doi: 10.1212/wnl.38.11.1682. [DOI] [PubMed] [Google Scholar]
- 10.Troncoso JC, Martin LJ, Dal Forno G, et al. Neuropathology in controls and demented subjects from the Baltimore Longitudinal Study of Aging. Neurobiol Aging. 1996;17:365–371. doi: 10.1016/0197-4580(96)00028-0. [DOI] [PubMed] [Google Scholar]
- 11.Price JL, Morris JC. Tangles and plaques in nondemented aging and preclinical Alzheimer’s disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 12.Knopman DS, Parisi JE, Salviati A, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003;62:1087–1095. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- 13.Mochizuki A, Peterson JW, Mufson EJ, et al. Amyloid load and neural elements in Alzheimer’s disease and nondemented individuals with high amyloid plaque density. Exp Neurol. 1996;142:89–102. doi: 10.1006/exnr.1996.0181. [DOI] [PubMed] [Google Scholar]
- 14.Arendt T. Alzheimer’s disease as a disorder of dynamic brain self-organization. Prog Brain Res. 2005;47:355–378. doi: 10.1016/S0079-6123(04)47025-3. [DOI] [PubMed] [Google Scholar]
- 15.Stern Y. The concept of cognitive reserve: A catalyst for research. J Clin Exp Neuropsychol. 2003;25:589–593. doi: 10.1076/jcen.25.5.589.14571. [DOI] [PubMed] [Google Scholar]
- 16.Scarmeas N, Stern Y. Cognitive reserve and lifestyle. J Clin Exp Neuropsychol. 2003;25:625–633. doi: 10.1076/jcen.25.5.625.14576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roe CM, Xiong C, Miller JP, Morris JC. Education and Alzheimer disease without dementia: Support for the cognitive reserve hypothesis. Neurology. 2007;68:223–228. doi: 10.1212/01.wnl.0000251303.50459.8a. [DOI] [PubMed] [Google Scholar]
- 18.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 19.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 20.Schmitt FA, Davis DG, Wekstein DR, et al. Preclinical AD revisited: Neuropathology of cognitively normal older adults. Neurology. 2000;55:370–376. doi: 10.1212/wnl.55.3.370. [DOI] [PubMed] [Google Scholar]
- 21.Benzing WC, Mufson EJ, Armstrong DM. Immunocytochemical distribution of peptidergic and cholinergic fibers in the human amygdala: Their depletion in Alzheimer’s disease and morphologic alteration in non-demented elderly with numerous senile plaques. Brain Res. 1993;625:125–138. doi: 10.1016/0006-8993(93)90145-d. [DOI] [PubMed] [Google Scholar]
- 22.Riudavets MA, Iacono D, Resnick SM, et al. Resistance to Alzheimer’s pathology is associated with nuclear hypertrophy in neurons. Neurobiol Aging. 2007;28:1484–1492. doi: 10.1016/j.neurobiolaging.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minoshima S, Giordani B, Berent S, et al. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- 24.Killiany RJ, Gomez-Isla T, Moss M, et al. Use of structural magnetic resonance imaging to predict who will get Alzheimer’s disease. Ann Neurol. 2000;47:430–439. [PubMed] [Google Scholar]
- 25.Resnick SM, Pham DL, Kraut MA, et al. Longitudinal magnetic resonance imaging studies of older adults: A shrinking brain. J Neurosci. 2003;23:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones BF, Barnes J, Uylings HB, et al. Differential regional atrophy of the cingulate gyrus in Alzheimer disease: A volumetric MRI study. Cereb Cortex. 2006;16:1701–1708. doi: 10.1093/cercor/bhj105. [DOI] [PubMed] [Google Scholar]
- 27.Barnes J, Godbolt AK, Frost C, et al. Atrophy rates of the cingulate gyrus and hippocampus in AD and FTLD. Neurobiol Aging. 2007;28:20–28. doi: 10.1016/j.neurobiolaging.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Devanand DP, Pradhaban G, Liu X, et al. Hippocampal and entorhinal atrophy in mild cognitive impairment: Prediction of Alzheimer disease. Neurology. 2007;68:828–836. doi: 10.1212/01.wnl.0000256697.20968.d7. [DOI] [PubMed] [Google Scholar]
- 29.Vogt BA, Nimchinsky EA, Vogt LJ, et al. Human cingulate cortex: Surface features, flat maps, and cytoarchitecture. J Comp Neurol. 1995;359:490–496. doi: 10.1002/cne.903590310. [DOI] [PubMed] [Google Scholar]
- 30.Vogt BA, Vogt L, Farber NB, et al. Architecture and neurocytology of monkey cingulate gyrus. J Comp Neurol. 2005;485:218–239. doi: 10.1002/cne.20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shock NW, Greulich RC, Andres R, et al. Washington, DC: U.S. Government Printing Office; Normal Human Aging: The Baltimore Longitudinal Study of Aging. 1984 NIH Publication No. 84-2450.
- 32.Kawas C, Gray S, Brookmeyer R, et al. Age-specific incidence rates of Alzheimer’s disease: The Baltimore Longitudinal Study of Aging. Neurology. 2000;54:2072–2077. doi: 10.1212/wnl.54.11.2072. [DOI] [PubMed] [Google Scholar]
- 33.Hughes CP, Berg L, Danzinger WL, et al. A new clinical scale for the staging of dementia. Br J Psychiatry. 1983;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 34.Morris JC, Ernesto C, Schafer K, et al. Clinical dementia rating training and reliability in multicenter studies: The Alzheimer’s disease cooperative study experience. Neurology. 1997;48:1508–1510. doi: 10.1212/wnl.48.6.1508. [DOI] [PubMed] [Google Scholar]
- 35.Silverman JM, Breitner JC, Mohs RC, et al. Reliability of the family history method in genetic studies of Alzheimer’s disease and related dementias. Am J Psychiatry. 1986;143:1279–1282. doi: 10.1176/ajp.143.10.1279. [DOI] [PubMed] [Google Scholar]
- 36.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral gray matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 37.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders-Third Edition-Revised (DSM-II-R) Washington, DC: American Psychiatric Press, Inc.; 1987. [Google Scholar]
- 38.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto T, Hirano A. A comparative study of modified Bielschowsky, Bodian and Thioflavin S stains on Alzheimer’s neurofibrillary tangles. Neuropathol Appl Neurobiol. 1986;12:3–9. doi: 10.1111/j.1365-2990.1986.tb00677.x. [DOI] [PubMed] [Google Scholar]
- 40.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 41.Howard C. Three Dimensional Measurements in Microscopy. New York, NY: BIOS Scientific Publishers Limited; 1998. Unbiased Stereology. [Google Scholar]
- 42.Baddeley AJ, Gundersen HJ, Cruz-Orive LM. Estimation of surface area from vertical sections. J Microsc. 1986;142:259–276. doi: 10.1111/j.1365-2818.1986.tb04282.x. [DOI] [PubMed] [Google Scholar]
- 43.Luna G. AFIP Manual of Histologic Staining Techniques. 3rd ed. New York, NY: McGraw-Hill Publications; 1968. [Google Scholar]
- 44.Lorente De Nó R. Studies on the structure of the cerebral cortex. I. The area entorhinalis. J Psychol Neurol. 1933;45:381–438. [Google Scholar]
- 45.StereoInvestigator System [computer program]. Version 7. Williston, VT: MicroBrightField, Inc. (MBF Bioscience); 2007. [Google Scholar]
- 46.Moller A, Strange P, Gundersen HJ. Efficient estimation of cell volume and number using the nucleator and the dissector. J Microsc. 1990;159:61–71. doi: 10.1111/j.1365-2818.1990.tb03019.x. [DOI] [PubMed] [Google Scholar]
- 47.Gundersen HJ. The nucleator. J Microsc. 1988;151:3–21. doi: 10.1111/j.1365-2818.1988.tb04609.x. [DOI] [PubMed] [Google Scholar]
- 48.SAS [computer program], Version 9.1. Cary, NC: SAS Institute; 2007. [Google Scholar]
- 49.Mann DM, Yates PO, Marcyniuk B. Some morphometric observations on the cerebral cortex and hippocampus in presenile Alzheimer’s disease, senile dementia of Alzheimer type and down’s syndrome in middle age. J Neurol Sci. 1985;69:139–159. doi: 10.1016/0022-510x(85)90129-7. [DOI] [PubMed] [Google Scholar]
- 50.Bundgaard MJ, Regeur L, Gundersen HJ, et al. Size of neocortical neurons in control subjects and in Alzheimer’s disease. J Anat. 2001;198:481–489. doi: 10.1046/j.1469-7580.2001.19840481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.West MJ, Kawas CH, Stewart WF, et al. Hippocampal neurons in pre-clinical Alzheimer’s disease. Neurobiol Aging. 2004;25:1205–1212. doi: 10.1016/j.neurobiolaging.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 52.Berciano MT, Novell M, Villagra NT, et al. Cajal body number and nucleolar size correlate with the cell body mass in human sensory ganglia neurons. J Struct Biol. 2007;158:410–420. doi: 10.1016/j.jsb.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 53.Moss T, Stefanovsky VY. At the center of eukaryotic life. Cell. 2002;109:545–558. doi: 10.1016/s0092-8674(02)00761-4. [DOI] [PubMed] [Google Scholar]
- 54.Raska I, Koberna K, Malinsky J, et al. The nucleolus and transcription of ribosomal genes. Biol Cell. 2004;96:579–594. doi: 10.1016/j.biolcel.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 55.Andersen JS, Lam YW, Leung AK, et al. Nucleolar proteome dynamics. Nature. 2005;433:77–83. doi: 10.1038/nature03207. [DOI] [PubMed] [Google Scholar]
- 56.Hermel EE, Ilha J, Xavier LL, et al. Influence of sex and estrous cycle, but not laterality, on the neuronal somatic volume of the posterodorsal medial amygdala of rats. Neurosci Lett. 2006;405:153–158. doi: 10.1016/j.neulet.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 57.Carrillo B, Pinos H, Guillamon A, et al. Morphometrical and neurochemical changes in the anteroventral subdivision of the rat medial amygdala during estrous cycle. Brain Res. 2007;1150:83–93. doi: 10.1016/j.brainres.2007.02.073. [DOI] [PubMed] [Google Scholar]
- 58.Jamieson SM, Liu JJ, Connor B, Dragunow M, McKeage MJ. Nucleolar enlargement, nuclear eccentricity and altered cell body immunostaining characteristics of large-sized sensory neurons following treatment of rats with paclitaxel. Neurotoxicology. 2007;28:1092–1098. doi: 10.1016/j.neuro.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 59.Vaughan DW. Effects of advancing age on the central response of rat facial neurons to axotomy: Light microscope morphometry. Anat Rec. 1990;228:211–219. doi: 10.1002/ar.1092280212. [DOI] [PubMed] [Google Scholar]
- 60.Farkas O, Povlishock JT. Cellular and subcellular change evoked by diffuse traumatic brain injury: A complex web of change extending far beyond focal damage. Prog Brain Res. 2007;161:43–59. doi: 10.1016/S0079-6123(06)61004-2. [DOI] [PubMed] [Google Scholar]
- 61.Markham JA, Greenough WT. Experience-driven brain plasticity: beyond the synapse. Neuron Glia Biol. 2004:351–363. doi: 10.1017/s1740925x05000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bell KF, Bennett DA, Cuello AC. Paradoxical upregulation of glutamatergic presynaptic during mild cognitive impairment. J Neurosci. 2007;27:10810–10817. doi: 10.1523/JNEUROSCI.3269-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woodard JL, Grafton ST, Votaw JR, et al. Compensatory recruitment of neural resources during overt rehearsal of word lists in Alzheimer’s disease. Neuropsychology. 1998;12:491–494. doi: 10.1037//0894-4105.12.4.491. [DOI] [PubMed] [Google Scholar]
- 64.Grady CL, McIntosh AR, Beig S, et al. Evidence from functional neuroimaging of a compensatory prefrontal network in Alzheimer’s disease. J Neurosci. 2003;23:986–993. doi: 10.1523/JNEUROSCI.23-03-00986.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buckner RL. Memory and executive function in aging and AD: Multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 66.Prvulovic D, Van de Ven V, Sack AT, et al. Functional activation imaging in aging and dementia. Psychiatry Res. 2005;140:97–113. doi: 10.1016/j.pscychresns.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 67.Dickerson BC, Salat DH, Greve DN, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Laferla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- 69.Yancopoulou D, Spillantini MG. Tau protein in familial and sporadic diseases. Neuromolecular Med. 2003;4:37–48. doi: 10.1385/NMM:4:1-2:37. [DOI] [PubMed] [Google Scholar]
- 70.Yang Y, Varvel NH, Lamb BT, et al. Ectopic cell cycle events link human Alzheimer’s disease and amyloid precursor protein transgenic mouse models. J Neurosci. 2006;26:775–784. doi: 10.1523/JNEUROSCI.3707-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arendt T, Bruckner MK. Linking cell-cycle dysfunction in Alzheimer’s disease to a failure of synaptic plasticity. Biochim Biophys Acta. 2007;1772:413–421. doi: 10.1016/j.bbadis.2006.12.005. [DOI] [PubMed] [Google Scholar]