Abstract
When blood is exposed to negatively charged surface materials such as glass, an enzymatic cascade known as the contact system becomes activated. This cascade is initiated by autoactivation of Factor XII and leads to both coagulation (via Factor XI) and an inflammatory response (via the kallikrein-kinin system). However, while Factor XII is important for coagulation in vitro, it is not important for physiological hemostasis, so the physiological role of the contact system remains elusive. Using patient blood samples and isolated proteins, we identified a novel class of Factor XII activators. Factor XII was activated by misfolded protein aggregates that formed by denaturation or by surface adsorption, which specifically led to the activation of the kallikrein-kinin system without inducing coagulation. Consistent with this, we found that Factor XII, but not Factor XI, was activated and kallikrein was formed in blood from patients with systemic amyloidosis, a disease marked by the accumulation and deposition of misfolded plasma proteins. These results show that the kallikrein-kinin system can be activated by Factor XII, in a process separate from the coagulation cascade, and point to a protective role for Factor XII following activation by misfolded protein aggregates.
Introduction
The contact system is an enzymatic cascade in blood that becomes activated when blood contacts surface materials. For example, this occurs during blood sampling in a glass or plastic vial. The system consists of the zymogens Factor XII (FXII), FXI, and prekallikrein (PK) and the nonenzymatic cofactor high-molecular-weight kininogen (HK). After activation of FXII into activated FXII (FXIIa), 2 strikingly different events can be triggered: (a) propagation of the intrinsic pathway of coagulation by activation of FXI; and (b) activation of the kallikrein-kinin system by activation of PK. Although the contact system is activated by a large number of mainly nonphysiological surfaces in vitro, the role of this system is still elusive.
Blood coagulation proceeds via extrinsic and intrinsic pathways; activation of the extrinsic pathway occurs via exposition of tissue factor at a site of injury and plays a critical role in physiological hemostasis. The intrinsic pathway of coagulation also has the formation of fibrin as its final consequence, and deficiencies of factors in this cascade (such as FVIII, FIX, and FXI) lead to bleeding disorders. This shows that the intrinsic pathway of coagulation contributes to physiological hemostasis. Paradoxically, deficiencies in the primary contact factors FXII, PK, or HK, which activate the intrinsic pathway of coagulation in vitro, are not associated with a bleeding phenotype. This suggests that these 3 factors do not contribute to the physiological role of the intrinsic pathway of coagulation. This contradiction was explained after the identification of an alternative route for the activation of FXI: thrombin, generated by the extrinsic pathway (resulting from exposure of tissue factor to plasma) activates FXI even in the absence of a negatively charged surface (1–3). The discovery that FXI is activated in an FXII-independent manner adds to our understanding of the intrinsic pathway of coagulation in hemostasis, but still leaves FXII without a physiological function.
Activation of the kallikrein-kinin system by FXIIa leads to release of the peptide hormone bradykinin (BK) from HK, which regulates inflammation, blood pressure control, and pain. The importance of this route is illustrated in vivo by observations in patients with hereditary angioedema (HAE). These patients experience painful swelling episodes in various tissues, in the absence of thrombosis. HAE can be caused by mutations in the C1 inhibitor gene, which encodes for the primary plasma inhibitor of a component of the complement system (C1) and the contact system components FXII (4), FXI (4), and kallikrein (5). An important role for the kallikrein-kinin system in HAE was revealed when it was found that fluids from blisters that were induced in HAE patients contained large amounts of kallikrein, whereas similar blister fluids obtained from control individuals did not (6). Additionally, a phenotype similar to HAE is seen with rare mutations in the FXII gene (7), which indicates a role for FXII in inflammatory processes (without thrombosis) in vivo.
Thus, although both the intrinsic pathway of coagulation and the kallikrein-kinin system are under the control of FXII and both are activated during surface-induced clotting, it is unclear how the 2 systems are activated in vivo and whether this occurs simultaneously.
Our aim here was to gain insight into the mechanism of FXII activation and delineate the routes to either coagulation (FXIa) and/or inflammation (kallikrein). Based on 3 lines of evidence, we hypothesized that FXII can be activated by misfolded proteins. First, FXII is activated by aggregated amyloid β peptide (Aβ) in vitro, and increased levels of activated FXII are observed in patients with Alzheimer’s disease (AD) (8–11). Second, it has become apparent over recent years that misfolded proteins, in contrast to native proteins, have structural and functional properties similar to those of amyloid (12). Third, adsorption of proteins to nonphysiological surfaces leads to structural perturbations and misfolding (13–20).
To test this hypothesis, we first investigated whether FXII was activated in patients affected by systemic amyloidosis, a disease in which misfolded plasma proteins of varying origin accumulate into widespread amyloid deposits. We next determined the capacity of misfolded proteins to stimulate FXII-dependent kallikrein generation with both purified proteins and plasma. Subsequently, we determined whether a misfolded protein intermediate is also required for kallikrein formation during contact activation by surfaces. We tested the procoagulant properties of misfolded proteins and determined their capacity to stimulate FXII-dependent FXIa generation. From these experiments, it became apparent that misfolded proteins predominantly stimulated FXII-dependent kallikrein generation, without generating FXIa, and this was verified in plasma of patients with systemic amyloidosis.
Results
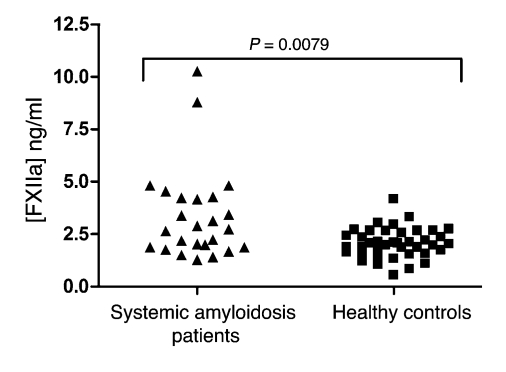
Elevated FXIIa levels in systemic amyloidosis.
The cerebrospinal fluid (CSF) of subjects with AD has been reported to contain elevated FXII activity (8, 9). FXII also colocalizes with amyloid plaques in the AD brain (11) and is activated by Aβ in vitro (10). Since we hypothesized that FXII is activated by misfolded proteins in general, we investigated whether elevated activation of FXII was limited to AD or also marked other protein misfolding diseases. Systemic amyloidosis is characterized by deposition of amyloid deposits in various sites of the body. These amyloid fibrils consist not of Aβ peptide, as is the case in AD, but of other amyloidogenic proteins, depending on the specific type of systemic amyloidosis. We found that the plasma levels of FXIIa, determined by ELISA, were significantly elevated in 25 subjects suffering from systemic amyloidosis (3.36 ± 2.18 ng/ml; expressed as mean ± SD) as compared with 40 age- and sex-matched healthy control subjects (2.07 ± 0.71 ng/ml; P = 0.0079; Figure 1). The significance of this finding was not influenced by the 2 extreme values in the patient population; omitting these values from the data set resulted in a P value of 0.0085 (data not shown). This finding indicates that FXII activation is not limited to the CSF of AD patients, but that it can also occur in plasma during another protein misfolding disease.
Figure 1. Elevated FXIIa levels in systemic amyloidosis.
Plasma samples from 25 patients with systemic amyloidosis (average age, 52 ± 11 years, 36% male) and of 40 healthy controls (average age, 49.4 ± 7.3 years, 37.5% male) were tested for levels of FXIIa by ELISA. Patients with systemic amyloidosis had significantly elevated levels of FXIIa, as compared by 2-tailed Student’s t test.
Misfolded protein aggregates, but not amyloid fibrils, induce FXII-dependent kallikrein generation in vitro.
Based on these findings in patients, we next investigated whether misfolded proteins other than Aβ are capable of inducing activation of FXII in vitro. To investigate this, we used an assay in which activation of FXII was indirectly detected via the capacity of FXIIa to convert PK into kallikrein. The generation of kallikrein was monitored via cleavage of the chromogenic substrate Chromozym PK. This assay was termed a FXII-dependent kallikrein generation assay, since omission of FXII led to complete loss of kallikrein generation in all our experiments (data not shown), indicating the critical role for FXII.
Misfolded proteins can occur in a variety of forms, such as amorphous aggregates and large amyloid fibrils (21, 22). Since both these forms are present in systemic amyloidosis patients, we also wanted to know which form of a misfolded protein induces FXII activation. The structural properties of protein preparations can be studied by their capacity to enhance fluorescence of the dyes thioflavin T (ThT) and Congo red (CR). Although ThT and CR are primarily known by their affinity for amyloid fibrils, these dyes are also known to display increased fluorescence in the presence of nonfibrillar misfolded protein aggregates (compared with native protein), which can therefore be said to have adopted amyloid-like properties (12, 23). To distinguish between fibrillar and nonfibrillar protein aggregates, protein preparations were also subjected to analysis by transmission electron microscopy (TEM).
We first prepared amyloid fibrils of Aβ (residues 1–42) and tested the ability of this preparation to stimulate FXII-dependent kallikrein generation, using an in vitro chromogenic assay that monitors the generation of active kallikrein from PK by FXIIa.
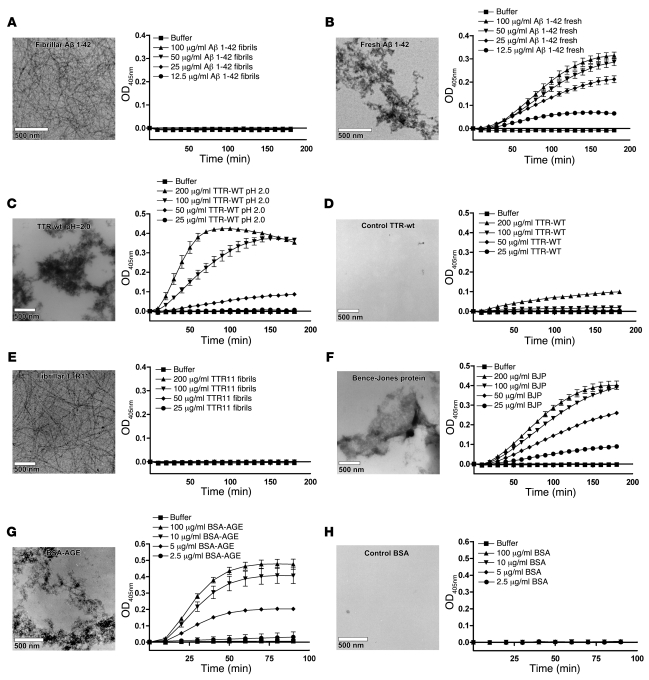
We found that amyloid fibrils of Aβ 1–42 had no capacity to induce FXII autoactivation (Figure 2A), whereas freshly dissolved Aβ 1–42 strongly induced FXII-dependent kallikrein generation (Figure 2B). Analysis by TEM revealed that freshly dissolved Aβ 1–42 contains a large amount of amorphous prefibrillar aggregates, which showed only limited fluorescence of ThT and CR (Supplemental Table 1; supplemental material available online with this article; doi: 10.1172/JCI35424DS1). The results of this experiment are in line with earlier reports on this topic (8, 10) (although no data on fibrillar Aβ was published at that time) and suggest that the FXII-activating properties of Aβ are lost upon fibril formation.
Figure 2. FXII-dependent kallikrein generation is induced by misfolded protein aggregates, but not by amyloid fibrils, in vitro.
FXII-dependent kallikrein generation was measured in vitro by a chromogenic assay. The conversion of 0.3 mM Chromozym PK in the presence of 7.7 nM PK was determined in the presence and absence of 0.97 nM FXII. None of the protein preparations tested was capable of converting Chromozym PK in the presence of PK without FXII present (data not shown). Amyloid fibrils of Aβ 1–42 did not stimulate FXII-dependent kallikrein generation (A), whereas amorphous aggregates of the freshly dissolved peptide did (B). Similarly, only amorphous aggregates of TTR (C), but not native TTR (D), or amyloid fibrils of TTR11 (E) could induce FXII-dependent kallikrein generation. Also, amorphous aggregates of Bence-Jones protein (BJP) (F) and BSA-AGE (G), but not freshly dissolved control BSA (H), could induce FXII-dependent kallikrein generation. The values in the graphs represent the mean ± SEM of duplicate determinations performed within 1 representative experiment of at least 3.
In addition to Aβ, we found that FXII-dependent kallikrein generation was also potently stimulated by misfolded aggregates of transthyretin (TTR; Figure 2C), which forms deposits in both senile and hereditary systemic amyloidosis, but not by the native protein (Figure 2D). Also, amyloid fibrils of the amyloid core fragment of TTR (TTR11; residues 105–115) were completely incapable of inducing FXII-dependent kallikrein generation (Figure 2E). Furthermore, in patients with primary systemic amyloidosis (AL), there is overproduction of immunoglobulin light chains that circulate in plasma and can be purified from urine as nonfibrillar aggregates (called Bence-Jones protein [BJP]). In line with our earlier findings, BJP aggregates were capable of activating FXII-dependent kallikrein generation (Figure 2F; 1 representative patient sample). These experiments show that FXII-dependent kallikrein generation can be initiated by pathogenic proteins in a misfolded aggregated state but not by those that have adopted an amyloid fibrillar structure.
We then studied this more extensively using various other proteins. Glycation of albumin has been reported to lead to formation of aggregated advanced glycation end products (AGEs) with amyloid-like properties (24, 25), and glycated albumin has been reported to be present in atherosclerotic plaques (26). Amorphous aggregates of glycated BSA (BSA-AGE) potently triggered FXII-dependent kallikrein generation, whereas freshly dissolved control BSA did not (Figure 2, G and H, respectively). We also prepared amorphous aggregates of glycated hemoglobin (Hb-AGE), fibrin peptide 13 (FP13), and denatured OVA (dOVA) and endostatin (dEndo), all of which had adopted properties of misfolded aggregates (Supplemental Table 1 and Supplemental Figure 1). All of them, but not their native control preparations, were capable of inducing FXII-dependent kallikrein generation (Supplemental Figure 2, A–H, respectively). Additionally, amyloid fibrils of islet amyloid polypeptide (IAPP; associated with type 2 diabetes) and glucagon were completely inert (Supplemental Figure 2, I and J).
Collectively, these experiments indicate that FXII interacts with a common binding site present in nonfibrillar misfolded protein aggregates but not in amyloid fibrils and that this interaction results in kallikrein generation.
FXII-dependent kallikrein activation by surfaces is modulated by cofactor proteins.
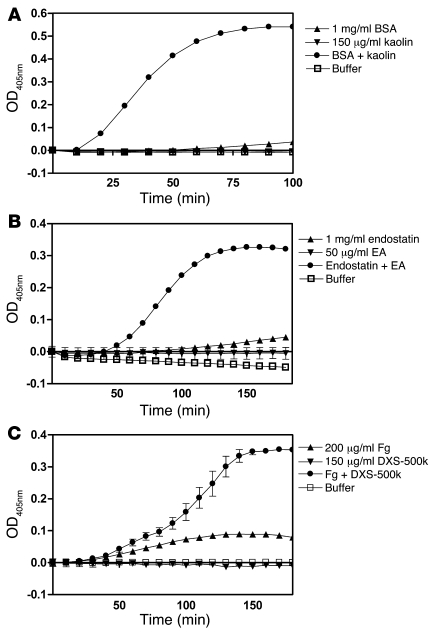
FXII autoactivates in plasma in the presence of surfaces such as kaolin, ellagic acid (EA), and dextran sulfate with an average Mr of 500 kDa (DXS-500k), leading to both coagulation and generation of kallikrein. It has been known for a long time that surfaces such as glass attract and adsorb a number of plasma proteins besides those of the contact system, most notably fibrinogen (Fg) and albumin (13, 18, 27). Given our earlier findings, we investigated whether kallikrein generation on surfaces could be mediated via generation of misfolded proteins. To test this hypothesis, we first empirically determined the surface concentrations at which the surfaces were unable to induce FXII-dependent kallikrein generation. Next, we preincubated the surfaces with freshly dissolved BSA, endostatin, and Fg in protein concentrations that gave little or no FXII-dependent kallikrein generation by themselves. In all experiments, FXII-dependent kallikrein generation drastically increased when surfaces were preincubated with either of these proteins. As shown in Figure 3A, BSA in the presence of kaolin potently induced kallikrein generation. Very similar effects were seen with DXS-500k and EA (Supplemental Figure 3, A and B). As shown in Figure 3B, endostatin in the presence of EA became a strong kallikrein generator. Again, addition of kaolin or DXS-500k to endostatin resulted in similar effects (Supplemental Figure 3, C and D). The combination of Fg and DXS-500k, compared with the individual components alone, also led to synergistic enhancement of kallikrein generation (Figure 3C), which was in agreement with our findings with the 2 other surfaces (Supplemental Figure 3, E and F). As before, no kallikrein generation was observed in any of the experiments in the absence of FXII (data not shown). Although the addition of HK was not necessary for activation of PK in the experiments discussed above (which were performed without HK), addition of HK accelerated the reactions (Supplemental Figure 4). In conclusion, all combinations of 3 contact activating surfaces with 3 proteins that are known to adsorb to negatively charged surfaces led to powerful induction of FXII-dependent kallikrein generation.
Figure 3. FXII-dependent kallikrein generation by surfaces is modulated by cofactor proteins.
Surfaces were incubated for 5 minutes at 37°C with 3 different proteins, BSA (A), endostatin (B) and Fg (C) and analyzed for their ability to induce FXII-dependent kallikrein generation. It was found that addition of these proteins was required for FXII activation by kaolin, EA, and DXS-500k. All other possible combinations of the above proteins with surfaces gave the same results (Supplemental Figure 3). The values in the graphs represent the mean ± SEM of duplicate determinations performed within 1 representative experiment of at least 3.
Proteins undergo structural perturbations in the presence of contact surfaces.
Since we had found that both misfolded protein aggregates alone and combinations of contact surfaces with cofactor proteins result in stimulation of FXII-dependent kallikrein generation, we hypothesized that proteins are being misfolded upon adsorption to contact surfaces. We therefore studied the structural effects that surfaces had on BSA using circular dichroism (CD) spectroscopy, a technique that can give insight into secondary and tertiary protein structure by making use of the optical behavior of (adsorbed) proteins under polarized light. These experiments revealed that immediate changes occur in the tertiary, but not in the secondary, structure of BSA when it binds to a surface material such as kaolin, suggesting that these changes are the minimal requirement for FXII autoactivation (Supplemental Figure 5). To substantiate this suggestion, we searched for and found proteins that did not meet these requirements (Supplemental Figure 6). Gelatin bound to kaolin without undergoing a conformational change and, as a consequence, did not activate FXII-dependent kallikrein generation. Peptides that failed to bind to kaolin (e.g., Roche Blocking Reagent) also failed to mediate FXII-dependent kallikrein generation on kaolin. It is important to note that proteins that have an altered secondary structure also induced FXII-dependent kallikrein generation (e.g., BSA-AGE; Figure 2G and Supplemental Figure 1A). However, alterations in secondary structure are always accompanied by changes in tertiary structure, thereby meeting the requirements for inducing activation of FXII.
Misfolded proteins induce kallikrein generation in plasma but are not procoagulant.
During our studies on activation of the contact system, we used a system of purified proteins to monitor FXII-dependent kallikrein generation. Based on the fact that the activation of PK is mediated by FXIIa, we first studied the possibility that the generation of FXIIa by misfolded proteins also resulted in a procoagulant effect in plasma. Although kaolin initiated a normal clotting time in an activated partial thromboplastin time–based (aPTT-based) clotting assay, no procoagulant activity of our misfolded proteins was detected under any circumstances (data not shown).
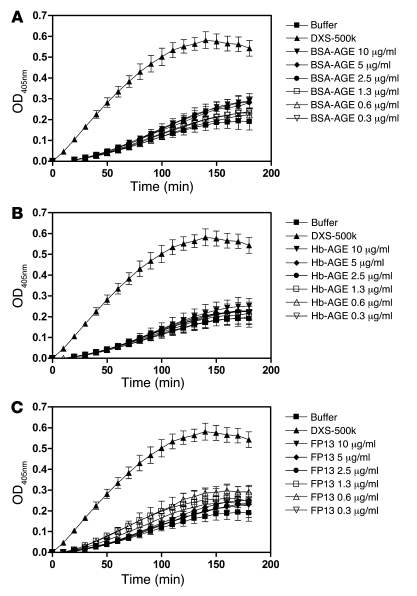
This observation raised the question of whether the FXII-dependent kallikrein generation observed earlier in our system using purified proteins was also abrogated in a plasma environment. We added misfolded or control proteins (selected by efficacy under in vitro conditions) to 1:10 diluted plasma and measured generation of kallikrein by conversion of Chromozym PK at 37°C chromogenically. These experiments showed that BSA-AGE, Hb-AGE, and FP13, but not their native controls (structural data are shown in Supplemental Figure 1), were capable of inducing kallikrein generation in a plasma environment (Supplemental Figure 7). In plasma that was deficient in FXII, no kallikrein generation could be observed, again indicating the critical role of FXII. These data showed that FXII-dependent kallikrein generation takes place when plasma is exposed to misfolded protein aggregates, but apparently this is not associated with coagulation.
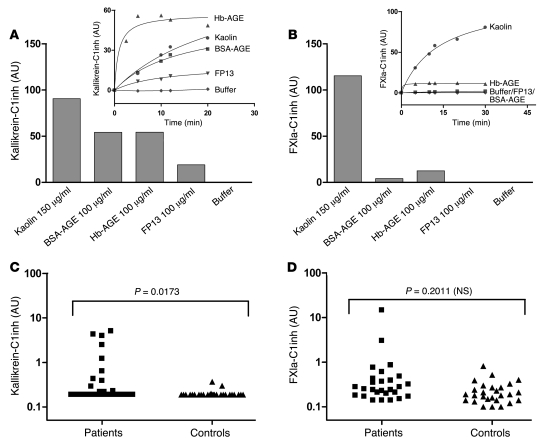
In an effort to explain the observed lack of procoagulant activity of our misfolded protein aggregates, we next studied the FXII-dependent activation of FXI. In brief, FXII that binds directly to a surface becomes activated, and this surface-bound FXIIa converts FXI into FXIa. The generation of FXIa can be monitored via cleavage of the chromogenic substrate Pefachrome XIa3371. We termed this assay a FXII-dependent FXIa generation assay, since omission of FXII led to complete loss of FXIa generation in all our experiments (data not shown), indicating the critical role for FXII. Although this assay is very similar to the kallikrein generation assay described earlier, we found it to be highly dependent on the presence of HK, whereas kallikrein generation was not. In this setup, we found that whereas DXS-500k was capable of generating FXIa in a FXII-dependent manner, none of our misfolded protein preparations had the same ability at any concentration tested (Figure 4). This experiment suggested that during exposure of plasma to misfolded proteins, kallikrein is activated by FXIIa, but this does not result in concurrent activation of FXI. This apparent discrepancy was investigated in further detail in plasma. Although determination of the activity of kallikrein and several other proteases in plasma can be performed using chromogenic substrates, amidolytic assays of FXIa activity in plasma are difficult, because of lack of sensitivity. However, activation of FXI and PK in plasma can also be measured indirectly by determination of the amount of enzyme that has been inactivated by serpins such as C1 esterase inhibitor (C1inh) (28). We looked at the amounts of both (FXIa-C1inh and kallikrein-C1inh) complexes that had been formed in citrated normal pooled plasma after it had been exposed to either kaolin or misfolded protein aggregates. Figure 5 shows that plasma that had been exposed to a contact surface, such as kaolin, contained elevated levels of both kallikrein-C1inh (Figure 5A) and FXIa-C1inh (Figure 5B) complexes, revealing activation of both coagulation and the kallikrein-kinin system. The bars in Figure 5, A and B, represent the maximal amounts of complexes that can be formed by an activator, determined by nonlinear regression of the time curves of complex formation shown in the insets. However, plasma that had been exposed to misfolded protein aggregates acted completely differently. Formation of kallikrein-C1inh complexes was observed when plasma was incubated with all 3 preparations (Figure 5A), whereas few or no FXIa-C1inh complexes were formed (Figure 5B). The generation of these complexes took place in a dose-dependent manner (Supplemental Figure 8). Together with the findings presented in Figure 4, this may explain the absence of procoagulant activity induced by misfolded proteins as measured in an aPTT-based assay and suggests that PK and FXI can be activated by FXIIa in different ways.
Figure 4. FXII-dependent activation of FXI, as measured by chromogenic assay in vitro, is not stimulated by misfolded protein aggregates.
Conversion of the chromogenic substrate Pefachrome XIa3371 in the presence of 1 nM FXII, 10 nM FXI, and 30 nM HK was measured. As a control, either FXII or FXI was omitted from the experiments. Although 2.5 μg/ml DXS-500k induced activation of FXIa in an FXII-dependent manner, as expected, misfolded protein aggregates of BSA-AGE (A), Hb-AGE (B), or FP13 (C) could not induce significant activation of FXI above buffer background at any concentration tested. Concentrations of these proteins up to 500 μg/ml were tested and found inactive (data not shown). The values in the graphs represent the mean ± SEM of duplicate determinations performed within 1 representative experiment of at least 3.
Figure 5. Misfolded proteins induce formation of kallikrein-C1inh complexes, but not FXIa-C1inh complexes, in plasma.
(A) The kallikrein-kinin system is activated in vitro when plasma is incubated with both contact surfaces and misfolded protein aggregates, as measured by levels of kallikrein-C1inh complexes. (B) Activation of coagulation, measured by plasma levels of FXIa-C1inh complexes, could only be determined in plasma that had been incubated with contact surfaces such as kaolin. The bars represent the maximal amounts of complexes that can be formed by an activator, as determined by nonlinear regression of the time curves of complex formation, shown in the insets. Determinations of these complexes in patients with systemic amyloidosis (average age, 51 ± 10 years, 40% male) show that in vivo kallikrein-C1inh complexes (C), but not FXIa-C1inh complexes (D), were significantly elevated as compared by 2-tailed Student’s t test with those in controls (average age, 49 ± 8 years, 40% male), indicating that circulating misfolded protein induces preferential activation of the kallikrein-kinin system via FXII.
Kallikrein formation, but not coagulation, is activated in protein misfolding disease.
Systemic amyloidosis is marked by a variety of hemostatic aberrances that contribute to its morbid pathology. Both thrombotic events and bleeding episodes have been described, but their origin has been poorly delineated. Since we had found that FXIIa levels are elevated in this disease (Figure 1), and FXI and kallikrein do not have to be activated simultaneously in plasma (Figure 5, A and B), we investigated the downstream effects of misfolded proteins on the activation of FXI and PK in vivo. In agreement with our in vitro studies (Figure 5A), elevated kallikrein-C1inh complex levels could be detected in 9 of 27 patients with systemic amyloidosis (0.83 ± 1.40 ng/ml; expressed as mean ± SD), compared with healthy control subjects (0.20 ± 0.04 ng/ml). This difference was statistically significant (P = 0.0173; Figure 5C). However, only 2 of 27 patients had elevated FXIa-C1inh complexes (0.94 ± 2.79 ng/ml), compared with controls (0.24 ± 0.15 ng/ml), and as a consequence, no significant difference in these complex levels could be demonstrated (P = 0.2011; Figure 5D).
Discussion
FXII was discovered in 1955 (29), when plasma from an asymptomatic individual, John Hageman (FXII is also known as Hageman factor), was found to have a profoundly prolonged clotting time in vitro due to a deficiency in this protease. After the discovery of FXII, the components and functions of the contact system were elucidated, mostly using in vitro studies. The absence of a bleeding diathesis in FXII, HK, and PK deficiencies contradicts the key role of these proteins in coagulation via the intrinsic pathway in vitro, and this has left the physiological significance of the contact system unclear.
Here, we demonstrate that FXII-dependent kallikrein generation is initiated by misfolded protein aggregates, which unexpectedly is not associated with FXIa generation, indicating that the misfolded proteins are not procoagulant (see schematic illustration, Figure 6). These observations were confirmed in vivo by the detection of elevated activation of both FXII and PK, but not FXI, in systemic amyloidosis patients. This finding implies that the kallikrein-kinin system and the intrinsic pathway of coagulation can be differentially regulated by FXII and can be activated independently of each other. However, it is in contrast to the simultaneous generation of kallikrein and FXIa when plasma is exposed to negatively charged surfaces such as kaolin that are commonly used in aPTT-based clotting assays. We show that adsorption of native proteins to surfaces such as kaolin leads to a conformational change (Supplemental Figure 5) that corresponds to the observed induction of FXII-dependent kallikrein generation (Figure 3), just as misfolded protein aggregates (in the absence of a surface) do (Figure 2). We propose that the kallikrein generation that is observed during aPTT-based clotting assays is the result of a misfolded protein cofactor; indeed, misfolded proteins in absence of a surface induce activation of PK in plasma (Figure 5 and Supplemental Figure 7). However, no such misfolded protein cofactor is required for or capable of inducing FXII-dependent FXIa formation (Figure 4).
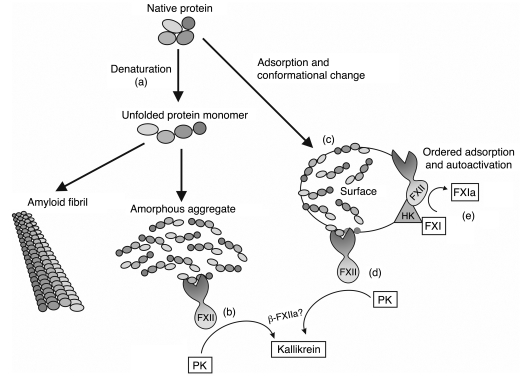
Figure 6. Activation of FXII by misfolded protein aggregates leads to specific activation of the kallikrein-kinin system.
A protein can lose its conformation in a number of ways, most notably by denaturation (a) (e.g., due to mechanical stress). Loss of conformation can lead to exposure of sites that are normally hidden within the protein molecule, e.g., due to electrostatic forces in an aqueous environment. As a result, the unfolded protein molecules will either aggregate into amorphous aggregates that can activate FXII-dependent kallikrein generation in the absence of a surface (b) or, in some cases, assemble into amyloid fibrils that do not activate this system independently. Apparently, amyloid fibrils have lost the epitope(s) required for activation of FXII. During adsorption of proteins to classical surface materials such as kaolin, several proteins also lose their native conformation (c), as was shown for BSA, Fg, and endostatin. These adsorbed proteins are able to stimulate FXII-dependent kallikrein generation (d). Misfolded proteins are not capable of inducing clotting, since they are unable to initiate FXII-dependent FXIa generation (e).
We have shown here that proteins adsorbed to a negatively charged surface induce FXII-dependent kallikrein generation and propose that this can also occur when plasma contacts a surface material. Adsorption of plasma proteins is of a transient nature, and changes can occur within a matter of seconds — a phenomenon referred to as the Vroman effect (27, 30). The sequence of adsorption and desorption events by different proteins on a surface is explained by the relative abundance and (in)stability of proteins, as has been postulated by Vroman and coworkers. The sequence is albumin, IgG, Fg/fibronectin, and HK/FXII. Based on our findings, we propose now that these transiently adsorbed proteins, such as albumin and Fg used in our studies, are responsible for the kallikrein generation that is seen within the time frame of surface-induced kallikrein formation, but not for clotting, whereas the direct adsorption of contact system proteins is responsible for the clotting response to a foreign surface.
Our findings demonstrate that FXII is able to activate either the intrinsic pathway of coagulation or the kallikrein-kinin system, based on the type activator that is present. But how can 1 protease activate these 2 proteolytic pathways separately? So far, 2 forms of activated FXII have been identified, called α-FXIIa and β-FXIIa. A recent publication elegantly demonstrates that adsorption of FXII to surfaces leads to changes in orientation and ordering of the molecules, which controls its activation (13). This activation of FXII leads to the formation of α-FXIIa, retaining its full-length molecular weight (80 kDa). In plasma, α-FXIIa can undergo a single cleavage by kallikrein, but this form remains surface bound and can activate FXI (resulting in clotting). A second form of FXIIa, called β-FXIIa, is formed when α-FXIIa is cleaved a second time by kallikrein. This 28-kDa fragment is not surface bound and has lost its capacity to induce FXIa formation but has a strong kallikrein-generating potential (31). Generation of different molecular forms of FXIIa could explain why FXII can control 2 distinct pathways in plasma, but this needs to be elucidated further.
What do our findings contribute to the understanding of the role of FXII in hemostasis? An upsurge in interest in the contact system and FXII took place recently when it was demonstrated that FXII has a role in pathological arterial thrombus formation in experimental mouse models (32), which is suggested to occur via direct interaction with platelets (33). This role of FXII in pathological thrombus formation is attributed to FXII-dependent generation of FXIa, since FXII- and FXI-knockout mice have the same phenotype (32). However, there also seems to be a role for HK in arterial thrombosis, since both kininogen (34) and BK receptor B2–deficient mice (35) are protected from thrombosis. Unfortunately, the role of plasma PK has not been elucidated in a similar fashion. Taken together, these reports suggest that there is still no clear role for the contact system (i.e., FXII, PK, and HK) in normal hemostasis. Our findings here do confirm, however, that the procoagulant role for the contact system lies in its direct interaction with a surface (at least, with respect to intrinsic coagulation), and although this system may not be physiologically relevant, it seems to play a role in thrombosis.
What are the implications of these findings? We propose that FXII-dependent activation of PK by misfolded proteins reflects a conserved protective response that is meant to perceive and clear damaged proteins in the extracellular compartment. Misfolded proteins are generated during injury and are detected by FXII, which leads to kallikrein and BK formation. Several downstream events, which include activation of ECs, are put in motion. First, BK is a potent stimulator of EC prostacyclin synthesis, NO formation, and smooth muscle hyperpolarization factor formation (36). These factors together make for a fast vasoactive and proinflammatory response. Second, the fibrinolytic system is activated by BK, which induces tissue-type plasminogen activator (tPA) release from ECs (36), while kallikrein activates both urokinase-type plasminogen activator (uPA) and plasminogen. We reported earlier that tPA-dependent plasmin formation is also stimulated by misfolded proteins (23, 37) and occurs in systemic amyloidosis (38). Activation of the fibrinolytic system has various consequences besides removal of fibrin polymers: active plasmin plays a role in inflammation (39) and wound regeneration (40). In addition, it has been reported that the crosstalk between the fibrinolytic and the kallikrein system (kallikrein-mediated plasmin formation) seems to be of importance for wound repair (41). Last, more long-term cellular events are triggered through recruitment and activation of several cell types, including macrophages as well as cells involved in adaptive immunity (42).
We here describe that misfolded protein aggregates, but not native, monomeric proteins, activate FXII, resulting in kallikrein formation. Considering the relatively wide variety of unrelated proteins that possess the capacity to trigger this system once they have adopted this aggregated structure, it is conceivable that they share a common conformation-dependent feature. Such features may resemble those of prefibrillar, oligomeric protein species that are currently held responsible for the toxicity seen in protein misfolding disease, for example, as has been described by Kayed et al. (43). It is therefore tempting to speculate that FXII may play a protective role by recognizing these potentially harmful protein species, thereby aiding in their clearance. Once proteins have assembled into amyloid fibrils, they have lost the ability to activate FXII-dependent kallikrein generation. This in turn may reflect the inability of the human body to cope with this pathological structure, which is seen in many protein misfolding diseases.
Excessive FXII-dependent kallikrein generation may have adverse consequences. A recent paper by Gao et al. elegantly demonstrates that diabetic retinopathy is caused by alkalinization of the vitreous, which in turn results in activation of the contact system (44). Generation of kallikrein and BK in turn leads to increased vascular permeability, followed by retinopathy. In similar fashion, lactic acidosis (which leads to low pH) has been described to induce activation of FXII and hypotension (45, 46). It is attractive to postulate that changes in pH lead to misfolding of proteins in vitreous, which would explain the consequent FXII and/or kallikrein generation and retinopathy. From a similar point of view, it would be of interest to investigate whether protein misfolding, FXII, and the kallikrein-kinin system are involved in the (unknown) etiology of inflammatory diseases. An example would be inflammatory bowel disease, which is in part mediated by BK and can be experimentally induced by oral administration of DXS to mice (47). Additionally, 2 recent publications have reported that the presence of the contaminant oversulfated chondroitin sulfate in certain preparations of heparin is responsible for serious adverse clinical events that are specifically attributed to activation of FXII and PK (48, 49). Our data suggests that the anaphylactoid reactions are possibly mediated via the generation of misfolded protein intermediates on this negatively charged molecule that resembles the DXS used in our study, but this remains to be elucidated.
Despite a number of recent reports on the role of FXII in (thrombotic) pathology, a number of epidemiological studies on the contact system show that FXII has a protective role in vivo (28, 50–52), which seems contradictory at first glance. In most of these studies, there seems to be a nonlinear relationship between FXII levels (or FXIIa-C1inh complexes) and disease risk. Such a phenomenon suggests that FXII fulfills more than one role in vivo: one is at risk for developing pathological problems when there is a low concentration of FXII (suggesting a protective role) but also when there is too much FXII (suggesting a pathological role). Since the pathological role of FXII has been elucidated by its crucial contribution to thrombosis (32), but not to normal hemostasis, we hereby propose that the protective role of FXII lies in its selective control over the kallikrein-kinin system. In this role, FXII recognizes misfolded proteins during tissue damage and infection and responds by activation of the kallikrein-kinin system.
In conclusion, FXII and the underlying kallikrein-kinin system are activated by misfolded proteins without induction of coagulation, which finally offers an explanation for the paradoxical role of FXII and the contact system.
Methods
Protein and sample preparations.
BSA (MP Biomedicals), endostatin (EntreMed), Fg (FIB3L; Enzyme Research Laboratories), gelatin (Merck; EMD), or Roche Blocking Reagent (Blocking Reagent for ELISA; Roche Diagnostics) were dissolved or diluted to 6 mg/ml in 10 mM HEPES, 137 mM NaCl, 4 mM KCl, pH 7.4 (HEPES-buffered saline [HBS]). The proteins were incubated for 15 minutes at 37°C before use. Kaolin (GenFarma), EA (Sigma-Aldrich), and DXS-500k (Amersham Biosciences; GE Healthcare) were dissolved in HBS to concentrations of 900, 300, and 900 μg/ml, respectively; all solutions had a neutral pH. In order to get a fine solution of EA, the suspension was incubated in a 15-ml polypropylene tube under constant rotation for 96 hours. OVA (Sigma-Aldrich) was dissolved at 1 mg/ml in HBS and incubated at 37°C for 15 minutes. Native OVA was freshly prepared for each experiment. Aggregates of misfolded OVA (dOVA) were prepared by gradual heating from 30°C to 85°C over 12 minutes, as previously published (23). Endostatin was aggregated, as previously published (53), by dialysis for 5 hours at 4°C against a 500-fold excess volume of 8 M urea in citrate phosphate buffer (17 mM citric acid, 66 mM Na2HPO4, 59 mM NaCl, pH 6.2) and dialysis against distilled H2O (3 times over 12 hours; ×2,500 excess volume). Consequently, the endostatin was dialyzed against citrate phosphate buffer twice. Due to this treatment, endostatin becomes a fine white solid that dissolves after brief incubation at 37°C. BSA was incubated at 100 mg/ml for 21 weeks in the presence of 1 M d-glucose-6-phosphate disodium salt hydrate and 0.05% NaN3. This treatment results in the formation of misfolded aggregates of BSA with amyloid-like properties (BSA-AGE) (24). After incubation, BSA-AGE was extensively dialyzed against distilled H2O. In identical fashion, 10 mg/ml of human hemoglobin (Sigma-Aldrich) was glycated for 38 weeks to yield misfolded hemoglobin with amyloid-like properties (25). Before use of Hb-AGE, all visible precipitates were removed by centrifugation at 16,000 g for 3 minutes. Misfolded protein aggregates were prepared from the fibrin-derived peptide FP13 K157D (KRLEVDIDIDIRS; synthesized by Pepscan Systems) by dissolving the peptide to 1 mg/ml in distilled water, with a similar peptide, FP10 (KRLEVDIDIK), functioning as control. Amyloid fibrils were prepared from residues 105–115 of human TTR (TTR11; YTIAALLSPYS, produced by the Peptide Synthesis Facility of the Netherlands Cancer Institute [NKI]), as previously published (54). Aβ (“Dutch type” E22Q, residues 1–42; DAEFRHDSGYEVHH-QKLVFFAEDVGSNKGAIIGLMVGGVVIA; produced by the Peptide Synthesis Facility of the NKI) was dissolved in DMSO at a concentration of 5 mM, aliquoted, and stored at –80°C. Aggregates of Aβ 1–42 were directly obtained by diluting the Aβ to 400 μg/ml in PBS. Amyloid fibrils were prepared by incubating Aβ 1–42 at 100 μM for 96 hours at pH 2.0 (adjusted with HCl) at 37°C. The solution was neutralized by dilution to 400 μg/ml in PBS. Amyloid fibrils of IAPP 1–37 were prepared as previously published (55) by incubating 1 mM IAPP, monomerized in a small volume of 1,1,1,3,3,3-hexafluoro-isopropanol (HFIP) prior to use, in HBS at 37°C for 24 hours. Amyloid fibrils of glucagon (Glucagen; Novo Nordisk) were prepared by incubation at 5 mg/ml at 37°C in 0.01 M HCl for 48 hours, as previously published (56). Recombinant human WT TTR (TTR-WT) was unfolded by dialysis versus 10 mM HCl in distilled H2O (TTR-WT pH 2.0) at 4°C for 96 hours. Aggregation was induced by addition of 25 μl 4 M NaCl to a 1,000-μl sample, giving a final NaCl concentration of 100 mM. Incubation of this sample for 24 hours results in protofibril formation (57). The aggregated TTR-WT pH 2.0 was dialyzed 3 times against HBS at 4°C.
Free immunoglobulin light chains, isolated from the urine of patients with myeloma-related amyloidosis, were kindly provided by B.J.E.G. Bast (University Medical Center, Utrecht, The Netherlands) and F.A.M. Redegeld (Utrecht Institute for Pharmaceutical Sciences, Utrecht University, Utrecht, The Netherlands). To exclude possible effects of LPS (58), protein preparations were routinely tested for endotoxin by Endosafe kit (Charles River Laboratories), according to the manufacturer’s instructions.
Chromogenic FXII-dependent kallikrein generation assay.
The conversion of the chromogenic substrate Chromozym PK (Roche Diagnostics) by kallikrein was measured at 405 nm and 37°C in a SpectraMax 340 microplate reader (Molecular Devices), in Costar 2595 microtiter plates. All measurements were performed in duplicate and repeated at least 3 times. The plates were blocked with 2% BSA in HBS 0.1% Tween-20 for 1 hour at room temperature where indicated. In all experiments, omission of FXII resulted in abrogated kallikrein generation. In the absence of PK, no conversion of the chromogenic substrate could be observed. Experiments contained 0.3 mM Chromozym PK and 5.8 μM ZnCl2 in the presence of 7.7 nM PK (Calbiochem; EMD Biosciences) and 0.97 nM FXII (Calbiochem; EMD Biosciences), buffered by HBS. The positive control was 100 μg/ml BSA-AGE, and the negative control was buffer alone.
Chromogenic FXII-dependent FXIa generation assay.
As in the kallikrein generation assay, conversion of the chromogenic substrate Pefachrome XIa3371 (Pentapharm) in the presence of 1 nM FXII, 10 nM FXI, and 30 nM HK was measured in Costar 2595 microtiter plates that were blocked with 2% BSA in HBS 0.1% Tween-20 for 1 hour at room temperature. As a control, either FXII or FXI were omitted from the experiments.
Plasma clotting assay.
aPTT-based clotting assays were performed in a KC-10 coagulometer (Amelung) by incubating citrated normal pooled plasma with proteins of interest (or a final concentration of 150 μg/ml kaolin) and 10 μM phospholipid vesicles for 3 minutes, after which we added 8.3 mM CaCl2 to initiate clotting.
Kallikrein generation in plasma.
The generation of plasma kallikrein was determined in triplicate in a 1:10 final dilution normal pooled plasma and FXII-deficient plasma (American Diagnostica Inc.) by chromogenic assay. All proteins and contact surfaces as well as plasma were prepared or diluted in HBS. Kallikrein generation, measured at 405 nm and 37°C in a SpectraMax 340 microplate reader, was determined by conversion of 0.36 mM Chromozym PK.
Plasma FXIIa ELISA.
Systemic amyloidosis patients were recruited at Groningen University Medical Center and Utrecht University Medical Center as described previously (38, 59, 60). The Institutional Review Boards of Groningen University Medical Center and Utrecht University Medical Center approved this study, and informed consent was obtained from all patients. Our patients included 11 individuals with primary systemic amyloidosis, 10 with secondary systemic amyloidosis (AA), and 4 with the hereditary form (ATTR). Plasma levels of FXIIa were determined in duplicate in citrated plasma by a commercially available ELISA kit (Axis-Shield).
Kallikrein-C1inh and FXIa-C1inh complexes in plasma.
Complexes of kallikrein and FXIa with C1inh were measured by ELISA in both pretreated normal pooled plasma and plasma from systemic amyloidosis patients, as previously published (28). In brief, C1inh was captured with a coated anti-C1inh antibody; subsequently, complexes were detected using antibodies against kallikrein or FXI. In experiments where plasma was incubated with contact surfaces or misfolded protein aggregates, a 1:1 dilution of plasma in HBS (containing the activator) was incubated in Eppendorf cups at 37°C. Samples were taken over time (2 volumes) and added to 3 volumes of PBS containing 100 μg/ml soybean trypsin inhibitor (Sigma-Aldrich) and 0.05% (w/v) Polybrene (Sigma-Aldrich). Data were expressed in arbitrary units as a percentage of positive control plasma and plotted in GraphPad Prism 4 for Windows; maximal complex formation by experimental activators was determined by nonlinear regression.
Statistics.
Statistical analysis of data was performed using Graphpad Instat 3.0. Normality of distributions was assessed by the Kolmogorov-Smirnov test, after which normally distributed data sets (FXIIa levels and FXIa-C1inh complex levels) were compared using unpaired 2-tailed t test with Welch correction and abnormally distributed data sets (kallikrein-C1inh complex levels) using a nonparametric 2-tailed t test (Mann-Whitney). Levels were considered elevated when they were greater than the sum of the mean value of age- and sex-matched healthy controls and 3 standard deviations. Data were plotted in GraphPad Prism 4 for Windows.
ThT and CR fluorescence assay.
Fluorescence of ThT (Sigma-Aldrich) and CR (Sigma-Aldrich) was measured on a Fluoroskan Ascent 2.5 Microplate Fluorimeter (Thermo Fisher Scientific) in black microtiter plates (Greiner Bio-One). The excitation wavelengths were 435 nm and 550 nm, whereas emission wavelengths were 485 nm and 595 nm, for ThT and CR respectively. Fluorescence of 25 μM ThT or 25 μM CR was measured in triplicate at a protein concentration of 100 μg/ml HBS. Background fluorescence of both protein in buffer and dye solution were subtracted from the total fluorescence signal. Fibrillar Aβ (“Dutch type” E22Q, residues 1–40; DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV; produced by the Peptide Synthesis Facility of the NKI) was used as a positive control in all fluorescence assays.
TEM.
Formvar/carbon-coated 100-mesh copper grids were placed on top of 5-μl drops of protein solution for 5 minutes. All amyloid fibrillar preparations were analyzed at stock solution concentrations (described above in Protein and sample preparations). For comparisons between native and treated protein solutions, TEM analyses were performed with grids that were prepared from solutions with equal protein concentrations. Unless described otherwise, this was done by diluting samples to 1 mg/ml in HBS. The grids were washed by placing them on a 100-μl drop of PBS and 3 drops of H2O, at each step incubating for 2 minutes. The grids were stained with 2% (mass/vol) methylcellulose with 0.4% uranyl acetate pH 4.0 for 2 minutes. Afterward, analyses were performed on a JEOL 1200 EX Transmission Electron Microscope, and electron micrographs were made at a ×10,000 enlargement. Control grids were made that had been incubated with buffer alone.
CD.
Far-UV CD was used to monitor changes in secondary structure induced by contact surfaces. Spectra were obtained using a Jasco J-810 Spectropolarimeter connected to a Jasco Peltier CDF-426S. The ellipticity was measured in a quartz cuvette with a pathway length of 1 mm (Hellma) between 190 and 260 nm in 1-nm steps (100 nm per minute), with a 1-second response time at standard sensitivity. To monitor changes in tertiary structure, near-UV CD was determined in a 1-cm quartz cuvette between 270 and 310 nm (Hellma) under the same conditions as used for far-UV CD. The experiments were performed by mixing 6 mg/ml of protein in a 1:1 ratio with 900 μg/ml of kaolin or DXS-500k or 300 μg/ml of EA in HBS. After mixing, samples were diluted in distilled water. For far-UV CD, a BSA and gelatin concentration of 250 μg/ml was used. For near-UV CD, 1.5 mg/ml of BSA or 6 mg/ml of gelatin was used. Spectra were recorded at ambient temperature for BSA and 37°C for gelatin. Before analysis of the spectra, a blank, containing all components except protein, was subtracted from the corresponding samples.
Kaolin binding experiment.
Kaolin was incubated with solutions of BSA, endostatin, Fg, gelatin, or Roche Blocking Reagent in Eppendorf cups for 5 minutes under conditions that support protein cofactor activity of albumin and Fg on kaolin (3 mg/ml protein, 450 μg/ml kaolin, buffered by HBS at 37°C). After incubation, kaolin was separated from the protein solution by centrifugation (3 minutes maximal speed), and the supernatant was removed by pipetting. Next, the kaolin pellets were washed by resuspension in 700 μl HBS, followed by centrifugation. This step was repeated 2 more times, after which the pellets were analyzed for protein binding by protein assay according to the manufacturer’s instructions (Cytoskeleton).
Supplementary Material
Acknowledgments
The authors would like to thank C. Weeterings, R. Urbanus, and M. Roest for valuable discussion. Additionally, we would like to thank B.J.E.G. Bast and F.A.M. Redegeld for providing Bence-Jones protein. This work was supported in part by a grant provided by the Dutch Thrombosis Foundation.
Footnotes
Nonstandard abbreviations used: Aβ, amyloid β peptide; AD, Alzheimer’s disease; AGE, advanced glycation end product; aPTT, activated partial thromboplastin time; BK, bradykinin; BSA-AGE, glycated BSA; C1inh, C1 esterase inhibitor; CD, circular dichroism; CR, Congo red; DXS, dextran sulfate; DXS-500k, DXS with an average Mr of 500 kDa; EA, ellagic acid; Fg, fibrinogen; FP, fibrin peptide; FXII, factor XII; FXIIa, activated FXII; HAE, hereditary angioedema; Hb-AGE, glycated hemoglobin; HBS, HEPES-buffered saline; HK, high-molecular-weight kininogen; IAPP, islet amyloid polypeptide; PK, prekallikrein; TEM, transmission electron microscopy; ThT, thioflavin T; TTR, transthyretin.
Conflict of interest: Barend Bouma and Martijn F.B.G. Gebbink are employees and shareholders of Crossbeta Biosciences BV, a biotechechnology company developing diagnostics and therapeutics for protein misfolding diseases. Bettina Schiks is an employee of Crossbeta Biosciences. Bonno N. Bouma is a shareholder of Crossbeta Biosciences.
Citation for this article: J. Clin. Invest. 118:3208–3218 (2008). doi:10.1172/JCI35424
See the related Commentary beginning on page 3006.
References
- 1.Bouma B.N., von dem Borne P.A., Meijers J.C. Factor XI and protection of the fibrin clot against lysis — a role for the intrinsic pathway of coagulation in fibrinolysis. Thromb. Haemost. 1998;80:24–27. [PubMed] [Google Scholar]
- 2.Gailani D., Broze G.J., Jr. Factor XI activation in a revised model of blood coagulation. Science. 1991;253:909–912. doi: 10.1126/science.1652157. [DOI] [PubMed] [Google Scholar]
- 3.Naito K., Fujikawa K. Activation of human blood coagulation factor XI independent of factor XII. Factor XI is activated by thrombin and factor XIa in the presence of negatively charged surfaces. J. Biol. Chem. 1991;266:7353–7358. [PubMed] [Google Scholar]
- 4.Forbes C.D., Pensky J., Ratnoff O.D. Inhibition of activated Hageman factor and activated plasma thromboplastin antecedent by purified serum C1 inactivator. J. Lab. Clin. Med. 1970;76:809–815. [PubMed] [Google Scholar]
- 5.van der Graaf F., Koedam J.A., Bouma B.N. Inactivation of kallikrein in human plasma. J. Clin. Invest. 1983;71:149–158. doi: 10.1172/JCI110743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curd J.G., Prograis L.J., Jr., Cochrane C.G. Detection of active kallikrein in induced blister fluids of hereditary angioedema patients. . J. Exp. Med. 1980;152:742–747. doi: 10.1084/jem.152.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cichon S., et al. Increased activity of coagulation factor XII (Hageman factor) causes hereditary angioedema type III. Am. J. Hum. Genet. 2006;79:1098–1104. doi: 10.1086/509899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergamaschini L., et al. Activation of the contact system in cerebrospinal fluid of patients with Alzheimer disease. Alzheimer Dis. Assoc. Disord. 1998;12:102–108. doi: 10.1097/00002093-199806000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Bergamaschini L., Donarini C., Gobbo G., Parnetti L., Gallai V. Activation of complement and contact system in Alzheimer’s disease. Mech. Ageing Dev. 2001;122:1971–1983. doi: 10.1016/S0047-6374(01)00311-6. [DOI] [PubMed] [Google Scholar]
- 10.Shibayama Y., et al. Zinc-dependent activation of the plasma kinin-forming cascade by aggregated beta amyloid protein. Clin. Immunol. 1999;90:89–99. doi: 10.1006/clim.1998.4621. [DOI] [PubMed] [Google Scholar]
- 11.Yasuhara O., Walker D.G., Mcgeer P.L. Hageman-factor and its binding-sites are present in senile plaques of Alzheimers-disease. Brain Res. 1994;654:234–240. doi: 10.1016/0006-8993(94)90484-7. [DOI] [PubMed] [Google Scholar]
- 12.Bucciantini M., et al. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature. 2002;416:507–511. doi: 10.1038/416507a. [DOI] [PubMed] [Google Scholar]
- 13.Chen X., et al. Ordered adsorption of coagulation factor XII on negatively charged polymer surfaces probed by sum frequency generation vibrational spectroscopy. Anal. Bioanal. Chem. 2007;388:65–72. doi: 10.1007/s00216-006-0999-8. [DOI] [PubMed] [Google Scholar]
- 14.Engel M.F., van Mierlo C.P., Visser A.J. Kinetic and structural characterization of adsorption-induced unfolding of bovine alpha -lactalbumin. J. Biol. Chem. 2002;277:10922–10930. doi: 10.1074/jbc.M106005200. [DOI] [PubMed] [Google Scholar]
- 15.Engel M.F., Visser A.J., van Mierlo C.P. Adsorption of bovine alpha-lactalbumin on suspended solid nanospheres and its subsequent displacement studied by NMR spectroscopy. Langmuir. 2004;20:5530–5538. doi: 10.1021/la049834b. [DOI] [PubMed] [Google Scholar]
- 16.Jungbauer A., Machold C., Hahn R. Hydrophobic interaction chromatography of proteins - III. Unfolding of proteins upon adsorption. J. Chromatogr. A. 2005;1079:221–228. doi: 10.1016/j.chroma.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Karlsson M., Ekeroth J., Elwing H., Carlsson U. Reduction of irreversible protein adsorption on solid surfaces by protein engineering for increased stability. J. Biol. Chem. 2005;280:25558–25564. doi: 10.1074/jbc.M503665200. [DOI] [PubMed] [Google Scholar]
- 18.Schmaier A.H., et al. The effect of high molecular weight kininogen on surface-adsorbed fibrinogen. Thromb. Res. 1984;33:51–67. doi: 10.1016/0049-3848(84)90154-3. [DOI] [PubMed] [Google Scholar]
- 19.Steenhoek I., Kooiman P. Adsorption of bacterial alpha-amylase on quartz and denaturation of adsorbed enzyme by shaking. Enzymologia. 1968;35:335–344. [PubMed] [Google Scholar]
- 20.Zhu M., Souillac P.O., Ionescu-Zanetti C., Carter S.A., Fink A.L. Surface-catalyzed amyloid fibril formation. J. Biol. Chem. 2002;277:50914–50922. doi: 10.1074/jbc.M207225200. [DOI] [PubMed] [Google Scholar]
- 21.Chiti F., Dobson C.M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 22.Herczenik E., Gebbink M.F.B.G. Molecular and cellular aspects of protein misfolding and disease. FASEB J. 2008;22:2115–2133. doi: 10.1096/fj.07-099671. [DOI] [PubMed] [Google Scholar]
- 23.Maas C., Hermeling S., Bouma B., Jiskoot W., Gebbink M.F. A role for protein misfolding in immunogenicity of biopharmaceuticals. J. Biol. Chem. 2007;282:2229–2236. doi: 10.1074/jbc.M605984200. [DOI] [PubMed] [Google Scholar]
- 24.Bouma B., et al. Glycation induces formation of amyloid cross-beta structure in albumin. J. Biol. Chem. 2003;278:41810–41819. doi: 10.1074/jbc.M303925200. [DOI] [PubMed] [Google Scholar]
- 25.Herczenik E., et al. Activation of human platelets by misfolded proteins. Arterioscler. Thromb. Vasc. Biol. 2007;27:1657–1665. doi: 10.1161/ATVBAHA.107.143479. [DOI] [PubMed] [Google Scholar]
- 26.Howlett G.J., Moore K.J. Untangling the role of amyloid in atherosclerosis. Curr. Opin. Lipidol. 2006;17:541–547. doi: 10.1097/01.mol.0000245260.63505.4f. [DOI] [PubMed] [Google Scholar]
- 27.Vroman L., Adams A.L., Fischer G.C., Munoz P.C. Interaction of high molecular weight kininogen, factor XII, and fibrinogen in plasma at interfaces. Blood. 1980;55:156–159. [PubMed] [Google Scholar]
- 28.Govers-Riemslag J.W., et al. The plasma kallikrein-kinin system and risk of cardiovascular disease in men. J. Thromb. Haemost. 2007;5:1896–1903. doi: 10.1111/j.1538-7836.2007.02687.x. [DOI] [PubMed] [Google Scholar]
- 29.Ratnoff O.D., Colopy J.E. A familial hemorrhagic trait associated with a deficiency of a clot-promoting fraction of plasma. J. Clin. Invest. 1955;34:602–613. doi: 10.1172/JCI103109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vroman L., Lukosevicius A. Ellipsometer recordings of changes in optical thickness of adsorbed films associated with surface activation of blood clotting. Nature. 1964;204:701–703. doi: 10.1038/204701b0. [DOI] [PubMed] [Google Scholar]
- 31.Revak S.D., Cochrane C.G., Bouma B.N., Griffin J.H. Surface and fluid phase activities of two forms of activated Hageman factor produced during contact activation of plasma. J. Exp. Med. 1978;147:719–729. doi: 10.1084/jem.147.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Renne T., Nieswandt B., Gailani D. The intrinsic pathway of coagulation is essential for thrombus stability in mice. Blood Cells Mol. Dis. 2006;36:148–151. doi: 10.1016/j.bcmd.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 33.Johne J., et al. Platelets promote coagulation factor XII-mediated proteolytic cascade systems in plasma. Biol. Chem. 2006;387:173–178. doi: 10.1515/BC.2006.023. [DOI] [PubMed] [Google Scholar]
- 34.Merkulov S., et al. Deletion of murine kininogen gene 1 (mKng1) causes loss of plasma kininogen and delays thrombosis. Blood. 2008;111:1274–1281. doi: 10.1182/blood-2007-06-092338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shariat-Madar Z., et al. Bradykinin B2 receptor knockout mice are protected from thrombosis by increased nitric oxide and prostacyclin. Blood. 2006;108:192–199. doi: 10.1182/blood-2006-01-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmaier A.H. Plasma kallikrein/kinin system: a revised hypothesis for its activation and its physiologic contributions. Curr. Opin. Hematol. 2000;7:261–265. doi: 10.1097/00062752-200009000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Kranenburg O., et al. Tissue-type plasminogen activator is a multiligand cross-beta structure receptor. Curr. Biol. 2002;12:1833–1839. doi: 10.1016/S0960-9822(02)01224-1. [DOI] [PubMed] [Google Scholar]
- 38.Bouma B., Maas C., Hazenberg B.P., Lokhorst H.M., Gebbink M.F. Increased plasmin-alpha2-antiplasmin levels indicate activation of the fibrinolytic system in systemic amyloidoses. . J. Thromb. Haemost. 2007;5:1139–1142. doi: 10.1111/j.1538-7836.2007.02457.x. [DOI] [PubMed] [Google Scholar]
- 39.Syrovets T., Simmet T. Novel aspects and new roles for the serine protease plasmin. Cell. Mol. Life Sci. 2004;61:873–885. doi: 10.1007/s00018-003-3348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J., Eriksson P.O., Hansson A., Hellstrom S., Ny T. Plasmin/plasminogen is essential for the healing of tympanic membrane perforations. Thromb. Haemost. 2006;96:512–519. [PubMed] [Google Scholar]
- 41.Lund L.R., et al. Plasminogen activation independent of uPA and tPA maintains wound healing in gene-deficient mice. EMBO J. 2006;25:2686–2697. doi: 10.1038/sj.emboj.7601173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schulze-Topphoff U., Prat A., Bader M., Zipp F., Aktas O. Roles of the kallikrein/kinin system in the adaptive immune system. Int. Immunopharmacol. 2008;8:155–160. doi: 10.1016/j.intimp.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Kayed R., et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 44.Gao B.B., et al. Extracellular carbonic anhydrase mediates hemorrhagic retinal and cerebral vascular permeability through prekallikrein activation. Nat. Med. 2007;13:181–188. doi: 10.1038/nm1534. [DOI] [PubMed] [Google Scholar]
- 45.Sonntag J., Emeis M., Strauss E., Obladen M. In vitro activation of complement and contact system by lactic acidosis. Mediators Inflamm. 1998;7:49–51. doi: 10.1080/09629359891388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sonntag J., Wagner M.H., Strauss E., Obladen M. Complement and contact activation in term neonates after fetal acidosis. Arch. Dis. Child. Fetal Neonatal Ed. 1998;78:F125–F128. doi: 10.1136/fn.78.2.f125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamata K., et al. Suppression of dextran sulfate sodium-induced colitis in kininogen-deficient rats and non-peptide B2 receptor antagonist-treated rats. Jpn. J. Pharmacol. 2002;90:59–66. doi: 10.1254/jjp.90.59. [DOI] [PubMed] [Google Scholar]
- 48.Guerrini M., et al. Oversulfated chondroitin sulfate is a contaminant in heparin associated with adverse clinical events. Nat. Biotechnol. 2008;26:669–675. doi: 10.1038/nbt1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kishimoto T.K., et al. Contaminated heparin associated with adverse clinical events and activation of the contact system. N. Engl. J. Med. 2008;358:2457–2467. doi: 10.1056/NEJMoa0803200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Doggen C.J., Rosendaal F.R., Meijers J.C. Levels of intrinsic coagulation factors and the risk of myocardial infarction among men: opposite and synergistic effects of factors XI and XII. Blood. 2006;108:4045–4051. doi: 10.1182/blood-2005-12-023697. [DOI] [PubMed] [Google Scholar]
- 51.Gallimore M.J., Harris S.L., Jones D.W., Winter M. Plasma levels of factor XII, prekallikrein and high molecular weight kininogen in normal blood donors and patients having suffered venous thrombosis. Thromb. Res. 2004;114:91–96. doi: 10.1016/j.thromres.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 52.Endler G., et al. Evidence of a U-shaped association between factor XII activity and overall survival. J. Thromb. Haemost. 2007;5:1143–1148. doi: 10.1111/j.1538-7836.2007.02530.x. [DOI] [PubMed] [Google Scholar]
- 53.Kranenburg O., et al. Recombinant endostatin forms amyloid fibrils that bind and are cytotoxic to murine neuroblastoma cells in vitro. FEBS Lett. 2003;539:149–155. doi: 10.1016/S0014-5793(03)00218-7. [DOI] [PubMed] [Google Scholar]
- 54.Jaroniec C.P., MacPhee C.E., Astrof N.S., Dobson C.M., Griffin R.G. Molecular conformation of a peptide fragment of transthyretin in an amyloid fibril. Proc. Natl. Acad. Sci. U. S. A. 2002;99:16748–16753. doi: 10.1073/pnas.252625999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Porat Y., Kolusheva S., Jelinek R., Gazit E. The human islet amyloid polypeptide forms transient membrane-active prefibrillar assemblies. Biochemistry. 2003;42:10971–10977. doi: 10.1021/bi034889i. [DOI] [PubMed] [Google Scholar]
- 56.Onoue S., et al. Mishandling of the therapeutic peptide glucagon generates cytotoxic amyloidogenic fibrils. Pharm. Res. 2004;21:1274–1283. doi: 10.1023/B:PHAM.0000033016.36825.2c. [DOI] [PubMed] [Google Scholar]
- 57.Lindgren M., Sorgjerd K., Hammarstrom P. Detection and characterization of aggregates, prefibrillar amyloidogenic oligomers, and protofibrils using fluorescence spectroscopy. Biophys. J. 2005;88:4200–4212. doi: 10.1529/biophysj.104.049700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morrison D.C., Cochrane C.G. Direct evidence for Hageman factor (factor XII) activation by bacterial lipopolysaccharides (endotoxins). J. Exp. Med. 1974;140:797–811. doi: 10.1084/jem.140.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Gameren I.I., Hazenberg B.P., Bijzet J., van Rijswijk M.H. Diagnostic accuracy of subcutaneous abdominal fat tissue aspiration for detecting systemic amyloidosis and its utility in clinical practice. Arthritis Rheum. 2006;54:2015–2021. doi: 10.1002/art.21902. [DOI] [PubMed] [Google Scholar]
- 60.Hazenberg B.P., Van Gameren I.I., Bijzet J., Jager P.L., van Rijswijk M.H. Diagnostic and therapeutic approach of systemic amyloidosis. Neth. J. Med. 2004;62:121–128. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.