Abstract
Physiologic hemostasis upon injury involves many plasma proteins in a well-regulated cascade of proteolytic reactions to form a clot. Deficiency of blood coagulation Factors VIII, IX, or XI is associated with hemophilia. Factor XII (FXII) autoactivates by contact with a variety of artificial or biologic negatively charged surfaces (contact activation), resulting in blood coagulation and activation of the inflammatory kallikrein-kinin and complement systems. However, surprisingly, individuals deficient in FXII rarely suffer from bleeding disorders. Most biologic surfaces that activate FXII become expressed in disease states. Investigators have long searched for physiologic activators of FXII and its role in vivo. In this issue of the JCI, Maas et al. show that misfolded protein aggregates produced during systemic amyloidosis allow for plasma FXIIa and prekallikrein activation and increased formation of kallikrein–C1 inhibitor complexes, without Factor XIa activation and coagulation (see the related article beginning on page 3208). This study describes a novel biologic surface for FXII activation and activity, which initiates inflammatory events independent of hemostasis.
In the early 1950s, Oscar Ratnoff and Joan Colopy observed a patient, John Hageman, whose blood, upon routine preoperative screening, was found to have prolonged clotting times in glass test tubes, even though Hageman had no history or symptoms of a bleeding disorder (1). The observation that something was missing in his blood, and that this factor changed upon exposure to glass, ushered in the notion that blood clotting factors circulate as inactive precursors that can be activated. Ratnoff, in collaboration with Earl Davie, identified that, in the disorder that became known as Hageman trait, a plasma serine protease later called Factor XII (FXII) was missing. Absence of FXII prevents the activation of the blood coagulation zymogen FXI that, when activated to become FXIa, leads to the formation of Factor IXa — a key intermediary in the intrinsic pathway of coagulation. These seminal studies contributed to the presentation of their waterfall cascade hypothesis for the blood coagulation system; a similar hypothesis was proposed that same year by Robert MacFarlane (2, 3). Ratnoff and his collaborators went on to show that FXII, which alters its physical properties during activation, induces vasodilatation and vascular permeability. These studies encapsulate the major known properties of FXII (Figure 1), a protein that autoactivates upon exposure to negatively charged surfaces to become the enzyme Factor XIIa (α-FXIIa), which then activates FXI, prekallikrein (PK), and C1 esterase (a subunit of the complement cascade). The consequence of FXI activation by α-FXIIa is the initiation of a series of proteolytic reactions resulting in thrombin generation, which precedes clot formation. α-FXIIa activation of PK forms plasma kallikrein that can reciprocally activate more FXII and liberate bradykinin from high-molecular-weight kininogen (HK). Bradykinin is a mediator of vasodilatation and increased vascular permeability (4). α-FXIIa when cleaved by plasma kallikrein forms Factor βXIIa (β-FXIIa), which then activates the macromolecular complex of the first component of complement, resulting in classic complement system activation; plasma kallikrein also directly activates complement components C3 and C5 (5, 6). Thus, the activation of FXII results in coagulation and complement activation with bradykinin liberation (Figure 1).
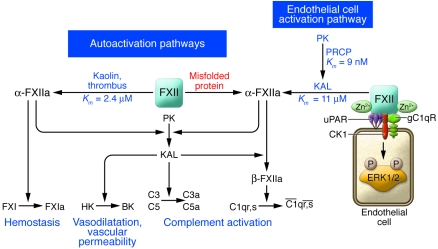
Figure 1. Mechanisms for FXII activation.
There are two pathways for FXII (Hageman factor) activation: autoactivation upon exposure to negatively charged surfaces and proteolytic activation on cell membranes. FXII autoactivates (Km = 2.4 μM) on an artificial or biologic surface such as kaolin or a thrombus to activate FXII to α-FXIIa. α-FXIIa then activates FXI to FXIa to initiate hemostasis and activates PK to form plasma kallikrein (KAL). KAL cleaves HK to liberate bradykinin, which induces vasodilatation and vascular permeability. KAL also activates the complement system by directly activating complement components C3 and C5 and cleaving α-FXIIa to form β-FXIIa (a soluble light chain enzymatic form [Hageman factor fragment]), which then activates the macromolecular C1qr,s complex to enzymatically active C1r and C1s. The results of the study by Maas et al. in this issue of the JCI (10) suggest that a second FXII autoactivation mechanism occurs upon exposure of FXII to aggregates of misfolded proteins and that this activation results in PK activation without FXI activation — showing that the kallikrein-kinin system can be activated separately from the coagulation cascade by FXII. A second pathway for FXII activation occurs on endothelial cells. PK bound to HK on endothelial cells is activated to plasma KAL by the serine protease prolylcarboxypeptidase (PRCP) (Km = 9 nM). KAL then activates FXII to α-FXIIa (Km = 11 μM). FXII also binds to endothelial cells in the presence of zinc ions and when bound stimulates ERK1/2 phosphorylation. CK1, cytokeratin 1; gC1qR, gC1q receptor; uPAR, urokinase plasminogen activator receptor.
Contact activation of Factor XII
Since FXII-deficient patients, along with PK- and HK-deficient patients, do not have a bleeding disorder, the relevance of the waterfall cascade hypothesis for blood coagulation hemostasis initiated by activated FXII has been questioned. Contact activation describes the unique property of FXII to undergo autoactivation and a change in shape when exposed to negatively charged artificial or biologic surfaces. Formation of FXIIa leads to PK activation in the presence of HK, hence the name contact activation system. However, the molecular basis for the formation of activated FXII remains unknown (7, 8). Over the last 4 decades, a growing list of “physiologic” negatively charged surfaces upon which FXII autoactivates have been recognized. In addition to nonphysiologic agents such as glass, polyethylene, and silicone rubber, FXII autoactivation occurs upon exposure to articular cartilage, skin, fatty acids, endotoxin, amyloid protein, and heparins, among others (9). It is in this context of identifying a biologic surface that supports FXII autoactivation that the study by Maas et al. in this issue of the JCI should be viewed (10). Although many biologic substances allow for FXII autoactivation, their exposure to this zymogen usually does not occur constitutively in the intravascular compartment in non-disease states. An alternative hypothesis, which bypasses any role for FXII in physiologic blood coagulation, was proposed by Gailani and Broze, who showed that, in vitro, in the presence of dextran sulfate and HK, thrombin activates FXI (11). Although questions have arisen as to whether thrombin activation of FXI can actually occur in plasma due to its high fibrinogen concentration (thrombin proteolyzes fibrinogen and FXI with the same affinity, but fibrinogen is 2 orders of magnitude more abundant than FXI in plasma), the hemostasis community has adopted this latter mechanism to explain the hemostatic activity of FXI but not FXII (12–14). This revised model of the blood coagulation cascade has left researchers puzzling as to the physiological role of FXII and how it is activated in vivo. The present report by Maas et al. (10) provides insight into a novel activity for FXII, i.e., its ability to initiate an inflammatory response in the presence of aggregates of abnormal, misfolded proteins in vivo.
Factor XII autoactivation initiated by misfolded or aggregated proteins
In the study in this issue by Maas et al. (10), the authors show that FXII autoactivation occurs in vitro in reaction to misfolded or amorphous protein aggregates of transthyretin (a homotetrameric protein in plasma and CSF), Bence-Jones protein (a monoclonal globulin protein found in blood or urine), glycated albumin, glycated hemoglobin, or the angiostatic drug endostatin (10). In individuals diagnosed with systemic amyloidosis, a condition characterized by the abnormal deposition of misfolded amyloid proteins in organs and/or tissues, the authors observed significantly elevated levels of activated FXII compared with controls, as assessed by one commercially available assay (10). The authors go on to show that contact activation initiated by FXII in reaction to these misfolded proteins is associated with elevated levels of plasma kallikrein–C1 inhibitor complexes, but not FXIa–C1 inhibitor complexes, both in vitro and in patients with systemic amyloidosis (10). This latter finding suggests that PK activation (and consequent activation of the inflammatory kallikrein-kinin and complement systems) triggered by FXII autoactivation in reaction to misfolded or amorphous protein aggregates can proceed independently of the intrinsic coagulation pathway (Figure 1).
Do the results of the Maas et al. (10) study reveal a major new pathway for FXII autoactivation and provide insight regarding a physiologic activity for FXII? What is intriguing about the current investigation is that it demonstrates constitutive FXII contact activation in the disease state of systemic amyloidosis. The data also suggest that FXII autoactivation in reaction to misfolded protein initiates an inflammatory response in reaction to these abnormal protein deposits. Activation of the so-called contact system proteins has been recognized in other pathologic states, such as acute attacks of hereditary angioedema, sepsis, diabetic retinopathy, induced arterial thrombosis, and acute myocardial infarction. However, the authors suggest that in systemic amyloidosis, there is PK activation without FXI activation (10). This contact activation–mediated disease phenotype also has been recognized in acute attacks of hereditary angioedema, where PK activation and bradykinin formation occur without FXI activation.
There are some methodological questions pertaining to the current study that should be raised (10). Since the second-order rate constants of plasma kallikrein and FXIa inhibition by C1 inhibitor are similar, but the plasma concentration of PK is 18-fold higher than that of FXI, many more subjects would have had to be studied in the FXI group to conclusively show that FXI was not activated significantly in systemic amyloidosis. Further, the higher coefficient of variation of the controls in the FXIa–C1 inhibitor assay also may have contributed to the lack of differences seen in these patient samples. The mechanism(s) by which PK, but not FXI, activation occurs in response to FXII autoactivation in reaction to misfolded proteins has not been elucidated in the current report (10). In future studies, it would be helpful to determine whether misfolded protein–triggered autoactivation of purified FXII results in reduced or absent activation of purified FXI compared with PK. Do misfolded proteins only bind HK and PK and not FXI? Finally, why do only aggregated proteins and not fibrillar proteins trigger FXII autoactivation?
FXII autoactivation in reaction to misfolded or amorphous protein aggregates in vivo, although a nonphysiologic event, may in fact constitute a type of detection or defense response to these structurally abnormal proteins. This pathway may constitute a means to regulate complement and inflammatory systems that involve coagulation protein S, thrombomodulin, and thrombin itself. Other biologic substances that have been reported to support FXII autoactivation and arise in disease states include RNA and sulfatides released after cell disruption and platelet polysomes present on a developing thrombus (15, 16). Unlike the present report (10), the previous two studies suggested that FXII autoactivation in reaction to a developing thrombus leads to FXI activation and thrombin formation (15, 16). The Maas et al. report suggests that certain biologic surfaces that trigger FXII autoactivation may lead to differential FXI or PK activation (10). Pathological contact activation in vivo is mostly associated with artificial substances interacting with the intravascular compartment. An extreme instance of this was reported recently: the infusion of unfractionated heparin adulterated with oversulfated chondroitin sulfate caused constitutional symptoms of nausea, dyspnea, and vomiting and 81 deaths from hypotension (17, 18). Oversulfated chondroitin sulfate, which in some heparin preparations was as high as 30% wt/wt, was associated with FXII autoactivation, plasma kallikrein formation, bradykinin liberation, and C3a and C5a formation, without evidence of FXI activation (18).
Physiologic activation and activities of FXII and PK
An alternative to the contact activation hypothesis for FXII activation has been proposed. On cultured endothelial cells, the serine protease prolylcarboxypeptidase produces kinetically favorable PK activation (Km = 9 nM) versus PK activation by α-FXIIa on negatively charged surfaces (Km = 2.4 μM) (Figure 1) (19, 20). Formed plasma kallikrein then leads to favorable FXII activation (Km = 11 μM). FXII activation by plasma kallikrein on cells occurs at a faster rate than autoactivation (20). FXII is a multidomain protein with a surface cell-binding region that has an EGF-like growth factor region and a catalytic domain. It is structurally similar to EGF, single chain urokinase, and tissue plasminogen activator. In the intravascular compartment, FXII binds to endothelial cell urokinase plasminogen activator receptor, cytokeratin 1, and the complement receptor gC1qR (21) (Figure 1). FXII binding to endothelial membranes is highly regulated by the plasma concentration of HK and Zn2+ (21). FXII, when bound to cells, stimulates ERK1/2 phosphorylation and [3H]thymidine uptake (22) (Figure 1).
Epidemiological studies in humans show that a polymorphism in FXII associated with lowered plasma FXII levels correlates with increased risk for myocardial infraction (23). These human clinical data do not correlate with the findings in FXII-deficient mice, which have shown reduced arterial thrombosis risk in several provocative mouse models (24). It has been proposed that the plasma kallikrein-kinin system influences thrombosis risk independent of hemostasis. Results from studies of both the kininogen- and bradykinin B2 receptor–knockout mice, in which the development of arterial thrombosis is delayed, support that assessment (25, 26).
In conclusion, the results of the present report by Maas et al. (10) indicate the existence of a pathway of FXII autoactivation upon its exposure to misfolded or aggregated proteins in the intravascular compartment or in tissues and that this pathway stimulates inflammatory systems without hemostasis activation. This pathway is not unlike that activated in acute attacks of hereditary angioedema, in which the absence of C1 inhibitor leads to increased contact system activation without hemostasis initiation. Alternatively, on a developing thrombus, FXII contributes to the size and strength of the occlusion. Additional studies are needed to better understand how there can be unique mechanisms of FXII autoactivation resulting in the formation of α-FXIIa that differentially activates its various substrates.
Acknowledgments
The author wishes to acknowledge the discoverer of Factor XII, Oscar D. Ratnoff, who died on May 20, 2008. This work is supported by grants HL052779-13, HL055709-05, and HL070766 from the National Heart, Lung, and Blood Institute.
Footnotes
Nonstandard abbreviations used: FXII, Factor XII; FXIIa, activated FXII; HK, high-molecular-weight kininogen; PK, prekallikrein.
Conflict of interest: The author has declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 118:3006–3009 (2008). doi:10.1172/JCI36617.
See the related article beginning on page 3208.
References
- 1.Ratnoff O.D., Colopy J.E. A familial hemorrhagic trait associated with a deficiency of a clot promoting fraction of plasma. J. Clin. Invest. 1955;34:602–613. doi: 10.1172/JCI103109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davie E.W., Ratnoff O.D. Waterfall sequence for intrinsic blood coagulation. Science. 1964;145:1310–1320. doi: 10.1126/science.145.3638.1310. [DOI] [PubMed] [Google Scholar]
- 3.Macfarlane R.G. An enzyme cascade in the blood clotting mechanism, and its function as a biochemical amplifier. Nature. . 1964;202:498–499. doi: 10.1038/202498a0. [DOI] [PubMed] [Google Scholar]
- 4.Rocha E., Silva M., Beraldo W.T., Rosenfeld G. Bradykinin, a hypotensive and smooth muscle stimulating factor released from plasma globulin by snake venoms and by trypsin. Am. J. Physiol. 1949;156:261–273. doi: 10.1152/ajplegacy.1949.156.2.261. [DOI] [PubMed] [Google Scholar]
- 5.Ghebrehiwet B., Silverberg M., Kaplan A.P. Activation of the classic pathway of complement by Hageman factor fragment. J. Exp. Med. 1981;153:665–676. doi: 10.1084/jem.153.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiScipio R.G. The activation of the alternative pathway C3 convertase by human plasma kallikrein. Immunology. 1982;45:587–595. [PMC free article] [PubMed] [Google Scholar]
- 7.Samuel M., Pixley R.A., Villanueva M.A., Colman R.W. Human factor XII (Hageman factor) autoactivation by dextran sulfate: circular dichroism, fluorescence, and ultraviolet difference spectroscopic studies. J. Biol. Chem. 1992;267:19691–19697. [PubMed] [Google Scholar]
- 8.Chen X., et al. Ordered adsorption of coagulation factor XII on negatively charged polymer surfaces probed by sum frequency generation vibrational spectroscopy. Anal. Bioanal. Chem. 2006;388:65–72. doi: 10.1007/s00216-006-0999-8. [DOI] [PubMed] [Google Scholar]
- 9. Schmaier, A.H. 1994. Contact activation. InThrombosis and hemorrhage. J. Loscalzo and A.I. Schafer, editors. Blackwell Publishing. Boston, Massachusetts, USA. 87–105. [Google Scholar]
- 10.Maas C., et al. Misfolded proteins activate Factor XII in humans, leading to kallikrein formation without initiating coagulation. J. Clin. Invest. . 2008;118:3208–3218. doi: 10.1172/JCI35424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gailani D., Broze G.J. Factor XI activation in a revised model of blood coagulation. Science. 1991;253:909–912. doi: 10.1126/science.1652157. [DOI] [PubMed] [Google Scholar]
- 12.Scott C.F., Colman R.W. Fibrinogen blocks the autoactivation and thrombin-mediated activation of factor XI on dextran sulfate. Proc. Natl. Acad. Sci. U. S. A. 1992;89:11189–11193. doi: 10.1073/pnas.89.23.11189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunnee T., et al. Activation of factor XI in plasma is dependent on factor XII. Blood. 1993;81:580–586. [PubMed] [Google Scholar]
- 14.Pedicord D.L., Seiffert D., Blat Y. Feedback activation of factor XI by thrombin does not occur in plasma. Proc. Natl. Acad. Sci. U. S. A. 2007;104:12855–12860. doi: 10.1073/pnas.0705566104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kannemeier C., et al. Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation. Proc. Natl. Acad. Sci. U. S. A. 2007;104:6388–6393. doi: 10.1073/pnas.0608647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith S.A., et al. Polyphosphate modulates blood coagulation and fibrinolysis. Proc. Natl. Acad. Sci. U. S. A. 2006;103:903–908. doi: 10.1073/pnas.0507195103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guerrini M., et al. Oversulfated chondroitin sulfate is a contaminant in heparin associated with adverse clinical events. Nat. Biotechnol. 2008;26:669–675. doi: 10.1038/nbt1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kishimoto T.K., et al. Contaminated heparin associated with adverse clinical events and activation of the contact system. N. Engl. J. Med. 2008;358:2457–2467. doi: 10.1056/NEJMoa0803200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shariat-Madar Z., Mahdi F., Schmaier A.H. Recombinant prolylcarboxpeptidase activates plasma prekallikrein. Blood. 2004;103:4554–4561. doi: 10.1182/blood-2003-07-2510. [DOI] [PubMed] [Google Scholar]
- 20.Rojkjaer R., Hasan A.A.K., Motta G., Schousboe I., and Schmaier A.H. Factor XII does not initiate prekallikrein activation on endothelial cells. Thromb. Haemost. 1998;80:74–81. [PubMed] [Google Scholar]
- 21.Mahdi F., Shariat-Madar Z., Figueroa C.D., Schmaier A.H. Factor XII interacts with the multiprotein assembly of urokinase plasminogen activator, gC1qR, and cytokeratin 1 on endothelial cell membranes. Blood. 2002;99:3585–3596. doi: 10.1182/blood.V99.10.3585. [DOI] [PubMed] [Google Scholar]
- 22.Gordon E.M., et al. Factor XII-induced mitogenesis is mediated via a distinct signal transduction pathway that activates a mitogen-activated protein kinase. Proc. Natl. Acad. Sci. U. S. A. 1996;93:2174–2179. doi: 10.1073/pnas.93.5.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zito F., et al. Association of the factor XII 46C>T polymorphism with risk of coronary heart disease in the WOSCOPS study. Atherosclerosis. 2002;165:153–158. doi: 10.1016/S0021-9150(02)00196-X. [DOI] [PubMed] [Google Scholar]
- 24.Renne T., et al. Defective thrombus formation in mice lacking coagulation factor XII. J. Exp. Med. 2005;202:271–281. doi: 10.1084/jem.20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merkulov S., et al. Deletion of murine kininogen gene 1 (mKng1) causes loss of plasma kininogen and delays thrombosis. Blood. 2008;111:1274–1281. doi: 10.1182/blood-2007-06-092338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shariat-Madar Z., et al. Bradykinin B2 receptor knockout mice are protected from thrombosis by increased nitric oxide and prostacyclin. Blood. 2006;108:192–199. doi: 10.1182/blood-2006-01-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]