Abstract
This review discusses various issues to consider when developing standard operating procedures for pre-clinical studies in the mdx mouse model of Duchenne muscular dystrophy (DMD). The review describes and evaluates a wide range of techniques used to measure parameters of muscle pathology in mdx mice and identifies some basic techniques that might comprise standardised approaches for evaluation. While the central aim is to provide a basis for the development of standardised procedures to evaluate efficacy of a drug or a therapeutic strategy, a further aim is to gain insight into pathophysiological mechanisms in order to identify other therapeutic targets. The desired outcome is to enable easier and more rigorous comparison of pre-clinical data from different laboratories around the world, in order to accelerate identification of the best pre-clinical therapies in the mdx mouse that will fast-track translation into effective clinical treatments for DMD.
Keywords: mdx mouse, muscular dystrophy, standard operating procedures, biological variation, muscle function, pre-clinical trials
Introduction
Therapeutic approaches to DMD
Duchenne muscular dystrophy (DMD) is a lethal X-linked muscle disease due to a defect in the sub-sarcolemmal protein dystrophin, that leads to membrane fragility, myofibre death (necrosis) and replacement of skeletal muscle by fibrous and fatty connective tissue (due to failed regeneration). This results in extensive wasting, weakness and loss of muscle function leading to death, often by the early 20s. DMD affects males although some carrier females can manifest and be severely affected (depending on the proportion of normal X-chromosomes that are inactivated during development) (Matthews et al., 1995; Wenger et al., 1992). While the genetic defect was identified in 1987, there is still no effective treatment for DMD. The therapeutic research approach that has received most attention to date is replacement of functional dystrophin by genetic, cell transplantation or molecular interventions, and there are many exciting developments in this field (Odom et al., 2007). In parallel, there is increasing interest in administration of exogenous factors (drugs or food supplements) to reduce the extent of myofibre necrosis, since promising protective effects have been reported for a variety of agents (Radley et al., 2007; Tidball and Wehling-Henricks, 2004) and combinations of such interventions present a daunting array of protocols to be tested. In addition it is feasible that pharmacotherapy may be necessary to increase the efficiency of genetic or molecular interventions, a factor that extends the importance of adequate pre-clinical tests for single or combined approaches.
During the last 20 years, various laboratories worldwide have focused on clarifying the pathogenic mechanisms and the eventual compensatory mechanisms, consequent to the primary defect in dystrophic muscles; this approach has led to the identification of new potential drug targets. Interestingly, different laboratories, using a variety of independent experimental approaches, generally obtain similar results in terms of factors aggravating the pathology and the potential efficacy of various drugs. However, comparing the relative efficacy of different drugs and interventions between laboratories is still difficult with consequent delay in data sharing and fragmentation of efforts. This observation pushes toward a concerted development of standard operating procedures (SOPs) for experiments in mdx mice that will simplify and hasten comparisons of data from different laboratories around the world and assist the research of scientists and pharmaceutical companies to optimise pre-clinical treatments for translation into clinical therapies.
Scope and limitations of the review
There are several animal models for DMD and all of these, like the human counterpart DMD, have defects in the sub sarcolemmal protein dystrophin that make the muscle membrane fragile and result in necrosis of skeletal muscle fibres along with cardiac and other problems (Collins and Morgan, 2003; McNally and MacLeod, 2005). Only the skeletal muscle situation is addressed in this review. The mdx mouse, first identified in 1984, is the most widely used model due to ease of breeding, genetic uniformity, economy, and convenience for laboratory experiments. Similar pathology to mdx is seen in mice lacking alpha-sarcoglycan, another protein in the dystrophin dystroglycoprotein sarcolemmal complex (Duclos et al., 1998). Dystrophic dog models of DMD were first identified in 1988 (Kornegay et al., 1988) in the dystrophic golden retriever (Collins and Morgan, 2003) which has a much more severe pathology than the mdx mouse and more closely resembles the human condition: whereas the smaller dystrophic beagles exhibit a less severe pathology (Shimatsu et al., 2003; Yugeta et al., 2006). The highly variable phenotype of dystrophic dogs combined with expense of maintaining colonies has limited their use for pre-clinical testing. Beyond these mammalian models, complementary use is being made of invertebrate models that are readily manipulated genetically and are relatively easy and inexpensive to breed and maintain, such as the dystrophic worm C. elegans (Collins and Morgan, 2003) and dystrophic zebra fish (Bassett and Currie, 2004; den Hertog, 2005); although the usefulness of these models for drug screening for human conditions is debated.
The review is comprised of two main parts. Part I is a description of the mdx mouse model and the high biological variation. In Part II the main parameters used to measure specific effects on the dystrophic muscles are discussed. Some basic protocols are proposed for both Parts I and II.
Part I. THE MDX MOUSE (AND BIOLOGICAL VARIATION)
Since the review is focussed on the mdx mouse model of DMD, it is pertinent to first outline the variations of the mdx model that are available.
Dmdmdx
The classical biochemical and genetic mouse model of DMD is the mdx mouse discovered in 1981 (Bulfield et al., 1984; Hoffman et al., 1987). Over the last 25 years, many elegant papers have been published on the mdx mouse and it is not our purpose to review this literature. In brief, there is an acute onset of pathology (increased myofibre necrosis and elevated blood CK) around 3 weeks of age, this reduces to a chronic low level of damage by 8 weeks which persists throughout life but is further decreased by one year of age (McGeachie et al., 1993). The mdx mutation occurred spontaneously due to a premature stop codon resulting in a termination in exon 23 of the dystrophin gene. The mdx mouse has no detectable dystrophin protein, although sporadic ‘revertant’ myofibres can express dystrophin: this can complicate interpretation of some studies especially those related to gene or cell replacement of dystrophin (Yokota et al., 2006).
Higher (~3.5 fold) mortality in mdx litters is reported (Torres and Duchen, 1987) and recent studies show increased (~ 45%) mortality before 7 days of age in mdx mice: this is not affected by litter size with some litters being unaffected and others totally lost [Radley and Grounds; De Luca et al., unpublished data 2007]. Mdx mice seem very susceptible to stress and this may be a contributing factor since avoiding all animal handling or routine cage cleaning reduces the neonatal mortality.
Dmdmdx-2Cv to Dmdmdx-5Cv
Elevated plasma CK levels were used to screen the progeny of chemical mutagen-treated male mice to identify four new mutations of Dmd, called Dmdmdx-2-5Cv (Chapman et al., 1989). Preliminary data showed that mice with mdx2Cv and mdx3Cv mutations have muscular dystrophic phenotypes that do not grossly differ from the characterized mdx mutation, and spontaneous revertant fibres occur less frequently in the Dmdmdx-4Cv and Dmdmdx-5Cv mutants than in Dmdmdx. These additional mdx mutations have not been widely used.
Mdx52 mice
Since the point mutation in exon 23 of the dystrophin gene in mdx mice allows the expression of four other shorter isoforms of the dystrophin gene through differential promoter usage, exon 52 knockout mice (mdx52) were generated to simulate the DMD phenotype commonly seen in human patients (Araki et al., 1997). A major advantage of this mutation (especially for studies designed to replace the missing dystrophin gene) is the complete absence of dystrophin since there are no revertant myofibres. The skeletal muscle pathology of mdx52 is similar to that of the mdx mouse for limb and diaphragm muscles, although hypertrophy was reported in mdx52 limb muscles.
1. Response of different skeletal muscles to muscular dystrophy
Fast-twitch muscles are generally the most susceptible to muscular dystrophy (and also ageing) in all species (Lynch et al., 2001). The progress of the dystropathology has been extensively described in mdx mice in the 1980s (Coulton et al., 1988b; Torres and Duchen, 1987) (reviewed in (Shavlakadze et al., 2004)). The absence of dystrophin results in Z-line streaming of sarcomeres by 1 day post-natal, rare isolated necrotic myofibres are seen by 5 days, by 10 days necrosis is evident in rostral muscles such as the head (masseter) and shoulder girdle (parascapular) (Torres and Duchen, 1987) and other muscles may show some pre-necrotic changes (Coulton et al., 1988b). There is an abrupt onset of skeletal muscle necrosis around 21 days of age in hind limb and many other muscles (from 20–80% of the muscle can be affected) that stimulates muscle regeneration (Whitehead et al., 2006b) (Fig. 1). Most groups report that myonecrosis in limb muscles is rare before 21 days of age (Shavlakadze et al., 2004), although a slightly earlier onset at 16 to 17 days has been reported in quadriceps muscles (Muntoni et al., 1993): these differences may reflect divergence between isolated mdx colonies in different countries. Necrosis peaks (30–60%, occasionally ~ 90%, of the TA muscle is affected during this acute damage phase) by 25–26 days (with many myotubes resulting from regeneration by day 28) and then decreases significantly to stabilize by 8 weeks of age to a relatively low level of active damage (~4–6%): the cyclic progression of necrosis (and regeneration) continues throughout life although reduces by one year (McGeachie et al., 1993). The acute onset of myofibre necrosis provides a good model to study therapeutic interventions designed to prevent or reduce necrosis, since a reduction in dystropathology is easily identified (Grounds and Torrisi, 2004; Radley and Grounds, 2006; Shavlakadze and Grounds, 2003; Stupka et al., 2001). However, drug interventions that may be toxic to the postnatal development of neuromuscular apparatus should be considered for such young mice. In contrast, reduced necrosis can be difficult to detect in adult mdx mice where there is little myofibre breakdown (~5%) and cumulative muscle pathology: for this reason exercise is often used to provoke myofibre damage in adult mdx mice.
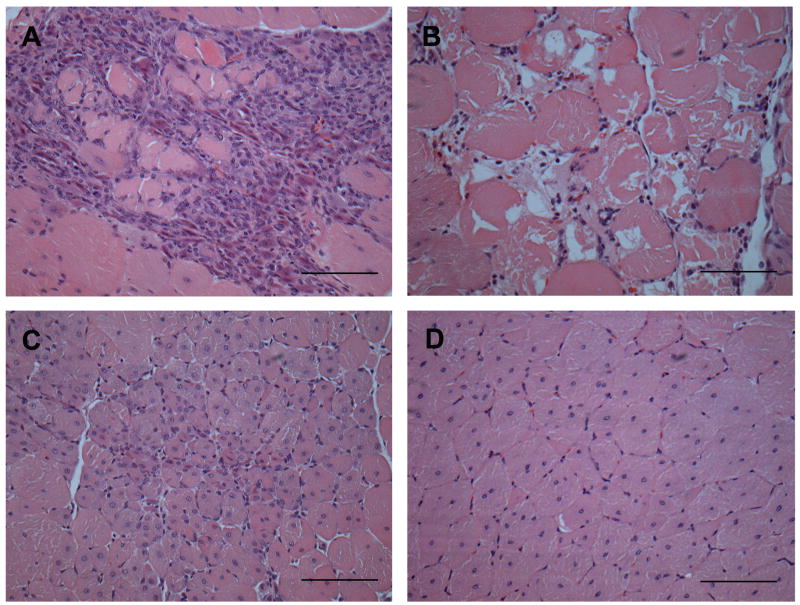
Fig 1. Histological features on a transverse section of exercised adult mdx quadriceps muscle stained with Haematoxylin and Eosin (all images are from the same muscle section).
A) Active muscle necrosis characterised by many inflammatory cells which have infiltrated dystrophic myofibres (sarcoplasm is barely visible). B) Active muscle necrosis characterized by fragmented sarcoplasm of dystrophic myofibres with irregular shape and few myonuclei; inflammatory cells are not conspicuous. C) Recent regeneration shown by small dystrophic myofibres (sometimes seen as smaller myotubes) with central nuclei. D) Regenerated muscle indicated by large mature dystrophic myofibres with central nuclei. Scale bar represents 100μm.
While young mdx mice at the acute phase of dystropathology show muscle weakness and the mdx muscles appear more susceptible to fatigue in vivo than control mice (De Luca et al., 2003), overall, adult mdx mice do not show in vivo functional muscle impairment up to 1 year of age (Coulton et al., 1988a; Muntoni et al., 1993). The symptoms of dystropathology are cumulative, with fibrosis becoming increasingly pronounced in older (15 months old) mdx mice (Lefaucheur et al., 1995): there are several different stages in the severity of the dystropathology between growing and mature mdx mice (Keeling et al., 2007) and these are affected by gender (Salimena et al., 2004). The limb and diaphragm are the 2 main groups of muscle that have been studied in muscular dystrophy.
In addition, some muscles of the head and chest region, including extraocular muscles (Fisher et al., 2005), masseter (Muller et al., 2001) and the laryngeal muscles (Marques et al., 2007), show a very mild pathology and are relatively spared from myonecrosis. The reasons for the mild dystropathology are not clear, although it is noted that these muscles may have an improved ability to regulate calcium homeostasis (Khurana et al., 1995) and selected mechanical properties that offer resistance to damage (Wiesen et al., 2007).
1a. Limb muscles
The hind limb muscles are the most widely studied and include the tibialis anterior (TA) and extensor digitorum longus (EDL), the gastrocnemius, quadriceps and the soleus muscle (Parry and Wilkinson, 1990; Wang and Kernell, 2001). The TA typically first manifests muscle necrosis from 21 days after birth and this is more pronounced than in the quadriceps (Radley and Grounds, 2006; Shavlakadze et al., 2004). Another study observed more necrosis in soleus than EDL at 24 days with greater cumulative muscle damage in soleus (~86%) than EDL (~36%) muscle at 34 days (Passaquin et al., 2002). The precise reason for the acute onset of dystropathology at 3 weeks of age is unresolved: it may relate to adult-type locomotor activity or to striking developmental changes in expression of various genes, including down-regulation of utrophin (Khurana et al., 1991) and of key genes involved in creatine synthesis (McClure et al., 2007) as well as in proteins involved in excitation-contraction coupling mechanisms (Bertocchini et al., 1997; De Luca et al., 1990; Schiaffino and Reggiani, 1996).
Exercise has a different impact on various limb muscles (depending on which muscles are recruited for the specific exercise regimes) and this needs to be taken into account e.g. 48 hours voluntary wheel running doubles necrosis in quadriceps muscles of adult mdx mice, but causes less damage to the TA and diaphragm (Archer et al., 2006; Hodgetts et al., 2006; Radley and Grounds, 2006) and the EDL is more affected than the plantaris and soleus muscles (Hayes and Williams, 1996). After 3 downhill running sessions (10m/min for 10min at a 15° decline) the muscles most affected are the diaphragm and triceps brachii, with little damage seen in either the TA or EDL (Brussee et al., 1997). A different exercise regimen, such as a protocol of chronic (at least 4 weeks) forced running on horizontal treadmill, increases muscle necrosis in the gastrocnemious muscle, with damage also being observed, although to a lesser extent, in TA and diaphragm (Burdi et al., 2006; Pierno et al., 2007) [Burdi & De Luca., data under review].
1b. Diaphragm
Over time, the diaphragm shows a more severe pathology than the limb muscles with extensive replacement of muscle fibres with fibrous connective tissue in mdx mice, more closely resembling the severe pathology of DMD where loss of diaphragm function is a major problem (Lynch et al., 1997; Stedman et al., 1991). Normal diaphragm muscle is composed mainly of type 2X and type 2A myofibres as shown by histochemical staining or antibodies specific for type 2A and 2B myosins (Gregorevic et al., 2002; Shavlakadze et al., 2004). Yet in dystrophic mdx muscles, type 2B myofibres increase over time as a result of bouts of regeneration in response to necrosis and this is conspicuous by 12 weeks of age (Shavlakadze et al., 2004). However, at early stages the pathology of the diaphragm is very mild and quite different to the severe acute onset in limb muscles: isolated necrotic myofibres are evident earlier, by 15 days after birth, and the damage is mild at least up to 30 days (Shavlakadze et al., 2004), with increasing severity of myofibre degeneration and increasing fibrous connective tissue over time (Gosselin and Williams, 2006; Krupnick et al., 2003; Niebroj-Dobosz et al., 1997; Stedman et al., 1991). Intrinsic differences in collagen metabolism have been demonstrated between functionally different normal skeletal muscles (Gosselin et al., 2007).
Impact of growth parameters
The much less severe dystropathology in mdx mice compared with DMD boys may be due in large part to vast difference in growth parameters between mice and humans, specifically to the much shorter time-scale of growth and maturation of mice (about 3 months compared with 20 years), much smaller body size (about 30g compared with 70kg) and consequent greatly reduced load on the smaller muscles in mice. In addition, there is stress on different muscle groups due to the use of 4 legs in mice compared with the vertical bipedal posture of humans. A comparison of developmental milestones for mice and men (see Box 1) suggests that as a very rough indication: 2 weeks (for mouse) may be equivalent to 3 months (for human); 3 weeks to 6 months; 4 weeks to 10 years; 8 weeks to 20 years and 12 weeks to 25 years.
Box 1. Developmental milestones in mice and men.
Composite data, compiled in close collaboration with Marta Fiorotto (Baylor College of Medicine, Houston, Texas)
When trying to draw parallels between the mouse and human for the study of muscle, the lines should be drawn on the basis of hormonal changes and physiological milestones beginning with muscle differentiation. It must be recognized that there is not a linear scaling; the proportion of the life-span taken to sexual maturity is only about 5% for mice, whereas for man it is approximately 20%. (The long childhood is a unique characteristic of man and the apes; rodents and many other mammals do not have a “childhood” and they effectively go from baby to teenager.) There is also a rostro-caudal pattern of development so that the muscles in the upper part of the body are likely to be at a slightly more advanced stage than that described below which is derived mainly from the study of (male) mouse hind limb muscles after birth.
Birth: skeletal muscle is poly-innervated, the tubular systems are rudimentary, and neonatal myosin heavy chain predominates. At the same time activity of thyroid hormone and the HPA axis are suppressed. Human muscle is at a similar stage of differentiation at about 18–22 weeks of gestation.
By 8–10 days post-natally: the tubular system is more mature, fibers have become mono-innervated and enable the coordinated contraction of muscles to promote balance and increased locomotor activity. With respect to locomotor function, mice begin weight bearing from 1–2 weeks of age, this contributes to synapse elimination (Minatel et al., 2003) and maturation (Missias et al., 1996), whereas in humans this is completed by birth (Hesselmans et al., 1993). In mice, activity of the thyroid (McArdle et al., 1998) and hypothalamic pituitary adrenal (HPA) (Schmidt et al., 2003) hormonal axes are starting to increase, and replacement of immature MHC by the adult myosin heavy chains is occurring (Allen and Leinwand, 2001). Mice are suckled on milk which is high fat/low carbohydrate. This roughly corresponds with a newborn human (Bronson, 2001; Elmlinger et al., 2001). [In mdx mice, blood CK levels at 7 days are the same as normal mice but by 10 days are elevated – indicating early symptoms of the disease]
14–16 days: Milk is gradually becoming limiting and insulin levels decrease substantially. The GI tract is not fully capable of digesting complex carbohydrates, and there is evidence from the inflexion in the normal muscle growth curves, that growth capacity is limited. At this time myostatin is also increasing rapidly. Muscle IGF-II mRNA and muscle IGF 1 receptor levels are approaching a nadir. In the human this represents about 3 months of age.
19–21 days: Mice are fully weaned: muscle is mature (Allen and Leinwand, 2001). Diet is now largely chow, and there is very rapid growth of the muscle. Growth hormone starts to increase from 21 days and peaks around 28–30 days (Alba and Salvatori, 2004). This represents approximately 6 months in humans (Butler-Browne et al., 1990; Mehta et al., 2005). [In mdx mice, acute muscle necrosis starts in hind limb muscles from 21 days: necrosis is seen in forelimb and other muscles at earlier ages]
4–5 weeks: pre-adolescence in mouse; gender differences are just beginning to emerge: equivalent to about 10 years of human age.
5–7 weeks: puberty in mice (Jean-Faucher et al., 1978), they are fully fertile and growth rate decreases: about 14–18 years of age in humans (Rodriguez et al., 2007).
Around 10–15 weeks: muscle has reached a maximum size in mice and growth in general has reached a plateau (Balice-Gordon and Lichtman, 1993): >20 years in the human. [In mdx mice, muscle necrosis stabilizes to a low persistence level of damage, <10% of muscle affected, that is increased by exercise]
12–18 months: in the mouse a gradual diminution in muscle mass becomes evident from 18 months, i.e. start of sarcopenia. In humans, this starts significantly after about 50 years of age (Shavlakadze and Grounds, 2003).
24+ months: lifespan in the mouse is approximately 30 months (varies between strains), that may correspond to about 80 years in humans. [In mdx mice, myofibre necrosis in hind limbs is further decreased and blood CK levels are very low by one year of age]
| SUMMARY of possible parallels for younger mdx mouse (during first 6 months) | ||||||||
| Mouse (weeks) | 1 | 2 | 3 | 4 | 6 | 8 | 12 | 26 |
| Human | newborn | 3m | 6m | 10yr | 16–18yr | 20 yr | 25yr | 35yr |
2. Biological variation between and within mice
2a. Factors influencing biological variation
Great variation in the timing and severity of the dystropathology can be seen between and within different mdx mouse colonies, between littermates and even between 2 legs of an individual mouse. Parameters affected include histological (e.g the extent of necrosis and muscle damage varies from massive [60–80%] to moderate necrosis [20–40%] at 23 days with some mice showing almost no damage even at 26 days (Radley and Grounds, 2006); this occurs between littermates and between TA muscles in both legs of an individual mouse e.g. 0% compared to 24% for one mouse at 21 days [Radley & Grounds, unpublished data].); cellular (e.g number of satellite cells (Schafer et al., 2005) and age-related increase in revertant myofibers (Yokota et al., 2006)); biochemical (serum creatine kinase (CK) levels in sedentary and exercised mice (Burdi et al., 2006; De Luca et al., 2005; Pierno et al., 2007; Vilquin et al., 1998) [see Section 5c]; molecular [gene expression profiles (Turk et al., 2006)] and functional aspects (participation in voluntary wheel exercise (Radley and Grounds, 2006) or forced treadmill running (De Luca et al., 2003; Vilquin et al., 1998) [discussed in Section 4a and 4b]. Such inherent variation necessitates constant conditions when grouping the animals, with large sample sizes (often at least 6–8 mice) to show statistically significant effects of treatments.
Epigenetic and genetic factors probably contribute to the wide range of phenotypic expression of muscular dystrophy (as discussed below). While it is clearly impractical to standardise many of these variables across laboratories globally, a deeper understanding of the many reasons for biological variation will help to initiate practices to minimise this problem.
2a. Developmental and in utero influences
It is now widely recognised that very early events before or at fertilisation, as well as in utero can have dramatic post-natal effects that contribute to large biological variation in anatomy, physiology, behaviour and the onset of disease (Gartner, 1990; Vandenbergh, 2004). Many of these are due to pre-natal hormone exposure; however environmental epigenetic also plays an important role in disease susceptibility (Jirtle and Skinner, 2007; Vandenbergh, 2004). Some of the in utero factors to consider that may influence the severity of the dystropathology in individual mdx mice (within a litter and between litters) are the size of the litter, the position within the uterus, the number of male siblings and the proximity of female pups to male littermates (this influences exposure to testosterone) along with a range of other factors (Vandenbergh, 2003). Some laboratories standardise litter size (e.g 4–8 pups) for all experiments to minimise variation in litter size that can affect initial body weights and thus biological variation. However, this may be considered wasteful and is unlikely to become a standard, plus it does not take into account the consequence of early neonatal mortality of mdx litters. One simple solution that is strongly recommended to reduce effects of inter-litter variation is to always select pups from a single litter for both test and control mice.
2b. Neonatal influences
The early post-natal environment is important with a wealth of data showing that maternal care mediates further variation in offspring phenotype and behaviour (Champagne et al., 2007) and catch-up post-natal growth by low birth weight humans and animals affects many metabolic and signalling events. Transmission of maternal behaviour (such as grooming and licking) and stress responses can occur from one generation to another by epigenetic modification of the chromatin around the glucocorticoid receptor gene (Francis et al., 1999; Weaver et al., 2004). The net result to the progeny is tighter regulation of stress hormone levels, an effect that is relayed to the next generation in subsequent maternal behaviour patterns.
These issues highlight some of the developmental variables that can influence the phenotype (severity of the dystropathology and potentially the propensity for voluntary exercise) of genetically equivalent inbred mice to contribute to the wide biological variation seem for mdx mice, both within and between litters.
2c. Gender
Major sex differences have been noted in skeletal muscles in relation to energy metabolism, fibre type composition and contractile speed. Generally, muscles from males tend to be faster and have higher maximum power output than from females, whereas muscles from females are more fatigue resistant, recover faster from repeated contractions and show less mechanical damage after exercise (Glenmark et al., 2004). Striking differences have been noted in the dystropathology between male and female mdx mice at different ages (Salimena et al., 2004), with less susceptibility to muscle damage in young (6 week) female mice but greater fibrosis in old females (1 and 2 years) suggesting a role for female hormones in the pattern of myonecrosis and repair. Other studies show that levels of blood serum creatine kinase are higher in older male mdx mice (Yoshida et al., 2006). Sex differences in muscle membrane damage in response to intense exercise have been shown in animals and humans, with females being less susceptible than males: this appears to be due to a reduced inflammatory response in females, with many aspects being influenced by gender (Stupka et al., 2000). It is widely recognised that both the innate and adaptive immune responses of females are heightened and more robust than in males (Verthelyi, 2006) and recent evidence confirms that the innate and adaptive arms of the immune system are different in post-pubertal male and female mice (Lamason et al., 2006). These gender differences are largely attributed to oestrogen levels and the regulation of nitric oxide by oestrogen increasingly appears to play a key role (Verthelyi, 2006). There are also gender differences in response to drugs (Franconi et al., 2007) and to diets (see 3c) and thus gender should be carefully considered when testing pharmaceutical or nutritional interventions.
While such gender-related issues appear to be important in the mdx mouse, they have barely been considered. Interpretation and data comparison is complicated when the gender of the mice used in experiments is either not specified (Hall et al., 2007; Kaczor et al., 2007) or mixed males and females are used (Granchelli et al., 2000). Ideally pre-clinical tests should be performed on groups of mdx mice of the same sex.
Males
Testosterone has many effects; male mice of some strains are particularly aggressive and can be difficult to cage as placing males together may lead to fighting and thus additional muscle damage. This is generally not a problem if males are either caged together at weaning, many mice are caged together (since 2 males alone in one cage are more likely to fight), and the males are not near a stud male or females (due to pheromones). The male response to female odours can affect behaviour and is influenced by the major histocompatibility type of the foetus of pregnant females (Beauchamp et al., 2000). In addition, age-related changes in pheromones in urine appear to relate to altered immune function (Osada et al., 2003). The effects of such odours within an animal house can be minimised by using individually ventilated cage systems (Section 3a). In addition, inter-male aggression in grouped housing is influenced by different kinds of environmental enrichment (Van Loo et al., 2004). Beyond the sex determining region on the Y-chromosome (SRY) and testosterone, there are multiple pathways that control sexual differentiation; e.g. sexual differences in motoneurones may be affected by the testicular hormone Mullerian Inhibitory Substance that is present in the blood of pre-pubertal males, but not females (Wang et al., 2005). Since DMD affects boys, it might be considered that it is more appropriate to use male mice, although the overall significance of this issue has yet to be proven.
Females
Hormonal changes during the estrus cycle influence immune status, stress, activity levels and age-related changes in cognitive function (Kopp et al., 2006) and this may be a significant additional variable when using female mdx mice.
2d. Genetics
Inbred mdx mice are homozygous and theoretically there is long-term genetic stability, but there will be a slow sub-line differentiation between colonies over many years (reviewed (Harris, 1997)). Ideally, colonies should be replaced periodically from an international source. Furthermore, if inbred mice are not kept under specific pathogen free (SPF) conditions, their phenotype variability can be higher than outbred mice (reviewed in (Biggers, 1958; Harris, 1997)). The genetic background strain can greatly influence the severity of the dystrophic phenotype as demonstrated for sarcoglycan deficient mice, although the gene loci that suppress the dystrophic phenotype remain to be identified (Heydemann et al., 2005).
While most mdx mice are maintained as homozygous/hemizygous colonies (where all X-chromosomes carry the gene mutation and thus all females and males are affected), maintenance as a heterozygous colony could more closely resemble the human situation and also provide appropriate negative littermate controls.
2e. Stress
Numerous factors can stress animals and this has effects on the brain, behaviour, hormones, and the immune system. Some of the well known stressors that produce physiological effects are social stress related to housing and dominance/subordination (reviewed in (Bartolomucci, 2007)), transport, restraint and handling, in addition to blood sampling, surgery and anaesthesia. For example, even simply transferring mice to a different room can increase cortisosterone levels and mice take about 4 days to acclimatize (Tuli et al., 1995). The rapid endocrine and metabolic response to the stress of handling is manifested by rapid changes in many blood components leading to the recommendation that any sampling be completed within 100 seconds of first touching an animal’s cage (Gartner et al., 1980) since some components are altered by 100% within 30 minutes. Considering that the in vivo procedures for drug treatment and evaluation cannot avoid a certain level of stress being experienced by the animals, it is essential to maintain standard handling procedures for all mice (test and controls) throughout the experimental procedure. Anecdotal evidence suggests that mdx mice are especially susceptible to stress.
3. Good husbandry practises to minimise biological variation
Environmental conditions such as housing and husbandry have a major impact on the laboratory animal throughout its life and will thereby influence the outcome of animal experiments. Much of the biological variation between and within mice (and in different laboratories) may reflect variation between aspects of animal husbandry (reviewed (Biggers, 1958; Harris, 1997; Reilly, 1998)). Issues to consider for standard laboratory practise include; caging, housing systems, space recommendations and microenvironment-enrichment, bedding; temperature and humidity; ventilation:-air quality, relative air pressure and individual cage ventilation; illumination:-photoperiod, intensity; noises; food: types of diet, quality assurance; water; sanitation and cleaning; identification and record keeping. There is a wealth of literature on these topics and it is well recognised that they have a major impact on phenotypic variation (Gartner, 1990). Inbred mice such as mdx are far more susceptible than outbred animals to phenotypic variation induced by minimal environmental variability (such as pathogens), simply because the population lacks genetic diversity (reviewed in (Harris, 1997)). Some of these factors are discussed below.
3a. Cage design
Cage design can modify the activity and behaviour of mice (Wurbel, 2001) with changes in the environments of animals, including environmental (supplemental) enrichment, having important effects on brain structure, physiology (including recovery from illness and injury), gene expression in various organs (Benefiel et al., 2005) and aggression between males (Van Loo et al., 2004). Individually ventilated cage systems generally help to maintain low ammonia and carbon dioxide concentrations; they support a low relative humidity, and reduce spread of allergens and infections. Additional benefits are that cages need to be cleaned less frequently (with reduced disturbance of the mice) and airborne pheromones (due to gender, stress, age) that can affect mouse hormonal responses are eliminated (Reeb-Whitaker et al., 2001).
3b. Night and Day (light and dark cycles)
Traditionally, mice are subjected to forced exercise and experiments are performed during the day (e.g. tissue samples collection and/or physiology experiments). Yet mice are nocturnal and normally active at night. Since the diurnal rhythms of mice and humans are out of phase, the nocturnal mouse more accurately corresponds to the daytime human (McLennan and Taylor-Jeffs, 2004). It is well documented that circadian rhythms profoundly influence many molecular, metabolic, endocrine, immunological and behavioural parameters (McCarthy et al., 2007; McLennan and Taylor-Jeffs, 2004) and thus it is important, within each laboratory, to maintain strictly the same time of day for exercising, treating, sampling or conducting physiological experiments on mice. One strategy to consider is the use of sodium lights to shift the day/night cycle in order and allow observations and interventions during the nocturnal phase with many scientific and welfare advantages (McLennan and Taylor-Jeffs, 2004).
3c. Food and Water
Diet can have a dramatic and rapid impact on cellular responses and gene expression, as illustrated by effects of soy or casein on cardiac hypertrophy (Stauffer et al., 2006) and how a diet enriched with omega-3 fatty acids can have potent post-natal effects on fetal programming (Wyrwoll et al., 2006): both of these studies emphasise the strong influence of gender. There is a huge literature on nutritional effects but these two examples illustrate the need for strictly standardised diets. Indeed nutritional interventions to ameliorate muscular dystrophy are being trialled in mdx mice and humans (Radley et al., 2007). It can be a challenge to rigorously control the quality of a well characterised diet (e.g. mouse chow) from a supplier, since even the standard ingredients may vary depending on the market source and the season. A further variable to consider is the impact of short-term (e.g. overnight) fasting on metabolic and cellular parameters. Awareness of such issues is important when evaluating variation between results from different laboratories and even within one laboratory over time.
Tap water that is traditionally used as drinking water in animal houses can vary very markedly in its mineral content and different additives such as chloride and fluoride, between different cities and may vary throughout the year (e.g. summer and winter) for the same location. Such differences may chemically interfere with some drugs. Whether such variations significantly influence the dystropathology is not known. To avoid such possible complications some laboratories, such as preclinical drug testing facility at CNMC in Washington (Nagaraju), now use purified water for all experimental mice.
4. Models of exercise-induced muscle damage to increase pathology in mdx mice
Endurance training produces many physiological, metabolic and vascular adaptations in skeletal muscle. This adaptive response may involve myokines, such as IL-6 and other cytokines, which are produced and released by contracting skeletal muscles and affect other organs of the body (Febbraio and Pedersen, 2005) combined with improvements in cardiac function. Beneficial effects of regular exercise on dystrophic mdx muscle are reported for free wheel running (Dupont-Versteegden et al., 1994; Hayes and Williams, 1996) and swimming that is a non-weight-bearing, low-intensity exercise (Hayes and Williams, 1998). The implications of such adaptation for strengthening dystrophic muscle, although still debated, are of much interest for physical therapy of DMD patients. However, here we will focus on exercise as an intervention to increase the severity of the phenotype in mdx mice, to enable more effective evaluation of drug treatment efficacy.
In adult mdx mice the muscle pathology is normally relatively mild and does not closely resemble the severity of DMD. The low level of damage in adult mdx mice can be elevated by exercise that increases myofibre necrosis and decreases muscle strength (Brussee et al., 1997; De Luca et al., 2003; Okano et al., 2005; Vilquin et al., 1998) enabling potential therapeutic interventions to be evaluated more rigorously throughout the in vivo treatment (Archer et al., 2006; De Luca et al., 2005; Granchelli et al., 2000; Payne et al., 2006; Radley et al., 2007). Muscles differ in their susceptibility to exercise-induced muscle damage, with fast fibres (Type 2) more likely to be damaged than slow (Type 1) fibres. Various models of exercise-induced muscle damage have been used to exacerbate the disease in mdx mice, including voluntary wheel running, treadmill running and swimming (also see Section 10), but only two widely used in vivo running models are described here.
4a. Voluntary wheel running
The simplest model is voluntary spontaneous exercise and this is generally well tolerated (Hayes and Williams, 1996). Voluntary wheel running allows the distance run by individual mice to be measured accurately and so relative activity can be related to the severity of the resultant muscle damage. Mice are voluntarily exercised using a metal mouse wheel placed (often suspended) inside the cage. Exercise data are collected via a small magnet attached to the mouse wheel and a sensor from a bicycle pedometer attached to the back of the cage. The sensor records single wheel revolutions, allowing total distance (km) and speed run by an individual mouse to be determined (Archer et al., 2006; Dupont-Versteegden et al., 1994; Hayes and Williams, 1996; Radley and Grounds, 2006). The majority of voluntarily running is done at night (12 hr dark cycle) (Hayes and Williams, 1996; Radley and Grounds, 2006). This exercise has the advantage that mice can be left with the exercise equipment continuously and their activity measured over many months. There can be wide variations in the amount of running between individual mice, one possible disadvantage is that mice are caged individually (even though the cages may have clear sides so they can see each other) and this lack of socialising may affect their behaviour. Another issue that is rarely considered is that dirt (e.g urine and faeces) can accumulate and increase resistance of the wheel rotation. This can also be influenced by the design of the wheel, and thus the amount of effort required to turn the wheel will vary as will the impact on the muscles. Such variations in wheel resistance may contribute to different results between mice and between laboratories and should be considered: indeed some designs deliberately increase the wheel resistance to increase the work load (Konhilas et al., 2005). More sophisticated computerised monitoring systems can be used to collect precise data on the patterns of running and stopping (Hara et al., 2002; Radley and Grounds, 2006) and have revealed that mdx mice run more intermittently than wild type mice (Hara et al., 2002).
Muscle necrosis is roughly doubled (increases from ~6 to 12%) in quadriceps muscle after 48 hours of voluntary exercise, although other muscles such as the TA are barely affected (Radley and Grounds, 2006) a finding reported by others even after a single night of running (Archer et al., 2006). Histological analysis after 48 hours allows induced necrosis to be assessed without the ensuing complication of new muscle formation (myotubes appear by 3 days after injury). Adaptation to voluntary wheel running occurs over about 2 months in normal adult C57Bl mice and varies between muscles (Konhilas et al., 2005). The capacity for voluntary wheel running over a long-period (Brunelli et al., 2007; Dupont-Versteegden et al., 1994; Radley et al., 2007) probably reflects the relative health of mdx mice. Therefore, voluntary exercise can provide an additional measure of the protective benefits of interventions. (The use of voluntary or treadmill running to measure improvements in exercise capacity is also mentioned in Section 9). When mdx mice voluntarily run greater distances, this puts additional strain on their muscles and so an improved muscle pathology under these conditions may represent an even greater protection than is immediately evident (Radley et al., 2007). Voluntary wheel running combined with forced treadmill running twice a week is another regimen to consider.
4b. Forced treadmill running
Voluntary exercise produces mild damage in mdx mice but the severity of damage can be increased by forced exercise running on a treadmill (Burdi et al., 2006; De Luca et al., 2005; Granchelli et al., 2000; Nakamura et al., 2003; Vilquin et al., 1998). This is usually done for convenience during the day. A protocol of 30 min running on a horizontal treadmill at a speed of 12m/min, twice a week for at least 4 weeks, starting at 4 weeks of age, i.e. soon after the first major bout of degeneration, causes significant weakness in the limb strength as measured by a grip strength meter (Granchelli et al., 2000). The in vivo weakness produced by such a protocol is observed exclusively in mdx mice with no similar effects in wild type mice: thus providing a reliable in vivo index with which to rapidly monitor potential drug efficacy (De Luca et al., 2003; De Luca et al., 2002; Granchelli et al., 2000). Due to concern about mdx mice being reluctant to run at the high speed of 12m/min, others have used slower speeds (6 m/min or 9.5m/min twice weekly) but also reported muscle damage and exhaustion with this forced running (Hudecki et al., 1993; Payne et al., 2006; Vilquin et al., 1998). Factors that can contribute to such differences in capability for treadmill running include slight divergence in mdx colonies, the age of the mdx mice, their husbandry and even calibration of treadmills. As chronic treadmill exercise is used mainly to exacerbate the pathology, the essential requirement is to maintain constant conditions during the protocol and to confirm muscle impairment.
When the protocol is started at 4–5 weeks of age, mdx mice (and rarely also wild type mice) may be reluctant to participate and sporadically stop running and rest for a few minutes (before being encouraged to start again) during a 30 minute session of forced exercise at 12 m/min (De Luca et al., 2003; De Luca et al., 2002). Slight increases or decreases in speed do not greatly change this attitude (at slower speeds all mice can lose interest in the activity and stop more frequently). The repeated stopping of the mdx mice may be due to increased fatigue or avoidance behaviour. A short warm-up at a slow speed (8 m/min) for 10 mins is a strategy to produce more consistent running over the 30 min run at 12 m/min (Payne et al., 2006): this helps to increase the proportion of mdx mice that complete the 30 min standard protocol, although 25% of 12 week old mdx mice may still fail to meet this requirement [Radley & Grounds, unpublished data]. A low-intensity electric shock is sometimes used to stimulate mouse running: however, this protocol is stressful and is not recommended for the mdx mice. A disadvantage of the treadmill exercise protocol is that it is time consuming, since dedicated and continuous supervision is required twice a week for a time depending on the total number of mice to be exercised. In mdx mice, the sustained forced exercise protocol significantly damages the triceps, gastrocnemius and quadriceps muscles. A significant increase in plasma CK is also generally observed in exercised mdx after 4–8 weeks, corroborating sarcolemmal damage in response to muscle work load (De Luca et al., 2005; Pierno et al., 2007).
Eccentric exercise (where activated muscles are forcibly lengthened) is most damaging to muscles of mdx mice, with more severe damage resulting from downhill running in comparison to horizontal running. With running downhill, mdx mice rapidly tire at a speed of 10 m/min after 30s, are unable to run continuously and often need encouragement after 2 minutes, with such exercise being limited to 5 minute bouts. In some cases this exhaustion can lead to death of mdx mice (Vilquin et al., 1998). Uphill running is less damaging and an example protocol of chronic running for mdx mice employed a speed of 15m/min, for 60 minutes twice a week for 5 weeks, followed by speeds of 23 m/min for a further 5 weeks (Okano et al., 2005).
Clearly a consistent exercise regime (either voluntary exercise, or forced exercise such as that of (De Luca et al., 2003; Granchelli et al., 2000; Okano et al., 2005; Payne et al., 2006) needs to be used across laboratories for accurate comparisons.
5. Part I. Conclusions and recommendations
Part I has outlined the factors that influence biological variation and emphasised the need to be aware of these and to standardise conditions where possible (e.g. related to breeding, husbandry and gender). It seems that 3 aspects that need to be addressed in order to help establish Standard Operating Procedures are:
Specify basic core experiments e.g. age of sampling, gender, exercise regime, onset of treatment (see Recommendation I in Box 1)
Define basic core methods of analysis (discussed in Part II)
Develop a scale to grade the efficacy of treatment (see Part II)
Part II. PARAMETERS TO MEASURE MUSCLE DYSTROPATHOLOGY AND FUNCTION IN MDX MICE
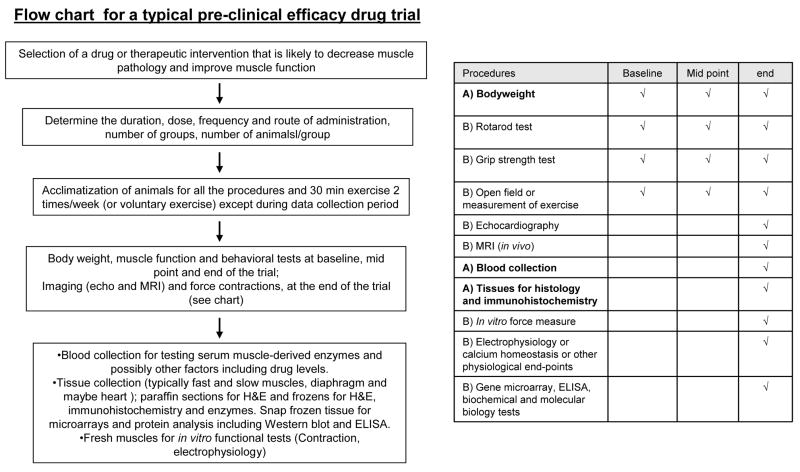
A range of histological analyses on muscle tissue sections, blood measurements and physiological parameters are used to assess the impact of various interventions on the pathology and function of muscles of mdx mice. Issues associated with these different measurements are discussed. It is noted that some of the measurements e.g. whole body imaging and functional and physiological assessments (Sections 6, 9 and 10) are done on intact animals, and often repeated throughout the study, prior to sacrifice.
5. Measuring leakiness of myofibres
Lack of functional dystrophin renders dystrophic myofibres susceptible to mechanical stresses, such as exercise induced damage that result in small disruptions of the muscle sarcolemma. These membrane lesions may be rapidly resealed or lead to further myofibre breakdown and necrosis. The exact mechanisms determining whether initial lesions result in resealing of the damaged sarcolemma (Doherty and McNally, 2003; McNeil and Kirchhausen, 2005) or alternatively result in myofibre necrosis are of considerable interest and underpin much current therapeutic research. There is good in vitro and in vivo evidence to support the hypothesis that the initial sarcolemmal damage is exacerbated by inflammatory cells and cytokines that result in further damage leading to myofibre necrosis (Brunelli et al., 2007; Grounds and Torrisi, 2004; Hodgetts et al., 2006; Radley et al., 2007; Radley and Grounds, 2006; Spencer et al., 2001; Tidball and Wehling-Henricks, 2005). Two main approaches are used to measure the extent of damaged (leaky or necrotic) myofibres. One uses labels (such as dyes or proteins) that rapidly enter through damaged sarcolemma into myofibres and remain in the sarcoplasm, and the other measures blood levels of various proteins that diffuse out of damaged myofibres.
5a. Evans Blue Dye (EBD) is used to identify blood vessel and cell membrane permeability in vivo since it is non-toxic and can be injected systemically as an intravital dye (Hamer et al., 2002). EBD uses albumin as a transporter molecule and diffuses into cells through membrane discontinuities to readily identify myofibres with permeable (leaky or necrotic) sarcolemma. Damaged myofibres which stain positive for EBD can be viewed in two different ways. Macroscopic examination of the whole mouse once the skin is removed shows dark blue stained myofibres that demonstrate the extent and pattern of damage for all muscles in the body (Matsuda et al., 1995; Straub et al., 1997). Microscopic examination of frozen muscle sections under green fluorescent light (Hamer et al., 2002) identifies EDB positive myofibres by red auto-fluorescence and has been widely used to quantitate myofibre damage (as a proportion of total myofibres) in many studies using mdx mice (Archer et al., 2006; Brussee et al., 1997; Shavlakadze et al., 2004; Straub et al., 1997). Once EBD enters the myofibre it diffuses along the length of the myofibre and thus may be visible at some distance (~150μm) from the initial site of damage (Hamer et al., 2002; Straub et al., 1997): this is an important consideration when viewing transverse muscle sections. EBD persists in vivo for at least 4 days after intraperitoneal injection (Hamer et al., 2002).
Administration of EBD is by intraperitoneal (IP) or intravenous (IV) injection. IV is widely used as it ensures rapid availability to all tissues, although IV injections through the tail vein of (black) mdx mice can be difficult for the inexperienced: therefore the easier route of administration is IP injection. Recommended protocols for EBD administration are: an IP injection, 16–24 hours prior to tissue sampling, of a 1% dye solution injected at 1% volume relative to body mass (Hamer et al., 2002) or IV injection (tail vein) of EBD that can be done within 3–6hrs prior to sampling (Straub et al., 1997). The choice between these protocols is probably not critical for many experiments.
It is agreed that myofibres with histologically distinct necrosis always stain positive for EBD (Archer et al., 2006; Brussee et al., 1997; Matsuda et al., 1995; Straub et al., 1997) and that myofibres with an intact sarcolemma do not stain (Matsuda et al., 1995; Straub et al., 1997). Some studies report that hypercontracted myofibres stain EBD positive (Brussee et al., 1997; Matsuda et al., 1995) whereas others do not support this conclusion (Straub et al., 1997). EBD can be present within myofibres that appear morphologically normal in H&E stained sections (Brussee et al., 1997; Hamer et al., 2002) and EBD uptake (into leaky cells) does not always reflect severe myofibre damage (necrosis) that will provoke regeneration and require myogenesis (Archer et al., 2006). Thus, the number of EBD positive myofibres can exceed and not accurately reflect the number of necrotic myofibres (Shavlakadze et al., 2004; Straub et al., 1997). This must be considered when quantitating spectrophotometrically the total EBD (in a section or extracted from dystrophic muscle) as a measure of overall damage (Hamer et al., 2002).
5b. Other markers (dyes and proteins) that enter damaged myofibres
Other techniques to measure sarcolemmal damage (reviewed in (Hamer et al., 2002)) include identification of proteins from the blood that have entered damaged myofibres, such as albumin (less sensitive than EBD bound to albumin but avoids the need for EBD administration) or fibronectin (Palacio et al., 2002); fluorescent labelled dextrans and Procion orange dye (POD). POD (FW 631g mol−1) is a smaller molecule than EBD and can be administered in vitro or in vivo (although there is some uncertainty about toxicity in the latter) (Pagel and Partridge, 1999; Palacio et al., 2002). POD is especially useful for post-sampling labelling of damaged myofibres by immersion of muscles in POD solution for 30–60 minutes (2% wt/volume in Ringer’s solution), which conveniently avoids the need for in vivo administration (Wehling et al., 2001). However, in vitro immersion is limited by the possibility of additional myofibre injury during the procedure (Consolino and Brooks, 2004; Palacio et al., 2002).
5c. Blood measurements of CK and other proteins that leak out of damaged myofibres
In blood samples (serum or plasma), a high level of activity of the enzyme creatine kinase (CK) (measured in a spectrophotometric assay) is another index of sarcolemmal fragility widely used as a diagnostic marker for muscular dystrophy (Zatz et al., 1991). It is assumed that the enzyme activity is a direct measure of the CK protein level in blood. The muscle (MM) isoform of CK is produced by both skeletal and heart muscle and total CK measurement in serum can include isoenzymes of CK derived from other tissues. Normally this is not a major issue and can be addressed by measuring the specific CK isoforms if required. The relationship between muscle damage and serum CK is not always straightforward and can be influenced by many factors. Exercise generally increases serum CK, suggesting a direct correlation between mechanical stress and sarcolemmal damage especially in dystrophic muscles (De Luca et al., 2005) and lower serum CK levels are an indication of reduced pathology and drug efficacy. CK levels in mdx mice increase between 7 and 10 days post-natally indicating the onset of muscle leakiness, are higher in older males than females (Yoshida et al., 2006) and decrease by one year of age (Coulton et al., 1988b). CK measurements at rest range from about 1,000 – 7,000U/L for females and up to17,000U/L for males and in exercised mdx mice range from 1,500 – 30,000U/L. Problems with CK measurements relate to high variability between individual mdx mice and changes related to age and the stage of disease. There is also variability between assay runs and possible interference of chemicals used for plasma preparation with the diagnostic kits, so it is recommended that control normal mouse blood is included for all assays. Collection of a sufficient volume of blood (about 200–500ul blood is required to yield approximately 100–200ul serum and at least 10–20ul is required per assay) is invasive and stressful. Thus, rather than multipoint evaluation in the same animals, most studies collect blood from the heart under terminal anaesthesia. Due to the high variation, CK analysis requires quite large number of mice (n=6–8) for unequivocal statistical analysis. Increased blood CK levels may also reflect increased muscle mass or increased CK within myofibres; it is noted that up-regulation (about double) of the creatine synthetic pathway is reported in mature muscles of mdx mice (McClure et al., 2007). Despite these issues, dramatic changes in blood CK levels can be a very useful measure of the severity, or correction, of dystropathology and this blood marker is used widely.
Other proteins that leak from damaged myofibres into the blood and have been used as a measure of muscle damage include the enzymes pyruvate kinase (Coulton et al., 1988b), aldolase, enolase, aspartate aminotransferase, and lactate dehydrogenase isoenzyme 5, as well as the muscle proteins myoglobin, troponin and alpha-actin (Martinez Amat et al., 2007) and some of these have been examined in mdx mice. In addition, leakage into serum of the soluble Ca(++)-binding protein parvalbumin that is very high in fast myofibres has been proposed as a useful diagnostic tool in mdx mice (Jockusch et al., 1990). To date, CK measurements remain the most widely used for monitoring muscle diseases but other more specific markers may emerge.
6. Whole body imaging to measure histopathology over time
Image capture technology aims to provide routine imaging of whole animals, body parts or whole muscles without the need for tissue biopsy or animal sacrifice, thus allowing repeat imaging from an individual mouse over time: this would be a great advantage. However, these techniques are still new and not yet established for routine laboratory use and they will face the same problems of standardisation to allow comparison of results between laboratory groups. A number of biomedical imaging modalities have been used to examine muscle tissue in vivo, including ultrasonography, magnetic resonance imaging (MRI) and confocal and multi-photon microscopy, with the potential of Optical Coherence Tomography (OCT) just starting to be investigated for mdx mice (Pasquesi et al., 2006) (reviewed in (Klyen et al., 2008)).
MRI can distinguish between healthy and damaged dystrophic muscles of mdx mice (McIntosh et al., 1998). The resolution is enhanced by combination with albumin–targeted contrast agents (taken into damaged cells) (Amthor et al., 2004), although another study in mice concluded that endogenous MR contrast was sufficient and did not require combination with a gadolinium based MRI contrast agent (Walter et al., 2005). MRI (without and with contrast agent) has recently been used in the dystrophic dog model where very high biological variation and the expense of dogs (Thibaud et al., 2007) makes 3-dimensional in vivo tissue analysis of an individual animal over time particularly attractive, especially for evaluating the effects of pre-clinical trials. The acquisition times for MRI are long and so motion due to cardiovascular-induced or breathing-induced artefacts can be an issue. The resolution of MRI is not very high (the resolution of 125 μm is insufficient to image the individual myofibres with an average diameter of 30–50 μm) but the development of MRI scanners specifically for mice, combined with enhanced image detail, may result in this technique becoming more widely used experimentally. MRI can also be used to monitor, non-invasively, transplanted stem cells pre-labelled by incubation with ferumoxide-polycation complexes that provide images with high spatial resolution (Cahill et al., 2004).
Confocal and multi-photon microscopy, with their superior resolution, can readily image individual myofibres in vivo under physiological and pathological conditions but depend on detection of fluorescent signals. For example, two-photon microscopy was used to characterize the topology and metabolic function of mitochondria within skeletal muscle of a living mouse (Rothstein et al., 2005). Whole animal imaging via fluorescent or luminescent labelling is a rapidly evolving and promising technique (reviewed in (Ntziachristos, 2006)). The power of this approach was elegantly demonstrated using transgenic α-sarcoglican null mice that emit a fluorescent calpain-activated signal from damaged muscle (Bartoli et al., 2006), but this is not readily applied to conventional mdx mice (that lack the vital transgenic label).
Whether non-invasive imaging will become a routine procedure to repeatedly monitor the status of muscles in an individual animal over time in response to drug and other therapeutic treatments, remains to be demonstrated.
7. Histological measurements to evaluate necrosis, regeneration and fibrosis
Dystrophic skeletal muscle pathology in mdx mice is typically assessed on haematoxylin and eosin (H&E) stained transverse sections, with the tibialis anterior muscle of the lower hindlimb being widely used, as it is readily accessible. When undertaking analysis it is important to consider the age of the mdx mice as different histological features change with age (discussed in Section 1).
7a. Young mdx mice (< 4 weeks)
The acute onset of myofibre necrosis occurs from around 21 days of age. The acute onset of dystropathology with high levels of necrosis provides a very sensitive assay to specifically evaluate therapeutic interventions designed to prevent or reduce myofibre necrosis. Normal (pre-necrotic) myofibres have peripheral nuclei, intact sarcolemma and non-fragmented sarcoplasm. Necrotic muscle is identified by the presence of infiltrating inflammatory cells (basophilic staining), hypercontracted myofibres and degenerating myofibres with fragmented sarcoplasm. Regenerating (recently necrotic) muscle is identified by activated myoblasts and, 2 –3 days later, small basophilic myotubes. These myotubes subsequently mature into plump myofibres with central nuclei (regenerated myofibres). Cumulative skeletal muscle damage in young mdx mice consists of active myofibre necrosis plus the areas of subsequent regeneration (new myofibres) (Fig 1). It is calculated that myonuclei of newly regenerated myofibres of mdx mice remain in a central location for about 50–100 days and thereafter 3–4% of myonuclei move to a peripheral subsarcolemmal position; i.e numbers of central myonuclei may decline after 100 days of age (McGeachie et al., 1993). Myofibre size can be measured as the cross sectional area but, while accurate for true transverse sections, values are distorted by myofibres cut obliquely (this is a major problem for clinical biopsies with variable myofibre orientation): this problem is avoided by instead measuring the minimal Feret’s diameter of myofibres (Briguet et al., 2004).
7b. Adult mdx mice (6 weeks +)
After the acute onset of myofibre necrosis in young mice, skeletal muscle from adult mdx mice consists of a low level of necrotic and regenerating (recently necrotic) tissue, regenerated myofibres (with central nuclei) and some unaffected (intact) myofibres. As described previously, necrotic and regenerating and regenerated muscles have distinct histological features (Fig 1). Unlike regenerated human myofibres, the nuclei of regenerated mouse myofibres stay central for many months. Therefore mdx myofibres with central nuclei are a reliable indicator of previously necrotic/regenerated tissue (although in other situations they might instead represent denervated myofibres). However, central nuclei do not indicate the ‘number’ of times that an individual myofibre has undergone necrosis and subsequent regeneration. In studies of older mdx mice, the area of muscle that has not succumbed to necrosis can be a useful measure, since this indicates resistance of the myofibres to damage: such unaffected intact myofibres look normal with peripheral nuclei (although it is noted that at a lower level the same myofibre might also contain central nuclei). Standardisation of key sampling times (e.g. 4 and 12 weeks and 6 months of age) greatly facilitates comparison of data between laboratories.
7c. Older mdx mice (6 months +) and fibrosis
Fibrosis and fatty connective tissue and myofibre atrophy can be pronounced in older mdx mice. Fibrosis is readily observed in H&E stained sections but can be emphasised by routine histochemical stains such as Van Gieson’s or Masson’s Trichrome. The onset of progressive replacement of muscle by fibrous connective tissue (mild fibrosis) is reported in limb muscles from 10–13 weeks, with extensive fibrous connective tissue (fibrosis) and some calcification from 16 to 20 months of age (Keeling et al., 2007; Lefaucheur et al., 1995; Salimena et al., 2004). Intrinsic differences in collagen content (measured by amount of hydroxyproline) and metabolism have been demonstrated between functionally different normal and mdx skeletal muscles (Gosselin et al., 2007). It is well documented that the progression of dystropathology is different in the mdx diaphragm with significant fibrosis by 9 months (discussed in Section 1b). With the diaphragm, extra care must be taken to cut sections at equivalent locations for histological comparisons, due to the complex structure and varying width of the diaphragm muscle (illustrated in (Shavlakadze et al., 2004)).
7d. Frozen vs Fixed/paraffin-embedded muscle tissue sections
Frozen sections are routinely used as they avoid problems of shrinkage due to fixation, can provide excellent histology and have the major advantage that the same tissue can be readily used for immunohistochemistry (since many antibodies do not work well on fixed muscle sections). Muscles are routinely frozen in isopentane cooled in liquid nitrogen, since the isopentane reduces surface tension and avoids trapping air around the muscle that can slow the freezing process. Disadvantages of frozen tissues are that skill is required to prepare (to avoid ice artefact) and to cut sections, the tissues must be stored at −80°C and they can deteriorate over time. In contrast, muscles that are fixed (in paraformaldehyde) and processed into paraffin blocks can simply be stored on the shelf indefinitely. Muscle sections from paraffin blocks are ideal for H&E analysis and for other routine histochemical stains, but can be limiting with respect to enzymatic or antibody staining.
Morphological features are usually identified manually by the researcher and quantified using various image analysis software. The analysis of dystropathology on histological muscle sections is highly interpretive and thus can vary slightly between individuals and laboratories: a standard set of reference images to emphasise the precise features that are measured (Fig 1) would help to reduce this variation. Scientific analysis that involves any degree of interpretation should be carried out as ‘blind’ analysis.
8. Immunohistochemical and molecular analyses
8a. Immunological analysis
Immunological determination can help to gain insight into tissue events accounting for the histological changes and a wealth of different antibodies can be used depending on the specific question being addressed (e.g as a consequence of drug or other treatment); only two specific antibodies are discussed below. Determination of dystrophin levels may help to evaluate the percentage of spontaneously and/or therapy induced revertant myofibres (Yokota et al., 2006) and similar approaches apply for components of the dystrophin-glycoprotein complex. Other than for gene therapies, these approaches are useful when considering drugs able to force premature stop codon mutations, or to exert exon skipping, or to inhibit proteasome activity (Alter et al., 2006; Bonuccelli et al., 2007; Welch et al., 2007): ideally this results in uniform distribution of dystrophin in most myofibres. Similarly, immunological determination of utrophin expression is an important assay to evaluate compensatory mechanism in dystrophic muscle induced by experimental protocols and/or drugs (Moghadaszadeh et al., 2003; Nowak and Davies, 2004). One cautionary note is that digital imaging of fluorescently labelled proteins or cells, in the hands of the inexperienced can sometimes result in false positives due to confusion with background fluorescence (that can be high in muscle tissue) and this can be exacerbated by image manipulation.
8b. Molecular analysis
Treatment of animals with compounds can change gene expression profiles. Gene expression can be measured at the mRNA level, using real-time quantitative RT-PCR or microarray, or at the protein level by measuring changes in levels of specific proteins using western blot, Elisa, proteomics (Ge et al., 2003) or phospho-protein profiling.. Global gene expression profiling using microarrays is increasingly popular to monitor many mRNAs of target tissues before and after drug therapy. Microarray analysis of mdx mice at different stages of the disease and muscle biopsies from DMD patients; provide considerable new insights into muscular dystrophies (Chen et al., 2005; Haslett and Kunkel, 2002; Porter et al., 2003), have identified pathways that are amenable for therapeutic intervention early in the disease process and large muscle biopsy microarray data sets are now in the public domain, (Bakay et al., 2002).
The powerful high-throughput tools of systems biology analysis (for RNA and protein) combined with biochemical techniques allow verification of the possible involvement of specific pathways in normal and diseased muscle (Hittel et al., 2007). However, gene expression patterns are not always easy to interpret, a change in gene expression does not always result in a change in protein expression and function, sub-threshold changes in both the gene and the protein may have a great impact for tissue function, and the relative (rather than absolute) amount of a protein may be the critical factor. Overall, the gene, protein and emerging non-protein-coding RNA (Pheasant and Mattick, 2007) array methods, which are difficult and expensive, require additional functional or biochemical analysis to detect real changes in gene product function.
9. In vivo measurements of whole body function and muscle strength in mice
Body weight is usually monitored (weekly or monthly) throughout chronic experiments as an index of general health, in addition to numerous tests on whole animals to evaluate overall functional capacity, muscle strength, muscle endurance and ability to fatigue and adapt. Apart from the measurements of activity (outlined in Section 9a), there are several simple non-invasive methods to evaluate muscle function in intact whole animals (Section 9b). The techniques based on behavioural testing (open field, exercise, grip meters etc.) may be biased by effect of drugs on tissues other than skeletal muscle, modulating either animal motivation or animal metabolism that can modify strength and capacity to participate in the test, with the possibility of false positive or false negative results. Therefore detailed analysis of functional parameters is required for validation of a benefit. Muscles can be further tested in whole animals by invasive in situ procedures with some possibility of repeated measurements on an individual mouse (Section 10) and, finally, many detailed measurements are made in vitro on muscles removed from animals in terminal experiments (Section 11).
9a. Whole animals: behavioural activity and response to exercise
Since activity (i.e. exercise) affects the amount of damage of dystrophic muscle, it is very important to determine whether a drug or treatment has any effect on mouse activity. The Digiscan open field apparatus measures exploratory locomotor activity of the animal. via a grid of invisible infrared light beams, with the position of the animal being determined when the beam is interrupted (Crawley, 1999; Hamann et al., 2003; Nagaraju et al., 2000). Such locomotion and behaviour is also clearly influenced by some drugs acting on cardiac and neurological systems. Behavioural tests are prone to variability and significant variation in absolute values can easily occur between different laboratories and between different experimenters within a same laboratory: therefore standard protocols must be used for these tests. Mdx mice at certain ages (e.g. between 10–28 weeks) show reduced locomotor activities in comparison to age and sex matched control normal mice [Nagaraju; unpublished data].
Other common ways to measure activity include monitoring voluntary wheel running or treadmill running (as outlined in Sections 4a and 4b). The ability of mdx mice to run in either of these situations (measured as distance run/day or week, or the time taken to cover a particular distance) is an indication of their general well-being and muscle function (Brunelli et al., 2007; Dupont-Versteegden et al., 1994; Radley et al., 2007).
9b. Whole animals: grip bar and rotarod strength and co-ordination measurements
Functional strength in mice has been widely measured by exploiting the animals’ tendency to grasp a horizontal metal bar while suspended by its tail. The bar is attached to a force transducer and the force produced during the pull on the bar can be measured regularly (e.g. weekly). This is a relatively simple way of measuring body strength and repeated measurement can be made on the same individual throughout the life of the mdx mouse. This grip bar strength dynamometer is the most commonly used (for simplicity and economy) in vivo test for monitoring impaired limb strength caused by chronic exercise in mdx mice and whether a specific intervention can reduce muscle weakness (Anderson et al., 2000; Connolly et al., 2001; De Luca et al., 2005; De Luca et al., 2003; Granchelli et al., 2000; Payne et al., 2006; Smith et al., 1995). Some studies suggest that strength of mdx mice decreases after 3 months of age, but there is some controversy (Keeling et al., 2007).
Grip strength determination has to be performed under strict experimental condition as it may be affected by many variables (e.g. volition, cognition and fatigue) independent of muscle dysfunction. Therefore, as for the other behavioural approaches, the benefit of an intervention may not be through direct effects on muscle per se. It is important that mouse strength is determined always by the same operator, using a constant protocol, i.e. a fixed number of determinations spaced by a fixed time, and possibly in a blind-fashion.
The Rotarod measures overall motor coordination; this is a motorised rotating treadmill which requires mice to maintain their grip and balance on a rotating drum, or simply lose their balance and fall off (Bogdanovich et al., 2002; Ozawa et al., 2006; Payne et al., 2006). Other tests for motor coordination involve coaxing an animal to walk along a narrow beam between two cages, to examine walking performance over successive weeks of treatment, or the wire hang-test where a mouse is placed on a wire cage lid, then held upside-down and latency to fall is recorded (Hamann et al., 2003).
Electromyographic studies are invasive but allow direct in vivo monitoring of electrical properties of muscles and disease progression in mdx mice (Han et al., 2006a). Other invasive procedures that directly measure muscle strength (without behavioural complications) are discussed below (Sections 10 and 11).
10. Whole animals: in situ nerve stimulated contraction with dynanometer to measure function of muscle groups
Measuring muscle function in situ in anesthetized laboratory mice usually involves measuring the strength of an entire muscle group, such as the ankle plantarflexors or dorsiflexors using a dynamometer (Ashton-Miller et al., 1992; Brooks et al., 2001; Hamer et al., 2002; Miller et al., 1998). This is an invasive technique since the (peroneal or tibial) nerve innervating the muscle group of the leg is stimulated via surface, hook, or needle electrodes, with the foot of one leg secured to a force plate. When the muscle group is electrically stimulated, the apparatus measures the moment developed about the ankle during isometric, isovelocity shortening, or isovelocity lengthening contractions (Ashton-Miller et al., 1992). One advantage of this precise in vivo measurement is that changes in the functional properties, e.g. adaptation, of a muscle group can be repeatedly evaluated in an individual mouse over time [Ridgley, Grounds et al., paper under review]. This technique involves minimal surgery and there are no complicating factors such as muscle injury/repair that could affect force production. The major disadvantage is that such measurements require the use of elaborate custom-built hardware that is not widely available.
11. Terminal physiological measurements of muscle function
In many physiological studies, muscles are removed from the animal and various parameters measured in vitro: therefore these experiments are terminal. Histological analysis can subsequently be carried out on whole muscles and these data reconciled with the physiological measurements.
11a. In situ nerve stimulated contraction of individual whole muscles
For in situ analysis the distal tendon of (usually) a hindlimb muscle such as EDL, soleus, medial gastrocnemius, or tibialis anterior is isolated and the tendon is sutured directly to the lever arm of a force/position controller while still attached to the muscle or muscle group (with the possibility of repeated experiments over time)(Brooks, 1998); alternatively the tendon is severed and then secured to the lever arm with suture (this is usually a terminal experiment). After the tendon is attached to the force–recording apparatus, the knee and body of the animal is secured, and the muscle in question stimulated by its nerve, e.g. the sciatic nerve (Brooks, 1998; Consolino and Brooks, 2004; Dellorusso et al., 2001; Schertzer et al., 2006; Stupka et al., 2006). The advantage of this technique (as for the in vivo evaluation in Section 10) is that the muscle sarcolemma, basement membrane and extracellular matrix connections remain intact, and the nerve and blood supply are undamaged which permit evaluation of larger muscles (e.g. tibialis anterior, gastrocnemius) that cannot be evaluated in vitro due to difficulties with perfusion of such large muscles. A potential disadvantage of the in situ approach is that since the muscle is stimulated via the nerve, the accuracy of the measurements relies upon there being no interference with normal innervation. Some neuromuscular conditions can obviously affect peripheral nerves which may thus interfere with normal neurotransmission and invalidate the in situ approach.
11b. Isolated whole muscles: in vitro measurements of function
Evaluation of muscle function in vitro requires careful surgical tendon-to-tendon excision of muscles from anaesthetized animals; these techniques take time to perfect. The slightest damage to muscle fibre integrity during surgery compromises muscle force-producing capacity. The isolated muscle (e.g. usually the whole EDL), is tied to a force recording apparatus which includes a fixed pin at one end and a lever arm of a dual-mode (force-length) servomotor or simply an isometric force transducer. The isolated muscle is stimulated by platinum plate electrodes that flank, but do not touch the preparation. The accurate measurement of maximum muscle force producing capacity (and power output) in vitro is dependent upon many factors including surgical dissection, the use of accurate force recording equipment (to ensure that the muscle is stimulated adequately to recruit all motor units within the muscle) and adequate muscle perfusion to prevent any part of the muscle becoming anoxic (Lynch et al., 2001). Muscles can be stimulated to contract with muscle length either maintained (isometric contractions), or shortened (“concentric” contractions), or lengthened (eccentric or lengthening contractions). Such in vitro analysis also allows physiological parameters of diaphragm muscle to be measured (Lynch et al., 1997; Petrof et al., 1993; Stedman et al., 1991). In most cases, evaluation of muscle function capacity in vivo (Section 10) or ex vivo (Section 11) should produce very similar maximum forces. If there is a significant discrepancy between these values, there are deficiencies in the methodology and/or equipment. If any of these parameters are overlooked, the measurement of the muscle’s true functional capacity will be inaccurate and therefore evaluation of the efficacy of the pharmaceutical or nutritional intervention will be compromised. The relative advantages and disadvantages of the in vitro preparation for assessing muscle function, depend on whether an intact nerve and blood supply is desirable for a specific evaluation. The in vitro preparation can be advantageous for assessing whether a treatment affects the contractile apparatus directly, without contributions from the nerve and blood supply. Ideally, measuring muscle function in situ and in vitro (using a contralateral muscle) would provide the most comprehensive assessment of treatment efficacy. In practice, however, such an approach is beyond the scope of most studies both in terms of the time constraints for investigation and the technical expertise required.
11c. Isolated individual myofibres in vitro
This technique uses isolated individual myofibres extracted from whole muscles, rather than assessment of isolated muscle fibre bundles or intact whole muscles. Experiments can be performed on membrane intact myofibres (Yeung et al., 2003) or on “skinned” myofibre preparations where the sarcolemma has been either mechanically peeled (Plant and Lynch, 2003) or chemically permeabilized (Lynch et al., 2000). This preparation allows for direct testing of the sensitivity of contractile filaments to activating Ca2+ (or other divalent cations, such as Sr2+) and an assessment of the effects of treatment on the contractile apparatus. Isolated myofibres can be attached directly between sensitive force transducers (at one end) and a length controller (at the other end) so that fibres can be activated (electrically or chemically) and fibre length either maintained, shortened or lengthened, as per assessments on intact muscle preparations. These experiments can be informative for assessing the cellular mechanisms of action of novel treatments since they permit examination of the contractile apparatus and of excitation-contraction coupling. As for all studies using single myofibres, it is important to recognise that only a limited number of myofibres can be sampled and these may or may not be representative of the entire population of fibres within a muscle. In dystrophic muscles, the incidence of myofibres with atypical morphologies such as complex branching also needs to be considered for these physiological assessments (Williams et al., 1993). This helps to ensure that the myofibre sampling methods do not skew findings due to the inclusion of only less damaged myofibres. The single fibre assessment of muscle function provides a more physiological approach than examination of myotubes formed in culture (Han et al., 2006b).
12. Calcium regulation and ion channels measurements
12a. Determination of calcium ion homeostasis
Altered Ca2+ homeostasis, linked to altered activity of various ion channels, has been proposed as a key consequence of dystrophin-deficiency contributing to the dystrophic pathology and to calcium-dependent proteolytic events (Alderton and Steinhardt, 2000; De Luca et al., 2003; Whitehead et al., 2006b). However, it is still debated whether dysregulation of Ca2+ is a primary consequence, or just another downstream player in the complex pathophysiological cascade. Changes in excitation-contraction coupling detected by electrophysiology are a functional integrated monitoring of altered calcium homeostasis (De Luca et al., 2003; De Luca et al., 2001). A variety of methods including the use of radiolabelled calcium and Ca2+-specific fluorescent dyes (like Fura-2 and FFP-18) in conjunction with microspectrofluorimetry or fluorescence digital imaging microscopy, have been used to measure alterations in resting intracellular free [Ca2+] ([Ca2+]i), sarcolemmal permeability and the events controlling Ca2+ homeostasis. These techniques are usually applied ex vivo on collagenase dissociated individual myofibres, myotubes (often of the C2C12 cell line) or intact tendon-to-tendon bundles of muscle fibres, but similar methods can be used to visualize Ca2+ events in peripherally located myofibres within intact muscles in vivo. The development of newer generation genetically encoded calcium biosensors offer the promise of improved Ca2+ sensitivity both in vitro and in vivo and will allow for powerful two-photon Ca2+ imaging of normal and dystrophic myofibres. A detailed discussion of these techniques is beyond the scope of this review although the determination of calcium homeostasis is an important end-point in pre-clinical testing. Despite different laboratories finding either dysregulation or no change in cytosolic Ca2+ levels in dystrophic muscle fibres (De Backer et al., 2002), attributed primarily to differences in fibre preparations and the dyes employed, there is a general consensus that dystrophic myofibres exhibit an increased sarcolemmal permeability to Ca2+, especially in response to osmotic and/or mechanical stress (Allen et al., 2005; Fraysse et al., 2004; Han et al., 2006b).
12b. Channels
Altered Ca2+permeability and homeostasis in dystrophic muscle may result from a greater activity/expression of specific subset of voltage-independent ion channels, rather than from physical breaks in the sarcolemma (Fraysse et al., 2004; Whitehead et al., 2006b). Attention has been focused on various ion channels involved in sarcolemmal permeability including stretch-activated and transient-receptor-potential (TRP) channels, as this may lead to a better understanding of the pathogenetic events as well as identification of possible target for pharmacological intervention (Iwata et al., 2003; Rolland et al., 2006; Whitehead et al., 2006a). A variety of techniques are used for characterization of the candidate channels, such as biophysical (see below), immunofluorescent and biochemical approaches (RT-PCR, western blotting etc) in parallel with toxins, antibodies and drugs able to target specific channels (Rolland et al., 2006; Suchyna and Sachs, 2007; Yeung et al., 2005).
12c. Other ion channels and biophysical recordings
Sarcolemmal ion channels, gated by voltage/mechanical/chemical stimuli, are important in determining excitation patterns and a proper contractile response. In dystrophic myofibers alterations in ion channels can result either directly from dystrophin-related sarcolemmal defects, or from downstream mechanisms modulating their function and/or expression. Electrophysiological techniques can measure ion channel function (voltage-clamp recordings) and membrane excitability characteristics (current-clamp recordings), by electrode-mediated recordings in isolated muscles or myofibres. A detailed description of these specialised techniques is beyond the scope of this review. However, patch clamp recordings in freshly isolated myofibers and/or cultured myotubes are highly versatile allowing detection of a single channel current for any sort of ion channel. Although a large number of native freshly dissociated myofibers have to be sampled to be representative of all muscles, alteration in Ca2+ permeable channels have been clearly described in mdx myofibers by this approach (Rolland et al., 2006; Vandebrouck et al., 2002; Yeung et al., 2005). Apart from over-activity of voltage-independent Ca2+ channels, the muscle chloride (Cl−) channels (CIC-1) are most affected by muscular dystrophy. These channels are responsible for the large conductance of Cl− (gCl), that provides electrical stability of the sarcolemma, and are monitored by the intracellular microelectrode current clamp method in intact isolated muscle. A decrease in gCl is a functional cellular sign of spontaneous (as in diaphragm) or exercised-induced (as in EDL) damage in mdx muscles, and leads to an altered excitability pattern (De Luca et al., 1997; De Luca et al., 2003; Pierno et al., 2007). This biophysical impairment can account for an increased excitability-induced stress of dystrophic muscle as well as for the EMG abnormalities detected in both mdx mice and DMD patients (Han et al., 2006a).
13. Part II. Conclusions, recommendation and grading of data
The many techniques that can be used to measure specific aspects of the pathophysiology of muscular dystrophy (e.g. cellular changes and function), to assess the efficacy of an intervention, have been outlined. A suite of core methods for analysis of pre-clinical experiments in the mdx mouse model are outlined in Recommendation II (see Box 3). Additional techniques will be used depending on this initial analysis and the specific questions being addressed by various experiments. A flow chart outlining a broader range of analyses is shown in Fig 2. One result of a recent Workshop on Pre-clinical testing for Duchenne dystrophy: End-points in the mdx mouse held in Washington, USA in October 2007, is that details of many Methods for Analysis will be collated and available in 2008 on the websites for TREAT-NMD (http://www.treat-nmd.eu/) and Wellstone-DC (http://www.wellstone-dc.org/) to help standardise the use of these procedures.
Box 3. Recommendation II. Basic methods to analyse the pre-clinical experiments.
A core of basic measurements that are possible for most laboratories is proposed. Clearly many other measurements are possible routinely in various laboratories but the core measurements should be made by all laboratories to help standardise basic comparison of data.
If an effect is found then many additional analyses can be undertaken often using the existing sampled tissues.. Therefore, need to ensure that tissues are collected in the correct way for such further analyses e.g.fresh myofibers for electrophysiology or calcium determination and frozen sections for immunohistochemistry, frozen tissues for protein and RNA.
Physiological measurements are very informative but may not be possible in all laboratories (therefore this is shown in italics). However some measure of muscle function and strength such as the capacity for exercise or grip strength should be included.
The minimal measurements are indicated below in the order in which they would be done for sampling. Items 3 and 4 are essential. However, it is highly recommended to try and include items 1 and 2. This simple suite of procedures is based on standard analyses along with the opportunity for more in-depth investigations. (An example of a flow chart for a pre-clinical drug trial is shown in Fig 2).
-
Ongoing measurements During the experiment, some simple non-invasive measurement of muscle function such as monitoring the exercise capacity and/or grip strength or rotarod activity can be undertaken regularly (Section 9). In addition, body weights should be measured regularly, at least at the beginning and end of the experiment.
Ideally, whole body imaging would also be performed but this is not yet optimised and specialised equipment is required (Section 6).
Muscle strength. Study of isolated EDL and other muscles ex vivo to measure specific force and strength is done immediately after sacrifice (Section 11a).
Blood At the termination of the experiment, blood should be taken, serum prepared and stored frozen, for analysis of serum CK enzyme activity (Section 5c).
Muscle histology Analysis of H&E stained transverse muscle sections (frozen or paraffin) is essential to quantitate areas of active muscle necrosis and regeneration, previous regeneration and (sometimes) unaffected myofibres, along with fibrosis and other features as required (Section 7).
Use of EBD (that involves injection of the dye prior to sampling) or post-sacrifice immersion of samples in procian orange to indicate leakiness are both optional (Section 5).
Fig 2. Example of flowchart for a typical 3–6 month preclinical drug trial in mdx mice.
based on protocols used by the authors. The items shown in bold (indicated by A) are essential for all pre-clinical trials. Many of the remaining procedures (indicated by the letter B) are highly recommended, with additional analyses also included.
Scale to grade efficacy of treatment
To enable efficient comparison of data a universal grade to rank the relative efficacy of each treatment is needed. Since different interventions may affect one aspect (e.g histology) more than another (e.g physiology) it seems appropriate to grade the outcome for each parameter, rather than simply use one overall final score. A scale with 5 grades is proposed for each parameter ranging from 1 (best) to 5 (worst), with ‘1’corresponding to the measurement in normal control C57Bl/10Scsn mice and ‘5’ to that in untreated mdx mice. (This grading might also be applied retrospectively to many published papers to try and facilitate some meaningful comparison of existing data).
Benchmarking of reference data for mdx and normal C57Bl/10Scsn mice is required to help standardise measurements. While it is appreciated that measurements may vary slightly between different laboratories (and this may be more of an issue for some techniques), such reference data for both strains of mice would be very useful to help validate a technique and to establish the range of values for the grading scale. Much of this information is already published in research papers, but needs to be collated and readily available on websites (as outlined above). Reference data for all parameters for both male and female mdx and control C57Bl/10ScSn mice at one standard age (e.g 12 weeks), plus a range of other ages, including measurements after exercise, would be most helpful.
The global availability of such detailed background information on various parameters for analysis that will emerge from new collaborative networks, combined with agreement on basic core standard procedures for analysis, should rapidly advance the comparison of data between laboratories worldwide and accelerate research progress.
SUMMARY
This review describes many factors that can influence the variation between similar experiments in different laboratories (Part I) and critically evaluates the technical and methodological approaches for analysis (Part II) to help co-ordinate pre-clinical tests in the mdx mouse. Although the mdx mouse is far from being the ideal model, its use allows us to gain more insight into the pathology and potential therapies for Duchenne muscular dystrophy. It is hoped that a more coordinated effort and the joining of different expertise may help to achieve greater and faster success in this important field. This review makes two major recommendations. The first relates to basic standardisation of experimental approaches (Recommendation I) and the second to minimal parameters to analyse to assist with comparison between laboratories (Recommendation II). These guide-lines are not intended as a limitation to experimental approach, but rather as a suggestion to help provide more precise answers in a field with so many expectations. It is hoped that this review and recommendations will serve as a basis for further discussion and refinements and as a starting point for the implementation of standard approaches for evaluating pre-clinical studies in mdx mice.
Box 2. Recommendation I: Basic standard experimental regimes for pre-clinical testing.
Basic regimes to assist with global standardisation of studies in mdx mice are suggested. (Other issues of standardisation related to animal husbandry and developmental and neonatal influences can be more challenging to address). Key issues to consider are: age of onset of treatment and sampling (influenced by the experimental aim); sampling at standard ages to facilitate comparison of much data; gender (specify gender; males may be more suitable for testing some drugs); each litter should be divided into test and control mice to help reduce variation; exercise –either voluntary wheel (the total distance run by each mouse must be measured) or forced treadmill running. (Protocols for analysis are outlines in Part II)
REGIME A To test the effects of interventions on the acute early stage of the disease treatments are started before the onset of necrosis (from about 14–17 days): two sampling regimes are outlined.
A1. Sample at 28 days. Treatment is started by day 17 with tissues sampled at 28 days (4 weeks) initially. This specifically targets the initial acute phase of myofibre necrosis.
A2. Sample at 12 weeks or later. Treatment can easily be extended to further sampling and analysis at 12 weeks (as for B1). Half of the mdx mice ideally should be exposed to exercise from 4 weeks of age (either voluntary wheel running or treadmill twice a week – or a combination) to increase the severity of the disease. Note: young mice before 4 weeks of age do not exercise well. (Longer term studies can build on this).
Advantages: treatment starts before the major initial bout of damage to mdx limb muscles. Interpretation is simplified due to absence of pre-existing background pathology. This young age relates to childhood events in DMD.
Disadvantages: this is an active period of growth and some drugs may have adverse effects or be difficult to administer to very young mice. Biological variation can be high due to differences in the time of onset and severity of pathology between young mice.
REGIME B To test the effects of interventions on adult and older mice where there is a relatively low background level of pathology. These regimes all involve exercise
B1. Short term (2 day) studies to specifically test the impact on myonecrosis. This is a quick ‘proof-of-concept test. Treatment of mice exercised with a single bout of treadmill exercise (day 0), or overnight wheel running and sampled on day 2. Use of 12 week old mice corresponds to a standard sampling time.
B2. Short term (one month) studies in young adult mice. Treatment of young adult mice (8 weeks) where active necrosis is low (but much pre-existing pathology exists). One month of treatment/exercise with sampling at 12 weeks is a simple protocol.
B3. Long term studies. Treatment/exercise starting at 4, 8 or 12 weeks and sampling at 12, 24 or 52 weeks.
B4. Fibrosis and later aspects of the disease. In some cases the treatment/exercise might start at 6 months. However, chronic exercise started at earlier stage can increase fibrosis, and thus some of these aspects can be investigated during B3 protocols.
Advantages: easy to obtain and work with older mice. Pathology has stabilised and is more standardised. Exercise exacerbates severity of the disease and can shorten the time required to show effects
Disadvantages: the pre-existing background pathology can complicate interpretation of results.
Acknowledgments
This review arose from discussions at The first Brazilian International Workshop on preclinical tests for drug therapies for muscular dystrophy held in Ribeirao Preto, Brazil in late 2006. MG thanks Ian McLennan (Dunedin, New Zealand) for stimulating discussions on animal husbandry. Current research support from various funding agencies is gratefully acknowledged with grants to: MG & HR, from the National Health & Medical Research Council (NH&MRC, Australia), L’Association Francaise contre les myopathies (France) and Parent Project, Germany (Akton Benni & co); KN, the US Department of Defence, National Institute of Health, Foundation to Eradicate Dystrophy and the Jain Foundation; G L, the Muscular Dystrophy Association (USA), NH&MRC (Australia) and the Australian Research Council; and ADL, Telethon-Italy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alba M, Salvatori R. A mouse with targeted ablation of the growth hormone-releasing hormone gene: a new model of isolated growth hormone deficiency. Endocrinology. 2004;145:4134–43. doi: 10.1210/en.2004-0119. [DOI] [PubMed] [Google Scholar]
- Alderton JM, Steinhardt RA. How calcium influx through calcium leak channels is responsible for the elevated levels of calcium-dependent proteolysis in dystrophic myotubes. Trends Cardiovascular Medicine. 2000;10:268–272. doi: 10.1016/s1050-1738(00)00075-x. [DOI] [PubMed] [Google Scholar]
- Allen DG, Whitehead NP, Yeung EW. Mechanisms of stretch-induced muscle damage in normal and dystrophic muscle: role of ionic changes. J Physiol. 2005;567:723–35. doi: 10.1113/jphysiol.2005.091694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DL, Leinwand LA. Postnatal myosin heavy chain isoform expression in normal mice and mice null for IIb or IId myosin heavy chains. Dev Biol. 2001;229:383–95. doi: 10.1006/dbio.2000.9974. [DOI] [PubMed] [Google Scholar]
- Alter J, Lou F, Rabinowitz A, Yin H, Rosenfeld J, Wilton SD, Partridge TA, Lu QL. Systemic delivery of morpholino oligonucleotide restores dystrophin expression bodywide and improves dystrophic pathology. Nat Med. 2006;12:175–7. doi: 10.1038/nm1345. [DOI] [PubMed] [Google Scholar]
- Amthor H, Egelhof T, McKinnell I, Ladd ME, Janssen I, Weber J, Sinn H, Schrenk HH, Forsting M, Voit T, Straub V. Albumin targeting of damaged muscle fibres in the mdx mouse can be monitored by MRI. Neuromuscul Disord. 2004;14:791–6. doi: 10.1016/j.nmd.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Anderson JE, Vargas C, Weber M. Deflazacort increases laminin expression and myogenic repair, and induces early persistent functional gain in mdx mouse muscular dystrophy. Cell Transplant. 2000;9:551–564. doi: 10.1177/096368970000900411. [DOI] [PubMed] [Google Scholar]
- Araki E, Nakamura K, Nakao K, Kameya S, Kobayashi O, Nonaka I, Kobayashi T, Katsuki M. Targeted disruption of exon 52 in the mouse dystrophin gene induced muscle degeneration similar to that observed in Duchenne muscular dystrophy. Biochem Biophys Res Commun. 1997;238:492–7. doi: 10.1006/bbrc.1997.7328. [DOI] [PubMed] [Google Scholar]
- Archer JD, Vargas CC, Anderson JE. Persistent and improved functional gain in mdx dystrophic mice after treatment with L-arginine and deflazacort. Faseb J. 2006;20:738–40. doi: 10.1096/fj.05-4821fje. [DOI] [PubMed] [Google Scholar]
- Ashton-Miller JA, He Y, Kadhiresan VA, McCubbrey DA, Faulkner JA. An apparatus to measure in vivo biomechanical behavior of dorsi-and plantarflexors of mouse ankle. Appl Physiol. 1992;72:1205–11. doi: 10.1152/jappl.1992.72.3.1205. [DOI] [PubMed] [Google Scholar]
- Bakay M, Zhao P, Chen J, Hoffman EP. A web-accessible complete transcriptome of normal human and DMD muscle. Neuromuscul Disord. 2002;12(Suppl 1):S125–41. doi: 10.1016/s0960-8966(02)00093-7. [DOI] [PubMed] [Google Scholar]
- Balice-Gordon RJ, Lichtman JW. In vivo observations of pre- and postsynaptic changes during the transition from multiple to single innervation at developing neuromuscular junctions. J Neurosci. 1993;13:834–55. doi: 10.1523/JNEUROSCI.13-02-00834.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli M, Bourg N, Stockholm D, Raynaud F, Delevacque A, Han Y, Borel P, Seddik K, Armande N, Richard I. A mouse model for monitoring calpain activity under physiological and pathological conditions. J Biol Chem. 2006;281:39672–80. doi: 10.1074/jbc.M608803200. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A. Social stress, immune functions and disease in rodents. Front Neuroendocrinol. 2007;28:28–49. doi: 10.1016/j.yfrne.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Bassett D, Currie PD. Identification of a zebrafish model of muscular dystrophy. Clin Exp Pharmacol Physiol. 2004;31:537–40. doi: 10.1111/j.1440-1681.2004.04030.x. [DOI] [PubMed] [Google Scholar]
- Beauchamp GK, Curran M, Yamazaki K. MHC-mediated fetal odourtypes expressed by pregnant females influence male associative behaviour. Anim Behav. 2000;60:289–295. doi: 10.1006/anbe.2000.1490. [DOI] [PubMed] [Google Scholar]
- Benefiel AC, Dong WK, Greenough WT. Mandatory “ enriched” housing of laboratory animals: the need for evidence-based evaluation. Ilar J. 2005;46:95–105. doi: 10.1093/ilar.46.2.95. [DOI] [PubMed] [Google Scholar]
- Bertocchini F, Ovitt CE, Conti A, Barone V, Scholer HR, Bottinelli R, Reggiani C, Sorrentino V. Requirement for the ryanodine receptor type 3 for efficient contraction in neonatal skeletal muscles. Embo J. 1997;16:6956–63. doi: 10.1093/emboj/16.23.6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggers JD. Variance control in the animal house. Nature. 1958;182:77–80. doi: 10.1038/182077a0. [DOI] [PubMed] [Google Scholar]
- Bogdanovich S, Krag TO, Barton ER, Morris LD, Whittemore LA, Ahima RS, Khurana TS. Functional improvement of dystrophic muscle by myostatin blockade. Nature. 2002;420:418–21. doi: 10.1038/nature01154. [DOI] [PubMed] [Google Scholar]
- Bonuccelli G, Sotgia F, Capozza F, Gazzerro E, Minetti C, Lisanti MP. Localized treatment with a novel FDA-approved proteasome inhibitor blocks the degradation of dystrophin and dystrophin-associated proteins in mdx mice. Cell Cycle. 2007;6:1242–8. doi: 10.4161/cc.6.10.4182. [DOI] [PubMed] [Google Scholar]
- Briguet A, Courdier-Fruh I, Foster M, Meier T, Magyar JP. Histological parameters for the quantitative assessment of muscular dystrophy in the mdx-mouse. Neuromuscul Disord. 2004;14:675–82. doi: 10.1016/j.nmd.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Bronson FH. Puberty in female mice is not associated with increases in either body fat or leptin. Endocrinology. 2001;142:4758–61. doi: 10.1210/endo.142.11.8495. [DOI] [PubMed] [Google Scholar]
- Brooks SV. Rapid recovery following contraction-induced injury to in situ skeletal muscles in mdx mice. J Muscle Res Cell Motil. 1998;19:179–87. doi: 10.1023/a:1005364713451. [DOI] [PubMed] [Google Scholar]
- Brooks SV, Opiteck JA, Faulkner JA. Conditioning of skeletal muscles in adult and old mice for protection from contraction-induced injury. J Gerontol A Biol Sci Med Sci. 2001;56:B163–71. doi: 10.1093/gerona/56.4.b163. [DOI] [PubMed] [Google Scholar]
- Brunelli S, Sciorati C, D’Antona G, Innocenzi A, Covarello D, Galvez BG, Perrotta C, Monopoli A, Sanvito F, Bottinelli R, Ongini E, Cossu G, Clementi E. Nitric oxide release combined with nonsteroidal antiinflammatory activity prevents muscular dystrophy pathology and enhances stem cell therapy. Proc Natl Acad Sci U S A. 2007;104:264–9. doi: 10.1073/pnas.0608277104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brussee V, Tardif F, Tremblay JP. Muscle fibers of mdx mice are more vulnerable to exercise than those of normal mice. Neuromuscular Disorders. 1997;7:487–92. doi: 10.1016/s0960-8966(97)00115-6. [DOI] [PubMed] [Google Scholar]
- Bulfield G, Siller WG, Wright PAL, Moore KJ. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdi R, Didonna MP, Pignol B, Nico B, Mangieri D, Rolland JF, Camerino C, Zallone A, Ferro P, Andreetta F, Confalonieri P, De Luca A. First evaluation of the potential effectiveness in muscular dystrophy of a novel chimeric compound, BN 82270, acting as calpain-inhibitor and anti-oxidant. Neuromuscular Disorders. 2006;16:237–48. doi: 10.1016/j.nmd.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Butler-Browne GS, Barbet JP, Thornell LE. Myosin heavy and light chain expression during human skeletal muscle development and precocious muscle maturation induced by thyroid hormone. Anat Embryol (Berl) 1990;181:513–22. doi: 10.1007/BF00174624. [DOI] [PubMed] [Google Scholar]
- Cahill KS, Gaidosh G, Huard J, Silver X, Byrne BJ, Walter GA. Noninvasive monitoring and tracking of muscle stem cell transplants. Transplantation. 2004;78:1626–33. doi: 10.1097/01.tp.0000145528.51525.8b. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Curley JP, Keverne EB, Bateson PP. Natural variations in postpartum maternal care in inbred and outbred mice. Physiol Behav. 2007;91:325–34. doi: 10.1016/j.physbeh.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Chapman VM, Miller DR, Armstrong D, Caskey CT. Recovery of induced mutations for X chromosome- linked muscular dystrophy in mice. Proceedings of National Academy of Science. 1989;86:1292–1296. doi: 10.1073/pnas.86.4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Nagaraju K, Bakay M, McIntyre O, Rawat R, Shi R, Hoffman EP. Early onset of inflammation and later involvement of TGFbeta in Duchenne muscular dystrophy. Neurology. 2005;65:826–34. doi: 10.1212/01.wnl.0000173836.09176.c4. [DOI] [PubMed] [Google Scholar]
- Collins CA, Morgan JE. Duchenne’s muscular dystrophy: animal models used to investigate pathogenesis and develop therapeutic strategies. International Journal of Experimental Pathology. 2003;84:165–172. doi: 10.1046/j.1365-2613.2003.00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly AM, Keeling RM, Mehta S, Pestronk A, Sanes JR. Three mouse models of muscular dystrophy: the natural history of strength and fatigue in dystrophin-, dystrophin/utrophin-, and laminin alpha2-deficient mice. Neuromuscul Disord. 2001;11:703–12. doi: 10.1016/s0960-8966(01)00232-2. [DOI] [PubMed] [Google Scholar]
- Consolino CM, Brooks SV. Susceptibility to sarcomere injury induced by single stretches of maximally activated muscles of mdx mice. Journal of Applied Physiology. 2004;96:633–8. doi: 10.1152/japplphysiol.00587.2003. [DOI] [PubMed] [Google Scholar]
- Coulton GR, Curtin NA, Morgan JE, Partridge TA. The mdx mouse skeletal muscle myopathy: II. Contractile properties. Neuropathology and Applied Neurobiology. 1988a;14:299–314. doi: 10.1111/j.1365-2990.1988.tb00890.x. [DOI] [PubMed] [Google Scholar]
- Coulton GR, Morgan JE, Partridge TA, Sloper JC. The mdx mouse skeletal muscle myopathy: I. A histological, morphometric and biochemical investigation. Neuropathology and Applied Neurobiology. 1988b;14:53–70. doi: 10.1111/j.1365-2990.1988.tb00866.x. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999;835:18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- De Backer F, Vandebrouck C, Gailly P, Gillis JM. Long-term study of Ca2+ homeostasis and of survival in collagenase-isolated muscle fibres from normal and mdx mice. J Physiol (Lond) 2002;542:855–865. doi: 10.1113/jphysiol.2002.020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca A, Conte Camerino D, Connold A, Vrbova G. Pharmacological block of chloride channels of developing rat skeletal muscle affects the differentiation of specific contractile properties. Pflugers Arch. 1990;416:17–21. doi: 10.1007/BF00370216. [DOI] [PubMed] [Google Scholar]
- De Luca A, Nico B, Liantonio A, Didonna MP, Fraysse B, Pierno S, Burdi R, Mangieri D, Rolland JF, Camerino C, Zallone A, Confalonieri P, Andreetta F, Arnoldi E, Courdier-Fruh I, Magyar JP, Frigeri A, Pisoni M, Svelto M, Conte Camerino D. A multidisciplinary evaluation of the effectiveness of cyclosporine a in dystrophic mdx mice. Am J Pathol. 2005;166:477–89. doi: 10.1016/S0002-9440(10)62270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca A, Pierno S, Camerino DC. Electrical properties of diaphragm and EDL muscles during the life of dystrophic mice. Am J Physiol. 1997;272:C333–40. doi: 10.1152/ajpcell.1997.272.1.C333. [DOI] [PubMed] [Google Scholar]
- De Luca A, Pierno S, Liantonio A, Cetrone M, Camerino C, Fraysse B, Mirabella M, Servidei S, Ruegg UT, Conte Camerino D. Enhanced dystrophic progression in mdx mice by exercise and beneficial effects of taurine and insulin-like growth factor-1. Journal of Pharmacological and Experimental Therapeutics. 2003;304:453–63. doi: 10.1124/jpet.102.041343. [DOI] [PubMed] [Google Scholar]
- De Luca A, Pierno S, Liantonio A, Cetrone M, Camerino C, Simonetti S, Papadia F, Camerino DC. Alteration of excitation-contraction coupling mechanism in extensor digitorum longus muscle fibres of dystrophic mdx mouse and potential efficacy of taurine. Br J Pharmacol. 2001;132:1047–54. doi: 10.1038/sj.bjp.0703907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca A, Pierno S, Liantonio A, Conte Camerino D. Pre-clinical trials in Duchenne dystrophy: what animal models can tell us about potential drug effectiveness. Neuromuscular Disorders. 2002;12(Suppl 1):S142–6. doi: 10.1016/s0960-8966(02)00100-1. [DOI] [PubMed] [Google Scholar]
- Dellorusso C, Crawford RW, Chamberlain JS, Brooks SV. Tibialis anterior muscles in mdx mice are highly susceptible to contraction-induced injury. J Muscle Res Cell Motil. 2001;22:467–75. doi: 10.1023/a:1014587918367. [DOI] [PubMed] [Google Scholar]
- den Hertog J. Chemical genetics:drug screens in zebrafish. Bioscience Reports. 2005;25:289–97. doi: 10.1007/s10540-005-2891-8. [DOI] [PubMed] [Google Scholar]
- Doherty KR, McNally EM. Repairing the tears: dysferlin in muscle membrane repair. Trends Mol Med. 2003;9:327–30. doi: 10.1016/s1471-4914(03)00136-9. [DOI] [PubMed] [Google Scholar]
- Duclos F, Straub V, Moore S, Venzke D, Hrstka R, Crosbie R, Durbeej M, Lebakken C, Ettinger A, van der Meulen J, Holt K, Lim L, Sanes J, Davidson B, Faulkner J, Williamson R, Campbell K. Progressive muscular dystrophy in α-sarcoglycan-deficient mice. Journal of Cell Biology. 1998;142:1461–1471. doi: 10.1083/jcb.142.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont-Versteegden EE, McCarter RJ, Katz MS. Voluntary exercise decreases progression of muscular dystrophy in diaphragm of mdx mice. J Appl Physiol. 1994;77:1736–41. doi: 10.1152/jappl.1994.77.4.1736. [DOI] [PubMed] [Google Scholar]
- Elmlinger MW, Kuhnel W, Lambrecht HG, Ranke MB. Reference intervals from birth to adulthood for serum thyroxine (T4), triiodothyronine (T3), free T3, free T4, thyroxine binding globulin (TBG) and thyrotropin (TSH. Clin Chem Lab Med. 2001;39:973–9. doi: 10.1515/CCLM.2001.158. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Pedersen BK. Contraction-induced myokine production and release: is skeletal muscle an endocrine organ? Exerc Sport Sci Rev. 2005;33:114–9. doi: 10.1097/00003677-200507000-00003. [DOI] [PubMed] [Google Scholar]
- Fisher MD, Budak MT, Bakay M, Gorospe JR, Kiellgren D, Pedrosa Donello F, Hoffman EP, Khurana TS. Definition of the unique human extraocular muscle allotype by expression profiling. Physiol Genomics. 2005;22:283–91. doi: 10.1152/physiolgenomics.00158.2004. [DOI] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–8. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Franconi F, Brunelleschi S, Steardo L, Cuomo V. Gender differences in drug responses. Pharmacol Res. 2007;55:81–95. doi: 10.1016/j.phrs.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Fraysse B, Liantonio A, Cetrone M, Burdi R, Pierno S, Frigeri A, Pisoni M, Camerino C, De Luca A. The alteration of calcium homeostasis in adult dystrophic mdx muscle fibers is worsened by a chronic exercise in vivo. Neurobiol Dis. 2004;17:144–54. doi: 10.1016/j.nbd.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Gartner K. A third component causing random variability beside environment and genotype. A reason for the limited success of a 30 year long effort to standardize laboratory animals? Lab Anim. 1990;24:71–7. doi: 10.1258/002367790780890347. [DOI] [PubMed] [Google Scholar]
- Gartner K, Buttner D, Dohler K, Friedel R, Lindena J, Trautschold I. Stress response of rats to handling and experimental procedures. Lab Anim. 1980;14:267–74. doi: 10.1258/002367780780937454. [DOI] [PubMed] [Google Scholar]
- Ge Y, Molloy MP, Chamberlain JS, Andrews PC. Proteomic analysis of mdx skeletal muscle: Great reduction of adenylate kinase 1 expression and enzymatic activity. Proteomics. 2003;3:1895–903. doi: 10.1002/pmic.200300561. [DOI] [PubMed] [Google Scholar]
- Glenmark B, Nilsson M, Gao H, Gustafsson JA, Dahlman-Wright K, Westerblad H. Difference in skeletal muscle function in males vs. females: role of estrogen receptor-beta. Am J Physiol Endocrinol Metab. 2004;287:E1125–31. doi: 10.1152/ajpendo.00098.2004. [DOI] [PubMed] [Google Scholar]
- Gosselin LE, Williams JE. Pentoxifylline fails to attenuate fibrosis in dystrophic (mdx) diaphragm muscle. Muscle Nerve. 2006;33:820–3. doi: 10.1002/mus.20523. [DOI] [PubMed] [Google Scholar]
- Gosselin LE, Williams JE, Personius K, Farkas GA. A comparison of factors associated with collagen metabolism in different skeletal muscles from dystrophic (mdx) mice: impact of pirfenidone. Muscle Nerve. 2007;35:208–16. doi: 10.1002/mus.20681. [DOI] [PubMed] [Google Scholar]
- Granchelli JA, Pollina C, Hudecki MS. Pre-clinical screening of drugs using the mdx mouse. Neuromusclular Disorders. 2000;10:235–239. doi: 10.1016/s0960-8966(99)00126-1. [DOI] [PubMed] [Google Scholar]
- Gregorevic P, Plant DR, Leeding KS, Bach LA, Lynch GS. Improved Contractile Function of the mdx Dystrophic Mouse Diaphragm Muscle after Insulin-Like Growth Factor-I Administration. Am J Pathol. 2002;161:2263–2272. doi: 10.1016/S0002-9440(10)64502-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grounds MD, Torrisi J. Anti-TNFα (Remicade) therapy protects dystrophic skeletal muscle from necrosis. FASEB Journal. 2004;18:676–682. doi: 10.1096/fj.03-1024com. [DOI] [PubMed] [Google Scholar]
- Hall JE, Kaczor JJ, Hettinga BP, Isfort RJ, Tarnopolsky MA. Effects of a CRF2R agonist and exercise on mdx and wildtype skeletal muscle. Muscle Nerve. 2007;36:336–41. doi: 10.1002/mus.20820. [DOI] [PubMed] [Google Scholar]
- Hamann M, Meisler MH, Richter A. Motor disturbances in mice with deficiency of the sodium channel gene Scn8a show features of human dystonia. Exp Neurol. 2003;184:830–8. doi: 10.1016/S0014-4886(03)00290-5. [DOI] [PubMed] [Google Scholar]
- Hamer PW, McGeachie JM, Davies MJ, Grounds MD. Evans blue dye as an in vivo marker of myofibre fragility and damage: optimising parameters for detecting initial myofibre permeability. J Anat. 2002;200:69–79. doi: 10.1046/j.0021-8782.2001.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JJ, Carter GT, Ra JJ, Abresch RT, Chamberlain JS, Robinson LR. Electromyographic studies in mdx and wild-type C57 mice. Muscle Nerve. 2006a;33:208–14. doi: 10.1002/mus.20455. [DOI] [PubMed] [Google Scholar]
- Han R, Grounds MD, Bakker AJ. Measurement of sub-membrane [Ca(2+)] in adult myofibers and cytosolic [Ca(2+)] in myotubes from normal and mdx mice using the Ca(2+) indicator FFP-18. Cell Calcium. 2006b;40:299–307. doi: 10.1016/j.ceca.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Hara H, Nolan PM, Scott MO, Bucan M, Wakayama Y, Fischbeck KH. Running endurance abnormality in mdx mice. Muscle Nerve. 2002;25:207–11. doi: 10.1002/mus.10023. [DOI] [PubMed] [Google Scholar]
- Harris I. Variables in Animal Based Research: Part 1. Phenotypic Variability in Experimental Research. ANZCCART News. 1997;10:1–8. http://www.adelaide.edu.au/ANZCCART/publications/facts.html.
- Haslett JN, Kunkel LM. Microarray analysis of normal and dystrophic skeletal muscle. International Journal of Developmental Neuroscience. 2002;20:359–365. doi: 10.1016/s0736-5748(02)00041-2. [DOI] [PubMed] [Google Scholar]
- Hayes A, Williams DA. Beneficial effects of voluntary wheel running on the properties of dystrophic mouse muscle. Journal of Applied Physiology. 1996;80:670–679. doi: 10.1152/jappl.1996.80.2.670. [DOI] [PubMed] [Google Scholar]
- Hayes A, Williams DA. Examining potential drug therapies for muscular dystrophy utilising the dy/dy mouse: 1. Clenbuterol. Journal of Neurological Sciences. 1998;157:122–128. doi: 10.1016/s0022-510x(98)00084-7. [DOI] [PubMed] [Google Scholar]
- Hesselmans LF, Jennekens FG, Van den Oord CJ, Veldman H, Vincent A. Development of innervation of skeletal muscle fibers in man: relation to acetylcholine receptors. Anat Rec. 1993;236:553–62. doi: 10.1002/ar.1092360315. [DOI] [PubMed] [Google Scholar]
- Heydemann A, Huber JM, Demonbreun A, Hadhazy M, McNally EM. Genetic background influences muscular dystrophy. Neuromuscul Disord. 2005;15:601–9. doi: 10.1016/j.nmd.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Hittel DS, Hathout Y, Hoffman EP. Proteomics and systems biology in exercise and sport sciences research. Exerc Sport Sci Rev. 2007;35:5–11. doi: 10.1097/jes.0b013e31802d744a. [DOI] [PubMed] [Google Scholar]
- Hodgetts S, Radley H, Davies M, Grounds MD. Reduced necrosis of dystrophic muscle by depletion of host neutrophils, or blocking TNFalpha function with Etanercept in mdx mice. Neuromuscul Disord. 2006;16:591–602. doi: 10.1016/j.nmd.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Hoffman EP, Monaco AP, Feener CC, Kunkel LM. Conservation of the Duchenne muscular dystrophy gene in mice and humans. Science. 1987;16:347–350. doi: 10.1126/science.3659917. [DOI] [PubMed] [Google Scholar]
- Hudecki MS, Pollina CM, Granchelli JA, Daly MK, Byrnes T, Wang JC, Hsiao JC. Strength and endurance in the therapeutic evaluation of prednisolone-treated MDX mice. Res Commun Chem Pathol Pharmacol. 1993;79:45–60. [PubMed] [Google Scholar]
- Iwata Y, Katanosaka Y, Arai Y, Komamura K, Miyatake K, Shigekawa M. A novel mechanism of myocyte degeneration involving the Ca2+-permeable growth factor-regulated channel. J Cell Biol. 2003;161:957–67. doi: 10.1083/jcb.200301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Faucher C, Berger M, de Turckheim M, Veyssiere G, Jean C. Developmental patterns of plasma and testicular testosterone in mice from birth to adulthood. Acta Endocrinol (Copenh) 1978;89:780–8. doi: 10.1530/acta.0.0890780. [DOI] [PubMed] [Google Scholar]
- Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nature. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockusch H, Friedrich G, Zippel M. Serum parvalbumin, an indicator of muscle disease in murine dystrophy and myotonia. Muscle Nerve. 1990;13:551–5. doi: 10.1002/mus.880130613. [DOI] [PubMed] [Google Scholar]
- Kaczor JJ, Hall JE, Payne E, Tarnopolsky MA. Low intensity training decreases markers of oxidative stress in skeletal muscle of mdx mice. Free Radic Biol Med. 2007;43:145–54. doi: 10.1016/j.freeradbiomed.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Keeling RM, Golumbek PT, Streif EM, Connolly AM. Weekly oral prednisolone improves survival and strength in male mdx mice. Muscle Nerve. 2007;35:43–8. doi: 10.1002/mus.20646. [DOI] [PubMed] [Google Scholar]
- Khurana TS, Prendergast RA, Alameddine HS, Tome FM, Fardeau M, Arahata K, Sugita H, Kunkel LM. Absence of extraocular muscle pathology in Duchenne’s muscular dystrophy: role for calcium homeostasis in extraocular muscle sparing. J Exp Med. 1995;182:467–75. doi: 10.1084/jem.182.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana TS, Watkins SC, Chafey P, Chelly J, Tome FM, Fardeau M, Kaplan JC, Kunkel LM. Immunolocalization and developmental expression of dystrophin related protein in skeletal muscle. Neuromuscul Disord. 1991;1:185–94. doi: 10.1016/0960-8966(91)90023-l. [DOI] [PubMed] [Google Scholar]
- Klyen BR, Armstrong JJ, Adie SG, Radley HG, Grounds MD, Sampson DD. Three-dimensional optical coherence tomography of whole-muscle autografts as a precursor to morphological assessment of muscular dystrophy in mice. J Biomed Opt. 2008;13:011003. doi: 10.1117/1.2870170. [DOI] [PubMed] [Google Scholar]
- Konhilas JP, Widegren U, Allen DL, Paul AC, Cleary A, Leinwand LA. Loaded wheel running and muscle adaptation in the mouse. Am J Physiol Heart Circ Physiol. 2005;289:H455–65. doi: 10.1152/ajpheart.00085.2005. [DOI] [PubMed] [Google Scholar]
- Kopp C, Ressel V, Wigger E, Tobler I. Influence of estrus cycle and ageing on activity patterns in two inbred mouse strains. Behav Brain Res. 2006;167:165–74. doi: 10.1016/j.bbr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Kornegay JN, Tuler SM, Miller DM, Levesque DC. Muscular dystrophy in a litter of golden retriever dogs. Muscle and Nerve. 1988;11:1056–1064. doi: 10.1002/mus.880111008. [DOI] [PubMed] [Google Scholar]
- Krupnick AS, Zhu J, Nguyen T, Kreisel D, Balsara KR, Lankford EB, Clark CC, Levine S, Stedman HH, Shrager JB. Inspiratory loading does not accelerate dystrophy in mdx mouse diaphragm: implications for regenerative therapy. J Appl Physiol. 2003;94:411–9. doi: 10.1152/japplphysiol.00689.2002. [DOI] [PubMed] [Google Scholar]
- Lamason R, Zhao P, Rawat R, Davis A, Hall JC, Chae JJ, Agarwal R, Cohen P, Rosen A, Hoffman EP, Nagaraju K. Sexual dimorphism in immune response genes as a function of puberty. BMC Immunol. 2006;7:2. doi: 10.1186/1471-2172-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur JP, Pastoret C, Sebille A. Phenotype of dystrophinopathy in old mdx mice. Anatom Rec. 1995;242:70–76. doi: 10.1002/ar.1092420109. [DOI] [PubMed] [Google Scholar]
- Lynch GS, Hinkle RT, Chamberlain JS, Brooks SV, Faulkner JA. Force and power output of fast and slow skeletal muscles from mdx mice 6–28 months old. J Physiol (Lond) 2001;535:591–600. doi: 10.1111/j.1469-7793.2001.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch GS, Rafael JA, Chamberlain JS, Faulkner JA. Contraction-induced injury to single permeabilized muscle fibres from mdx, transgenic mdx, and control mice. American Journal of Physiological Cell Physiology. 2000;279:C1290–C1294. doi: 10.1152/ajpcell.2000.279.4.C1290. [DOI] [PubMed] [Google Scholar]
- Lynch GS, Rafael JA, Hinkle RT, Cole NM, Chamberlain JS, Faulkner JA. Contractile properties of diaphragm muscle segments from old mdx and old transgenic mdx mice. Am J Physiol. 1997;272:C2063–8. doi: 10.1152/ajpcell.1997.272.6.C2063. [DOI] [PubMed] [Google Scholar]
- Marques MJ, Ferretti R, Vomero VU, Minatel E, Neto HS. Intrinsic laryngeal muscles are spared from myonecrosis in the mdx mouse model of Duchenne muscular dystrophy. Muscle Nerve. 2007;35:349–53. doi: 10.1002/mus.20697. [DOI] [PubMed] [Google Scholar]
- Martinez Amat A, Marchal Corrales JA, Rodriguez Serrano F, Boulaiz H, Prados Salazar JC, Hita Contreras F, Caba Perez O, Carrillo Delgado E, Martin I, Aranega Jimenez A. Role of alpha-actin in muscle damage of injured athletes in comparison with traditional markers. Br J Sports Med. 2007;41:442–6. doi: 10.1136/bjsm.2006.032730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda R, Nishikawa A, Tanaka H. Visualization of dystrophic muscle fibers in mdx mouse by vital staining with evans blue: evidence of apoptosis in dystrophin-deficient muscle. Journal of Biochemistry. 1995;118:959–964. doi: 10.1093/jb/118.5.959. [DOI] [PubMed] [Google Scholar]
- Matthews PM, Benjamin D, Van Bakel I, Squier MV, Nicholson LV, Sewry C, Barnes PR, Hopkin J, Brown R, Hilton-Jones D, et al. Muscle X-inactivation patterns and dystrophin expression in Duchenne muscular dystrophy carriers. Neuromuscul Disord. 1995;5:209–20. doi: 10.1016/0960-8966(94)00057-g. [DOI] [PubMed] [Google Scholar]
- McArdle A, Helliwell TR, Beckett GJ, Catapano M, Davis A, Jackson MJ. Effect of propylthiouracil-induced hypothyroidism on the onset of skeletal muscle necrosis in dystrophin-deficient mdx mice. Clin Sci (Lond) 1998;95:83–9. [PubMed] [Google Scholar]
- McCarthy JJ, Andrews JL, McDearmon EL, Campbell KS, Barber BK, Miller BH, Walker JR, Hogenesch JB, Takahashi JS, Esser KA. Identification of the circadian transcriptome in adult mouse skeletal muscle. Physiol Genomics. 2007;31:86–95. doi: 10.1152/physiolgenomics.00066.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure WC, Rabon RE, Ogawa H, Tseng BS. Upregulation of the creatine synthetic pathway in skeletal muscles of mature mdx mice. Neuromuscul Disord. 2007;17:639–650. doi: 10.1016/j.nmd.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeachie JK, Grounds MD, Partridge TA, Morgan JE. Age-related changes in replication of myogenic cells in mdx mice: quantitative autoradiographic studies. J Neurol Sci. 1993;119:169–179. doi: 10.1016/0022-510x(93)90130-q. [DOI] [PubMed] [Google Scholar]
- McIntosh LM, Baker RE, Anderson JE. Magnetic resonance imaging of regenerating and dystrophic mouse muscle. Biochemistry and Cell Biology. 1998;76:532–541. doi: 10.1139/bcb-76-2-3-532. [DOI] [PubMed] [Google Scholar]
- McLennan IS, Taylor-Jeffs J. The use of sodium lamps to brightly illuminate mouse houses during their dark phases. Laboratory Animals (London) 2004;38:384–392. doi: 10.1258/0023677041958927. [DOI] [PubMed] [Google Scholar]
- McNally EM, MacLeod H. Therapy insight: cardiovascular complications associated with muscular dystrophies. Nat Clin Pract Cardiovasc Med. 2005;2:301–8. doi: 10.1038/ncpcardio0213. [DOI] [PubMed] [Google Scholar]
- McNeil PL, Kirchhausen T. An emergency response team for membrane repair. Nat Rev Mol Cell Biol. 2005;6:499–505. doi: 10.1038/nrm1665. [DOI] [PubMed] [Google Scholar]
- Mehta A, Hindmarsh PC, Stanhope RG, Turton JP, Cole TJ, Preece MA, Dattani MT. The role of growth hormone in determining birth size and early postnatal growth, using congenital growth hormone deficiency (GHD) as a model. Clin Endocrinol (Oxf) 2005;63:223–31. doi: 10.1111/j.1365-2265.2005.02330.x. [DOI] [PubMed] [Google Scholar]
- Miller SW, Hassett CA, Faulkner JA. Recovery of muscle transfer replacing the total plantar flexor muscle group in rats. Journal of Applied Physiology. 1998;84:1865–1871. doi: 10.1152/jappl.1998.84.6.1865. [DOI] [PubMed] [Google Scholar]
- Minatel E, Neto HS, Marques MJ. Acetylcholine receptor distribution and synapse elimination at the developing neuromuscular junction of mdx mice. Muscle Nerve. 2003;28:561–9. doi: 10.1002/mus.10416. [DOI] [PubMed] [Google Scholar]
- Missias AC, Chu GC, Klocke BJ, Sanes JR, Merlie JP. Maturation of the acetylcholine receptor in skeletal muscle: regulation of the AChR gamma-to-epsilon switch. Dev Biol. 1996;179:223–38. doi: 10.1006/dbio.1996.0253. [DOI] [PubMed] [Google Scholar]
- Moghadaszadeh B, Albrechtsen R, Guo L, Zaik M, Kawaguchi N, Borup RHF, Kronquist P, Schroder HD, Davies KE, Voit T, Neielson FC, Engvall E, Wewer UM. Compensation for dystrophin-deficiency: ADAM 12 overexpression in skeletal muscle results in increased alpha 7 integrin, utrophin, and associated glycoproteins. Human Molecular Genetics. 2003;12:2467–2493. doi: 10.1093/hmg/ddg264. [DOI] [PubMed] [Google Scholar]
- Muller J, Vayssiere N, Royuela M, Leger ME, Muller A, Bacou F, Pons F, Hugon G, Mornet D. Comparative evolution of muscular dystrophy in diaphragm, gastrocnemius and masseter muscles from old male mdx mice. Journal of Muscle Research & Cell Motility. 2001;22:133–139. doi: 10.1023/a:1010305801236. [DOI] [PubMed] [Google Scholar]
- Muntoni F, Mateddu A, Marchei F, Clerk A, Serra G. Muscular weakness in the mdx mouse. Journal of the Neurological Sciences. 1993;120:71–7. doi: 10.1016/0022-510x(93)90027-v. [DOI] [PubMed] [Google Scholar]
- Nagaraju K, Raben N, Loeffler L, Parker T, Rochon PJ, Lee E, Danning C, Wada R, Thompson C, Bahtiyar G, Craft J, Hooft Van Huijsduijnen R, Plotz P. Conditional up-regulation of MHC class I in skeletal muscle leads to self-sustaining autoimmune myositis and myositis-specific autoantibodies. Proc Natl Acad Sci U S A. 2000;97:9209–14. doi: 10.1073/pnas.97.16.9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y-N, Iwamoto H, Ono Y, Shiba N, Nishimura S, Tabata S. Relationship among collagen amount, distribution and architecture in the M. longissimus thoracis and M. pectoralis profundus from pigs. Meat Science. 2003;64:43–50. doi: 10.1016/s0309-1740(02)00135-3. [DOI] [PubMed] [Google Scholar]
- Niebroj-Dobosz I, Fidzianska A, Glinka Z. Comparitive studies of hind limb and diaphragm muscles of mdx mice. Basic and Applied Myology. 1997;7:381–386. [Google Scholar]
- Nowak KJ, Davies KE. Duchenne muscular dystrophy and dystrophin: pathogenesis and opportunaties for treatment. EMBO (European Molecular Biology Organization) Journal. 2004;5:872–6. doi: 10.1038/sj.embor.7400221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntziachristos V. Fluorescence molecular imaging. Annu Rev Biomed Eng. 2006;8:1–33. doi: 10.1146/annurev.bioeng.8.061505.095831. [DOI] [PubMed] [Google Scholar]
- Odom GL, Gregorevic P, Chamberlain JS. Viral-mediated gene therapy for the muscular dystrophies: Successes, limitations and recent advances. Biochim Biophys Acta. 2007;1772:243–62. doi: 10.1016/j.bbadis.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano T, Yoshida K, Nakamura A, Sasazawa F, Oide T, Takeda S, Ikeda S. Chronic exercise accelerates the degeneration-regeneration cycle and downregulates insulin-like growth factor-1 in muscle of mdx mice. Muscle Nerve. 2005;32:191–9. doi: 10.1002/mus.20351. [DOI] [PubMed] [Google Scholar]
- Osada K, Yamazaki K, Curran M, Bard J, Smith BP, Beauchamp GK. The scent of age. Proc Biol Sci. 2003;270:929–33. doi: 10.1098/rspb.2002.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa R, Hayashi YK, Ogawa M, Kurokawa R, Matsumoto H, Noguchi S, Nonaka I, Nishino I. Emerin-lacking mice show minimal motor and cardiac dysfunctions with nuclear-associated vacuoles. Am J Pathol. 2006;168:907–17. doi: 10.2353/ajpath.2006.050564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagel CN, Partridge TA. Covert persistence of mdx mouse myopathy is revealed by acute and chronic effects of irradiation. Journal of the Neurological Sciences. 1999;164:103–116. doi: 10.1016/s0022-510x(99)00061-1. [DOI] [PubMed] [Google Scholar]
- Palacio J, Galdiz JB, Alvarez FJ, Orozco-Levi M, Lloreta J, Gea J. Procion orange tracer dye technique vs. identification of intrafibrillar fibronectin in the assessment of sarcolemmal damage. Eur J Clin Invest. 2002;32:443–7. doi: 10.1046/j.1365-2362.2002.01005.x. [DOI] [PubMed] [Google Scholar]
- Parry DJ, Wilkinson RS. The effect of reinnervation on the distribution of muscle fibre types in the tibialis anterior muscle of the mouse. Can J Physiol Pharmacol. 1990;68:596–602. doi: 10.1139/y90-086. [DOI] [PubMed] [Google Scholar]
- Pasquesi JJ, Schlacter SC, Boppart MD, Chaney E, Kaufman SJ, Boppart SA. In vivo detection of exercise-induced ultrastructural changes in genetically-altered murine skeletal muscle using polarization-sensitive optical coherence tomography. Optics Express. 2006;14:1547–1556. doi: 10.1364/oe.14.001547. [DOI] [PubMed] [Google Scholar]
- Passaquin AC, Renard M, Kay L, Challet C, Mokhtarian A, Wallimann T, Ruegg UT. Creatine supplementation reduces skeletal muscle degeneration and enhances mitochondrial function in mdx mice. Neuromuscul Disord. 2002;12:174–182. doi: 10.1016/s0960-8966(01)00273-5. [DOI] [PubMed] [Google Scholar]
- Payne ET, Yasuda N, Bourgeois JM, Devries MC, Rodriguez MC, Yousuf J, Tarnopolsky MA. Nutritional therapy improves function and complements corticosteroid intervention in mdx mice. Muscle Nerve. 2006;33:66–77. doi: 10.1002/mus.20436. [DOI] [PubMed] [Google Scholar]
- Petrof BJ, Stedman HH, Shrager JB, Eby J, Sweeney HL, Kelly AM. Adaptations in myosin heavy chain expression and contractile function in dystrophic mouse diaphragm. Am J Physiol. 1993;265:C834–41. doi: 10.1152/ajpcell.1993.265.3.C834. [DOI] [PubMed] [Google Scholar]
- Pheasant M, Mattick JS. Raising the estimate of functional human sequences. Genome Res. 2007;17:1245–53. doi: 10.1101/gr.6406307. [DOI] [PubMed] [Google Scholar]
- Pierno S, Nico B, Burdi R, Liantonio A, Didonna MP, Cippone V, Fraysse B, Rolland JF, Mangieri D, Andreetta F, Ferro P, Camerino C, Zallone A, Confalonieri P, De Luca A. Role of tumour necrosis factor alpha, but not of cyclo-oxygenase-2-derived eicosanoids, on functional and morphological indices of dystrophic progression in mdx mice: a pharmacological approach. Neuropathol Appl Neurobiol. 2007;33:344–59. doi: 10.1111/j.1365-2990.2007.00798.x. [DOI] [PubMed] [Google Scholar]
- Plant DR, Lynch GS. Depolarization-induced contraction and SR function in mechanically skinned muscle fibers from dystrophic mdx mice. Am J Physiol Cell Physiol. 2003;285:C522–8. doi: 10.1152/ajpcell.00369.2002. [DOI] [PubMed] [Google Scholar]
- Porter JD, Merriam AP, Leahy P, Gong B, Khanna S. Dissection of temporal gene expression signatures of affected and spared muscle groups in dystrophin-deficient (mdx) mice. Human Molecular Genetics. 2003;12:1813–21. doi: 10.1093/hmg/ddg197. [DOI] [PubMed] [Google Scholar]
- Radley HG, De Luca A, Lynch GS, Grounds MD. Duchenne muscular dystrophy: Focus on pharmaceutical and nutritional interventions. Int J Biochem Cell Biol. 2007;39:469–77. doi: 10.1016/j.biocel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Radley HG, Grounds MD. Cromolyn administration (to block mast cell degranulation) reduces necrosis of dystrophic muscle in mdx mice. Neurobiol Dis. 2006;23:387–97. doi: 10.1016/j.nbd.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Reeb-Whitaker CK, Paigen B, Beamer WG, Bronson RT, Churchill GA, Schweitzer IB, Myers DD. The impact of reduced frequency of cage changes on the health of mice housed in ventilated cages. Lab Anim. 2001;35:58–73. doi: 10.1258/0023677011911381. [DOI] [PubMed] [Google Scholar]
- Reilly J. Variables in Animal Based Research: Part 2. Variability associcated with experimental conditions and techniques. ANZCCART News. 1998;11:1–12. http://www.adelaide.edu.au/ANZCCART/publications/facts.html.
- Rodriguez A, Muller DC, Metter EJ, Maggio M, Harman SM, Blackman MR, Andres R. Aging, androgens, and the metabolic syndrome in a longitudinal study of aging. J Clin Endocrinol Metab. 2007;92:3568–72. doi: 10.1210/jc.2006-2764. [DOI] [PubMed] [Google Scholar]
- Rolland JF, De Luca A, Burdi R, Andreetta F, Confalonieri P, Conte Camerino D. Overactivity of exercise-sensitive cation channels and their impaired modulation by IGF-1 in mdx native muscle fibers: Beneficial effect of pentoxifylline. Neurobiol Dis. 2006;24:466–74. doi: 10.1016/j.nbd.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Rothstein EC, Carroll S, Combs CA, Jobsis PD, Balaban RS. Skeletal muscle NAD(P)H two-photon fluorescence microscopy in vivo: topology and optical inner filters. Biophys J. 2005;88:2165–76. doi: 10.1529/biophysj.104.053165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimena MC, Lagrota-Candido J, Quirico-Santos Tl. Gender dimorphism influences extracellular matrix expression and regeneration of muscular tissue in mdx dystrophic mice. Histochemical Cell Biology. 2004;122:435–444. doi: 10.1007/s00418-004-0707-8. [DOI] [PubMed] [Google Scholar]
- Schafer R, Zweyer M, Knauf U, Mundegar RR, Wernig A. The ontogeny of soleus muscles in mdx and wild type mice. Neuromuscul Disord. 2005;15:57–64. doi: 10.1016/j.nmd.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Schertzer JD, Ryall JG, Lynch GS. Systemic administration of IGF-I enhances oxidative status and reduces contraction-induced injury in skeletal muscles of mdx dystrophic mice. Am J Physiol Endocrinol Metab. 2006;291:E499–505. doi: 10.1152/ajpendo.00101.2006. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev. 1996;76:371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Enthoven L, van der Mark M, Levine S, de Kloet ER, Oitzl MS. The postnatal development of the hypothalamic-pituitary-adrenal axis in the mouse. Int J Dev Neurosci. 2003;21:125–32. doi: 10.1016/s0736-5748(03)00030-3. [DOI] [PubMed] [Google Scholar]
- Shavlakadze T, Grounds MD. Therapeutic interventions for age-related muscle wasting: importance of innervation and exercise for preventing sarcopenia. In: Rattan S, editor. Modulating aging and longevity. Kluwer Academic publisher; The Netherlands: 2003. pp. 139–166. [Google Scholar]
- Shavlakadze T, White J, Hoh JF, Rosenthal N, Grounds MD. Targeted expression of insulin-like growth factor-1 reduces the necrosis of dystrophic mdx muscle. Mol Therapy. 2004;10:829–843. doi: 10.1016/j.ymthe.2004.07.026. [DOI] [PubMed] [Google Scholar]
- Shimatsu Y, Katagiri K, Furuta T, Nakura M, Tanioka Y, Yuasa K, Tomohiro M, Kornegay JN, Nonaka I, Takeda S. Canine X-linked muscular dystrophy in Japan (CXMDJ) Exp Anim. 2003;52:93–7. doi: 10.1538/expanim.52.93. [DOI] [PubMed] [Google Scholar]
- Smith JP, Hicks PS, Ortiz LR, Martinez MJ, Mandler RN. Quantitative measurement of muscle strength in the mouse. J Neurosci Methods. 1995;62:15–19. doi: 10.1016/0165-0270(95)00049-6. [DOI] [PubMed] [Google Scholar]
- Spencer MJ, Montecino-Rodriguez E, Dorshkind K, Tidball JG. Helper (CD4+) and cytotoxic (CD8+) T cells promote the pathology of dystrophin-deficient muscle. Clin Immunol. 2001;98:235–243. doi: 10.1006/clim.2000.4966. [DOI] [PubMed] [Google Scholar]
- Stauffer BL, Konhilas JP, Luczak ED, Leinwand LA. Soy diet worsens heart disease in mice. The Journal of Clinical Investigation. 2006;116:209–216. doi: 10.1172/JCI24676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedman HH, Sweeny HL, Shrager JB, Maguire HC, Panettieri RA, Petrof B, Narusawa M, Leferovich JM, Sladky JT, Kelly AM. The MDX mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature. 1991;352:536–538. doi: 10.1038/352536a0. [DOI] [PubMed] [Google Scholar]
- Straub V, Rafael JA, Chamberlain JS, Campbell KP. Animal models for muscular dystrophy show different patterns of sarcolemmal disruption. J Cell Biol. 1997;139:375–385. doi: 10.1083/jcb.139.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupka N, Lowther S, Chorneyko K, Bourgeois JM, Hogben C, Tarnopolsky MA. Gender differences in muscle inflammation after eccentric exercise. J Appl Physiol. 2000;89:2325–32. doi: 10.1152/jappl.2000.89.6.2325. [DOI] [PubMed] [Google Scholar]
- Stupka N, Plant DR, Schertzer JD, Emerson T, Bassel-Duby R, Olson E, Lynch G. Activated calcineurin ameliorates contraction-induced injury in mdx dystrophic mice. J Physiol (Lond) 2006;575:645–56. doi: 10.1113/jphysiol.2006.108472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupka N, Tarnopolsky MA, Yardley NJ, Phillips SM. Cellular adaptation to repeated eccentric exercise-induced muscle damage. J Appl Physiol. 2001;91:1669–78. doi: 10.1152/jappl.2001.91.4.1669. [DOI] [PubMed] [Google Scholar]
- Suchyna TM, Sachs F. Mechanosensitive channel properties and membrane mechanics in mouse dystrophic myotubes. The Journal of Physiology. 2007;15:369–87. doi: 10.1113/jphysiol.2006.125021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaud JL, Monnet A, Bertoldi D, Barthelemy I, Blot S, Carlier PG. Characterization of dystrophic muscle in golden retriever muscular dystrophy dogs by nuclear magnetic resonance imaging. Neuromuscul Disord. 2007;17:575–84. doi: 10.1016/j.nmd.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Tidball JG, Wehling-Henricks M. Evolving therapeutic strategies for Duchenne muscular dystrophy: targeting downstream events. Pediatr Res. 2004;56:831–41. doi: 10.1203/01.PDR.0000145578.01985.D0. [DOI] [PubMed] [Google Scholar]
- Tidball JG, Wehling-Henricks M. Damage and inflammation in muscular dystrophy: potential implications and relationships with autoimmune myositis. Current Opinion in Rheumatology. 2005;17:707–713. doi: 10.1097/01.bor.0000179948.65895.1a. [DOI] [PubMed] [Google Scholar]
- Torres LBF, Duchen LW. The mutant mdx: Inherited myopathy in the mouse. Brain. 1987;110:269–299. doi: 10.1093/brain/110.2.269. [DOI] [PubMed] [Google Scholar]
- Tuli JS, Smith JA, Morton DB. Stress measurements in mice after transportation. Lab Anim. 1995;29:132–8. doi: 10.1258/002367795780740249. [DOI] [PubMed] [Google Scholar]
- Turk R, Sterrenburg E, van der Wees CG, de Meijer EJ, de Menezes RX, Groh S, Campbell KP, Noguchi S, van Ommen GJ, den Dunnen JT, t Hoen PA. Common pathological mechanisms in mouse models for muscular dystrophies. FASEB J. 2006;20:127–9. doi: 10.1096/fj.05-4678fje. [DOI] [PubMed] [Google Scholar]
- Van Loo PL, Van der Meer E, Kruitwagen CL, Koolhaas JM, Van Zutphen LF, Baumans V. Long-term effects of husbandry procedures on stress-related parameters in male mice of two strains. Lab Anim. 2004;38:169–77. doi: 10.1258/002367704322968858. [DOI] [PubMed] [Google Scholar]
- Vandebrouck C, Martin D, Colson-Van Schoor M, Debaix H, Gailly P. Involvement of TRPC in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle fibers. J Cell Biol. 2002;158:1089–96. doi: 10.1083/jcb.200203091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbergh JG. Prenatal hormone exposure and sexual variation. American Scientist. 2003;91:218–225. [Google Scholar]
- Vandenbergh JG. Animal models and studies of in utero endocrine disruptor effects. Ilar J. 2004;45:438–42. doi: 10.1093/ilar.45.4.438. [DOI] [PubMed] [Google Scholar]
- Verthelyi D. Female’s heightened immune status: estrogen, T cells, and inducible nitric oxide synthase in the balance. Endocrinology. 2006;147:659–61. doi: 10.1210/en.2005-1469. [DOI] [PubMed] [Google Scholar]
- Vilquin JT, Brussee V, Asselin I, Kinoshita I, Gingras M, Tremblay JP. Evidence of mdx mouse skeletal muscle fragility in vivo by eccentric running exercise. Muscle Nerve. 1998;21:567–76. doi: 10.1002/(sici)1097-4598(199805)21:5<567::aid-mus2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Walter G, Cordier L, Bloy D, Sweeney HL. Noninvasive monitoring of gene correction in dystrophic muscle. Magn Reson Med. 2005;54:1369–76. doi: 10.1002/mrm.20721. [DOI] [PubMed] [Google Scholar]
- Wang LC, Kernell D. Fibre type regionalisation in lower hindlimb muscles of rabbit, rat and mouse: a comparative study. J Anat. 2001;199:631–43. doi: 10.1046/j.1469-7580.2001.19960631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PY, Koishi K, McGeachie AB, Kimber M, Maclaughlin DT, Donahoe PK, McLennan IS. Mullerian inhibiting substance acts as a motor neuron survival factor in vitro. Proc Natl Acad Sci U S A. 2005;102:16421–5. doi: 10.1073/pnas.0508304102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–54. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Wehling M, Spencer MJ, Tidball JG. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. Journal of Cell Biology. 2001;155:123–31. doi: 10.1083/jcb.200105110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch EM, Barton ER, Zhuo J, Tomizawa Y, Friesen WJ, Trifillis P, Paushkin S, Patel M, Trotta CR, Hwang S, Wilde RG, Karp G, Takasugi J, Chen G, Jones S, Ren H, Moon YC, Corson D, Turpoff AA, Campbell JA, Conn MM, Khan A, Almstead NG, Hedrick J, Mollin A, Risher N, Weetall M, Yeh S, Branstrom AA, Colacino JM, Babiak J, Ju WD, Hirawat S, Northcutt VJ, Miller LL, Spatrick P, He F, Kawana M, Feng H, Jacobson A, Peltz SW, Sweeney HL. PTC124 targets genetic disorders caused by nonsense mutations. Nature. 2007;447:87–91. doi: 10.1038/nature05756. [DOI] [PubMed] [Google Scholar]
- Wenger SL, Steele MW, Hoffman EP, Barmada MA, Wessel HB. X inactivation and dystrophin studies in a t(X;12) female: evidence for biochemical normalization in Duchenne muscular dystrophy carriers. Am J Med Genet. 1992;43:1012–5. doi: 10.1002/ajmg.1320430619. [DOI] [PubMed] [Google Scholar]
- Whitehead NP, Streamer M, Lusambili LI, Sachs F, Allen DG. Streptomycin reduces stretch-induced membrane permeability in muscles from mdx mice. Neuromuscul Disord. 2006a;16:845–54. doi: 10.1016/j.nmd.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Whitehead NP, Yeung EW, Allen DG. Muscle damage in mdx (dystrophic) mice: role of calcium and reactive oxygen species. Clin Exp Pharmacol Physiol. 2006b;33:657–662. doi: 10.1111/j.1440-1681.2006.04394.x. [DOI] [PubMed] [Google Scholar]
- Wiesen MH, Bogdanovich S, Agarkova I, Perriard JC, Khurana TS. Identification and characterization of layer-specific differences in extraocular muscle m-bands. Invest Ophthalmol Vis Sci. 2007;48:1119–27. doi: 10.1167/iovs.06-0701. [DOI] [PubMed] [Google Scholar]
- Williams DA, Head SI, Lynch GS, Stephenson DG. Contractile properties of skinned muscle fibres from young and adult normal and dystrophic (mdx) mice. J Physiol. 1993;460:51–67. doi: 10.1113/jphysiol.1993.sp019458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurbel H. Ideal homes? Housing effects on rodent brain and behaviour. Trends Neurosci. 2001;24:207–11. doi: 10.1016/s0166-2236(00)01718-5. [DOI] [PubMed] [Google Scholar]
- Wyrwoll CS, Mark PJ, Mori TA, Puddey IB, Waddell BJ. Prevention of programmed hyperleptinemia and hypertension by postnatal dietary omega-3 fatty acids. Endocrinology. 2006;147:599–606. doi: 10.1210/en.2005-0748. [DOI] [PubMed] [Google Scholar]
- Yeung EW, Head SI, Allen DG. Gadolinium reduces short-term stretch-induced muscle damage in isolated mdx mouse muscle fibres. J Physiol. 2003;552:449–58. doi: 10.1113/jphysiol.2003.047373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung EW, Whitehead NP, Suchyna TM, Gottlieb PA, Sachs F, Allen DG. Effects of stretch-activated channel blockers on [Ca2+]i and muscle damage in the mdx mouse. J Physiol. 2005;562:367–80. doi: 10.1113/jphysiol.2004.075275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T, Lu QL, Morgan JE, Davies KE, Fisher R, Takeda S, Partridge TA. Expansion of revertant fibers in dystrophic mdx muscles reflects activity of muscle precursor cells and serves as an index of muscle regeneration. J Cell Sci. 2006;119:2679–87. doi: 10.1242/jcs.03000. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Yonetani A, Shirasaki T, Wada K. Dietary NaCl supplementation prevents muscle necrosis in a mouse model of Duchenne muscular dystrophy. Am J Physiol Regul Integr Comp Physiol. 2006;290:R449–55. doi: 10.1152/ajpregu.00684.2004. [DOI] [PubMed] [Google Scholar]
- Yugeta N, Urasawa N, Fujii Y, Yoshimura M, Yuasa K, Wada MR, Nakura M, Shimatsu Y, Tomohiro M, Takahashi A, Machida N, Wakao Y, Nakamura A, Takeda S. Cardiac involvement in Beagle-based canine X-linked muscular dystrophy in Japan (CXMDJ): electrocardiographic, echocardiographic, and morphologic studies. BMC Cardiovasc Disord. 2006;6:47. doi: 10.1186/1471-2261-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatz M, Rapaport D, Vainzof M, Passos-Bueno MR, Bortolini ER, Pavanello Rde C, Peres CA. Serum creatine-kinase (CK) and pyruvate-kinase (PK) activities in Duchenne (DMD) as compared with Becker (BMD) muscular dystrophy. J Neurol Sci. 1991;102:190–6. doi: 10.1016/0022-510x(91)90068-i. [DOI] [PubMed] [Google Scholar]