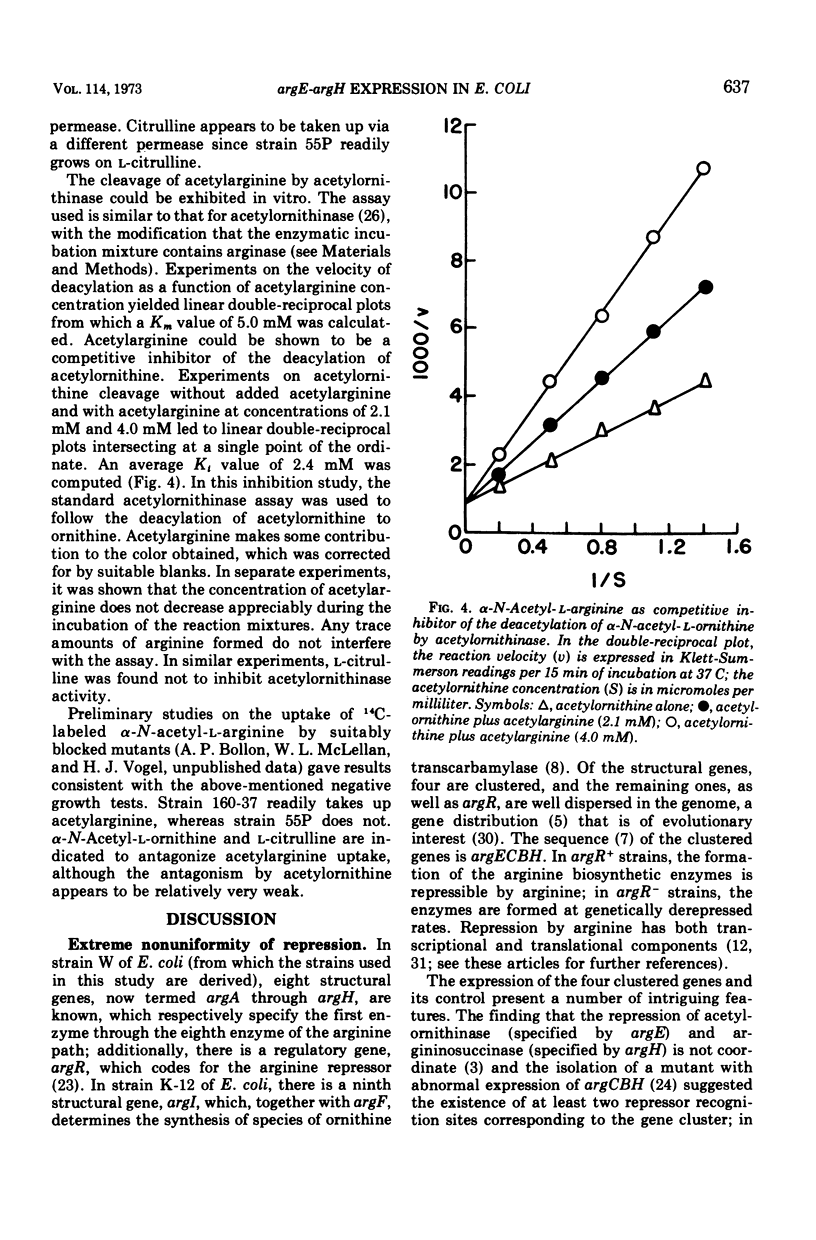
Abstract
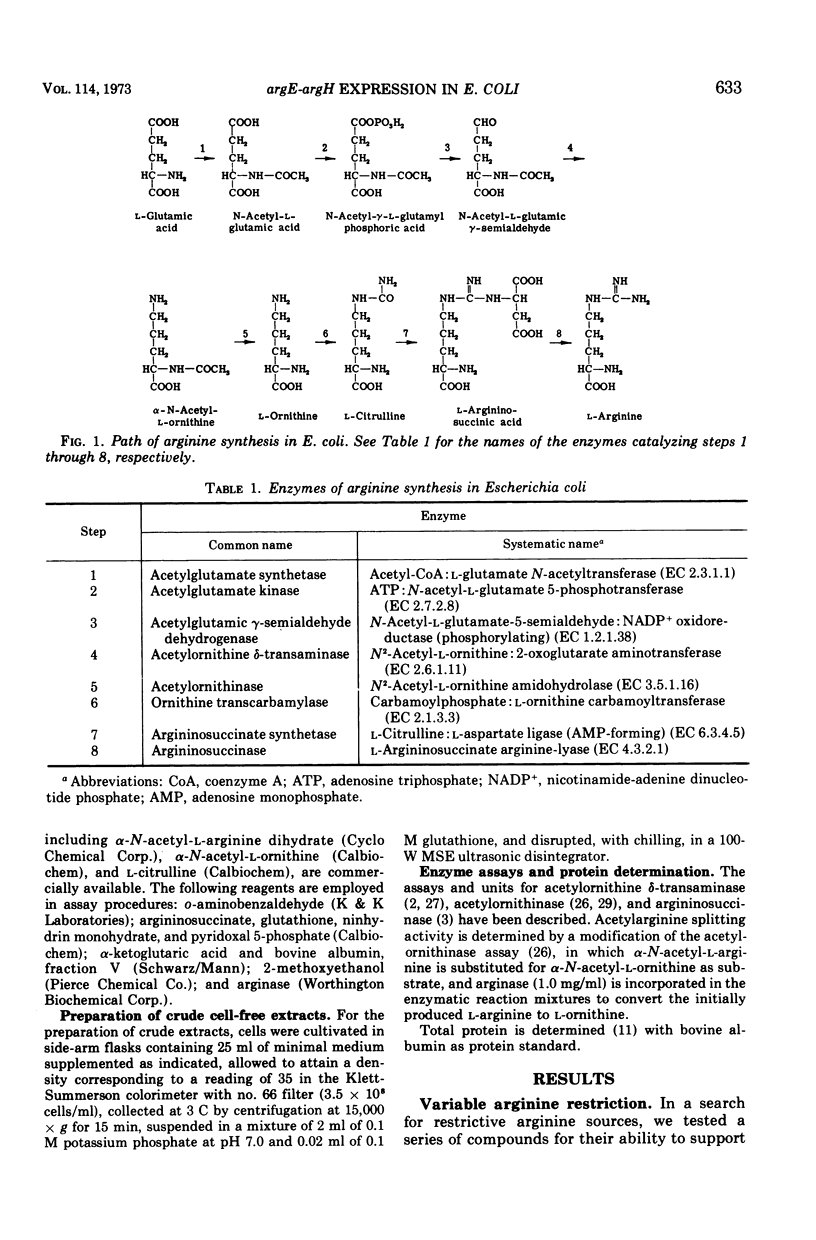
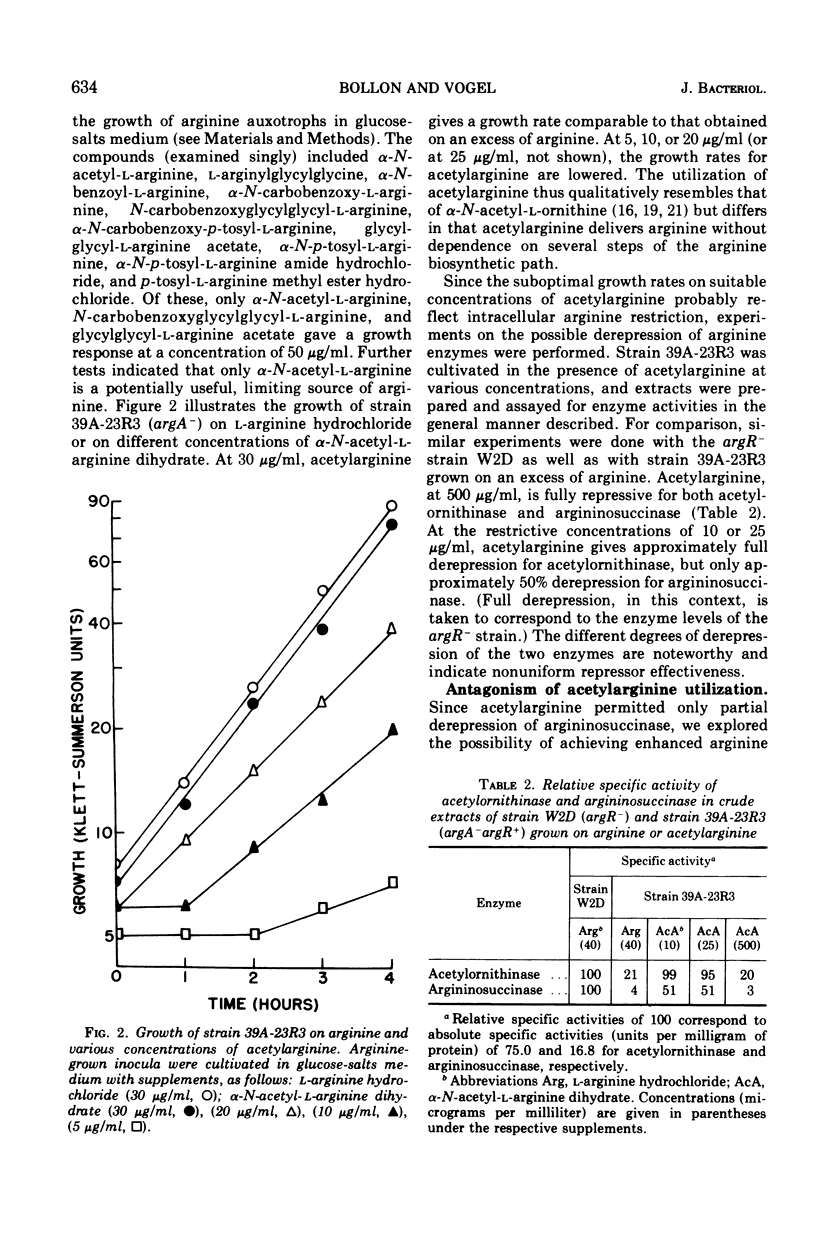
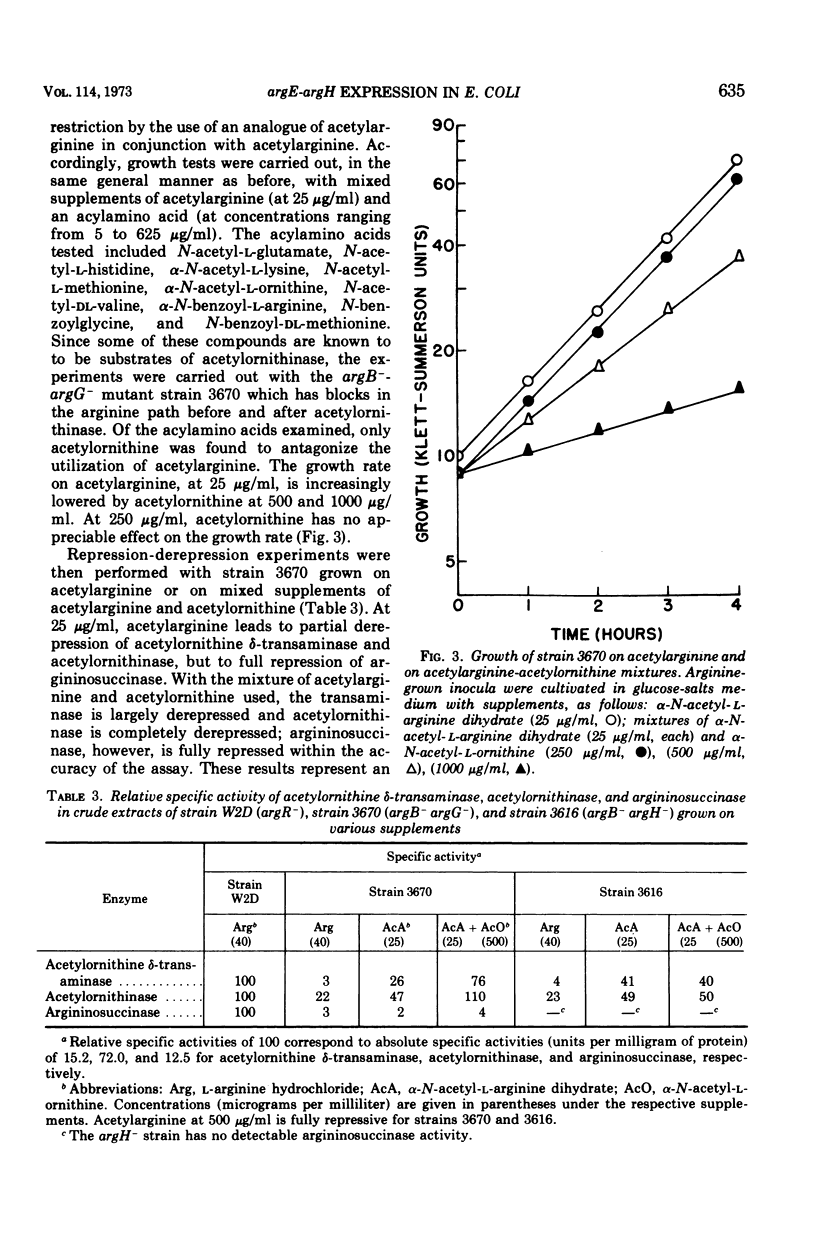
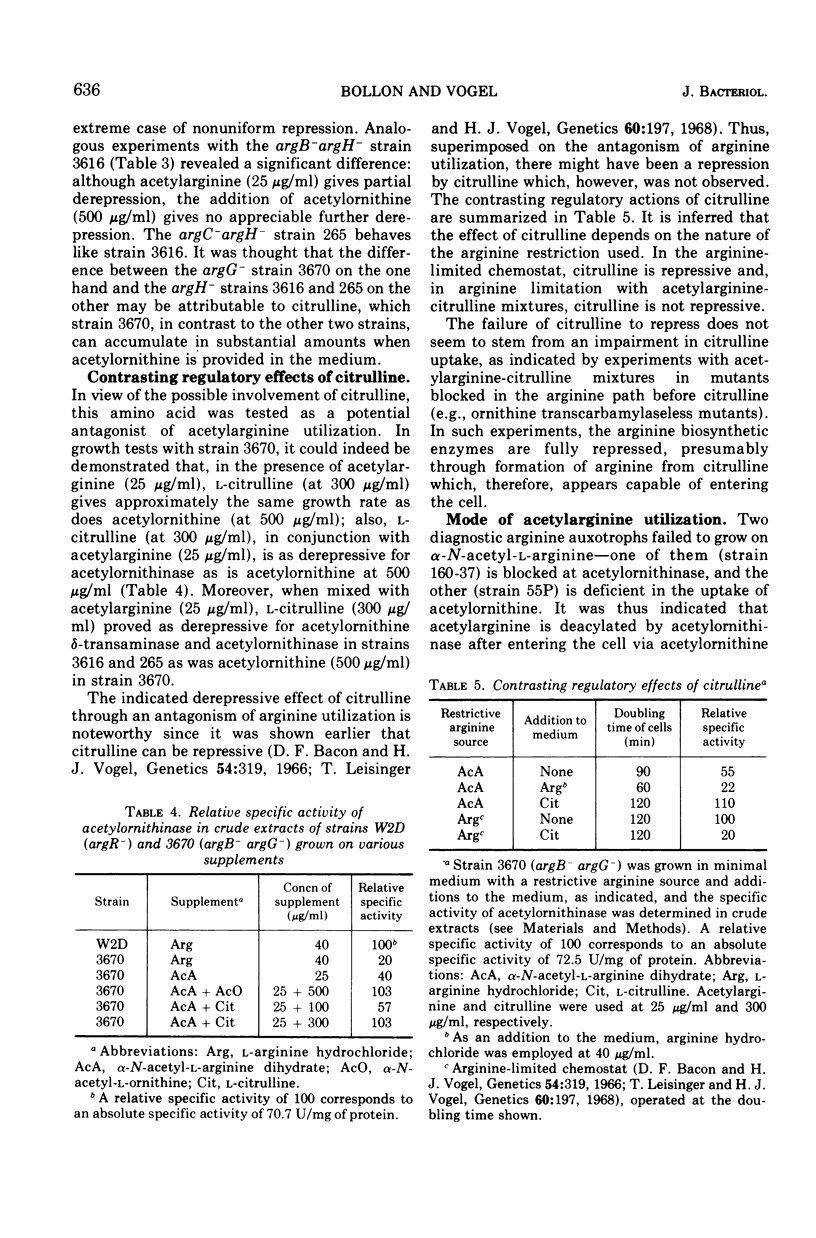
In regulatory studies of the arginine biosynthetic system of Escherichia coli, α-N-acetyl-l-arginine (AcA) is a useful restrictive arginine source. In strain 39A-23R3 (argA−), at 25 μg/ml, AcA gives suboptimal growth rates and is fully derepressive for acetylornithinase (specified by argE) and approximately 50% derepressive for argininosuccinase (specified by argH). At 10 μg/ml, the growth rate decreases, whereas the extent of derepression is unchanged; at 500 μg/ml, full repression results. In strain 3670 (argB−argG−), AcA (25 μg/ml) leads to partial derepression of acetylornithinase but full repression of argininosuccinase. Thus, the repression patterns for both strains, although not identical, are nonuniform. AcA utilization is antagonized by α-N-acetyl-l-ornithine (AcO). In strain 3670 (blocked before and after acetylornithinase), the growth rate on AcA (25 μg/ml) is lowered by AcO (500 μg/ml); acetylornithinase is completely derepressed, whereas argininosuccinase is fully repressed. This difference in regulatory behavior represents extreme nonuniform repression. Unexpectedly, the effect of AcO is attributable to the conversion of AcO to citrulline (Cit). In strain 3670, mixtures of AcA (25 μg/ml) and Cit (300 μg/ml) permit complete derepression of acetylornithinase; there is evidence that Cit enters the cell. In contrast, in the arginine-limited chemostat, Cit represses acetylornithinase. These opposite regulatory effects of Cit appear to stem from the difference in arginine restriction. AcA enters the cell via AcO permease and is deacylated by acetylornithinase (Km, 5.0 mM). AcA competitively inhibits AcO cleavage (Ki, 2.4 mM), but Cit is not inhibitory. The antagonism of AcA utilization by AcO or Cit is thought to be exerted at the AcO permease.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABELSON P. H., VOGEL H. J. Amino acid biosynthesis in Torulopsis utilis and Neurospora crassa. J Biol Chem. 1955 Mar;213(1):355–364. [PubMed] [Google Scholar]
- ALBRECHT A. M., VOGEL H. J. ACETYLORNITHINE DELTA-TRANSAMINASE. PARTIAL PURIFICATION AND REPRESSION BEHAVIOR. J Biol Chem. 1964 Jun;239:1872–1876. [PubMed] [Google Scholar]
- Baumberg S., Bacon D. F., Vogel H. J. Individually repressible enzymes specified by clustered genes of arginine synthesis. Proc Natl Acad Sci U S A. 1965 May;53(5):1029–1032. doi: 10.1073/pnas.53.5.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elseviers D., Cunin R., Glansdorff N. Control regions within the argECBH gene cluster of Escherichia coli K12. Mol Gen Genet. 1972;117(4):349–366. doi: 10.1007/BF00333028. [DOI] [PubMed] [Google Scholar]
- GLANSDORFF N. TOPOGRAPHY OF COTRANSDUCIBLE ARGININE MUTATIONS IN ESCHERICHIA COLI K-12. Genetics. 1965 Feb;51:167–179. doi: 10.1093/genetics/51.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glansdorff N., Sand G., Verhoef C. The dual genetic control of ornithine transcarbamylase synthesis in Escherichia coli K12. Mutat Res. 1967 Nov-Dec;4(6):743–751. doi: 10.1016/0027-5107(67)90083-8. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A. Control of the argECBH cluster in Escherichia coli. Mol Gen Genet. 1972;117(4):337–348. doi: 10.1007/BF00333027. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A., Gorini L. A unitary account of the repression mechanism of arginine biosynthesis in Escherichia coli. I. The genetic evidence. J Mol Biol. 1969 Jan 14;39(1):73–87. doi: 10.1016/0022-2836(69)90334-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McLellan W. L., Vogel H. J. Translational and transcriptional components of repression by arginine in Escherichia coli. Biochem Biophys Res Commun. 1972 Sep 5;48(5):1027–1033. doi: 10.1016/0006-291x(72)90811-x. [DOI] [PubMed] [Google Scholar]
- SERCARZ E. E., GORINI L. DIFFERENT CONTRIBUTION OF EXOGENOUS AND ENDOGENOUS ARGININE TO REPRESSOR FORMATION. J Mol Biol. 1964 Feb;8:254–262. doi: 10.1016/s0022-2836(64)80135-2. [DOI] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger L., Bacon D. F., Vogel H. J. Enzyme repressibility and repressor effectiveness in phenotypic revertants of arginine auxotrophs. Genetics. 1969 Sep;63(1):53–61. doi: 10.1093/genetics/63.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J. A "pace-setting" phenomenon in derepressed enzyme formation. Biochem Biophys Res Commun. 1960 Oct;3:373–376. doi: 10.1016/0006-291x(60)90047-4. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Vogel H. J., Bacon D. F. Gene aggregation: evidence for a coming together of functionally related, not closely linked genes. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1456–1459. doi: 10.1073/pnas.55.6.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel H. J., Bonner D. M. ON THE GLUTAMATE-PROLINE-ORNITHINE INTERRELATION IN NEUROSPORA CRASSA. Proc Natl Acad Sci U S A. 1954 Aug;40(8):688–694. doi: 10.1073/pnas.40.8.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel H. J. Path of Ornithine Synthesis in Escherichia Coli. Proc Natl Acad Sci U S A. 1953 Jul;39(7):578–583. doi: 10.1073/pnas.39.7.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel H. J. REPRESSION OF AN ACETYLORNITHINE PERMEATION SYSTEM. Proc Natl Acad Sci U S A. 1960 Apr;46(4):488–494. doi: 10.1073/pnas.46.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel R. H., Knight G. J., Vogel H. J. Altered translational or transcriptional components of repression in argR mutants of Escherichia coli: evidence from a translation retarder. Biochem Biophys Res Commun. 1972 Sep 5;48(5):1034–1040. doi: 10.1016/0006-291x(72)90812-1. [DOI] [PubMed] [Google Scholar]


